Freestanding Translucent ZnO–Cellulose Nanocomposite Films for Ultraviolet Sensor Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
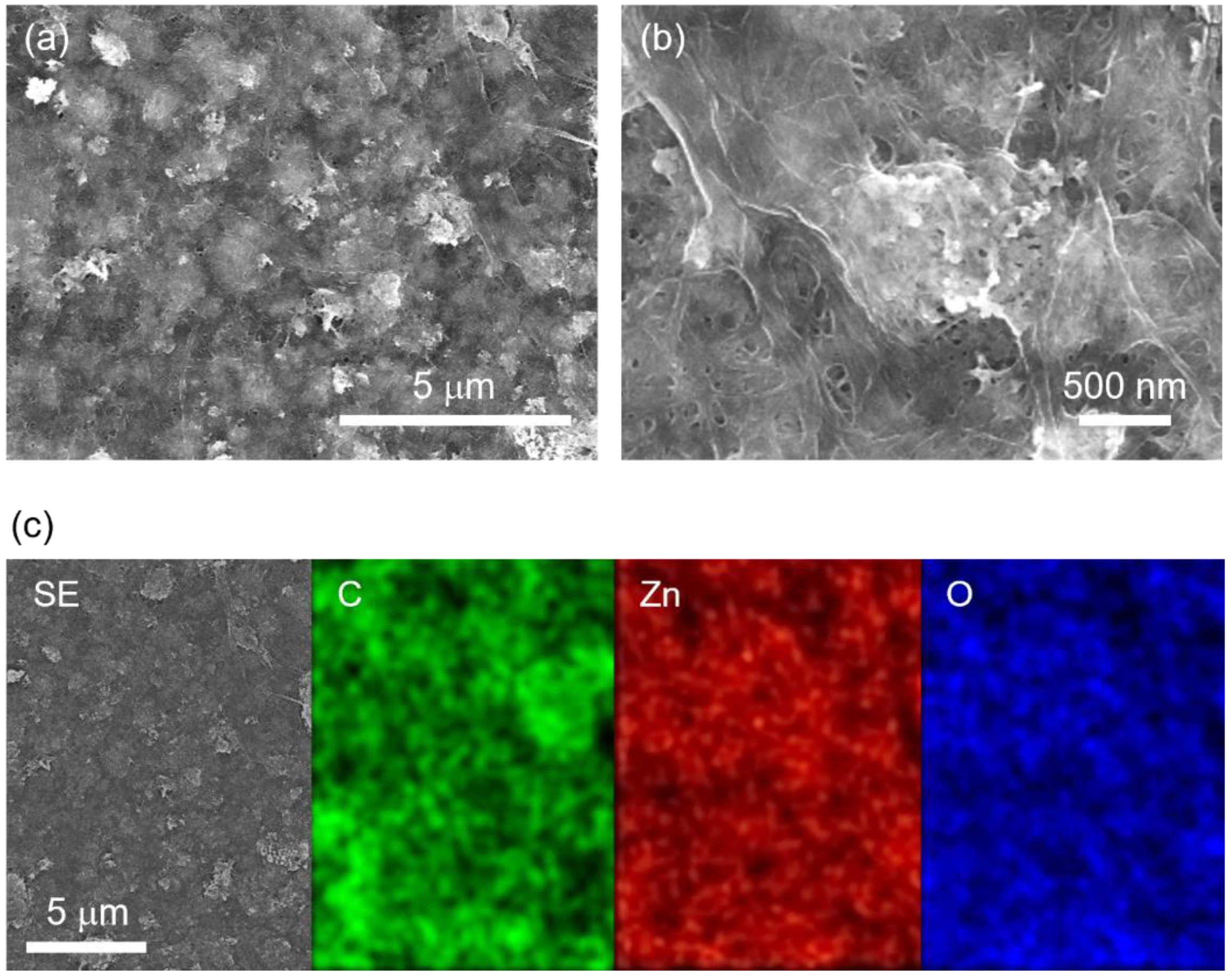
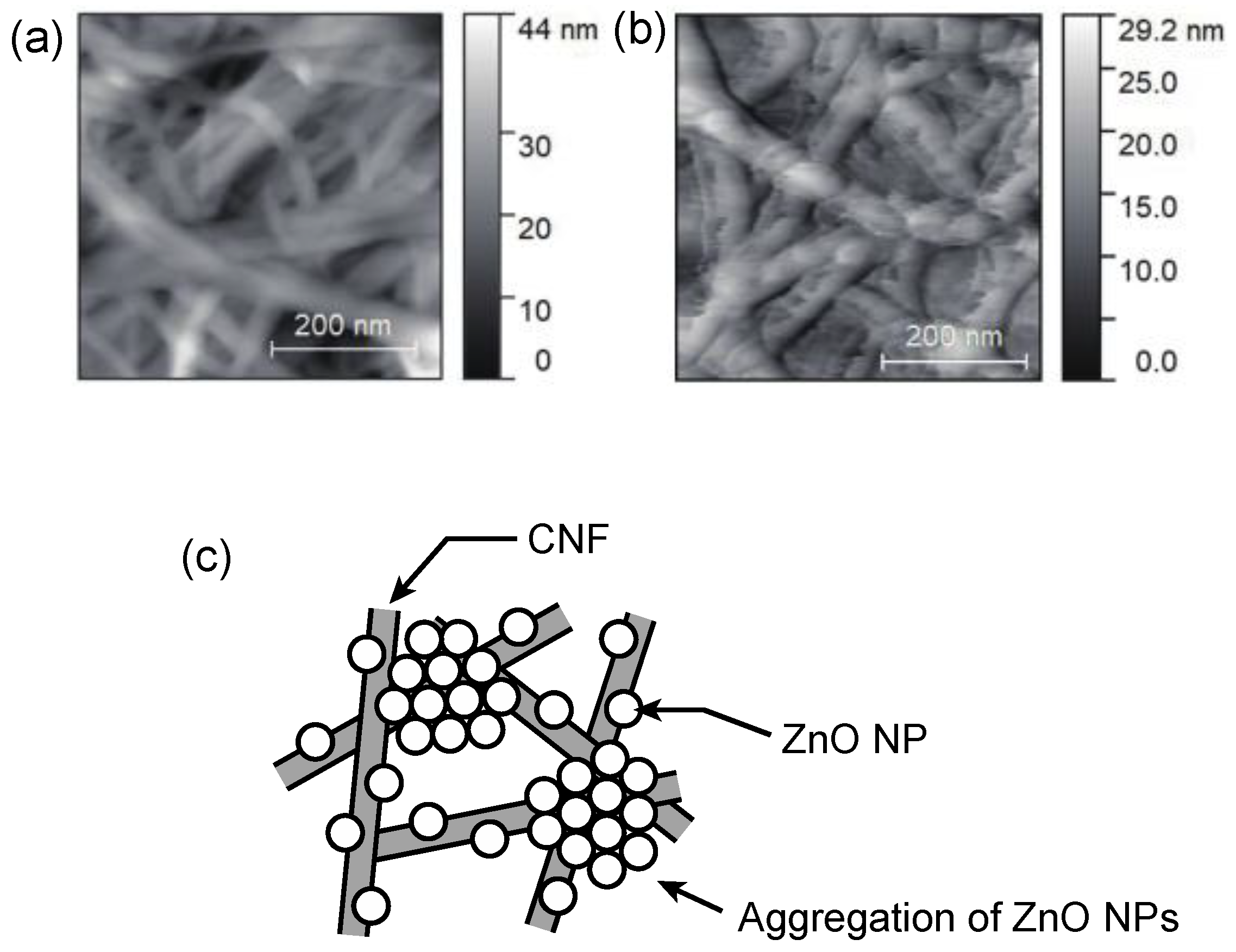
3.1. Characterization of Freestanding ZnO–CNF Nanocomposite Films
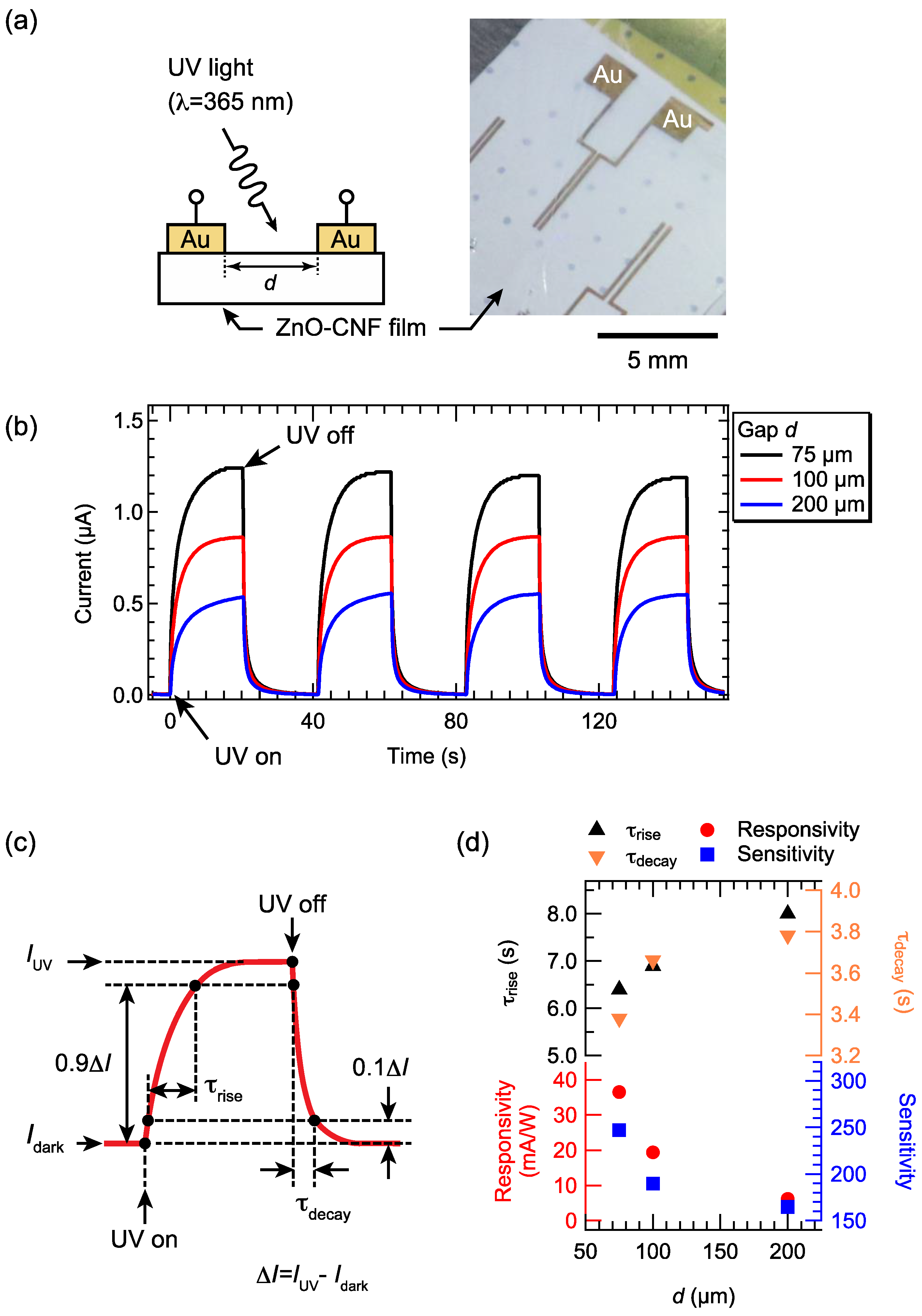
3.2. Electrical Properties of ZnO–CNF Nanocomposite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Q.; Zhang, Y.; Li, C.; He, Z.; Choy, W.C.H.; Low, P.J.; Sonar, P.; Kyaw, A.K.K. Biodegradable Materials and Green Processing for Green Electronics. Adv. Mater. 2020, 32, 2001591. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, K.; Lin, X.; Li, H.; Tao, T.; Wu, Q.; Zheng, Q.; Huang, L.; Ni, Y.; Chen, L.; et al. Transparent and conductive cellulose film by controllably growing aluminum doped zinc oxide on regenerated cellulose film. Cellulose 2020, 27, 4847–4855. [Google Scholar] [CrossRef]
- Jin, J.; Lee, D.; Im, H.G.; Han, Y.C.; Jeong, E.G.; Rolandi, M.; Choi, K.C.; Bae, B.S. Chitin Nanofiber Transparent Paper for Flexible Green Electronics. Adv. Mater. 2016, 28, 5169–5175. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Brenckle, M.A.; Yang, M.; Zhang, J.; Liu, M.; Siebert, S.M.; Averitt, R.D.; Mannoor, M.S.; McAlpine, M.C.; Rogers, J.A.; et al. Silk-based conformal, adhesive, edible food sensors. Adv. Mater. 2012, 24, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Tobjork, D.; Osterbacka, R. Paper electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A. Wood nanocelluloses: Fundamentals and applications as new bio-based nanomaterials. J. Wood Sci. 2013, 59, 449–459. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Nogi, M.; Komoda, N.; Nge, T.T.; Sugahara, T.; Suganuma, K. Uniformly connected conductive networks on cellulose nanofiber paper for transparent paper electronics. NPG Asia Mater. 2014, 6, e93. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, K.; Koga, H.; Celano, U.; Zhuge, F.; Kanai, M.; Rahong, S.; Meng, G.; He, Y.; De Boeck, J.; Jurczak, M.; et al. Cellulose nanofiber paper as an ultra flexible nonvolatile memory. Sci. Rep. 2014, 4, 5532. [Google Scholar] [CrossRef] [Green Version]
- Inui, T.; Koga, H.; Nogi, M.; Komoda, N.; Suganuma, K. A miniaturized flexible antenna printed on a high dielectric constant nanopaper composite. Adv. Mater. 2015, 27, 1112–1116. [Google Scholar] [CrossRef]
- Gimenez, A.J.; Yanez-Limon, J.M.; Seminario, J.M. ZnO–Cellulose Composite for UV Sensing. IEEE Sens. J. 2013, 13, 1301–1306. [Google Scholar] [CrossRef]
- Gullapalli, H.; Vemuru, V.S.; Kumar, A.; Botello-Mendez, A.; Vajtai, R.; Terrones, M.; Nagarajaiah, S.; Ajayan, P.M. Flexible piezoelectric ZnO-paper nanocomposite strain sensor. Small 2010, 6, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Ghule, K.; Ghule, A.V.; Chen, B.-J.; Ling, Y.-C. Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem. 2006, 8, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, K.; Nayak, J. ZnO-cellulose nanocomposite powder for application in UV sensors. In Proceedings of the AIP Conference Proceeding, Bhubaneswar, India, 26–30 December 2016; AIP Publishing LLC: Melville, NY, USA, 2017. [Google Scholar]
- Pimentel, A.; Samouco, A.; Nunes, D.; Araujo, A.; Martins, R.; Fortunato, E. Ultra-Fast Microwave Synthesis of ZnO Nanorods on Cellulose Substrates for UV Sensor Applications. Materials 2017, 10, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.H.; Cunha, I.; Pinto, J.V.; Neto, J.P.; Pereira, L.; Fortunato, E.; Martins, R. UV-Responsive Screen-Printed Porous ZnO Nanostructures on Office Paper for Sustainable and Foldable Electronics. Chemosensors 2021, 9, 192. [Google Scholar] [CrossRef]
- Yin, D.; Chen, Z.-Y.; Jiang, N.-R.; Liu, Y.-F.; Bi, Y.-G.; Zhang, X.-L.; Han, W.; Feng, J.; Sun, H.-B. Highly transparent and flexible fabric-based organic light emitting devices for unnoticeable wearable displays. Org. Electron. 2020, 76, 105494. [Google Scholar] [CrossRef]
- Fan, F.R.; Lin, L.; Zhu, G.; Wu, W.; Zhang, R.; Wang, Z.L. Transparent triboelectric nanogenerators and self-powered pressure sensors based on micropatterned plastic films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef] [Green Version]
- Yun, T.G.; Park, M.; Kim, D.H.; Kim, D.; Cheong, J.Y.; Bae, J.G.; Han, S.M.; Kim, I.D. All-Transparent Stretchable Electrochromic Supercapacitor Wearable Patch Device. ACS Nano 2019, 13, 3141–3150. [Google Scholar] [CrossRef]
- Tsuboi, K.; Yokota, S.; Kondo, T. Difference between bamboo- and wood-derived cellulose nanofibers prepared by the aqueous counter collision method. Nord. Pulp Pap. Res. J. 2014, 29, 69–76. [Google Scholar] [CrossRef]
- Fox, M. Optical Properties of Solids; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Nickel, N.H.; Terukov, E. (Eds.) Zinc Oxide—A Material for Micro- and Optoelectronic Applications; Springer: St. Petersburg, Russia, 2005; Volume 194. [Google Scholar]
- ASTM D1003-11; Standard Test Method for Haze and Luminous Transmittance of Transparent Plastics. ASTM International: West Conshohocken, PA, USA, 2011. [CrossRef]
- Zhao, S.-W.; Zheng, M.; Zou, X.-H.; Guo, Y.; Pan, Q.-J. Self-Assembly of Hierarchically Structured Cellulose@ZnO Composite in Solid–Liquid Homogeneous Phase: Synthesis, DFT Calculations, and Enhanced Antibacterial Activities. ACS Sustain. Chem. Eng. 2017, 5, 6585–6596. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; Taylor & Francis: London, UK, 1994. [Google Scholar]
- Kawashima, T.; Hishinuma, M. Analysis of Electrical Conduction Paths in Ni/YSZ Particulate Composites Using Percolation Theory. Mater. Trans. JIM 1996, 37, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- Boruah, B.D. Zinc oxide ultraviolet photodetectors: Rapid progress from conventional to self-powered photodetectors. Nanoscale Adv. 2019, 1, 2059–2085. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, H.; Kawamoto, Y.; Ikuno, T. Freestanding Translucent ZnO–Cellulose Nanocomposite Films for Ultraviolet Sensor Applications. Nanomaterials 2022, 12, 940. https://doi.org/10.3390/nano12060940
Komatsu H, Kawamoto Y, Ikuno T. Freestanding Translucent ZnO–Cellulose Nanocomposite Films for Ultraviolet Sensor Applications. Nanomaterials. 2022; 12(6):940. https://doi.org/10.3390/nano12060940
Chicago/Turabian StyleKomatsu, Hiroaki, Yurika Kawamoto, and Takashi Ikuno. 2022. "Freestanding Translucent ZnO–Cellulose Nanocomposite Films for Ultraviolet Sensor Applications" Nanomaterials 12, no. 6: 940. https://doi.org/10.3390/nano12060940





