Preparation of the Nanostructured Ni-Mg-O Oxide System by a Sol–Gel Technique at Varied pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Ni-Mg-OH and Ni-Mg-O Samples
2.2. Characterization of the Ni-Mg-OH and Ni-Mg-O Samples
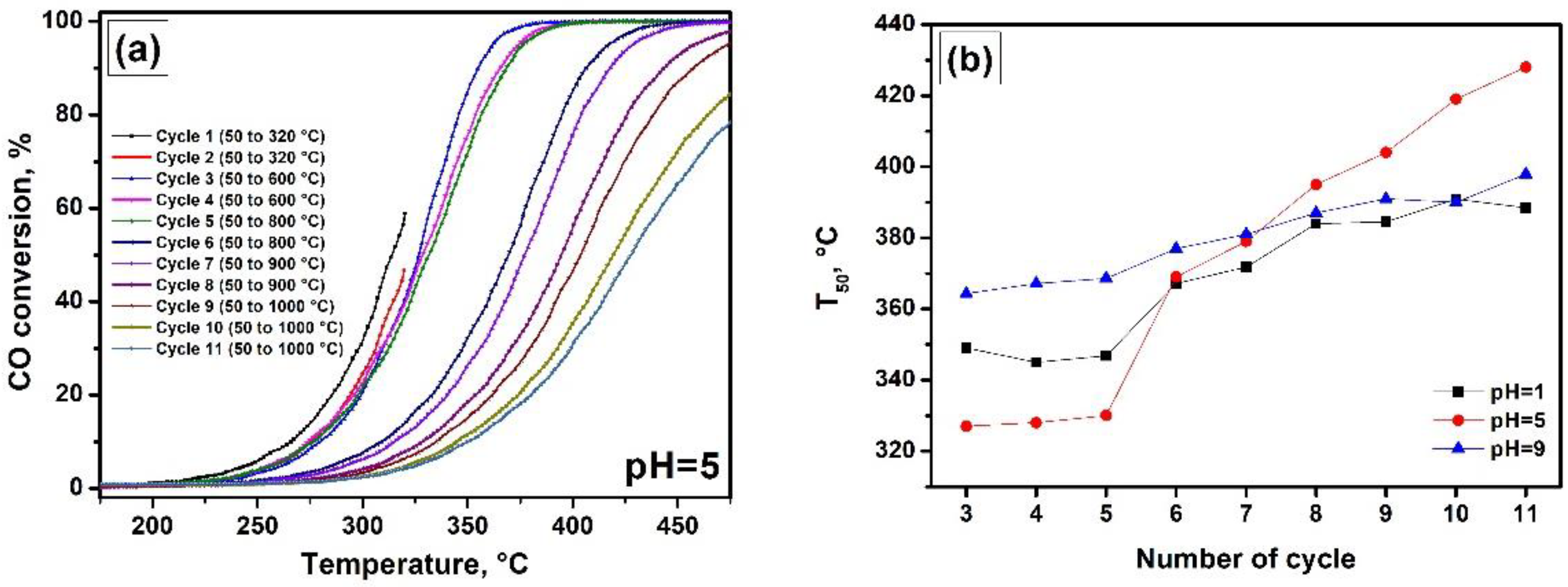
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adán-Más, A.; Silva, T.M.; Guerlou-Demourgues, L.; Bourgeois, L.; Labrugere-Sarroste, C.; Montemor, M.F. Nickel-cobalt oxide modified with reduced graphene oxide: Performance and degradation for energy storage applications. J. Power Sources 2019, 419, 12–26. [Google Scholar] [CrossRef]
- Isacfranklin, M.; Deepika, C.; Ravi, G.; Yuvakkumar, R.; Velauthapillai, D.; Saravanakumar, B. Nickel, bismuth, and cobalt vanadium oxides for supercapacitor applications. Ceram. Int. 2020, 46, 28206–28210. [Google Scholar] [CrossRef]
- Tu, B.; Yin, Y.; Zhang, F.; Su, X.; Lyu, X.; Cheng, M. High performance of direct methane-fuelled solid oxide fuel cell with samarium modified nickel-based anode. Int. J. Hydrog. Energy 2020, 45, 27587–27596. [Google Scholar] [CrossRef]
- Yadav, H.M.; Ramesh, S.; Kumar, K.A.; Shinde, S.; Sandhu, S.; Sivasamy, A.; Shrestha, N.K.; Kim, H.S.; Kim, H.-S.; Bathula, C. Impact of polypyrrole incorporation on nickel oxide@multi walled carbon nanotube composite for application in supercapacitors. Polym. Test. 2020, 89, 106727. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Hillie, K.T.; Swart, H.C.; Leshabane, N.; Tshilongo, J.; Motaung, D.E. Fabrication of a propanol gas sensor using p-type nickel oxide nanostructures: The effect of ramping rate towards luminescence and gas sensing characteristics. Mater. Chem. Phys. 2020, 253, 123316. [Google Scholar] [CrossRef]
- Anand, G.T.; Nithiyavathi, R.; Ramesh, R.; John Sundaram, S.; Kaviyarasu, K. Structural and optical properties of nickel oxide nanoparticles: Investigation of antimicrobial applications. Surf. Interf. 2020, 18, 100460. [Google Scholar] [CrossRef]
- Fuku, X.; Matinise, N.; Masikini, M.; Kasinathan, K.; Maaza, M. An electrochemically active green synthesized polycrystalline NiO/MgO catalyst: Use in photo-catalytic applications. Mater. Res. Bull. 2018, 97, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kang, E.; Son, S.U.; Park, H.M.; Lee, M.K.; Kim, J.; Kim, K.W.; Noh, H.J.; Park, J.H.; Bae, C.J.; et al. Monodisperse Nanoparticles of Ni and NiO: Synthesis, Characterization, Self-Assembled Superlattices, and Catalytic Applications in the Suzuki Coupling Reaction. Adv. Mater. 2005, 17, 429–434. [Google Scholar] [CrossRef]
- Wei, W.; Cui, B.; Jiang, X.; Lu, L. The catalytic effect of NiO on thermal decomposition of nitrocellulose. J. Therm. Anal. Calorim. 2010, 102, 863–866. [Google Scholar] [CrossRef]
- Gayán, P.; Dueso, C.; Abad, A.; Adanez, J.; de Diego, L.F.; García-Labiano, F. NiO/Al2O3 oxygen carriers for chemical-looping combustion prepared by impregnation and deposition–precipitation methods. Fuel 2009, 88, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Mattisson, T.; Järdnäs, A.; Lyngfelt, A. Reactivity of Some Metal Oxides Supported on Alumina with Alternating Methane and OxygenApplication for Chemical-Looping Combustion. Energy Fuels 2003, 17, 643–651. [Google Scholar] [CrossRef]
- Mattisson, T.; Johansson, M.; Lyngfelt, A. The use of NiO as an oxygen carrier in chemical-looping combustion. Fuel 2006, 85, 736–747. [Google Scholar] [CrossRef]
- Shen, L.; Wu, J.; Xiao, J. Experiments on chemical looping combustion of coal with a NiO based oxygen carrier. Combust. Flame 2009, 156, 721–728. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Ilyina, E.V.; Bedilo, A.F.; Vedyagin, A.A. Nanocrystalline aerogel VOx/MgO as a catalyst for oxidative dehydrogenation of propane. Reac. Kinet. Catal. Lett. 2009, 97, 355–361. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Vedyagin, A.A.; Bedilo, A.F.; Zaikovskii, V.I.; Klabunde, K.J. Aerogel VOx/MgO catalysts for oxidative dehydrogenation of propane. Catal. Today 2009, 144, 278–284. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A.; Cherepanova, S.V.; Nadeev, A.N.; Bedilo, A.F.; Klabunde, K.J. Synthesis and characterization of mesoporous VOx/MgO aerogels with high surface area. Micropor. Mesopor. Mater. 2012, 160, 32–40. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A.; Bedilo, A.F. Aerogel method for preparation of nanocrystalline CoOx MgO and VOx MgO catalysts. J. Sol-Gel Sci. Technol. 2013, 68, 423–428. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A. Preparation of nanocrystalline VMg(OH)x and VOx MgO from organometallic precursors. Inorg. Mater. 2009, 45, 1267–1270. [Google Scholar] [CrossRef]
- Karnaukhov, T.M.; Vedyagin, A.A.; Cherepanova, S.V.; Rogov, V.A.; Stoyanovskii, V.O.; Mishakov, I.V. Study on reduction behavior of two-component Fe Mg O oxide system prepared via a sol-gel technique. Int. J. Hydrog. Energy 2017, 42, 30543–30549. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Mishakov, I.V.; Karnaukhov, T.M.; Krivoshapkina, E.F.; Ilyina, E.V.; Maksimova, T.A.; Cherepanova, S.V.; Krivoshapkin, P.V. Sol–gel synthesis and characterization of two-component systems based on MgO. J. Sol-Gel Sci. Technol. 2017, 82, 611–619. [Google Scholar] [CrossRef]
- Karnaukhov, T.M.; Vedyagin, A.A.; Cherepanova, S.V.; Rogov, V.A.; Mishakov, I.V. Sol–gel synthesis and characterization of the binary Ni–Mg–O oxide system. J. Sol-Gel Sci. Technol. 2019, 92, 208–214. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Karnaukhov, T.M.; Cherepanova, S.V.; Stoyanovskii, V.O.; Rogov, V.A.; Mishakov, I.V. Synthesis of binary Co–Mg–O oxide system and study of its behavior in reduction/oxidation cycling. Int. J. Hydrog. Energy 2019, 44, 20690–20699. [Google Scholar] [CrossRef]
- Veselov, G.B.; Karnaukhov, T.M.; Bauman, Y.I.; Mishakov, I.V.; Vedyagin, A.A. Sol-Gel-Prepared Ni-Mo-Mg-O System for Catalytic Transformation of Chlorinated Organic Wastes into Nanostructured Carbon. Materials 2020, 13, 4404. [Google Scholar] [CrossRef]
- Diao, Y.; Walawender, W.P.; Sorensen, C.M.; Klabunde, K.J.; Ricker, T. Hydrolysis of Magnesium Methoxide. Effects of Toluene on Gel Structure and Gel Chemistry. Chem. Mater. 2001, 14, 362–368. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Guan, B.H.; Zaid, H.M.; Soleimani, H.; Ching, D.L.C. Impact of pH on zinc oxide particle size by using sol-gel process. AIP Conf. Proc. 2016, 1787, 050011. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Sreekantan, S. Effect of pH on TiO2 Nanoparticles via Sol-Gel Method. Adv. Mater. Res. 2010, 173, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, S.; Razavi, R.S.; Loghman-Estarki, M.R.; Alhaji, A. Development of MgO–Y2O3 Composite Nanopowder by Pechini Sol–Gel Method: Effect of Synthesis Parameters on Morphology, Particle Size, and Phase Distribution. J. Clust. Sci. 2017, 28, 1523–1539. [Google Scholar] [CrossRef]
- Bokhimi, X.; Morales, A.; Lopez, T.; Gomez, R. Crystalline Structure of MgO Prepared by the Sol-Gel Technique with Different Hydrolysis Catalysts. J. Solid State Chem. 1995, 115, 411–415. [Google Scholar] [CrossRef]
- Lopez, T.; Garcia-Cruz, I.; Gomez, R. Synthesis of magnesium oxide by the sol-gel method: Effect of the pH on the surface hydroxylation. J. Catal. 1991, 127, 75–85. [Google Scholar] [CrossRef]
- Boehm, H.-P.; Knözinger, H. Nature and estimation of functional groups on solid surfaces. In Catalysis; Springer: Berlin/Heidelberg, Germany, 1983; pp. 39–207. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Kenzhin, R.M.; Plyusnin, P.E.; Shubin, Y.V.; Mishakov, I.V. Effect of Alumina Phase Transformation on Stability of Low-Loaded Pd-Rh Catalysts for CO Oxidation. Top. Catal. 2016, 60, 152–161. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Stoyanovskii, V.O.; Plyusnin, P.E.; Shubin, Y.V.; Slavinskaya, E.M.; Mishakov, I.V. Effect of metal ratio in alumina-supported Pd-Rh nanoalloys on its performance in three way catalysis. J. Alloy. Compd. 2018, 749, 155–162. [Google Scholar] [CrossRef]
- Femitha, R.D.; Vaithyanathan, C. Preparation of Magnesium Hydroxide Nanoparticles from Bittern. Green Chem. Technol. Lett. 2016, 2, 87–90. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Meinhold, R.H. Thermal decomposition of brucite, Mg(OH)2: A 25Mg MAS NMR study. Thermochim. Acta 1993, 230, 339–343. [Google Scholar] [CrossRef]
- Madarász, J.; Varga, P.P.; Pokol, G. Evolved gas analyses (TG/DTA–MS and TG–FTIR) on dehydration and pyrolysis of magnesium nitrate hexahydrate in air and nitrogen. J. Anal. Appl. Pyrol. 2007, 79, 475–478. [Google Scholar] [CrossRef]
- Małecka, B.; Łącz, A.; Drożdż, E.; Małecki, A. Thermal decomposition of d-metal nitrates supported on alumina. J. Therm. Anal. Calorim. 2014, 119, 1053–1061. [Google Scholar] [CrossRef] [Green Version]
- Rejitha, K.S.; Ichikawa, T.; Mathew, S. Investigations on the thermal behaviour of [Ni(NH3)6](NO3)2 and [Ni(en)3](NO3)2 using TG–MS and TR-XRD under inert condition. J. Therm. Anal. Calorim. 2011, 107, 887–892. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, M.; Wang, Z. Theoretical studies on the acid-catalyzed decompositions of HCHO and HCOOH: Mechanism and thermochemistry. Comput. Theor. Chem. 2021, 1206, 113482. [Google Scholar] [CrossRef]
- Sietsma, J.R.A.; Friedrich, H.; Broersma, A.; Versluijs-Helder, M.; Jos van Dillen, A.; de Jongh, P.E.; de Jong, K.P. How nitric oxide affects the decomposition of supported nickel nitrate to arrive at highly dispersed catalysts. J. Catal. 2008, 260, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Liu, C.; Chen, W. Hydrogen production from steam reforming of ethanol over Ni/MgO-CeO2 catalyst at low temperature. J. Rare Earths 2009, 27, 948–954. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Mesbah, A.W.; Muzart, J.; Sahebdelfar, S. NiO–MgO Solid Solution Prepared by Sol–Gel Method as Precursor for Ni/MgO Methane Dry Reforming Catalyst: Effect of Calcination Temperature on Catalytic Performance. Catal. Lett. 2015, 146, 238–248. [Google Scholar] [CrossRef]
- Zanganeh, R.; Rezaei, M.; Zamaniyan, A. Preparation of nanocrystalline NiO–MgO solid solution powders as catalyst for methane reforming with carbon dioxide: Effect of preparation conditions. Adv. Powder Technol. 2014, 25, 1111–1117. [Google Scholar] [CrossRef]
- Stankic, S.; Sterrer, M.; Hofmann, P.; Bernardi, J.; Diwald, O.; Knözinger, E. Novel Optical Surface Properties of Ca2+-Doped MgO Nanocrystals. Nano Lett. 2005, 5, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
- Hüfner, S. Electronic structure of NiO and related 3d-transition-metal compounds. Adv. Phys. 1994, 43, 183–356. [Google Scholar] [CrossRef]
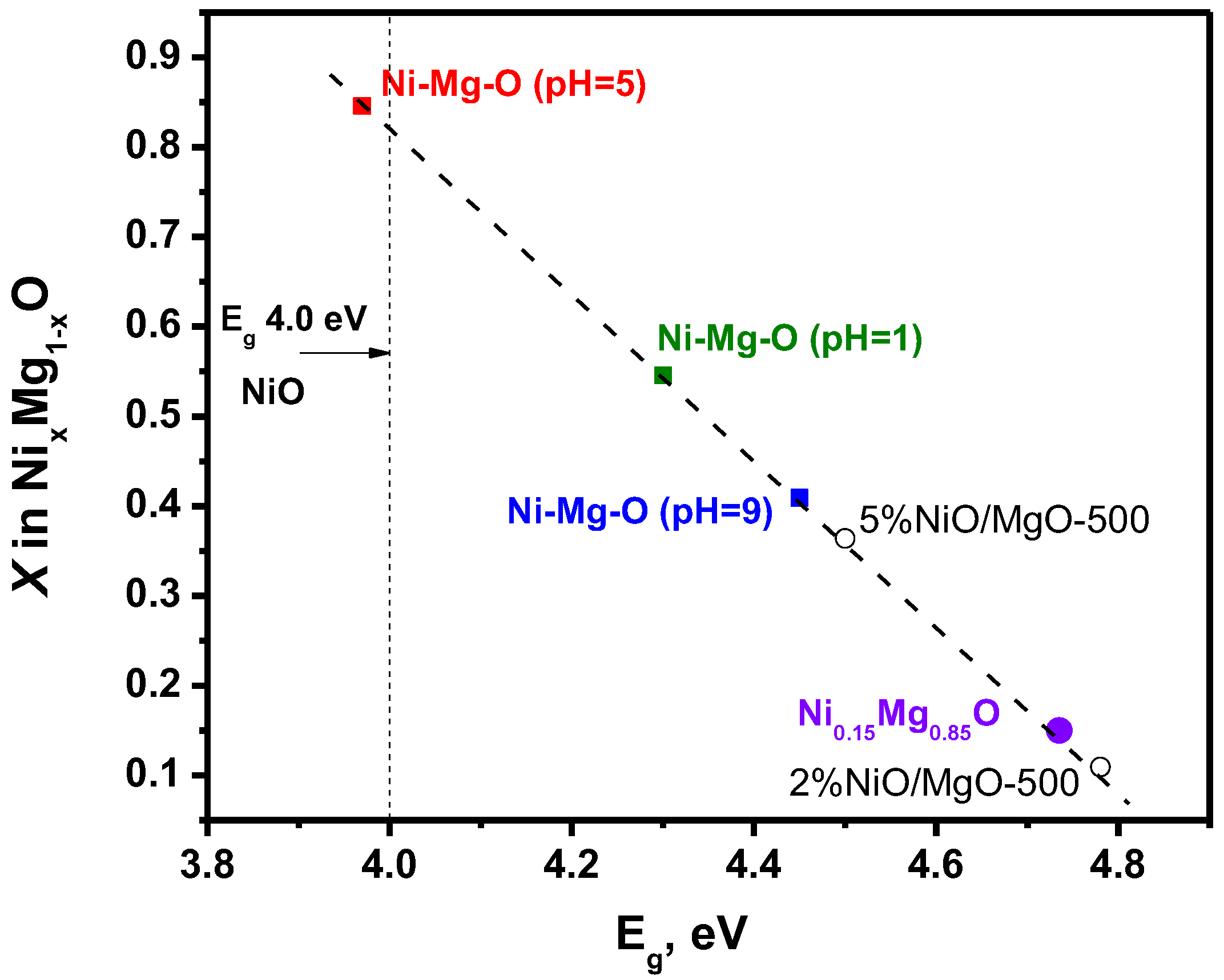
- Niedermeier, C.A.; Råsander, M.; Rhode, S.; Kachkanov, V.; Zou, B.; Alford, N.; Moram, M.A. Band gap bowing in NixMg1−xO. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Qiu, T.; Tang, B.; Zhang, G.; Yao, R.; Xu, W.; Chen, J.; Fu, X.; Ning, H.; Peng, J. Temperature-Controlled Crystal Size of Wide Band Gap Nickel Oxide and Its Application in Electrochromism. Micromachines 2021, 12, 80. [Google Scholar] [CrossRef]
- Barati Dalenjan, M.; Rashidi, A.; Khorasheh, F.; Ardjmand, M. Effect of Ni ratio on mesoporous Ni/MgO nanocatalyst synthesized by one-step hydrothermal method for thermal catalytic decomposition of CH4 to H2. Int. J. Hydrog. Energy 2022, 47, 11539–11551. [Google Scholar] [CrossRef]
- Jiang, S.; Lu, Y.; Wang, S.; Zhao, Y.; Ma, X. Insight into the reaction mechanism of CO2 activation for CH4 reforming over NiO-MgO: A combination of DRIFTS and DFT study. Appl. Surf. Sci. 2017, 416, 59–68. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, Q.; Zhang, J.; Sun, Y.; Zhu, Y. NiO-MgO nanoparticles confined inside SiO2 frameworks to achieve highly catalytic performance for CO2 reforming of methane. Mol. Catal. 2017, 432, 31–36. [Google Scholar] [CrossRef]
- Feng, J.; Ding, Y.; Guo, Y.; Li, X.; Li, W. Calcination temperature effect on the adsorption and hydrogenated dissociation of CO2 over the NiO/MgO catalyst. Fuel 2013, 109, 110–115. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Liu, W.; Chen, W.; Ran, R.; Li, M.; Weng, D. Soot oxidation over CeO2 and Ag/CeO2: Factors determining the catalyst activity and stability during reaction. J. Catal. 2016, 337, 188–198. [Google Scholar] [CrossRef]
- Wang, H.; Luo, S.; Zhang, M.; Liu, W.; Wu, X.; Liu, S. Roles of oxygen vacancy and O−in oxidation reactions over CeO2 and Ag/CeO2 nanorod model catalysts. J. Catal. 2018, 368, 365–378. [Google Scholar] [CrossRef]
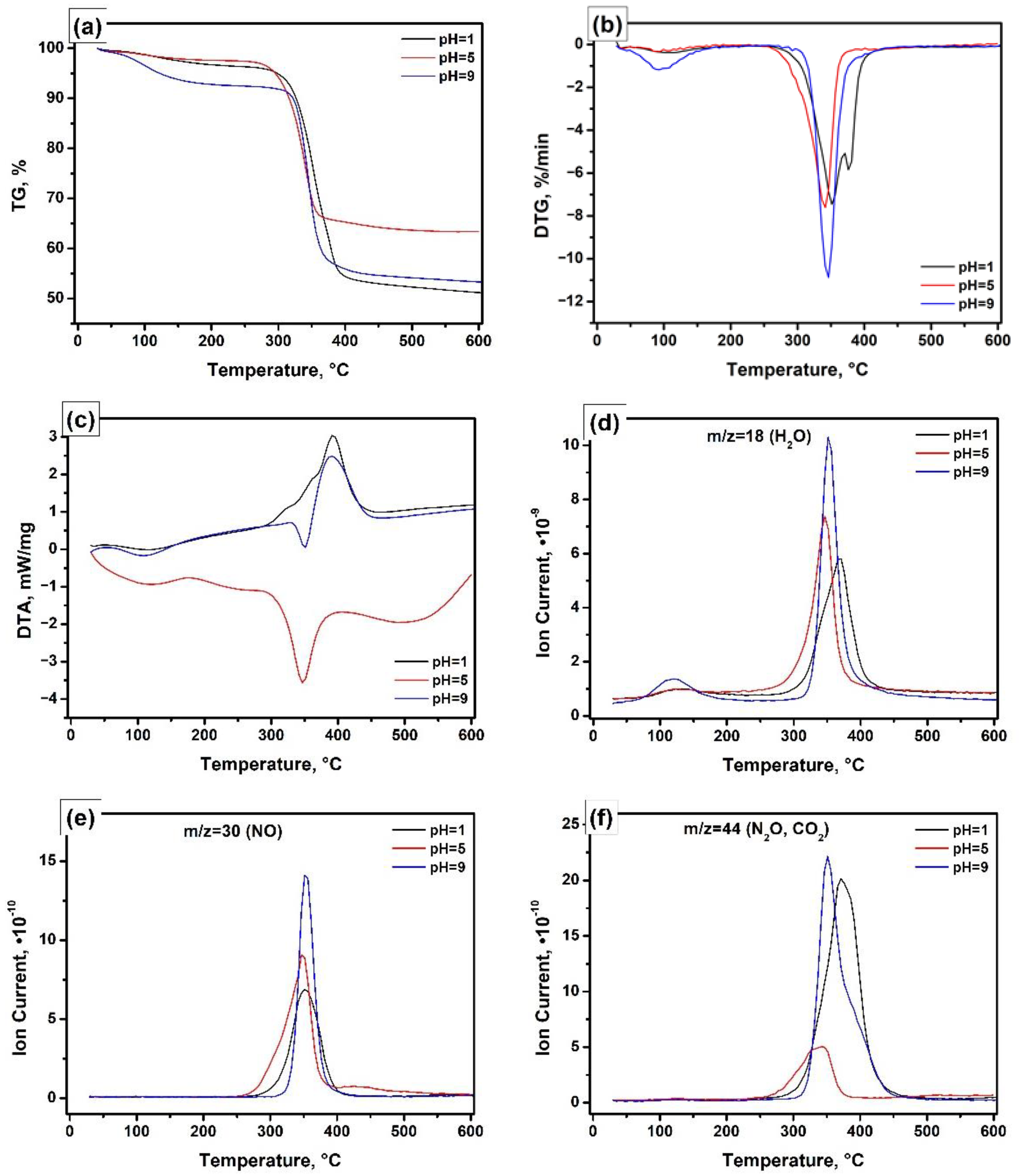
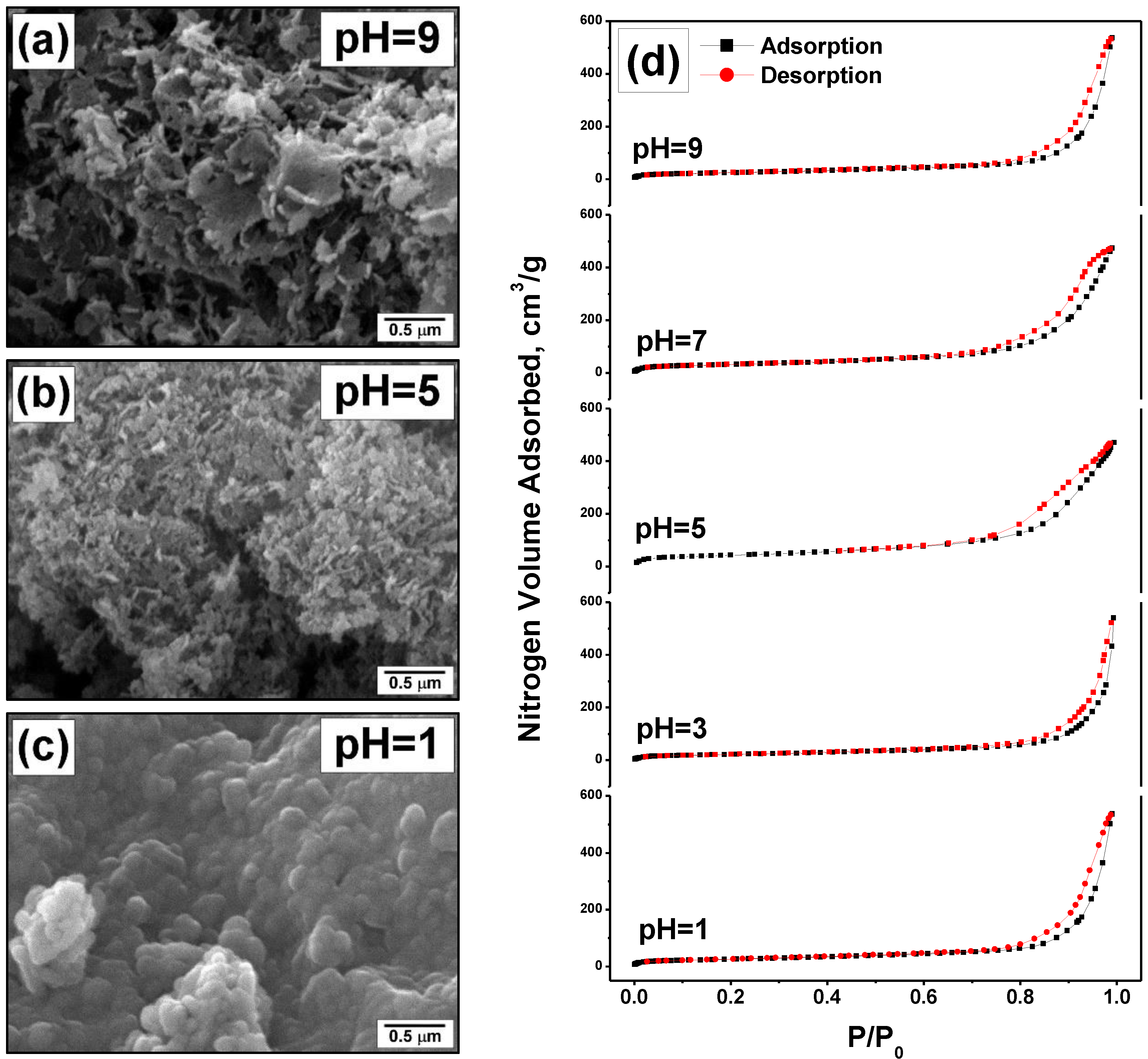


| pH | SSA, m2/g | Vpore, cm3/g | Dav, nm |
|---|---|---|---|
| 1 | 103 | 1.05 | 41 |
| 3 | 81 | 0.67 | 33 |
| 5 | 154 | 0.72 | 19 |
| 7 | 115 | 0.73 | 26 |
| 9 | 90 | 0.83 | 37 |
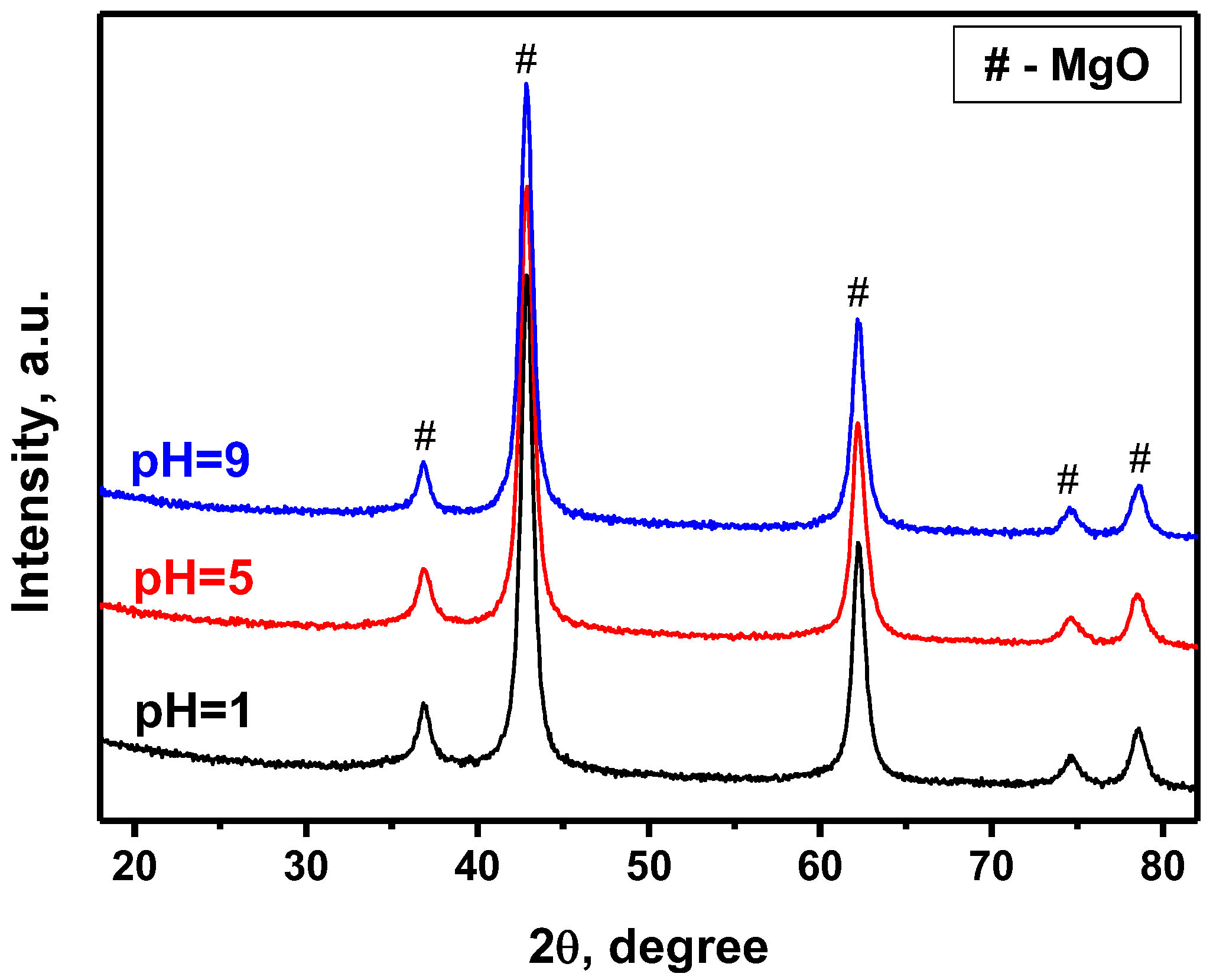
| # | Sample | Lattice Parameter a, Å | D, nm |
|---|---|---|---|
| 1 | Ni-Mg-O (pH = 1) | 4.220(1) | 7 |
| 2 | Ni-Mg-O (pH = 5) | 4.220(1) | 8 |
| 3 | Ni-Mg-O (pH = 9) | 4.219(1) | 8 |
| 4 | NiO (PDF#47-1049) | 4.177 | - |
| 5 | MgO (PDF#45-0946) | 4.211 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselov, G.B.; Karnaukhov, T.M.; Stoyanovskii, V.O.; Vedyagin, A.A. Preparation of the Nanostructured Ni-Mg-O Oxide System by a Sol–Gel Technique at Varied pH. Nanomaterials 2022, 12, 952. https://doi.org/10.3390/nano12060952
Veselov GB, Karnaukhov TM, Stoyanovskii VO, Vedyagin AA. Preparation of the Nanostructured Ni-Mg-O Oxide System by a Sol–Gel Technique at Varied pH. Nanomaterials. 2022; 12(6):952. https://doi.org/10.3390/nano12060952
Chicago/Turabian StyleVeselov, Grigory B., Timofey M. Karnaukhov, Vladimir O. Stoyanovskii, and Aleksey A. Vedyagin. 2022. "Preparation of the Nanostructured Ni-Mg-O Oxide System by a Sol–Gel Technique at Varied pH" Nanomaterials 12, no. 6: 952. https://doi.org/10.3390/nano12060952
APA StyleVeselov, G. B., Karnaukhov, T. M., Stoyanovskii, V. O., & Vedyagin, A. A. (2022). Preparation of the Nanostructured Ni-Mg-O Oxide System by a Sol–Gel Technique at Varied pH. Nanomaterials, 12(6), 952. https://doi.org/10.3390/nano12060952








