Gold Nanorod Substrate for Rat Fetal Neural Stem Cell Differentiation into Oligodendrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
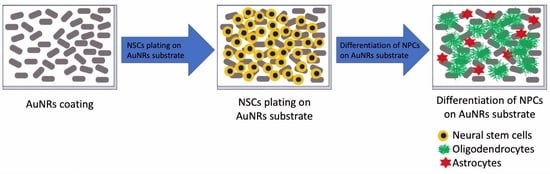
2.2. Fabrication and Functionalization of AuNR Surfaces
2.3. Fabrication of Functionalized Substrate Using Functional Silane Compounds
2.4. Amino-Functionalized Coverslip Coating with Functionalized AuNRs
2.5. Characterization of AuNRs
2.6. Rat NSC Culture
2.7. Differentiation of NPCs
2.8. Immunofluorescence Staining of Differentiated Cells
2.9. Statistical Analysis
3. Results
3.1. AuNR Characterization
3.2. Differentiation of NPCs on AuNR and PDL Surfaces
3.3. Evaluation of Myelin Basic Protein (MBP) and Integrin Expressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salzer, J.L. Polarized domains of myelinated axons. Neuron 2003, 40, 297–318. [Google Scholar] [CrossRef] [Green Version]
- Pryjmaková, J.; Kaimlová, M.; Hubáček, T.; Švorčík, V.; Siegel, J. Nanostructured materials for artificial tissue replacements. Int. J. Mol. Sci. 2020, 21, 2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shefi, O.; Prinz, C.; Wheeler, B.C.; Ballerini, L.; Rauti, R.; Pampaloni, N.P.; Giugliano, M.; Scaini, D. Advances in Nano Neuroscience: From Nanomaterials to Nanotools. Front. Neurosci. 2019, 1, 953. [Google Scholar] [CrossRef]
- Ma, D.; Zhao, Y.; Huang, L.; Xiao, Z.; Chen, B.; Shi, Y.; Shen, H.; Dai, J. A novel hydrogel-based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials 2020, 237, 119830. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-de-Souza, J.L.; Treger, J.S.; Dang, B.; Kent, S.B.H.; Pepperberg, D.R.; Bezanilla, F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 2015, 86, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Pandanaboina, S.C.; Alghazali, K.M.; Nima, Z.A.; Alawajji, R.A.; Sharma, K.D.; Watanabe, F.; Saini, V.; Biris, A.S.; Srivatsan, M. Plasmonic nano surface for neuronal differentiation and manipulation. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102048. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Alghazali, K.M.; Newby, S.D.; Nima, Z.A.; Hamzah, R.N.; Watanabe, F.; Bourdo, S.E.; Masi, T.J.; Stephenson, S.M.; Anderson, D.E.; Dhar, M.S.; et al. Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitors. Sci. Rep. 2017, 7, 16654. [Google Scholar] [CrossRef] [Green Version]
- Nima, Z.A.; Mahmood, M.; Xu, Y.; Mustafa, T.; Watanabe, F.; Nedosekin, D.A.; Juratli, M.A.; Fahmi, T.; Galanzha, E.I.; Nolan, J.P.; et al. Circulating tumor cell identification by functionalized silver-gold nanorods with multicolor, super-enhanced SERS and photothermal resonances. Sci. Rep. 2014, 4, 4752. [Google Scholar] [CrossRef]
- Vang, K.B.; Safina, I.; Darrigues, E.; Nedosekin, D.; Nima, Z.A.; Majeed, W.; Watanabe, F.; Kannarpady, G.; Kore, R.A.; Casciano, D.; et al. Modifying Dendritic Cell Activation with Plasmonic Nano Vectors. Sci. Rep. 2017, 7, 5513. [Google Scholar] [CrossRef] [Green Version]
- Darrigues, E.; Nima, Z.A.; Nedosekin, D.A.; Watanabe, F.; Alghazali, K.M.; Zharov, V.P.; Biris, A.S. Tracking Gold Nanorods’ Interaction with Large 3D Pancreatic-Stromal Tumor Spheroids by Multimodal Imaging: Fluorescence, Photoacoustic, and Photothermal Microscopies. Sci. Rep. 2020, 10, 3362. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Nima, Z.A.; Honda, T.; Mitsuhara, M.; Nishida, M.; Biris, A.S. X-ray photoelectron spectroscopy and transmission electron microscopy analysis of silver-coated gold nanorods designed for bionanotechnology applications. Nanotechnology 2017, 28, 025704. [Google Scholar] [CrossRef] [PubMed]
- Benters, R.; Niemeyer, C.M.; Wöhrle, D. Dendrimer-activated solid supports for nucleic acid and protein microarrays. ChemBioChem 2001, 2, 686–694. [Google Scholar] [CrossRef]
- Sharma, K.D.; Pandanaboina, S.C.; Srivatsan, M.; Xie, J.Y. Predominant differentiation of rat fetal neural stem cells into functional oligodendrocytes in vitro. Neurosci. Lett. 2020, 736, 135264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.D.; Schaal, D.; Kore, R.A.; Hamzah, R.N.; Pandanaboina, S.C.; Hayar, A.; Griffin, R.J.; Srivatsan, M.; Reyna, N.S.; Xie, J.Y. Glioma-derived exosomes drive the differentiation of neural stem cells to astrocytes. PLoS ONE 2020, 15, e0234614. [Google Scholar] [CrossRef]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef]
- O’meara, R.W.; Michalski, J.-P.; Kothary, R. Integrin Signaling in Oligodendrocytes and Its Importance in CNS Myelination. J. Signal Transduct. 2011, 2011, 354091. [Google Scholar] [CrossRef] [Green Version]
- Willison, A.G.; Smith, S.; Davies, B.M.; Kotter, M.R.N.; Barnett, S.C. A scoping review of trials for cell-based therapies in human spinal cord injury. Spinal Cord 2020, 58, 844–856. [Google Scholar] [CrossRef]
- McGinley, L.M.; Kashlan, O.N.; Bruno, E.S.; Chen, K.S.; Hayes, J.M.; Kashlan, S.R.; Raykin, J.; Johe, K.; Murphy, G.G.; Feldman, E.L. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer’s disease. Sci. Rep. 2018, 8, 14776. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgari, V.; Landarani-Isfahani, A.; Salehi, H.; Amirpour, N.; Hashemibeni, B.; Kazemi, M.; Bahramian, H. Direct Conjugation of Retinoic Acid with Gold Nanoparticles to Improve Neural Differentiation of Human Adipose Stem Cells. J. Mol. Neurosci. 2020, 70, 1836–1850. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Yi, X.; El-Sayed, M.A. Gold nanoparticles: Catalyst for the oxidation of NADH to NAD+. J. Photochem. Photobiol. B Biol. 2005, 81, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.P.; Zhang, J.Z.; Titus, H.E.; Karl, M.; Merzliakov, M.; Dorfman, A.R.; Karlik, S.; Stewart, M.G.; Watt, R.K.; Facer, B.D.; et al. Nanocatalytic activity of clean-surfaced, faceted nanocrystalline gold enhances remyelination in animal models of multiple sclerosis. Sci. Rep. 2020, 10, 1936. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.; Liu, D.; Fong, C.C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Zhang, J.; Fong, C.; Yang, M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater. Sci. Eng. C 2014, 42, 70–77. [Google Scholar] [CrossRef]
- Zhou, J.; Han, Y.; Lu, S. Direct role of interrod spacing in mediating cell adhesion on Sr-HA nanorod-patterned coatings. Int. J. Nanomed. 2014, 9, 1243–1260. [Google Scholar] [CrossRef] [Green Version]
- Teo, B.K.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.S.; Yap, C.H.; Ramagopal, Y.; Romer, L.H.; Yim, E.K.F. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef]
- Lourenço, T.; Grãos, M. Modulation of oligodendrocyte differentiation by mechanotransduction. Front. Cell. Neurosci. 2016, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Jagielska, A.; Lowe, A.L.; Makhija, E.; Wroblewska, L.; Guck, J.; Franklin, R.J.M.; Shivashankar, G.V.; Van Vliet, K.J. Mechanical strain promotes oligodendrocyte differentiation by global changes of gene expression. Front. Cell. Neurosci. 2017, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, T.; Paes De Faria, J.; Bippes, C.A.; Maia, J.; Lopes-Da-Silva, J.A.; Relvas, J.B.; Graõs, M. Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues. Sci. Rep. 2016, 6, 21563. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, K.D.; Alghazali, K.M.; Hamzah, R.N.; Pandanaboina, S.C.; Nima Alsudani, Z.A.; Muhi, M.; Watanabe, F.; Zhou, G.-L.; Biris, A.S.; Xie, J.Y. Gold Nanorod Substrate for Rat Fetal Neural Stem Cell Differentiation into Oligodendrocytes. Nanomaterials 2022, 12, 929. https://doi.org/10.3390/nano12060929
Sharma KD, Alghazali KM, Hamzah RN, Pandanaboina SC, Nima Alsudani ZA, Muhi M, Watanabe F, Zhou G-L, Biris AS, Xie JY. Gold Nanorod Substrate for Rat Fetal Neural Stem Cell Differentiation into Oligodendrocytes. Nanomaterials. 2022; 12(6):929. https://doi.org/10.3390/nano12060929
Chicago/Turabian StyleSharma, Krishna Deo, Karrer M. Alghazali, Rabab N. Hamzah, Sahitya Chetan Pandanaboina, Zeid A. Nima Alsudani, Malek Muhi, Fumiya Watanabe, Guo-Lei Zhou, Alexandru S. Biris, and Jennifer Yanhua Xie. 2022. "Gold Nanorod Substrate for Rat Fetal Neural Stem Cell Differentiation into Oligodendrocytes" Nanomaterials 12, no. 6: 929. https://doi.org/10.3390/nano12060929
APA StyleSharma, K. D., Alghazali, K. M., Hamzah, R. N., Pandanaboina, S. C., Nima Alsudani, Z. A., Muhi, M., Watanabe, F., Zhou, G.-L., Biris, A. S., & Xie, J. Y. (2022). Gold Nanorod Substrate for Rat Fetal Neural Stem Cell Differentiation into Oligodendrocytes. Nanomaterials, 12(6), 929. https://doi.org/10.3390/nano12060929