Flexible Cu3(HHTP)2 MOF Membranes for Gas Sensing Application at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu3(HHTP)2 MOF
2.3. Fabrication of the Cu3(HHTP)2–MOF/PVA/IL Membranes
2.4. Characterization
2.5. Sensor Fabrication and H2S Gas Sensing Tests
3. Results and Discussion
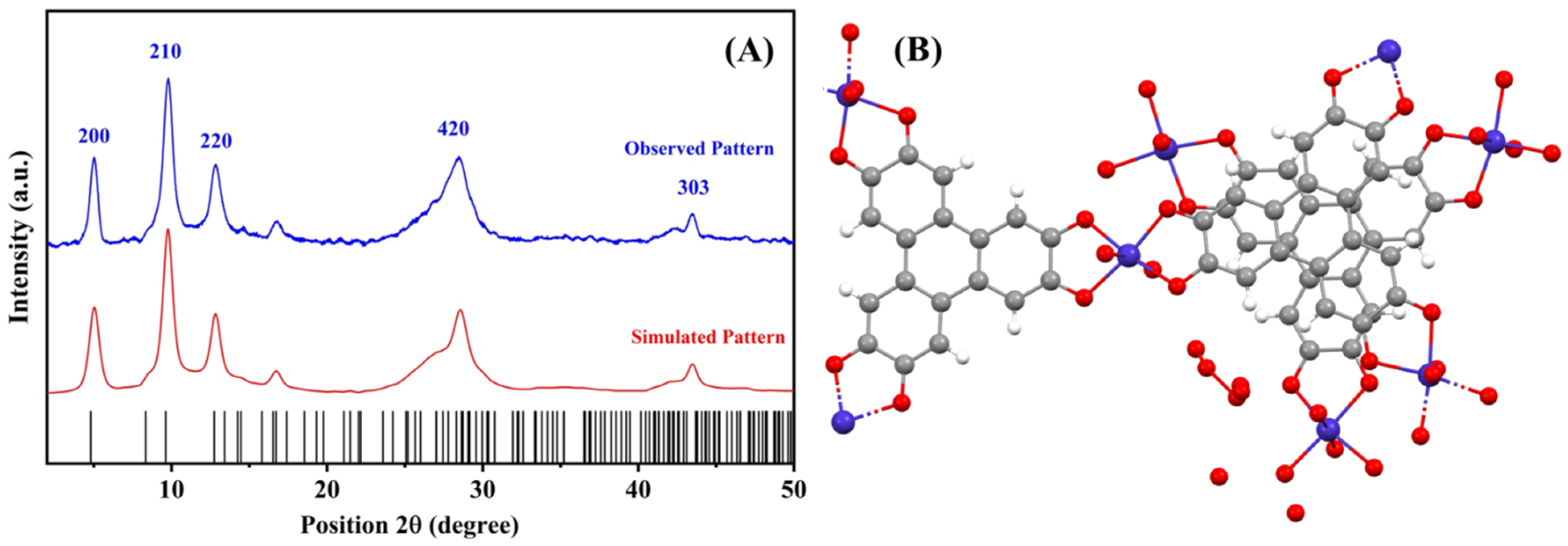
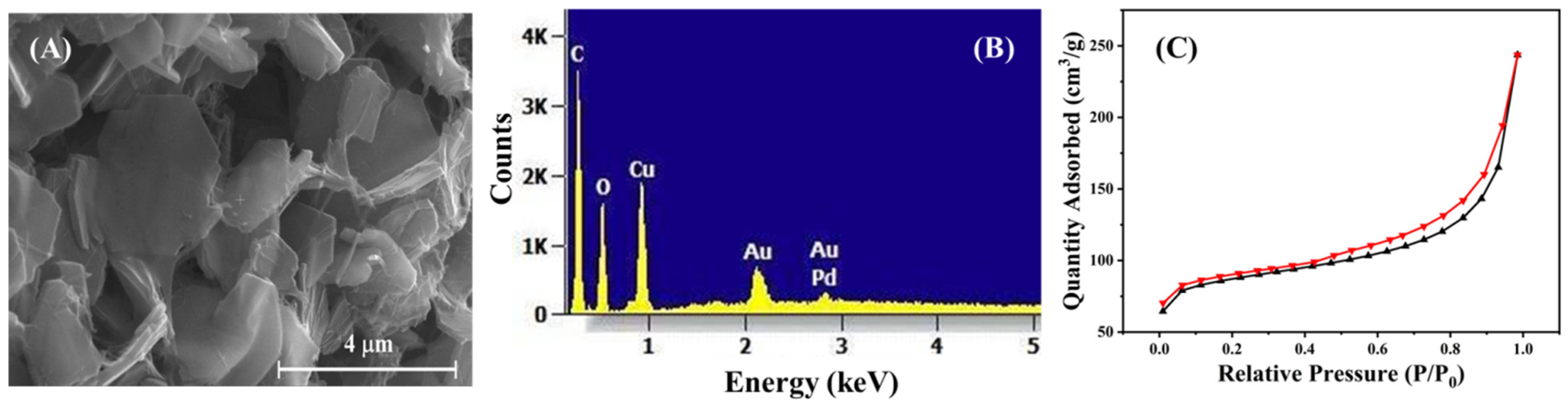
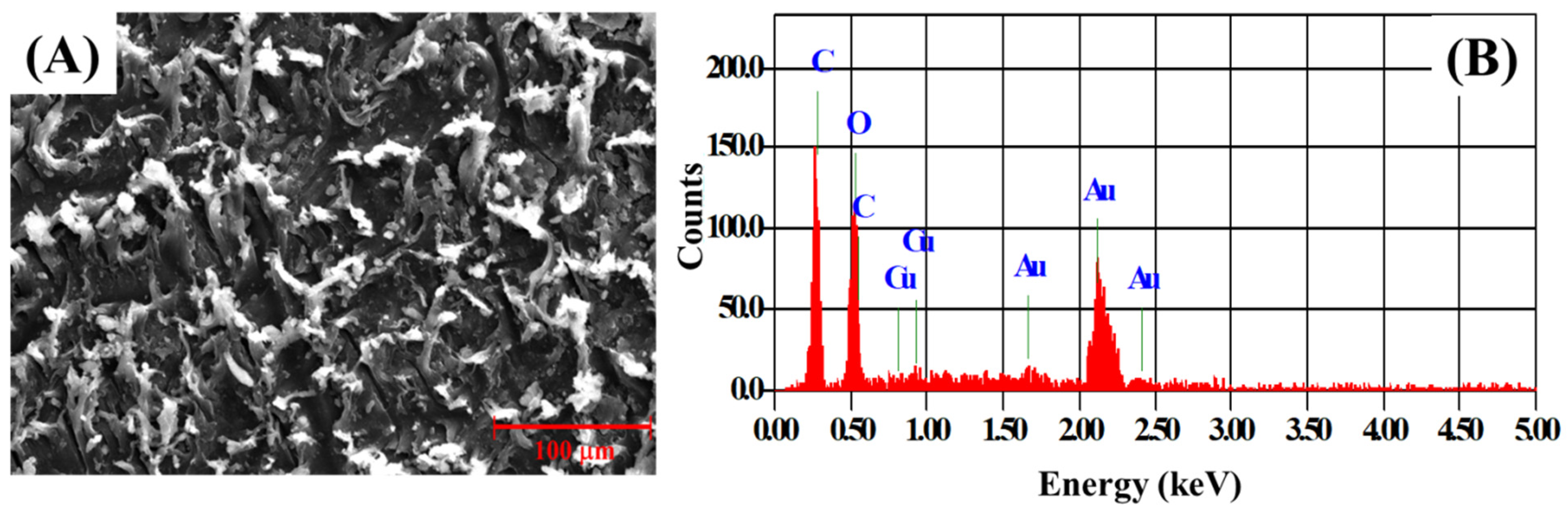
3.1. Structural and Morphological Characterization of Cu-MOF and Cu-MOF/PVA/IL Membrane
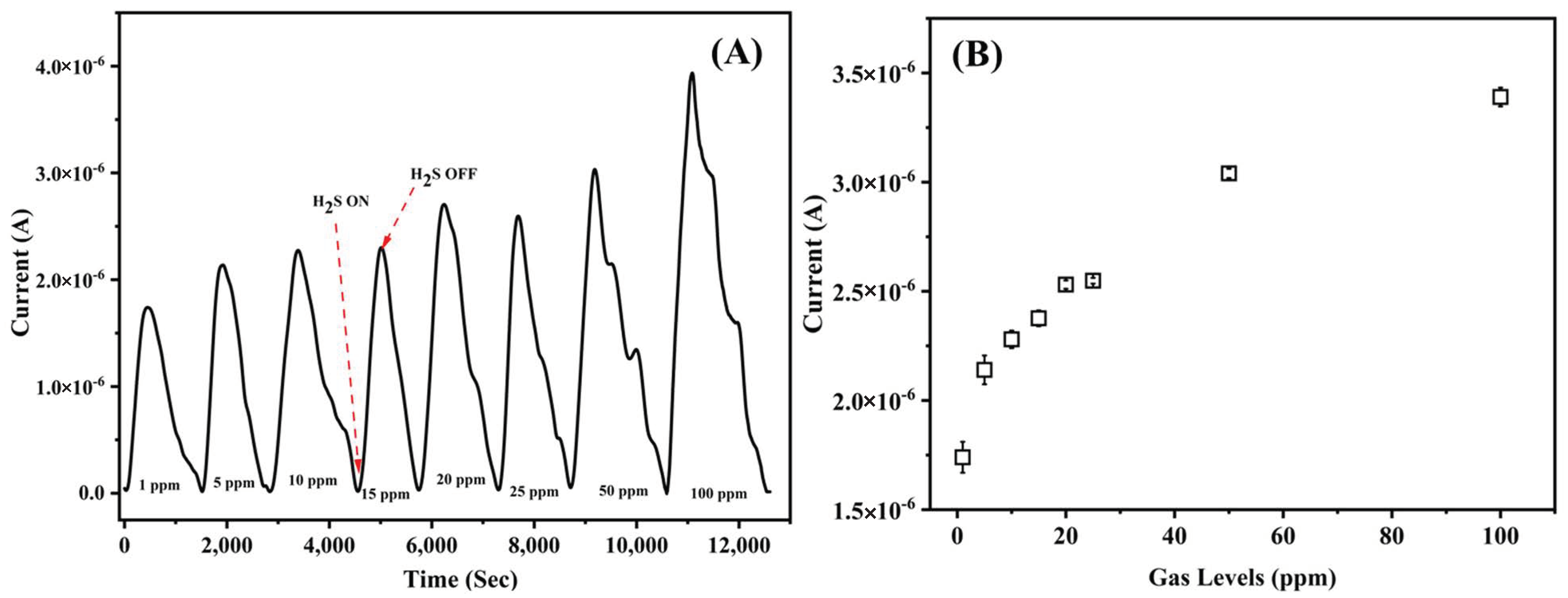
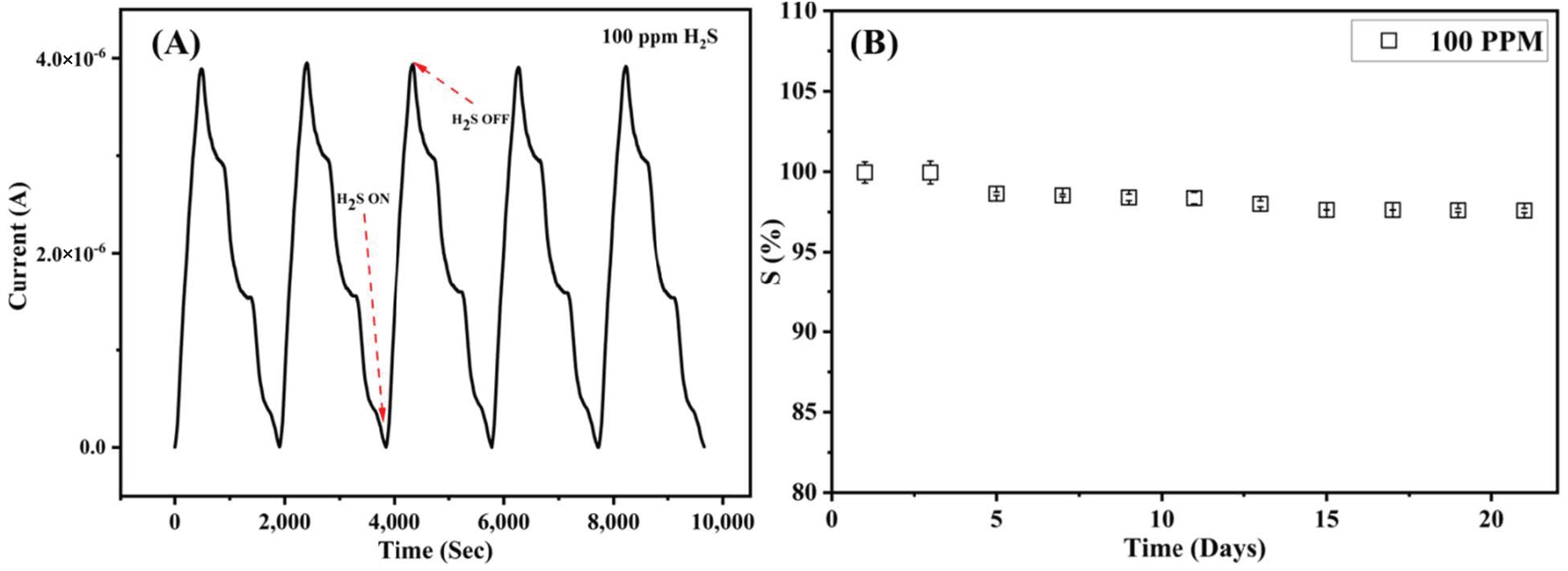
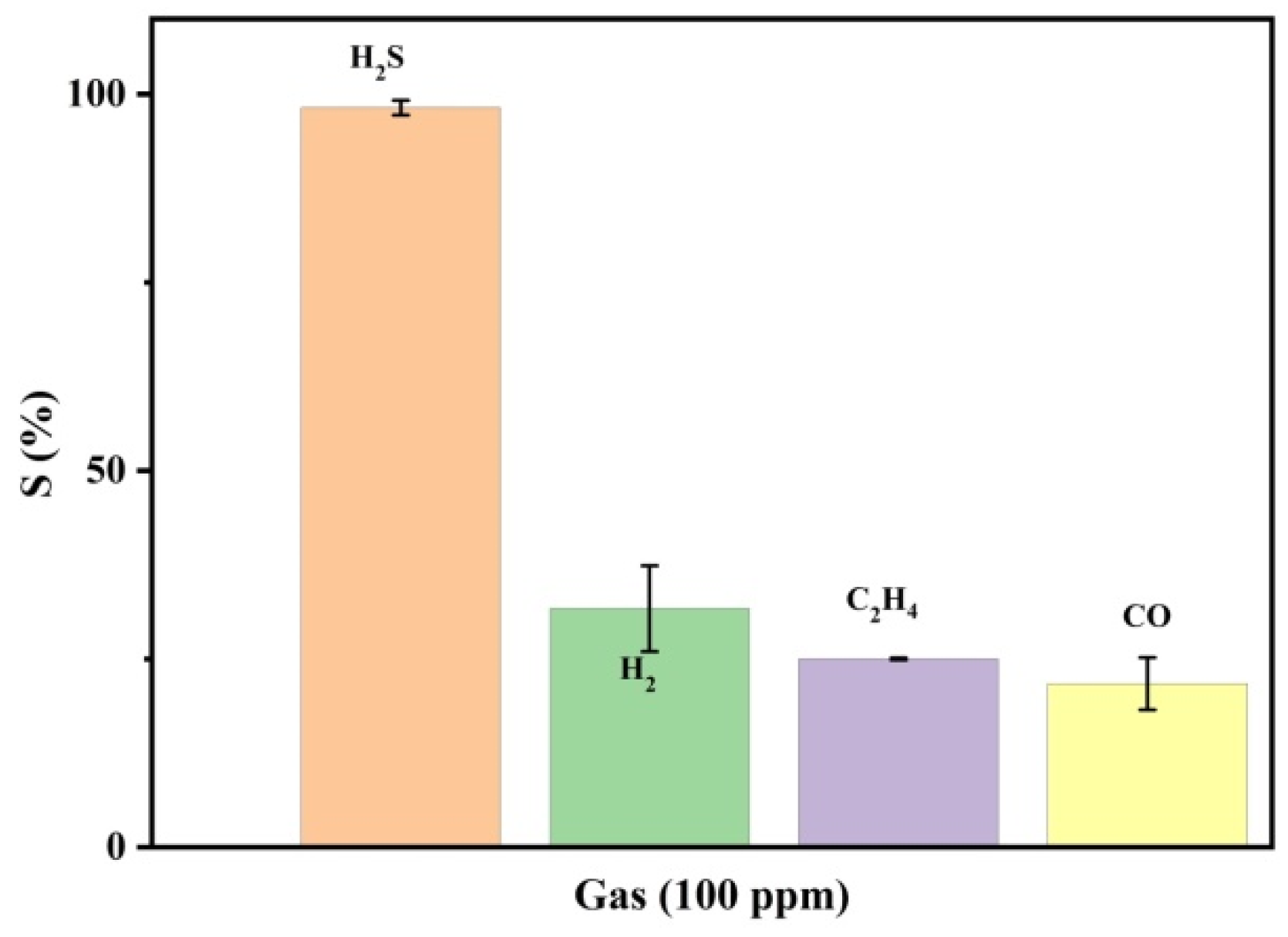
3.2. Gas Sensing Performance
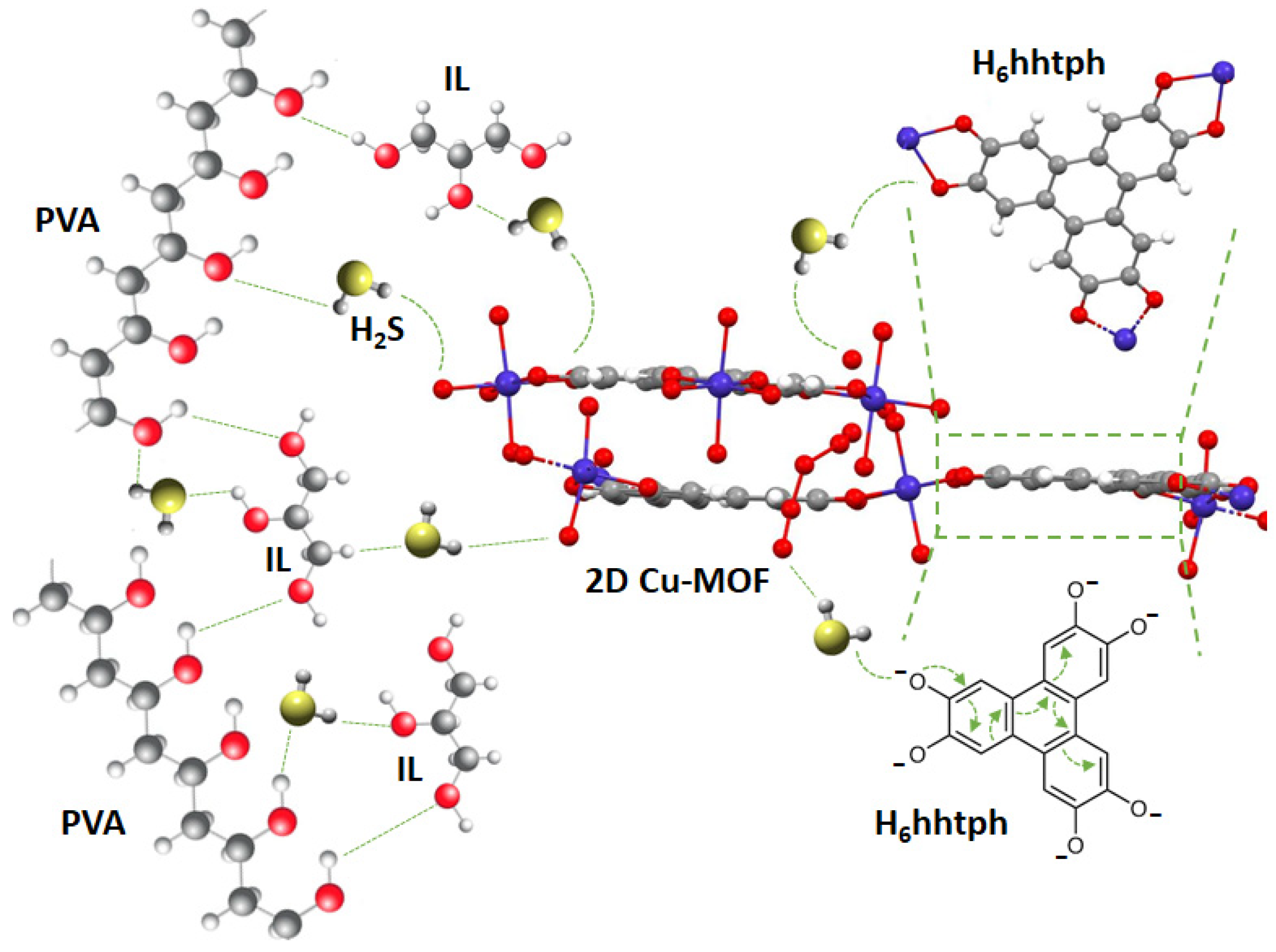
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Liu, D.; Lin, W. Metal-organic Frameworks as A Tunable Platform for Designing Functional Molecular Materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Yin, L.; Low, N.; Ji, D.; Liu, Y.; Yao, J.; Zhong, Z.; Xing, W. Zeolitic-imidazolate-framework filled hierarchical porous nanofiber membrane for air cleaning. J. Membr. Sci. 2020, 594, 117467. [Google Scholar] [CrossRef]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Petit, C.; Mendoza, B.; Bandosz, T.J. Hydrogen Sulfide Adsorption on MOFs and MOF/Graphite Oxide Composites. ChemPhysChem 2010, 11, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Townsend, T.; Bitton, G. Inhibition of hydrogen sulfide generation from disposed gypsum drywall using chemical inhibitors. J. Hazard. Mater. 2011, 191, 204–211. [Google Scholar] [CrossRef]
- Xu, Q.; Townsend, T. Factors affecting temporal H2S emission at construction and demolition (C&D) debris landfills. Chemosphere 2014, 96, 105–111. [Google Scholar] [CrossRef]
- Panza, D.; Belgiorno, V. Hydrogen sulphide removal from landfill gas. Process Saf. Environ. Prot. 2010, 88, 420–424. [Google Scholar] [CrossRef]
- Eun, S.; Reinhart, D.R.; Cooper, C.D.; Townsend, T.G.; Faour, A. Hydrogen sulfide flux measurements from construction and demolition debris (C&D) landfills. Waste Manag. 2007, 27, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Sun, Z.; Zhu, S.; Lou, Z.; Zhu, N.; Feng, L. The identification and health risk assessment of odor emissions from waste landfilling and composting. Sci. Total Environ. 2019, 649, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.; Schwartz, J.; Spengler, J.D. Air pollution and daily mortality: Associations with particulates and acid aerosols. Environ. Res. 1992, 59, 362–373. [Google Scholar] [CrossRef]
- Doujaiji, B.; Al-Tawfiq, J.A. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med. 2010, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Koe, L.C.C.; Tan, N.C. Field Performance of Activated Carbon Adsorption for Sewage Air. J. Environ. Eng. 1990, 116, 721–734. [Google Scholar] [CrossRef]
- Chen, X.; Behboodian, R.; Bagnall, D.; Taheri, M.; Nasiri, N. Metal-Organic-Frameworks: Low Temperature Gas Sensing and Air Quality Monitoring. Chemosensors 2021, 9, 316. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical Sensors Based on Metal-Organic Frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [Green Version]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Pohle, R.; Tawil, A.; Davydovskaya, P.; Fleischer, M. Metal Organic Frameworks as Promising High Surface Area Material for Work Function Gas Sensors. Procedia Eng. 2011, 25, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Zheng, J.; Wu, A.; Xu, G.; Nagarkar, S.S.; Zhang, G.; Tsujimoto, M.; Sakaki, S.; Horike, S.; Otake, K.-I.; et al. A Dual-Ligand Porous Coordination Polymer Chemiresistor with Modulated Conductivity and Porosity. Angew. Chem. Int. Ed. 2020, 59, 172–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Kim, I.-D. Bimetallic Nanoparticles-Stabilized Conductive Metal-Organic Frameworks for Superior Chemiresistors. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2020; p. 2431. [Google Scholar]
- Hoppe, B.; Hindricks, K.D.J.; Warwas, D.P.; Schulze, H.A.; Mohmeyer, A.; Pinkvos, T.J.; Zailskas, S.; Krey, M.R.; Belke, C.; König, S.; et al. Graphene-like metal-organic frameworks: Morphology control, optimization of thin film electrical conductivity and fast sensing applications. CrystEngComm 2018, 20, 6458–6471. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3(hexaiminotriphenylene)2: An electrically conductive 2D metal-organic framework for chemiresistive sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Liu, S.F.; Swager, T.M.; Dincă, M. Chemiresistive sensor arrays from conductive 2D metal-organic frameworks. J. Am. Chem. Soc. 2015, 137, 13780–13783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.K.; Jensen, K.E.; Pivak, P.A.; Mirica, K.A. Direct Self-Assembly of Conductive Nanorods of Metal–Organic Frameworks into Chemiresistive Devices on Shrinkable Polymer Films. Chem. Mater. 2016, 28, 5264–5268. [Google Scholar] [CrossRef]
- Yao, M.-S.; Lv, X.-J.; Fu, Z.-H.; Li, W.-H.; Deng, W.-H.; Wu, G.-D.; Xu, G. Layer-by-Layer Assembled Conductive Metal-Organic Framework Nanofilms for Room-Temperature Chemiresistive Sensing. Angew. Chem. 2017, 129, 16737–16741. [Google Scholar] [CrossRef]
- Arul, C.; Moulaee, K.; Donato, N.; Iannazzo, D.; Lavanya, N.; Neri, G.; Sekar, C. Temperature modulated Cu-MOF based gas sensor with dual selectivity to acetone and NO2 at low operating temperatures. Sens. Actuators B Chem. 2021, 329, 129053. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Xue, Q.; Wen, Y.; Hong, X.; Ullah, K. TiO2/Cu-MOF/PPy composite as a novel photocatalyst for decomposition of organic dyes. J. Mater. Sci. Mater. Electron. 2021, 32, 4097–4109. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, T.; Wang, W.; Bi, S.; Jiang, M.; Zhang, K.Y.; Liu, S.; Huang, W.; Zhao, Q. Layer-by-Layer 2D Ultrathin Conductive Cu3(HHTP)2 Film for High-Performance Flexible Transparent Supercapacitors. Adv. Mater. Interfaces 2021, 8, 2100308. [Google Scholar] [CrossRef]
- Chen, E.-X.; Yang, H.; Zhang, J. Zeolitic Imidazolate Framework as Formaldehyde Gas Sensor. Inorg. Chem. 2014, 53, 5411–5413. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Abu-Hani, A.F.; Mahmoud, S.T.; Haik, Y. Selective H2S sensor based on CuO nanoparticles embedded in organic membranes. Sens. Actuators B Chem. 2016, 231, 593–600. [Google Scholar] [CrossRef]
- Abu-Hani, A.F.; Awwad, F.; Greish, Y.E.; Ayesh, A.; Mahmoud, S.T. Design, fabrication, and characterization of low-power gas sensors based on organic-inorganic nano-composite. Org. Electron. 2017, 42, 284–292. [Google Scholar] [CrossRef]
- Haik, Y.; Ayesh, A.I.; Mohsin, M.A. Semiconducting Polymer. U.S. Patent US8796673B2, 5 August 2014. [Google Scholar]
- Josh, V.; Haik, M.Y.; Ayesh, A.I.; Mohsin, M.A.; Haik, Y. Electrical properties of sorbitol-doped poly(vinyl alcohol)-poly(acrylamide-co-acrylic acid) polymer membranes. J. Appl. Polym. Sci. 2013, 128, 3861–3869. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Mohsin, M.A.; Haik, M.Y.; Haik, Y. Investigations on electrical properties of poly(vinyl alcohol) doped with 1-methyl-3-n-decyl-imidazolium bromide ionic liquid. Curr. Appl. Phys. 2012, 12, 1223–1228. [Google Scholar] [CrossRef]
- Allam, M.; Ayesh, A.I.; Mohsin, M.A.; Haik, Y. Physical properties of PVA doped with algal glycerol. J. Appl. Polym. Sci. 2013, 130, 4482–4489. [Google Scholar] [CrossRef]
- Ali, A.; Alzamly, A.; E Greish, Y.; Bakiro, M.; Nguyen, H.L.; Mahmoud, S.T. A Highly Sensitive and Flexible Metal–Organic Framework Polymer-Based H2S Gas Sensor. ACS Omega 2021, 6, 17690–17697. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Park, S.S.; Dos Reis, R.; Dravid, V.P.; Kim, H.; Mirkin, C.A.; Stoddart, J.F. Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries. Nat. Commun. 2019, 10, 4948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.S.; Kumar, S.S.; Kulandainathan, M.A. Efficient electrosynthesis of highly active Cu3(BTC)2-MOF and its catalytic application to chemical reduction. Microporous Mesoporous Mater. 2013, 168, 57–64. [Google Scholar] [CrossRef]
- Rawool, C.R.; Srivastava, A.K. A dual template imprinted polymer modified electrochemical sensor based on Cu metal organic framework/mesoporous carbon for highly sensitive and selective recognition of rifampicin and isoniazid. Sens. Actuators B Chem. 2019, 288, 493–506. [Google Scholar] [CrossRef]
- Koo, W.; Kim, S.; Jang, J.-S.; Kim, D.; Kim, I. Catalytic Metal Nanoparticles Embedded in Conductive Metal-Organic Frameworks for Chemiresistors: Highly Active and Conductive Porous Materials. Adv. Sci. 2019, 6, 1900250. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sensor/Material | Optimum Operating Temperature (°C) | Gas | Detection Limit | Refs. |
|---|---|---|---|---|
| Cu3(HHTP)2/PVA/IL | 23 °C | H2S | 1 ppm | This Work |
| Cu/Ni (HHTP) | RT | H2S/NO | 40–80 ppm | [26] |
| Cu3(HHTP)2 | 250 °C | Acetone | 50 ppm | [28] |
| H2/CO | 20 ppm | |||
| CO2/NH3 | 200 ppm | |||
| CH4 | 500 ppm | |||
| NO2 | 1 ppm | |||
| PVA-Semiconducting nanoparticles (CuO, ZnFe2O4, CuFe2O4, and WO3) based sensors | 80 °C | H2S | 10 ppm | [32,33] |
| Pd and Pt NPs doped Cu3(HHTP)2 | RT | NO2 | 1 ppm | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; AlTakroori, H.H.D.; Greish, Y.E.; Alzamly, A.; Siddig, L.A.; Qamhieh, N.; Mahmoud, S.T. Flexible Cu3(HHTP)2 MOF Membranes for Gas Sensing Application at Room Temperature. Nanomaterials 2022, 12, 913. https://doi.org/10.3390/nano12060913
Ali A, AlTakroori HHD, Greish YE, Alzamly A, Siddig LA, Qamhieh N, Mahmoud ST. Flexible Cu3(HHTP)2 MOF Membranes for Gas Sensing Application at Room Temperature. Nanomaterials. 2022; 12(6):913. https://doi.org/10.3390/nano12060913
Chicago/Turabian StyleAli, Ashraf, Husam H. D. AlTakroori, Yaser E. Greish, Ahmed Alzamly, Lamia A. Siddig, Naser Qamhieh, and Saleh T. Mahmoud. 2022. "Flexible Cu3(HHTP)2 MOF Membranes for Gas Sensing Application at Room Temperature" Nanomaterials 12, no. 6: 913. https://doi.org/10.3390/nano12060913
APA StyleAli, A., AlTakroori, H. H. D., Greish, Y. E., Alzamly, A., Siddig, L. A., Qamhieh, N., & Mahmoud, S. T. (2022). Flexible Cu3(HHTP)2 MOF Membranes for Gas Sensing Application at Room Temperature. Nanomaterials, 12(6), 913. https://doi.org/10.3390/nano12060913







