Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances
Abstract
:| Table of Content | |
| 1. Introduction | 2 |
| 2. Au nanomaterials | 4 |
| 2.1 Au nanoparticles | 5 |
| 2.2 Au nanocages | 6 |
| 2.3 Nanoporous gold | 6 |
| 2.4 Au-based nanomaterials | 7 |
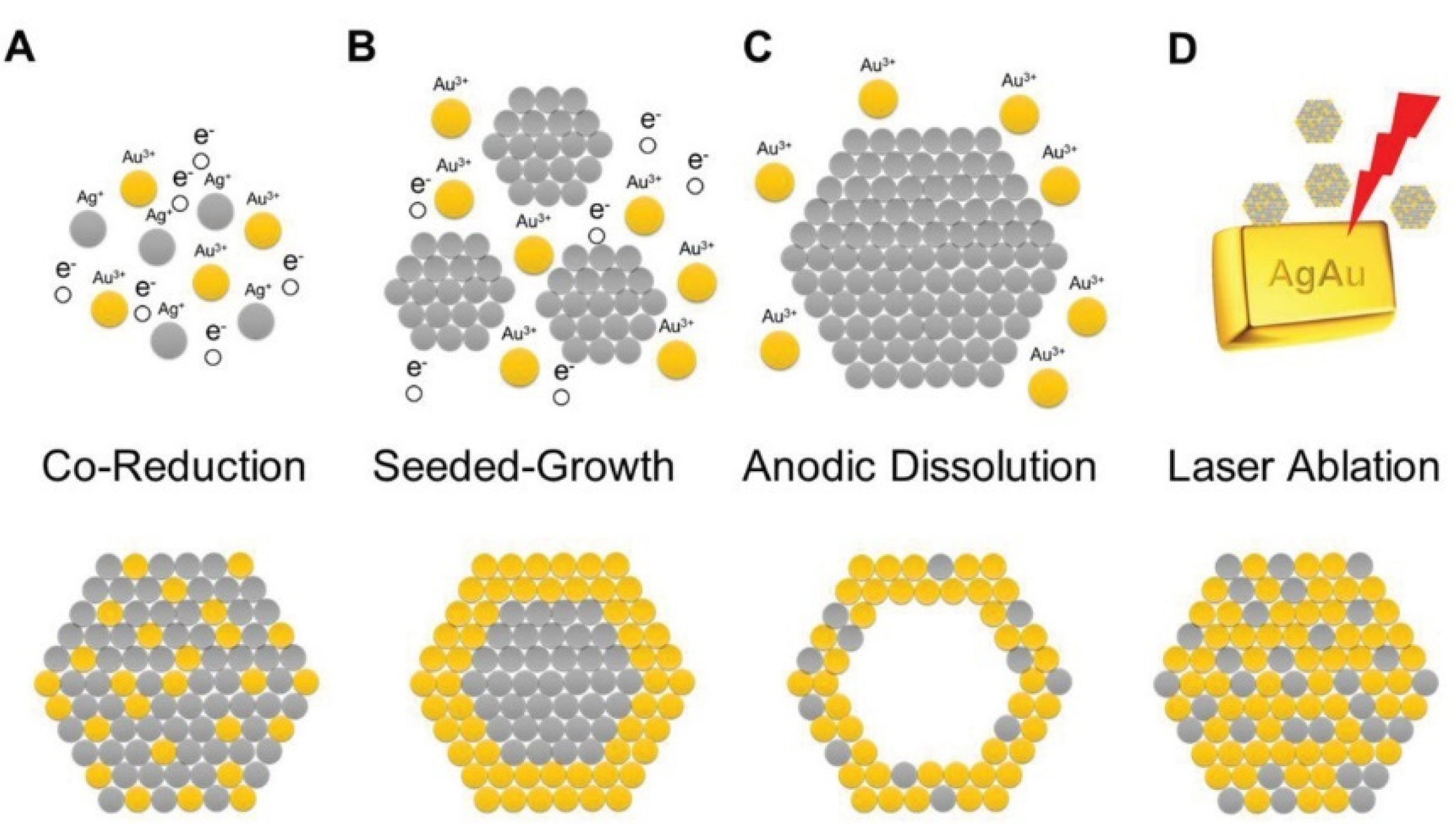
| 2.4.1 Bimetallic nanoparticles | 7 |
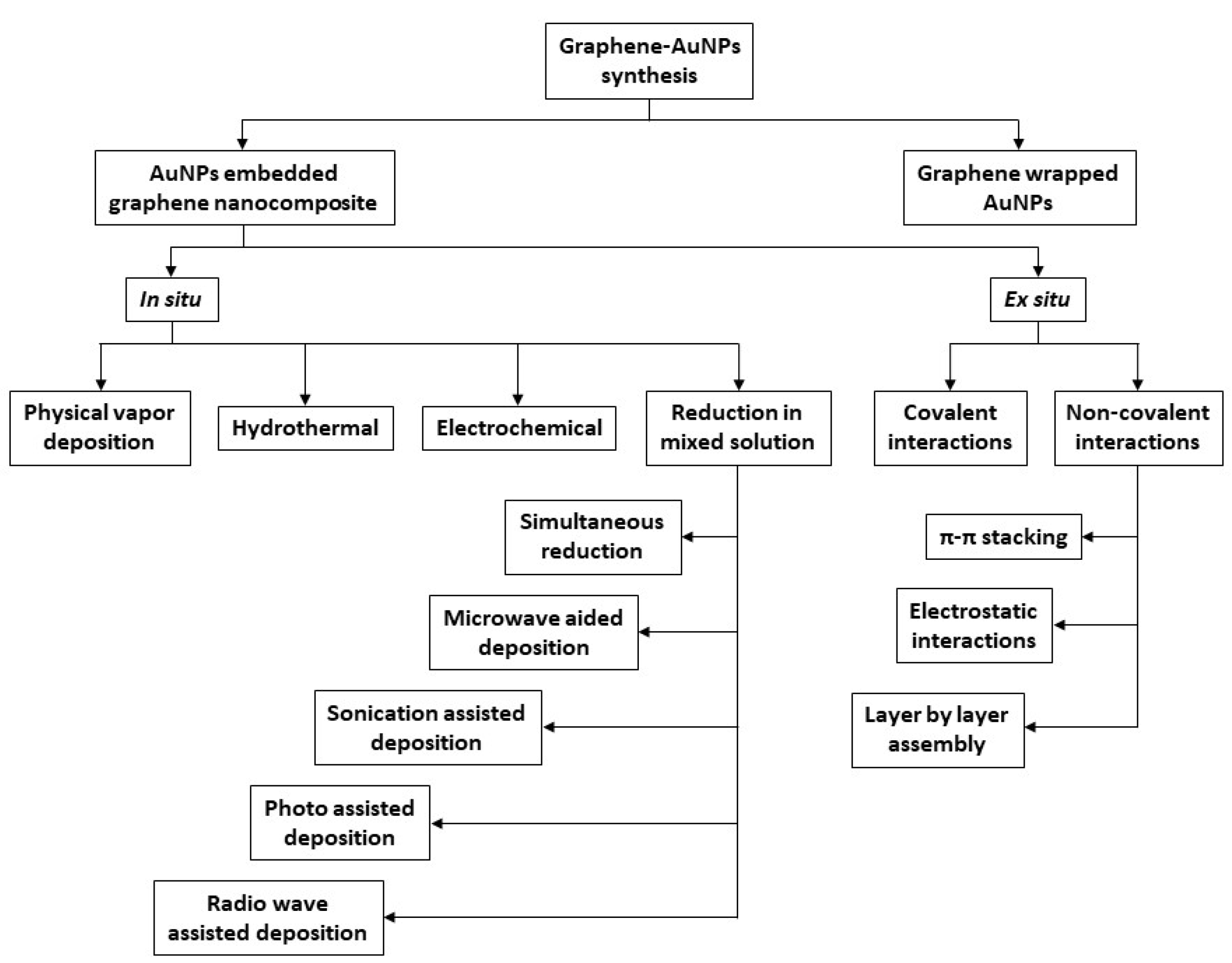
| 2.4.2 Au-based nanocomposites | 8 |
| 3. Gold nanomaterials applications to electrochemical sensors for phenolic antioxidants detection: some examples | 11 |
| 3.1. Phenolic acids | 11 |
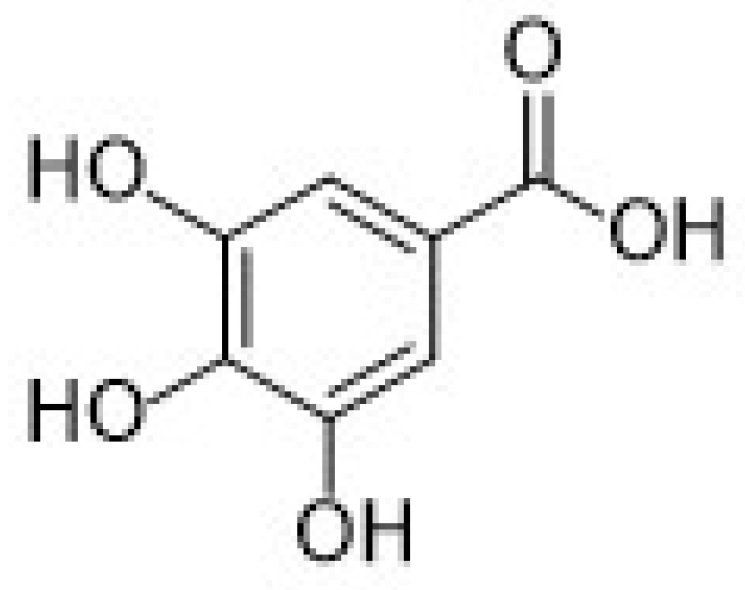
| 3.1.1 Gallic acid | 11 |
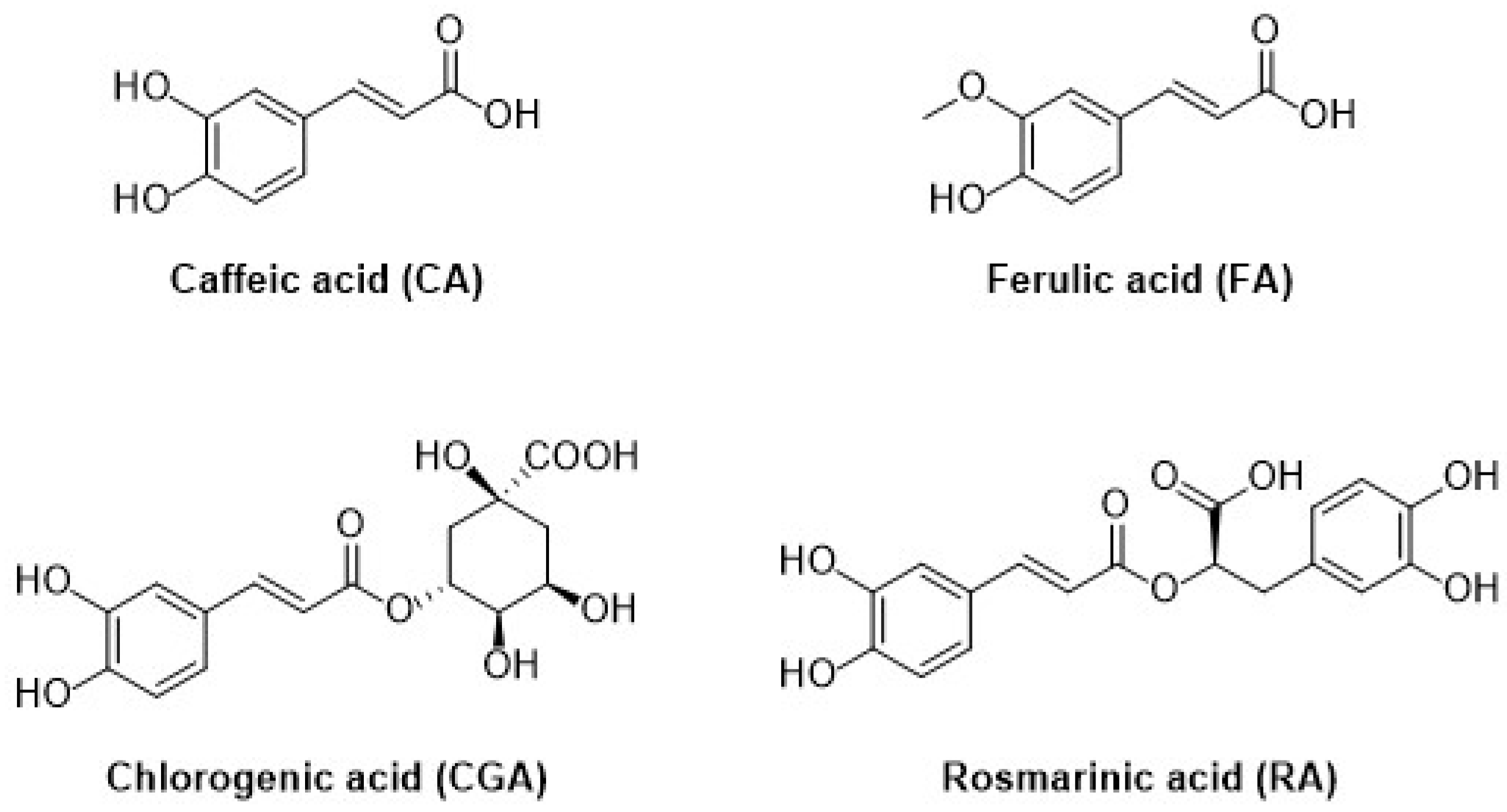
| 3.1.2. Hydroxycinnamic acids | 13 |
| 3.1.3. Some consideration on phenolic acids (bio)sensors base on Au nanomaterials | 24 |
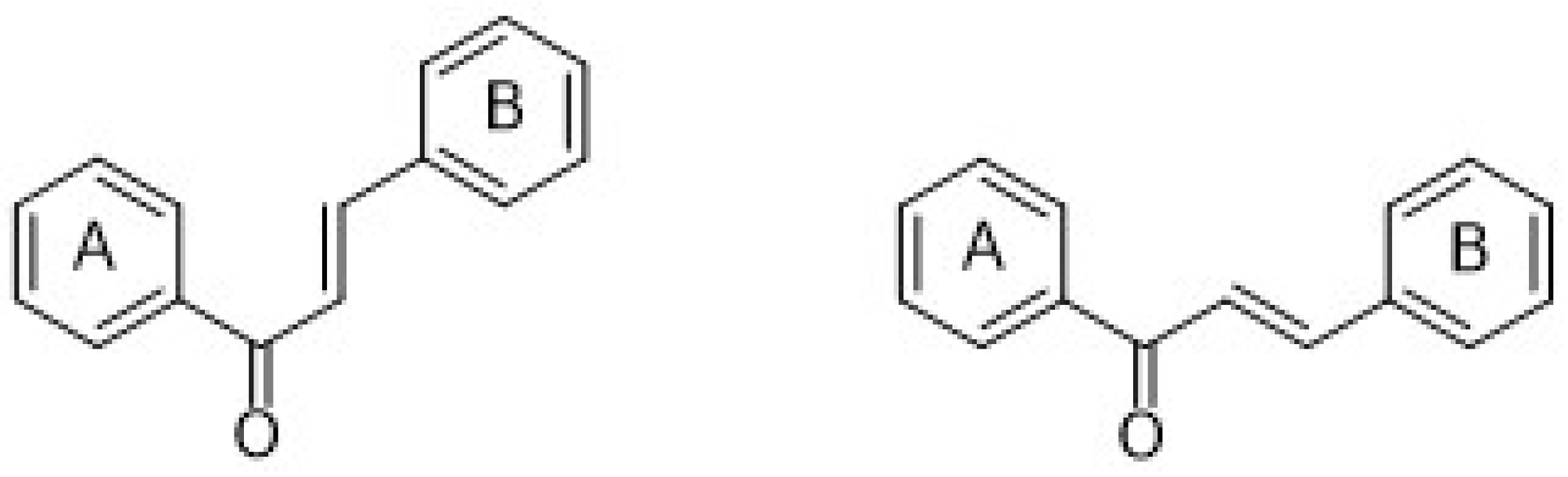
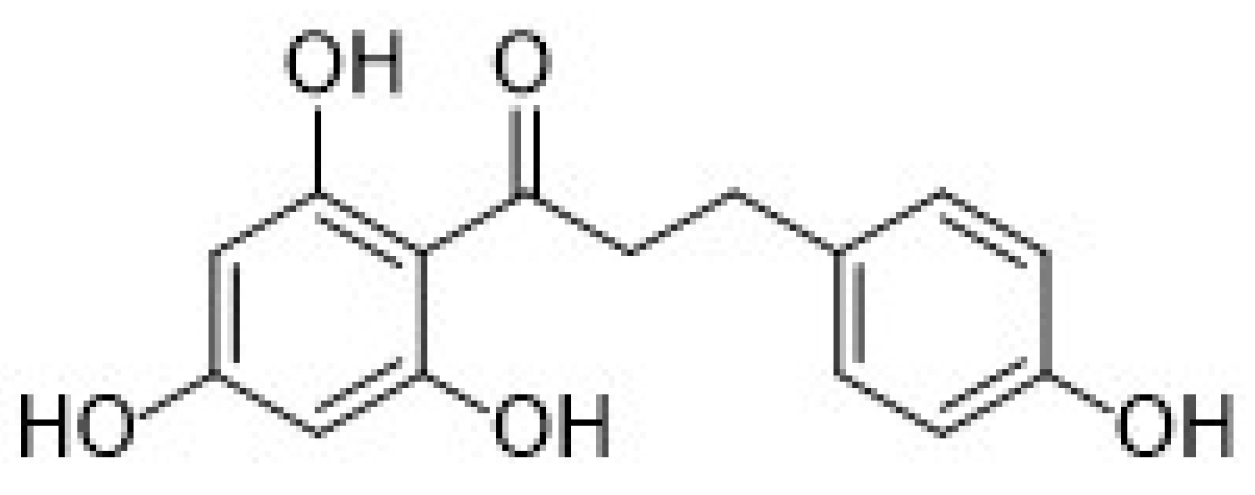
| 3.2. Stilbenes | 27 |
| 3.3. Flavonoids | 29 |
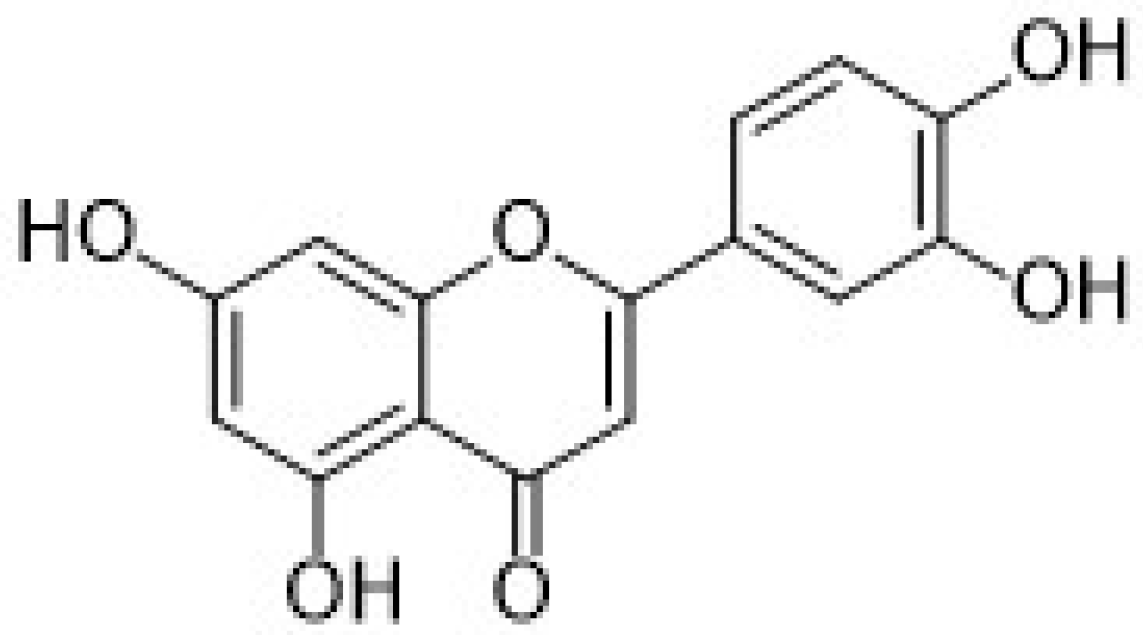
| 3.3.1. Luteolin | 32 |
| 3.3.2. Myricetin | 34 |
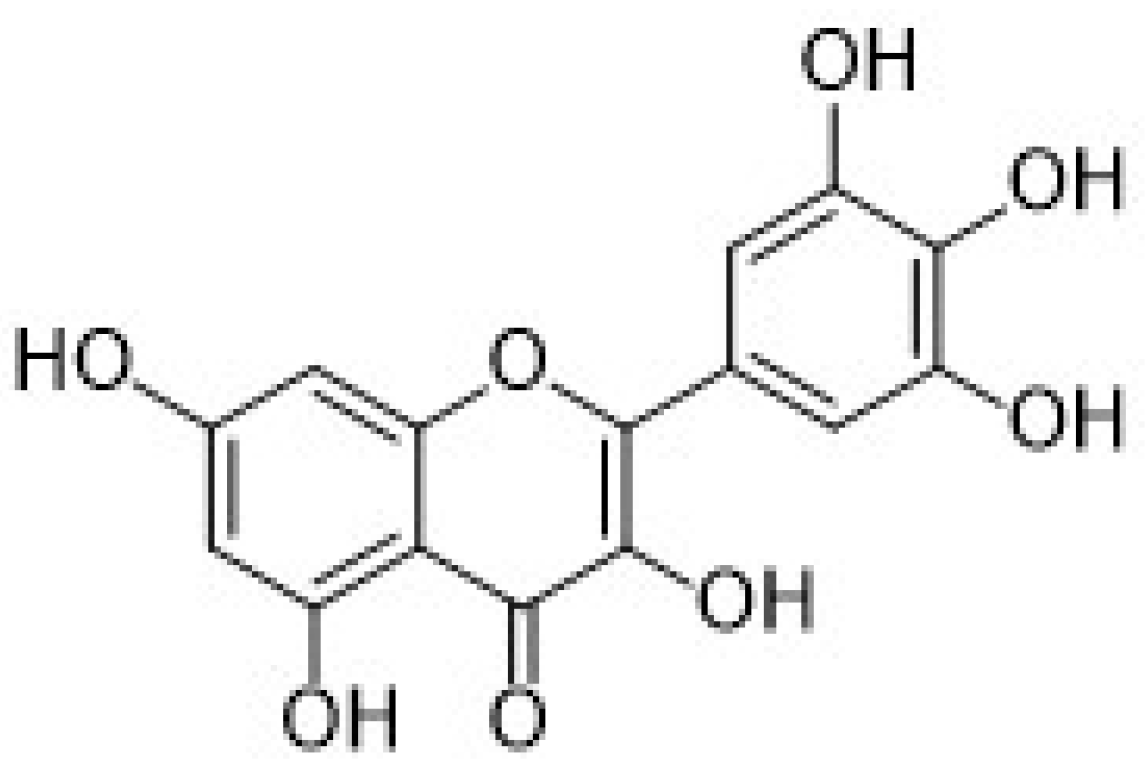
| 3.3.3. Quercetin | 36 |
| 3.3.4. Rutin | 42 |
| 3.3.5. Catechin | 47 |
| 3.3.6. Some considerations on flavonoids (bio)sensors based on Au nanomaterials | 49 |
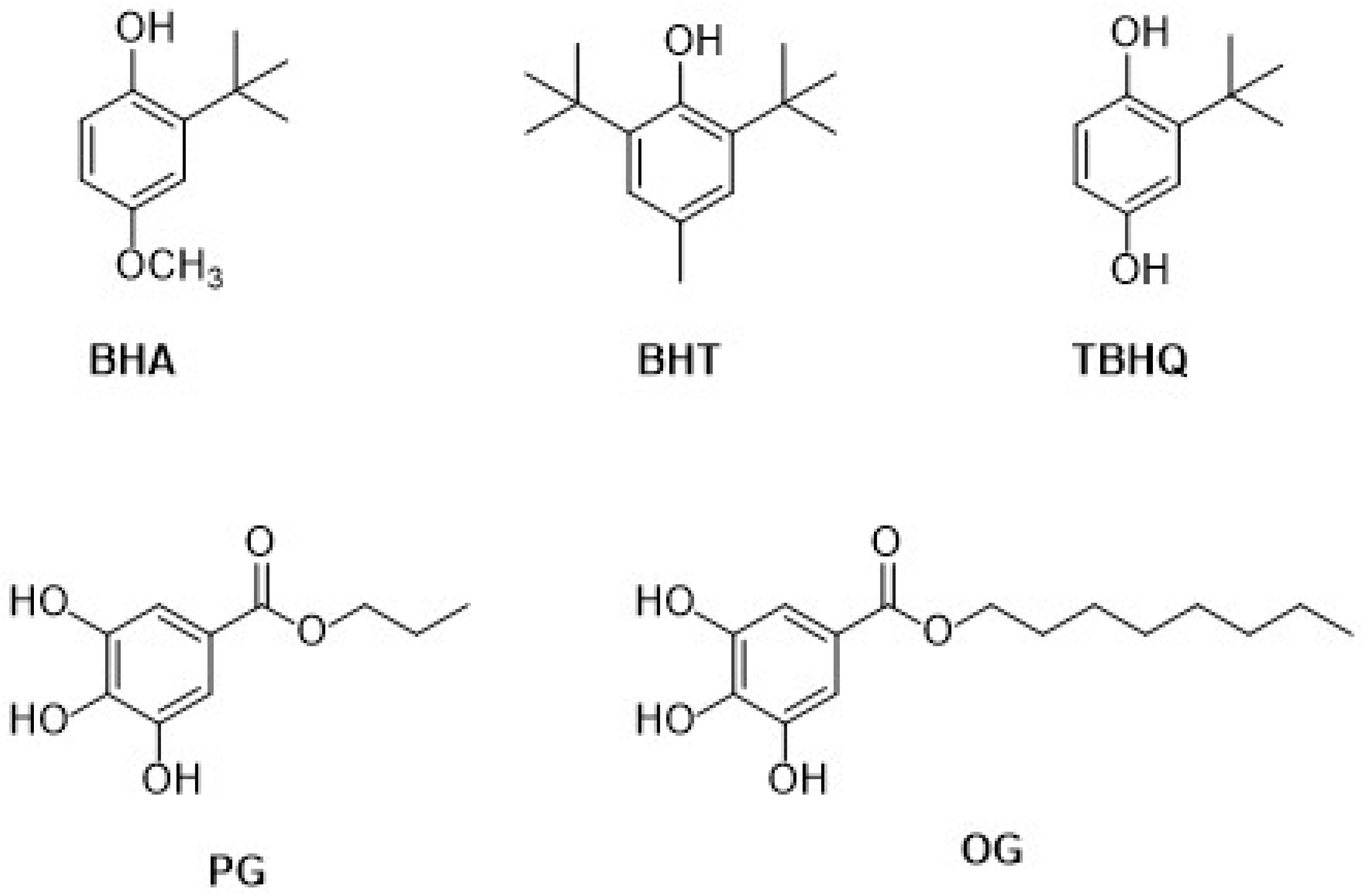
| 3.4. Synthetic phenolic antioxidants | 52 |
| 3.4.1. Butylated hydroxyanisole | 53 |
| 3.4.2. Tert-butylhydroquinone | 55 |
| 3.4.3. Propyl gallate and octyl gallate | 57 |
| 3.4.4. Some considerations on synthetic phenolic antioxidants (bio)sensors based on Au nanomaterials | 58 |
| 4. Conclusions | 60 |
| References | 63 |
1. Introduction
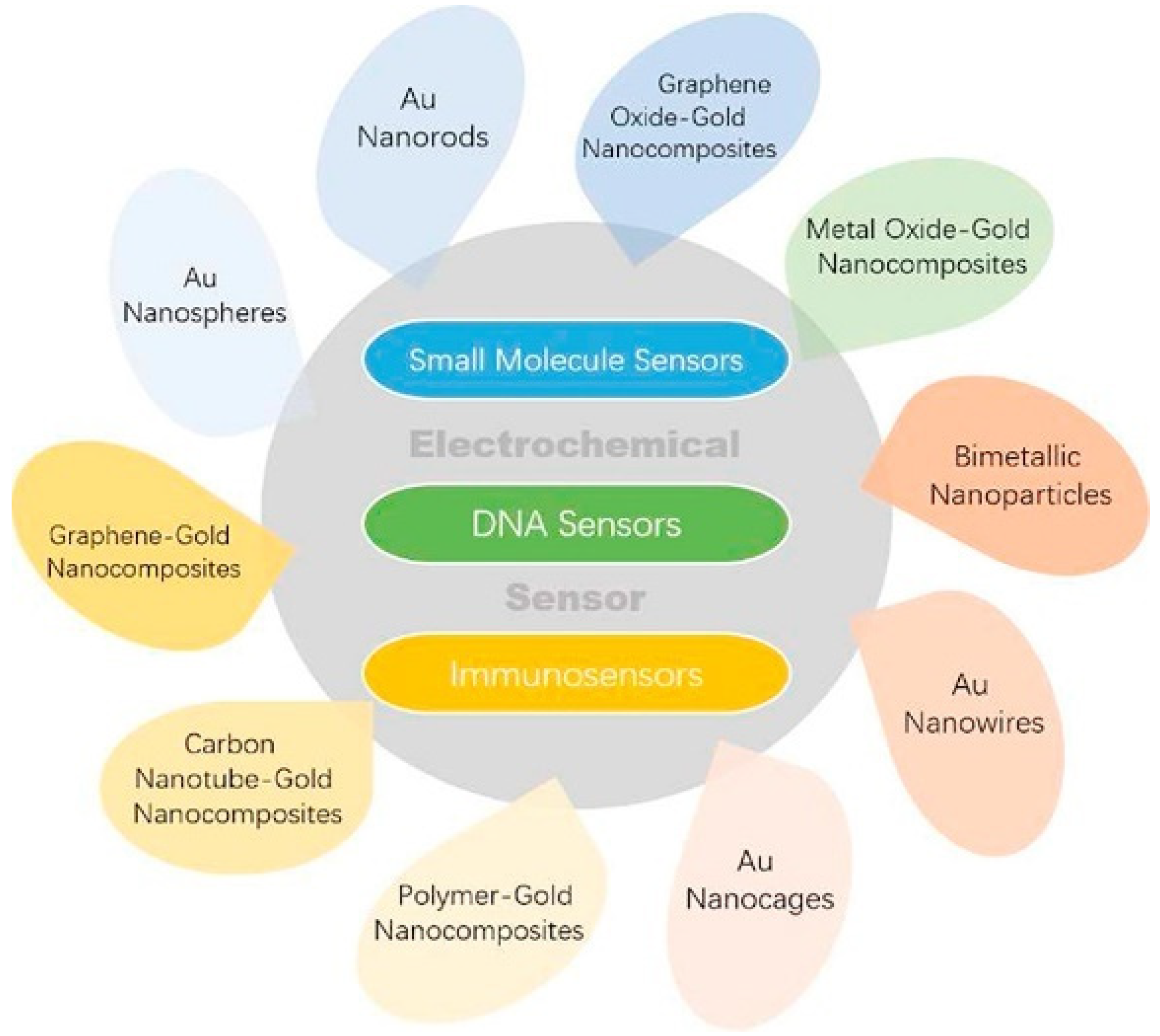
2. Au Nanomaterials
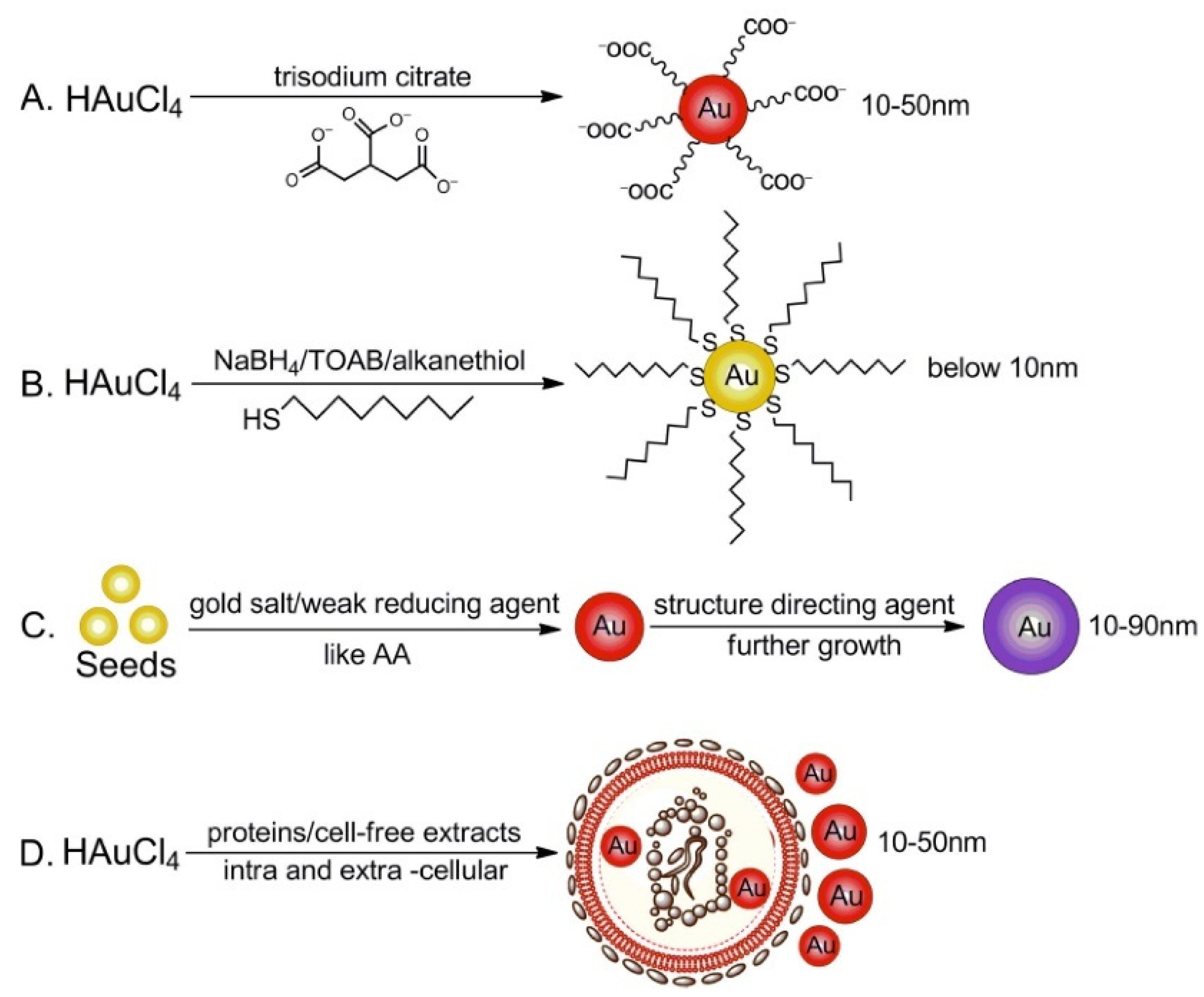
2.1. Au Nanoparticles
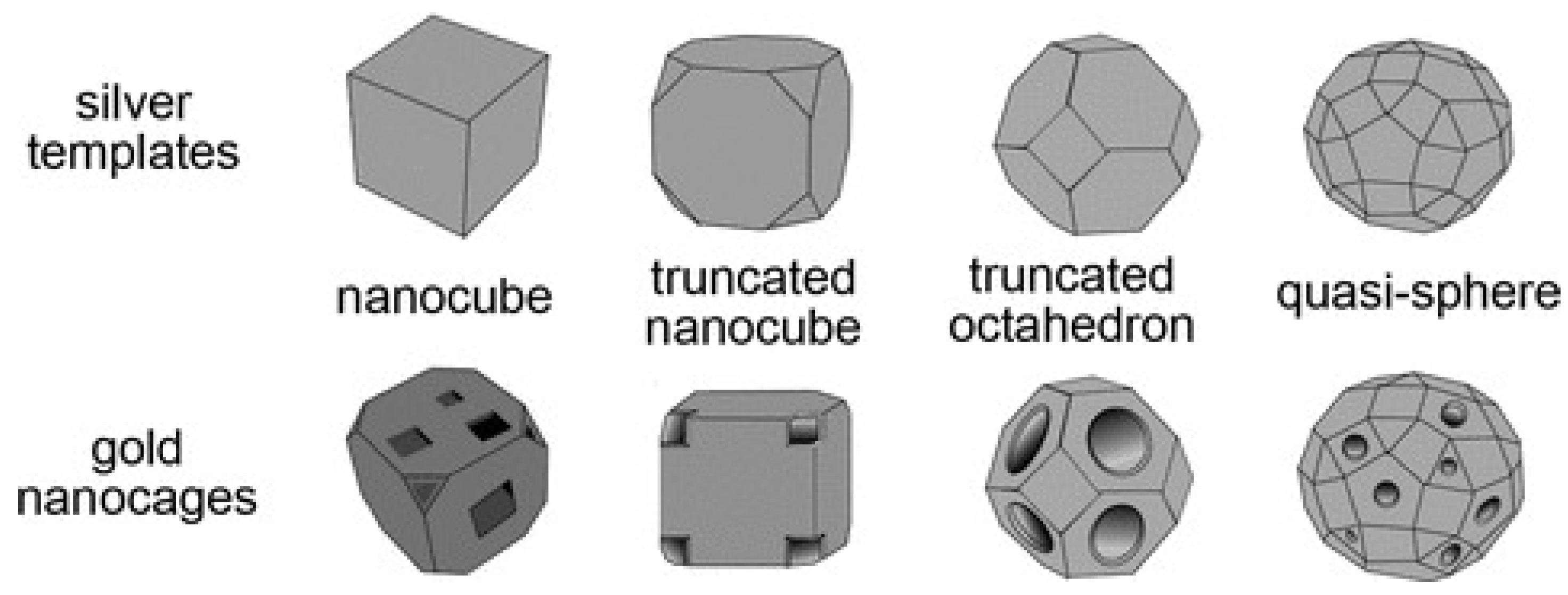
2.2. Au Nanocages
2.3. Nanoporous Gold
2.4. Au-Based Nanomaterials
2.4.1. Bimetallic Nanoparticles
2.4.2. Au-Based Nanocomposites
3. Gold Nanomaterials Applications to Electrochemical Sensors for Phenolic Antioxi-Dants Detection: Some Examples
3.1. Phenolic Acids
3.1.1. Gallic Acid
3.1.2. Hydroxycinnamic Acids
3.1.3. Some Considerations on Phenolic Acids (Bio)sensors Based on Au Nanomaterials
3.2. Stilbenes
3.3. Flavonoids
3.3.1. Luteolin
3.3.2. Myricetin
3.3.3. Quercetin
3.3.4. Rutin
3.3.5. Catechin
3.3.6. Some Considerations on Flavonoids (Bio)sensors Based on Au Nanomaterials
3.4. Synthetic Phenolic Antioxidants
3.4.1. Butylated Hydroxyanisole
3.4.2. Tert-Butylhydroquinone
3.4.3. Propyl Gallate and Octyl Gallate
3.4.4. Some Considerations on Synthetic Phenolic Antioxidants (Bio)sensors Based on Au Nanomaterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 3–44. [Google Scholar]
- Chiorcea-Paquim, A.-M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef]
- Della Pelle, F.; Compagnone, D. Nanomaterial-Based Sensing and Biosensing of Phenolic Compounds and Related Antioxidant Capacity in Food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [Green Version]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits-an overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1924/2006 of the European Parliament and of the Council. Off. J. Eur. Union 2006, L404, 9–40. [Google Scholar]
- Patanè, P.; Laganà, P.; Devi, P.; Vig, A.; Haddad, M.A.; Natalello, S.; Cava, M.A.; Ameen, S.M.; Hashim, H.A. Polyphenols and Functional Foods from the Regulatory Viewpoint. J. AOAC Int. 2019, 102, 1373–1377. [Google Scholar] [CrossRef]
- EU Register of Nutrition and Health Claims Made on Foods. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/ (accessed on 5 November 2021).
- European Community. Council Regulation No. 432/2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Communities 2012, L136, 1–40. [Google Scholar]
- Mastralexi, A.; Nenadis, N.; Tsimidou, M.Z. Addressing Analytical Requirements to Support Health Claims on “Olive Oil Polyphenols” (EC Regulation 432/2012). J. Agric. Food Chem. 2014, 62, 2459–2461. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation, and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Panel on Food additives and Nutrient Sources added to Food (ANS), Statement on the refined exposure assessment of tertiary-butyl hydroquinone (E 319). EFSA J. 2016, 14, 4363–4389. [Google Scholar]
- EFSA. Panel on Food additives and Nutrient Sources added to Food (ANS), Statement on the refined exposure assessment of butylated hydroxyanisole-BHA (E 320) as a food additive. EFSA J. 2011, 9, 2392–2441. [Google Scholar]
- EFSA. Panel on Food additives and Nutrient Sources added to Food (ANS), Statement on the refined exposure assessment of propyl gallate (E 310) as a food additive. EFSA J. 2014, 12, 3642–3688. [Google Scholar]
- EFSA. Panel on Food additives and Nutrient Sources added to Food (ANS), Statement on the refined exposure assessment of octyl gallate (E 311) as a food additive. EFSA J. 2015, 13, 4248–4287. [Google Scholar]
- Nejadmansouri, M.; Majdinasab, M.; Nunes, G.S.; Marty, J.L. An Overview of Optical and Electrochemical Sensors and Biosensors for Analysis of Antioxidants in Food during the Last 5 Years. Biosensors 2020, 10, 1176. [Google Scholar] [CrossRef]
- Vilela, D.; Gonzalez, M.C.; Escarpa, A. Nanoparticles as analytical tools for in-vitro antioxidant-capacity assessment and beyond. Trends Anal. Chem. 2015, 64, 1–16. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Kazakov, Y.E. Electrochemical Hybrid Methods and Sensors for Antioxidant/Oxidant Activity Monitoring and Their Use as a Diagnostic Tool of Oxidative Stress Future Perspectives and Challenges. Chemosensors 2020, 8, 90. [Google Scholar] [CrossRef]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical Methods to Evaluate the Antioxidant Activity and Capacity of Foods: A Review. Electroanalysis 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.-B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of nanomaterials: A crucial step in biosensor fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-M.; Hu, Y.; Yange, Y.-K.; Liu, H.; Fang, G.-Z.; Lu, X. Emerging functional nanomaterials for the detection of food contaminants. Trends Food Sci. Technol. 2018, 71, 94–106. [Google Scholar] [CrossRef]
- Shuo Wanga Malhotra, B.D.; Ali, M.A. Nanomaterials in biosensors: Fundamentals and applications. In Nanomaterials for Biosensors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–75. [Google Scholar]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Curulli, A. Nanomaterials in Electrochemical Sensing Area Applications and Challenges in Food Analysis. Molecules 2020, 25, 5759. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review-Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Ge, L.; Li, S.-P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef]
- Petrucci, R.; Pasquali, M.; Scaramuzzo, F.A.; Curulli, A. Recent Advances in Electrochemical Chitosan-Based Chemosensors and Biosensors: Applications in Food Safety. Chemosensors 2021, 9, 254. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Bartlett, P.N. Bioelectrochemistry 45: Fundamentals, Experimental Techniques, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zoski, C. Handbook of Electrochemistry, 1st ed.; Cynthia, Z., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Suni, I.I. Impedance methods for electrochemical sensors using nanomaterials. TrAC Trends Anal. Chem. 2008, 27, 604–611. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zeng, G.; Lai, C.; Huang, D.; Xu, P.; Zhang, C.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; et al. ‘’Gold rush’’ in modern science: Fabrication strategies and typical advanced applications of gold nanoparticles in sensing. Coord. Chem. Rev. 2018, 359, 1–31. [Google Scholar] [CrossRef]
- Sayadi, K.; FAkbarzadeh, F.; Pourmardan, V.; Saravani-Aval, M.; Sayadi, J.; Pal Singh Chauhan, N.; Sargazi, G. Methods of green synthesis of Au NCs with emphasis on their morphology: A mini-review. Heliyon 2021, 7, e07250. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47–59. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Ortiz-Castillo, J.E.; Gallo-Villanueva, R.C.; Marc, M.J.; Perez-Gonzalez, V.H. Anisotropic gold nanoparticles: A survey of recent synthetic methodologies. Coord. Chem. Rev. 2020, 425, 213489. [Google Scholar] [CrossRef]
- Shinde, S.K.; Kim, D.-Y.; Saratale, R.G.; Kadam, A.A.; Saratale, G.D.; Syed, A.; Bahkali, A.H.; Ghodake, G.S. Histidine Functionalized Gold Nanoparticles for Screening Aminoglycosides and Nanomolar Level Detection of Streptomycin in Water, Milk, and Whey. Chemosensors 2021, 9, 358. [Google Scholar] [CrossRef]
- Falahati, M.; Attar, F.; Sharifi, M.; Saboury, A.A.; Salihi, A.; Aziz, F.M.; Kostova, I.; Burda, C.; Priecel, P.; Lopez-Sanchez, J.A.; et al. Gold nanomaterials as key suppliers in biological and chemical sensing, catalysis, and medicine. BBA-Gen. Subj. 2020, 1864, 129435. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Nanocages Gold, From synthesis to theranostic applications. Accounts Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, M.; Zhang, Q.; Cho, E.C.; Cobley, C.M.; Kim, C.; Glaus, C.; Wang, L.V.; Welch, M.J.; Xia, Y. Gold nanocages: A novel class of multifunctional nanomaterials for theranostic applications. Adv. Funct. Mater. 2010, 20, 3684–3694. [Google Scholar] [CrossRef]
- Raveendran, S.; Sen, A.; Maekawa, T.; Kumar, D.S. Ultra-fast microwave aided synthesis of gold nanocages and structural maneuver studies. Nano Res. 2017, 10, 1078–1091. [Google Scholar] [CrossRef]
- Kim, S.H. Nanoporous gold: Preparation and applications to catalysis and sensors. Curr. Appl. Phys. 2018, 18, 810–818. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, S.; Wang, X.; Xu, P. Nanoporous gold: A review and potentials in biotechnological and biomedical applications. Nano Sel. 2021, 2, 1437–1458. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Nanoporous Gold-Based Sensing. Coatings 2020, 10, 899. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Prakash Dwivedi, R.; ALOthman, Z.A.; Tessema Mola, G. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, S.; Doherty, W.; Amani Babadi, A.; Syamim Akmal Che Mansor, M.; Muhd Julkapli, N.; Hessel, V.; Ostrikov, K. Gold–Carbon Nanocomposites for Environmental Contaminant Sensing. Micromachines 2021, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A.; Ullah, H. Preparation, Functionalization, Modification, and Application of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Khalil, I.; Muhd Julkapli, N.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–Gold Nanoparticles Hybrid—Synthesis, Functionalization, and Application in an Electrochemical and Surface-Enhanced Raman Scattering Biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Haque, E.; Kim, J.; Malgras, V.; Reddy, K.R.; Ward, A.C.; You, J.; Bando, Y.; Shahriar, H.M.d.A.; Yamauchi, Y. Recent Advances in Graphene Quantum Dots: Synthesis, Properties, and Applications. Small Methods 2018, 2, 1800050. [Google Scholar] [CrossRef]
- Fu, Y.; Zeng, G.; Lai, C.; Huang, D.; Qin, L.; Yi, H.; Xigui Liu, X.; Zhang, M.; Li, B.; Liu, S.; et al. Hybrid architectures based on noble metals and carbon-based dots nanomaterials: A review of recent progress in synthesis and applications. Chem. Eng. J. 2020, 399, 125743. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Josowicz, M. Composites of Intrinsically Conducting Polymers as Sensing Nanomaterials. Chem. Rev. 2008, 108, 746–769. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical-Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- John, A.; Benny, L.; Cherian, A.R.; Yethadka Narahari, S.; Varghese, A.; Hegde, G. Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: A review. J. Nanostructure Chem. 2021, 11, 1–31. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polym. Plast. Technol. Mater. 2021, 60, 504–521. [Google Scholar] [CrossRef]
- Bommegowda Rashmi, H.; Singh Negi, P. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Liang, Z.; Zhai, H.; Chen, Z.; Wang, H.; Wang, S.; Zhou, O.; Huang, X. A simple, ultrasensitive sensor for gallic acid and uric acid based on gold microclusters/sulfonate functionalized graphene modified glassy carbon electrode. Sens. Actuators B 2016, 224, 915–925. [Google Scholar] [CrossRef]
- Shahamirifard, S.A.; Ghaedi, M.; Razmi, Z.; Hajati, S. A simple ultrasensitive electrochemical sensor for simultaneous determination of gallic acid and uric acid in human urine and fruit juices based on zirconia-choline chloride-gold nanoparticles-modified carbon paste electrode. Biosens. Bioelectron. 2018, 114, 30–36. [Google Scholar] [CrossRef]
- Datta, S.; Kanjilal, B.; Sarkara, P. Electrochemical Sensor for Detection of Polyphenols in Tea and Wine with Differential Pulse Voltammetry and Electrochemical Impedance Spectroscopy Utilizing Tyrosinase and Gold Nanoparticles Decorated Biomembrane. J. Electrochem. Soc. 2017, 164, B118–B126. [Google Scholar] [CrossRef]
- Hashkavayi, A.; Hashemnia, S.; Osfouri, S. Investigations of antioxidant potential and protective effect of Acanthophora algae on DNA damage: An electrochemical approach. Microchem. J. 2020, 159, 105455. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic Acids and Derivatives Formulations for Skin Damages and Disorders: A Review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives: A Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Voltamperometric Sensors and Biosensors Based on Carbon Nanomaterials Used for Detecting Caffeic Acid-A Review. Int. J. Mol. Sci. 2020, 21, 9275. [Google Scholar] [CrossRef]
- Di Carlo, G.; Curulli, A.; Toro, R.G.; Bianchini, C.; De Caro, T.; Padeletti, G.; Zane, D.; Ingo, G.M. Green Synthesis of Gold–Chitosan Nanocomposites for Caffeic Acid Sensing. Langmuir 2012, 28, 5471–5479. [Google Scholar] [CrossRef]
- Curulli, A.; Di Carlo, G.; Ingo, G.M.; Riccucci, C.; Zane, D.; Bianchini, C. Chitosan Stabilized Gold Nanoparticle-Modified Au Electrodes for the Determination of Polyphenol Index in Wines: A Preliminary Study. Electroanalysis 2012, 24, 897–904. [Google Scholar] [CrossRef]
- Bottari, D.; Pigani, L.; Zanardi, C.; Terzi, F.; Paturca, S.V.; Grigorescu, S.D.; Matei, C.; Lete, C.; Lupu, S. Electrochemical Sensing of Caffeic Acid Using Gold Nanoparticles Embedded in Poly(3,4-ethylenedioxythiophene) Layer by Sinusoidal Voltage Procedure. Chemosensors 2019, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Sawut, N.; Jamal, R.; Abdiryim, T.; Kadir, A.; Zhang, R.; Helil, Z.; Niyaz, M. Preparation of thiol-grafted poly(3,4-ethylenedioxythiophene)/yolk–shell carbon sphere/Au composites for the simultaneous detection of caffeic acid and levofloxacin. J. Mater. Chem. C 2021, 9, 13876. [Google Scholar] [CrossRef]
- Mohtar, L.G.; Aranda, P.; Messina, G.A.; Nazareno, M.A.; Pereira, S.V.; Raba, J.; Bertolino, F.A. Amperometric biosensor based on laccase immobilized onto a nanostructured screen-printed electrode for determination of polyphenols in propolis. Microchem. J. 2019, 144, 13–18. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, H.; Wang, J.; Li, S.; Xiong, Z.; Yan, B.; Caiqin Wang, C.; Du, Y. Visible light enhanced electrochemical detection of caffeic acid with waxberry-like PtAuRu nanoparticles modified GCE. Sens. Actuators B. Chem. 2018, 272, 135–138. [Google Scholar] [CrossRef]
- Vinoth, S.; Shalini Devi, K.S.; Pandikumar, A. A comprehensive review on graphitic carbon nitride based electrochemical and biosensors for environmental and healthcare applications. Trends Anal. Chem. 2021, 140, 116274. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kokulnathan, T.; Tsai, P.-C.; Valan Arasu, M.; Al-Dhabi, N.A.; Prakasham, K.; Kumar Ponnusamy, V. Ultrasonication-assisted synthesis of gold nanoparticles decorated ultrathin graphitic carbon nitride nanosheets as a highly efficient electrocatalyst for sensitive analysis of caffeic acid in food samples. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Della Pelle, F.; Rojas, D.; Silveri, F.; Ferraro, G.; Fratini, E.; Scroccarello, A.; Escarpa, A.; Compagnone, D. Class-selective voltammetric determination of hydroxycinnamic acids structural analogs using a WS2/catechin-capped AuNPs/carbon black–based nanocomposite sensor. Microchim. Acta 2020, 187, 296. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.C.; Ravanini, A.E.; Figueiredo-Filho, L.C.S.; Iniesta, J.; Banks, C.E.; Fatibello-Filho, O. Imparting improvements in electrochemical sensors: Evaluation of different carbon blacks that give rise to significant improvement in the performance of electroanalytical sensing platforms. Electrochim. Acta 2015, 157, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon black as an outstanding and affordable nanomaterial for electrochemical (bio)sensor design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; He, J.; Pang, P.; Gao, Y.; Hu, Q. Electrochemical Behavior of Caffeic Acid Assayed with Gold Nanoparticles/Graphene Nanosheets Modified Glassy Carbon Electrode. Electroanalysis 2013, 25, 1230–1236. [Google Scholar] [CrossRef]
- Favero, G.; Fusco, G.; Mazzei, F.; Tasca, F.; Antiochia, R. Electrochemical Characterization of Graphene and MWCNT Screen-Printed Electrodes Modified with AuNPs for Laccase Biosensor Development. Nanomaterials 2015, 5, 1995–2006. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lu, B.; Gao, Y.; Yang, T.; Yue, R.; Xu, J.; Gao, L. Facile one-pot preparation of Pd–Au/PEDOT/graphene nanocomposites and their high electrochemical sensing performance for caffeic acid detection. RSC Adv. 2016, 6, 89157. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Yue, R.; Yang, T.; Gao, L. Facile one-pot synthesis of Au–PEDOT/rGO nanocomposite for highly sensitive detection of caffeic acid in red wine sample. Electrochim. Acta 2016, 196, 1–12. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Raja, N.; Chen, S.-M.; Liao, W.-C. Nanomolar electrochemical detection of caffeic acid in fortified wine samples based on gold/palladium nanoparticles decorated graphene flakes. J. Colloid Interface Sci. 2017, 501, 77–85. [Google Scholar]
- Bharath, G.; Alhseinat, E.; Madhu, R.; Mugo, S.M.; Alwasel, S.; Harrath, A.H. Facile synthesis of Au@α-Fe2O3@RGO ternary nanocomposites for enhanced electrochemical sensing of caffeic acid toward biomedical applications. J. Alloys Compd. 2018, 750, 819–827. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Li, X.; He, J.; Huang, W.; Cai, Y.; Wang, J.; Chen, T.; Du, Y.; Yao, Y. Au-Nitrogen-Doped Graphene Quantum Dot Composites as “On-Off” Nanosensors for Sensitive Photo-Electrochemical Detection of Caffeic Acid. Nanomaterials 2020, 10, 1972. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Chen, T.; Hu, L.; Du, Y.; Yao, Y.; Goh, M.C. Simply synthesized nitrogen-doped graphene quantum dot (NGQD)-modified electrode for the ultrasensitive photoelectrochemical detection of dopamine. Nanophotonics 2020, 9, 3831–3839. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, M.; Zhang, Q.; Wang, Y.; Kong, X.; Wang, L.; Wang, H.; Zhang, Y. Sensitive determination of chlorogenic acid in pharmaceutical products based on the decoration of 3D macroporous carbon with Au nanoparticles via polyoxometalates. Analyst 2017, 142, 2603–2610. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Yao, Q.; Yan, F.; Cui, C.; Sun, M.; Zhang, H. Facile and green decoration of Pd nanoparticles on macroporous carbon by polyoxometalate with enhanced electrocatalytic ability. RSC Adv. 2016, 6, 39618. [Google Scholar] [CrossRef]
- Song, Y.; Lu XYi Li Guo, Q.; Chen, S.; Mao, L.; Hou, H.; Wang, L. Nitrogen-Doped Carbon Nanotubes Supported by Macroporous Carbon as an Efficient Enzymatic Biosensing Platform for Glucose. Anal. Chem. 2016, 88, 1371–1377. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Huang, W.; Wang, Y.; Hu, X. A novel AuNPs-doped COFs composite as electrochemical probe for chlorogenic acid detection with enhanced sensitivity and stability. Sens. Actuators B. Chem. 2018, 276, 362–369. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, B.; Liu, G.; Zhang, D. Electrochemical Sensors Based on Covalent Organic Frameworks: A Critical Review. Front. Chem. 2020, 8, 601044. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec MLiu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of New, Nanoporous Carbon with Hexagonally Ordered Mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Zhu, R.; Ding, J.; Jin, L.; Pang, H. Interpenetrated structures appeared in supramolecular cages, MOFs, COFs. Coord. Chem. Rev. 2019, 389, 119–140. [Google Scholar] [CrossRef]
- Shi, X.; Yao, Y.; Xu, Y.; Liu, K.; Zhu, G.; Chi, L.; Lu, G. Imparting Catalytic Activity to a Covalent Organic Framework Material by Nanoparticle Encapsulation. ACS Appl. Mater. Interfaces 2017, 9, 7481–7488. [Google Scholar] [CrossRef] [PubMed]
- Chauhana, P.; Annu Nitin Raja, A.; Jain, R. Nanogold modified glassy carbon sensor for the quantification of phytoestrogenchlorogenic acid. Surf. Interfaces 2020, 19, 100536. [Google Scholar] [CrossRef]
- Munteanu, I.-G.; Apetrei, C. Electrochemical Determination of Chlorogenic Acid in Nutraceuticals Using Voltammetric Sensors Based on Screen-Printed Carbon Electrode Modified with Graphene and Gold Nanoparticles. Int. J. Mol. Sci. 2021, 22, 8897. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K.-C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Anselmi, C.; Centini, M.; Ricci, M.; Buonocore, A.; Granata, P.; Tsuno, T.; Maffei Facino, R. Analytical characterization of a ferulic acid/γ-cyclodextrin inclusion complex. J. Pharm. Biomed. Anal. 2006, 40, 875–881. [Google Scholar] [CrossRef]
- Brito, L.G.; Leite, G.Q.; Duarte, F.Í.C.; Ostrosky, E.A.; Ferrari, M.; de Lima, A.A.N.; Nogueira, F.H.A.; Aragão, C.F.S.; de Lelis Ferreira, B.D.; de Freitas Marques, M.B.; et al. Thermal behavior of ferulic acid employing isoconversional models and artificial neural network. J. Therm. Anal. Calorim. 2019, 138, 3715–3726. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Silva-Xavier, W.; Monte-Alto-Costa, A. Topical application of a commercially available formulation of vitamin C stabilized by vitamin E and ferulic acid reduces tissue viability and protein synthesis in ex vivo human normal skin. Cosmet Dermatol. 2020, 19, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.-G.; Apetrei, C. Development of a Novel Electrochemical Biosensor Based on Carbon Nanofibers-Gold Nanoparticles–Tyrosinase for the Detection of Ferulic Acid in Cosmetics. Sensors 2020, 20, 6724. [Google Scholar]
- Buffon, E.; Stradiotto, N.R. A molecularly imprinted polymer on reduced graphene oxide-gold nanoparticles modified screen-printed electrode for selective determination of ferulic acid in orange peels. Microchem. J. 2021, 167, 106339. [Google Scholar] [CrossRef]
- Azevedo Beluomini, M.; da Silva, J.L.; Cardoso de Sa, A.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: A review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Buleandra, M.; Cheregi, M.C. Electrochemical Methods and (Bio) Sensors for Rosmarinic Acid Investigation. Chemosensors 2020, 8, 74. [Google Scholar] [CrossRef]
- Brondani, D.; Zapp, E.; Cruz Vieira, I.; Dupont, J.; Weber Scheeren, C. Gold nanoparticles in an ionic liquid phase supported in a biopolymeric matrix applied in the development of a rosmarinic acid biosensor. Analyst 2011, 136, 2495–2505. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Prakash Mishra, A.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Valere Tsouh Fokou, P.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Komorowska, J.; Wątroba, M.; Szukiewicz, D. Review of beneficial effects of resveratrol in neurodegenerative diseases such as Alzheimer’s disease. Adv. Med. Sci. 2020, 65, 415–423. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Zhang, J.; Li, Y.; Zhang, Y.; Li, Y.; Ye, B.-C. Novel electrochemical sensing platform based on integration of molecularly imprinted polymer with Au@Ag hollow nanoshell for determination of resveratrol. Talanta 2019, 196, 479–485. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Q.; Yang, J.; Chen, S.; Hu, F.; Hu, Y.; Lin, H. Synergistic effects of layer-by-layer films for highly selective and sensitive electrochemical detection of trans-resveratrol. Food Chem. 2021, 338, 127851. [Google Scholar] [CrossRef]
- Huang, S.; Yang, J.; Li, S.; Qin, Y.; Mo, Q.; Chen, L.; Li, X. Highly sensitive molecular imprinted voltammetric sensor for resveratrol assay in wine via polyaniline/gold nanoparticles signal enhancement and polyacrylamide recognition. J. Electroanal. Chem. 2021, 895, 115455. [Google Scholar] [CrossRef]
- Huang, W.; Seehafer, K.; Bunz, U.H.F. Discrimination of Flavonoids by a Hypothesis Free Sensor Array. ACS Appl. Polym. Mater. 2019, 1, 1301–1307. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z. Application of Electrochemical Sensors Based on Carbon Nanomaterials for Detection of Flavonoids. Nanomaterials 2020, 10, 2020. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Ye, Y.; Huang, Z.; Ruan, Z.; Jin, N. Phloretin as both a substrate and inhibitor of tyrosinase: Inhibitory activity and mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117642. [Google Scholar] [CrossRef]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, J.; Pi, F.; Yang, H.; Liu, J.; Zhang, Y.; Xia, S.; Wang, J.; Xu, D.; Sun, X. A novel electrochemical biosensor for antioxidant evaluation of phloretin based on cell-alginate/ʟ-cysteine/gold nanoparticle-modified glassy carbon electrode. Biosens. Bioelectron. 2018, 119, 119–125. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Wozniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Erdogan Orhan, I.; Rizwani, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, Z.; Huang, Y.; Lai, G.; Zhang, H.; Su, W.; Yu, J.; Wang, A.; Lin, C.-T.; Yu, A. Square wave voltammetric quantitative determination of flavonoid luteolin in peanut hulls and Perilla based on Au NPs loaded boron nitride nanosheets. J. Electroanal. Chem. 2018, 817, 128–133. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, R.; Niu, Y.; Sun, W.; Shao, T.; Chen, X. Gold Nanocage-Based Electrochemical Sensing Platform for Sensitive Detection of Luteolin. Sensors 2018, 18, 2309. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Zeng, P.; Ma, C.; Peng, H.; Yang, J.; Huang, J.; Zhang, M.; Cheng, F. Electrochemical sensor for sensitive detection of luteolin based on multi-walled carbon nanotubes/poly(3,4-ethylenedioxythiophene)-gold nanocomposites. New J. Chem. 2020, 44, 1953–1961. [Google Scholar] [CrossRef]
- Niu, X.; Huang, Y.; Zhang, W.; Yan, L.; Wang, L.; Li, Z.; Sun, W. Synthesis of gold nanoflakes decorated biomass-derived porous carbon and its application in electrochemical sensing of luteolin. J. Electroanal. Chem. 2021, 880, 114832. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Z.; Guo, Y.; Dong, C. Electrochemical sensor for facile detection of trace luteolin based on thio-β-cyclodextrin functionalized graphene/gold nanoparticles hybrids. J. Electroanal. Chem. 2015, 759, 137–143. [Google Scholar] [CrossRef]
- Wen, Z.; Li, X.; Niu, X.; Zhao, W.; Cheng, Y.; Ma, Q.; Li, X.; Sun, W.; Li, G. Application of Gold Nanoparticle and Three-Dimensional Graphene Based Electrode for Sensitive Voltammetric Analysis of Luteolin. Int. J. Electrochem. Sci. 2017, 12, 4847–4855. [Google Scholar] [CrossRef]
- Tang, J.; Huang, R.; Zheng, S.; Jiang, S.; Yu, H.; Li, Z.; Wang, J. A sensitive and selective electrochemical sensor based on graphene quantum dots/gold nanoparticles nanocomposite modified electrode for the determination of luteolin in peanut hulls. Microchem. J. 2019, 145, 899–907. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Hajian, R.; Azah Yusof, N.; Faragi, T.; Shams, N. Fabrication of an Electrochemical Sensor Based on Gold Nanoparticles/Carbon Nanotubes as Nanocomposite Materials: Determination of Myricetin in Some Drinks. PLoS ONE 2014, 9, e96686. [Google Scholar] [CrossRef]
- Loon Ng, K.; Mun Lee, S.; Mei Koor, S.; Tuan, G.H. Electrochemical preparation and characterization of a gold nanoparticles graphite electrode: Application to myricetin antioxidant analysis. Anal. Sci. 2015, 31, 1075–1081. [Google Scholar]
- Xing, R.; Tong, L.; Zhao, X.; Liu, H.; Ma, P.; Zhao, J.; Liu, X.; Liu, S. Rapid and sensitive electrochemical detection of myricetin based on polyoxometalates/SnO2/gold nanoparticles ternary nanocomposite film electrode. Sens. Actuators B. Chem. 2019, 283, 35–41. [Google Scholar] [CrossRef]
- Rauf, A.; Imran MKhan, I.A.; Rehman, M.; Gilani, S.G.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef]
- Lutfi Yola, M.; Atar, N. A novel voltammetric sensor based on gold nanoparticles involved in p-aminothiophenol functionalized multi-walled carbon nanotubes: Application to the simultaneous determination of quercetin and rutin. Electrochim. Acta 2014, 119, 24–31. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.-C.; Dhenadhayalan, N.; Lin, K.-C. Light-Controlled Photochemical Synthesis of Gelatin-Capped Gold Nanoparticles for Spectral Activity and Electro-oxidation of Quercetin. ChemElectroChem 2017, 4, 2842–2851. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ibrahim, H.; Almandil, N.B.; Sayed, M.A.; Kawde, A.-N. A new hybrid nanocomposite electrode based on Au/CeO2-decorated functionalized glassy carbon microspheres for the voltammetric sensing of quercetin and its interaction with DNA. Anal. Methods 2020, 12, 2846. [Google Scholar] [CrossRef]
- Ibrahim, H.; Temerk, Y. A novel electrochemical sensor based on B doped CeO2 nanocubesmodified glassy carbon microspheres paste electrode for individual and simultaneous determination of xanthine and hypoxanthine. Sens. Actuators B 2016, 232, 125–137. [Google Scholar] [CrossRef]
- Temerk, Y.M.; Ibrahim, M.S.; Kotb, M.; Schuhmann, W. Interaction of antitumor flavonoids with dsDNA in the absence and presence of Cu (II). Anal. Bioanal. Chem. 2013, 405, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Saljooqi, A.; Shamspur, T.; Mostafavi, A. Fe3O4@SiO2-PANI-Au Nanocomposite Prepared for Electrochemical Determination of Quercetin in Food Samples and Biological Fluids. Electroanalysis 2020, 32, 581–587. [Google Scholar] [CrossRef]
- Biswa, A.; Minakshi, M.; Tripathy, B.C. Probing the electrochemical properties of biopolymer modified EMD nanoflakes through electrodeposition for high performance alkaline batteries. Dalton Trans. 2016, 45, 5557. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Deng, Y.; Zhu, L.; Liu, J.; Zhang, B.; Zhang, Y.; Sun, W.; Li, G. Au-Co nanoparticles-embedded N-doped carbon nanotube hollow polyhedron modified electrode for electrochemical determination of quercetin. Microchim. Acta 2020, 187, 546. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Zhu, P.; Tian, Y.; Chen, Y.; Wu, C. Applications of Functional Metal-Organic Frameworks in Biosensors. Biotechnol. J. 2021, 16, 1900424. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, R.; Yang, P.; Lu, L.; Shen, L.; Tao, J.; Liu, Z.; Zhao, P. An Excellent Electrochemical Sensor Based on Highly Porous Gold Film Modified Gold Electrode for Detecting Quercetin in Food and Medicine. J. Electrochem. Soc. 2020, 167, 047514. [Google Scholar] [CrossRef]
- Nasrollahi, S.; Ghoreishi, S.M.; Khoobi, A. Nanoporous gold film: Surfactant-assisted synthesis, anodic oxidation and sensing application in electrochemical determination of quercetin. J. Electroanal. Chem. 2020, 864, 114097. [Google Scholar] [CrossRef]
- Yola, M.Y.; Atar, N.; Ustundag, Z.; Solak, A.O. A novel voltammetric sensor based on p-aminothiophenol functionalized graphene oxide/gold nanoparticles for determining quercetin in the presence of ascorbic acid. J. Electroanal. Chem. 2013, 698, 9–16. [Google Scholar] [CrossRef]
- Li, J.; Qu, J.; Yang, R.; Qu, L.; Harrington, P. A Sensitive and Selective Electrochemical Sensor Based on Graphene Quantum Dot/Gold Nanoparticle Nanocomposite Modified Electrode for the Determination of Quercetin in Biological Samples. Electroanalysis 2016, 28, 1322–1330. [Google Scholar] [CrossRef]
- Zhou, Z.; Gu, C.; Chen, C.; Zhao, P.; Xie, Y.; Fei, J. An ultrasensitive electrochemical sensor for quercetin based on 1-pyrenebutyrate functionalized reduced oxide graphene /mercapto-β-cyclodextrin /Au nanoparticles composite film. Sens. Actuators B. Chem. 2019, 288, 88–95. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, P.; Wang, C.; Yang, P.; Xie, Y.; Fei, J. Ultra-sensitive amperometric determination of quercetin by using a glassy carbon electrode modified with a nanocomposite prepared from aminated graphene quantum dots, thiolated β-cyclodextrin and gold nanoparticles. Microchim. Acta 2020, 187, 130. [Google Scholar] [CrossRef]
- Stefanov, C.; Cioates Negut, C.; Dinu Gugoasa, L.A.; van Staden, J.F. Gold nanoparticle-graphene quantum dots nanozyme for the wide range and sensitive electrochemical determination of quercetin in plasma droplets. Microchim. Acta 2020, 187, 611. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin-A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar]
- Brugnerotto, P.; Silva, T.R.; Brondani, D.; Zapp, E.; Cruz Vieira, I. Gold Nanoparticles Stabilized in β-Cyclodextrin and Decorated with Laccase Applied in the Construction of a Biosensor for Rutin. Electroanalysis 2017, 29, 1031–1037. [Google Scholar] [CrossRef]
- Yang, H.; Li, B.; Cui, R.; Xing, R.; Liu, S. Electrochemical sensor for rutin detection based on Au nanoparticle-loaded helical carbon nanotubes. J. Nanopart. Res. 2017, 19, 354. [Google Scholar] [CrossRef]
- Cui, R.; Han, Z.; Zhu, J.J. Helical carbon nanotubes: Intrinsic peroxidase catalytic activity and its application for biocatalysis and biosensing. Chem. A Eur. J. 2011, 17, 9377–9384. [Google Scholar] [CrossRef]
- Liu, J.; Weng, W.; Yin, C.; Li XNiu, Y.; Li, G.; Sun, W. A sensitive electrochemical sensor for detection of rutin based on a gold nanocage-modified electrode. J. Chin. Chem. Soc. 2019, 66, 1336–1340. [Google Scholar] [CrossRef]
- Zhong, T.; Guo, Q.; Yin, Z.; Zhu, X.; Liu, R.; Liu, A.; Huang, S. Polyphenol oxidase/gold nanoparticles/mesoporous carbon-modified electrode as an electrochemical sensing platform for rutin in dark teas. RSC Adv. 2019, 9, 2152. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Chen, H.; Zhan, X.; Qin, X.; Kuang, Y.; Li, M.; Liang, Z.; Yang, J.; Su, Z. One-pot electrodeposition of metal organic frameworks composites accelerated by electroreduced graphene oxide and gold nanoparticles for rutin electroanalysis. J. Electroanal. Chem. 2021, 897, 115590. [Google Scholar] [CrossRef]
- Chen, X.; Yang, G.; Feng, S.; Shi, L.; Huang, Z.; Pan, H.; Liu, W. Au@AuPt nanoparticles embedded in B-doped graphene: A superior electrocatalyst for determination of rutin. Appl. Surf. Sci. 2017, 402, 232–244. [Google Scholar] [CrossRef]
- Niu, X.; Wen, Z.; Li, X.; Zhao, W.; Li, X.; Huang, Y.; Li, Q.; Li, G.; Sun, W. Fabrication of graphene and gold nanoparticle modified acupuncture needle electrode and its application in rutin analysis. Sens. Actuators B 2018, 255, 471–477. [Google Scholar] [CrossRef]
- Yang, B.; Bin, D.; Zhang, K.; Dua, Y.; Majima, T. A seed-mediated method to design N-doped graphene supported gold silver nanothorns sensor for rutin detection. J. Colloid Interface Sci. 2018, 512, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, I.M.; Apetrei, C. A modified nanostructured graphene-gold nanoparticle carbon screen-printed electrode for the sensitive voltammetric detection of rutin. Measurement 2018, 114, 37–43. [Google Scholar] [CrossRef]
- Guo, Q.; Hu, F.; Yang, X.; Yang, J.; Yang, S.; Chen, X.; Wu, F.; Minteer, S.D. In-situ and controllable synthesis of graphene-gold nanoparticles/molecularly imprinted polymers composite modified electrode for sensitive and selective rutin detection. Microchem. J. 2020, 158, 105254. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Tang, Y.; Zhao, F.; Zeng, B. Fabrication and application of a rutin electrochemical sensor based on rose-like AuNPs-MoS2-GN composite and molecularly imprinted chitosan. Microchem. J. 2021, 168, 106505. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, R.; Dong, C.; Cheng, F.; Guo, Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosens. Bioelectron. 2019, 142, 111529. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, M. Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46, 1994–1998. [Google Scholar] [CrossRef]
- Li, S.; Yang, B.; Wang, C.; Wang, J.; Feng, Y.; Yan, B.; Xiong, Z.; Du, Y. A facile and green fabrication of Cu2O-Au/NG nanocomposites for sensitive electrochemical determination of rutin. J. Electroanal. Chem. 2017, 786, 20–27. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Li, R.-F.; Zhang, S.-F.; Wang, Z.-X.; Li, H.-Y.; Hai-Lin Fang, H.-L.; Wang, W. Nitrogen and sulphur co-doped reduced graphene oxide-gold nanoparticle composites for electrochemical sensing of rutin. Microchem. J. 2021, 160, 105684. [Google Scholar] [CrossRef]
- Yuann, J.-M.P.; Lee, S.-Y.; Yang, M.-J.; Huang, S.-T.; Cheng, C.-W.; Liang, J.-Y. A Study of Catechin Photostability Using Photolytic Processing. Processes 2021, 9, 293. [Google Scholar] [CrossRef]
- Sabaghi, M.; Hoseyni, S.Z.; Tavasoli, S.; Mozafari, M.R.; Katouzian, I. Strategies of confining green tea catechin compounds in nano-biopolymeric matrices: A review. Colloids Surf. B Biointerfaces 2021, 204, 111781. [Google Scholar] [CrossRef]
- Singh, S.; Jain, D.V.S.; Singla, M.L. One step electrochemical synthesis of gold nanoparticles–polypyrrole composite for application in catechin electrochemical biosensor. Anal. Methods 2013, 5, 1024–1032. [Google Scholar] [CrossRef]
- Datta, S.; Banerjee, S.; Sarkar, P. Electrochemical Biosensing of Tea Polyphenols by Modified Enzyme Electrode via Gold Nanoparticles Produced by Green Technology. Int. J. Adv. Inf. Sci. Technol. 2014, 23, 435–443. [Google Scholar]
- Feng, S.; Hu, S.; Chen, X.; Zhang, G.; Liu, G.; Liu, W. Au@Pt Nanoparticles Embedded in N-doped Graphene as sensor for Determination of Catechin. Int. J. Electrochem. Sci. 2020, 15, 6778–6789. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. Glassy carbon electrodes modified with gold nanoparticles for the simultaneous determination of three food antioxidants. Anal. Chim. Acta 2013, 765, 54–62. [Google Scholar] [CrossRef]
- Fan, L.; Kan, X. Sensitive detection of butylated hydroxyanisole based on free-standing paper decorated with gold and NiO nanoparticles. Microchem. J. 2020, 159, 105511. [Google Scholar] [CrossRef]
- Motia, S.; Bouchikhi, B.; El Bari, N. An electrochemical molecularly imprinted sensor based on chitosan capped with gold nanoparticles and its application for highly sensitive butylated hydroxyanisole analysis in foodstuff products. Talanta 2021, 223, 121689. [Google Scholar] [CrossRef]
- Yue, X.; Song, W.; Zhu, W.; Wang, J.; Wang, Y. In situ surface electrochemical co-reduction route towards controllable construction of AuNPs/ERGO electrochemical sensing platform for simultaneous determination of BHA and TBHQ. Electrochim. Acta 2015, 182, 847–855. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Wang, H.; Li, J.; Qu, L.; Harrington, P. High-selective and sensitive voltammetric sensor for butylated hydroxyanisole based on AuNPs–PVP–graphene nanocomposites. Talanta 2015, 138, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yin, W.; Tang, K.; Li, D.; Shao, K.; Zuo, Y.; Ma, J.; Liu, J.; Han, H. Enzymatic biosensor of horseradish peroxidase immobilized on Au-Pt nanotube/Au-graphene for the simultaneous determination of antioxidants. Anal. Chim. Acta 2016, 933, 89–96. [Google Scholar] [CrossRef]
- Du, Y.; Gao, X.; Ye, X.; Zheng, Z.; Feng, Q.; Wang, C.; Wu, K. Composition, and architecture-engineered Au–SnO2/GNs-SWCNTs nanocomposites as ultrasensitive and robust electrochemical sensor for antioxidant additives in foods. Sens. Actuators B 2014, 203, 926–934. [Google Scholar] [CrossRef]
- Fan, L.; Hao, Q.; Kan, Q. Three-dimensional graphite paper based imprinted electrochemical sensor for tertiary butylhydroquinone selective recognition and sensitive detection. Sens. Actuators B 2018, 256, 520–527. [Google Scholar] [CrossRef]
- Chen, T.; Xu, J.; Yang, P.; Sheng, Q.; Zheng, J.; Cao, W.; Yue, T.; Zhou, M.; Wang, C. Facile controlled synthesis of AuPd and AuPt bimetallic nanocrystals for enhanced electrocatalytic sensing. Sens. Actuators B. Chem. 2019, 298, 126724. [Google Scholar] [CrossRef]
- Yue, X.; Luo, X.; Zhou, Z.; Bai, Y. Selective electrochemical determination of tertiary butylhydroquinone in edible oils based on an in-situ assembly molecularly imprinted polymer sensor. Food Chem. 2019, 289, 84–94. [Google Scholar] [CrossRef]
- Ezhil Vilian, A.T.; Umapathi, R.; Hwang, S.-K.; Lee, M.J.; Huh, Y.S.; Han, Y.-K. Simple synthesis of a clew-like tungsten carbide nanocomposite decorated with gold nanoparticles for the ultrasensitive detection of tert-butylhydroquinone. Food Chem. 2021, 348, 128936. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Cho, J.-M.; Kim, I.; Jeong, D.S.; Lee, K.-S.; Park, J.-K.; Baik, Y.-J.; Choi, H.-J.; Lee, W.-S. Tungsten carbide nanowalls as electrocatalyst for hydrogen evolution reaction: New approach to durability issue. Appl. Catal. B Environ. 2017, 203, 684–691. [Google Scholar] [CrossRef]
- Cui, M.; Huang, J.; Wang, Y.; Wu, Y.; Luo, X. Molecularly imprinted electrochemical sensor for propyl gallate based on PtAu bimetallic nanoparticles modified graphene-carbon nanotube composites. Biosens. Bioelectron. 2015, 68, 563–569. [Google Scholar] [CrossRef]
- Cyriac, S.T.; Thomas, D.; Vikraman, A.E.; Kumar, K.G. Electrochemical Sensor for Propyl Gallate, Based on Synergic Effect of Gold Nanoparticle and Poly(p-aminobenzenesulfonic Acid). J. Electrochem. Soc. 2016, 163, B683–B688. [Google Scholar] [CrossRef]
- Sivasankaran, U.; Vikraman, A.E.; Thomas, D.; Kumar, K.G. Nanomolar Level Determination of Octyl Gallate in Fats and Oils. Food Anal. Methods 2016, 9, 2115–2123. [Google Scholar] [CrossRef]
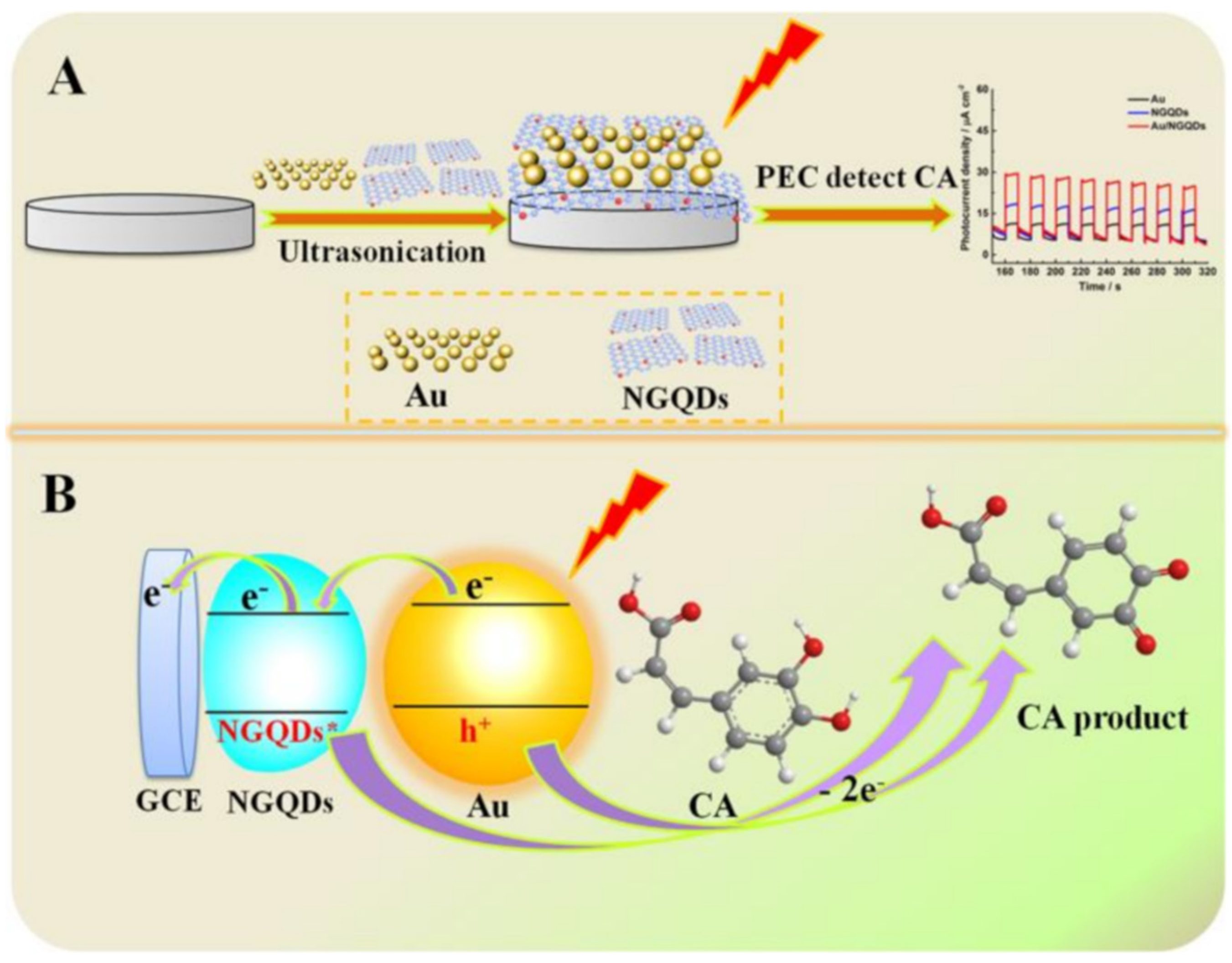
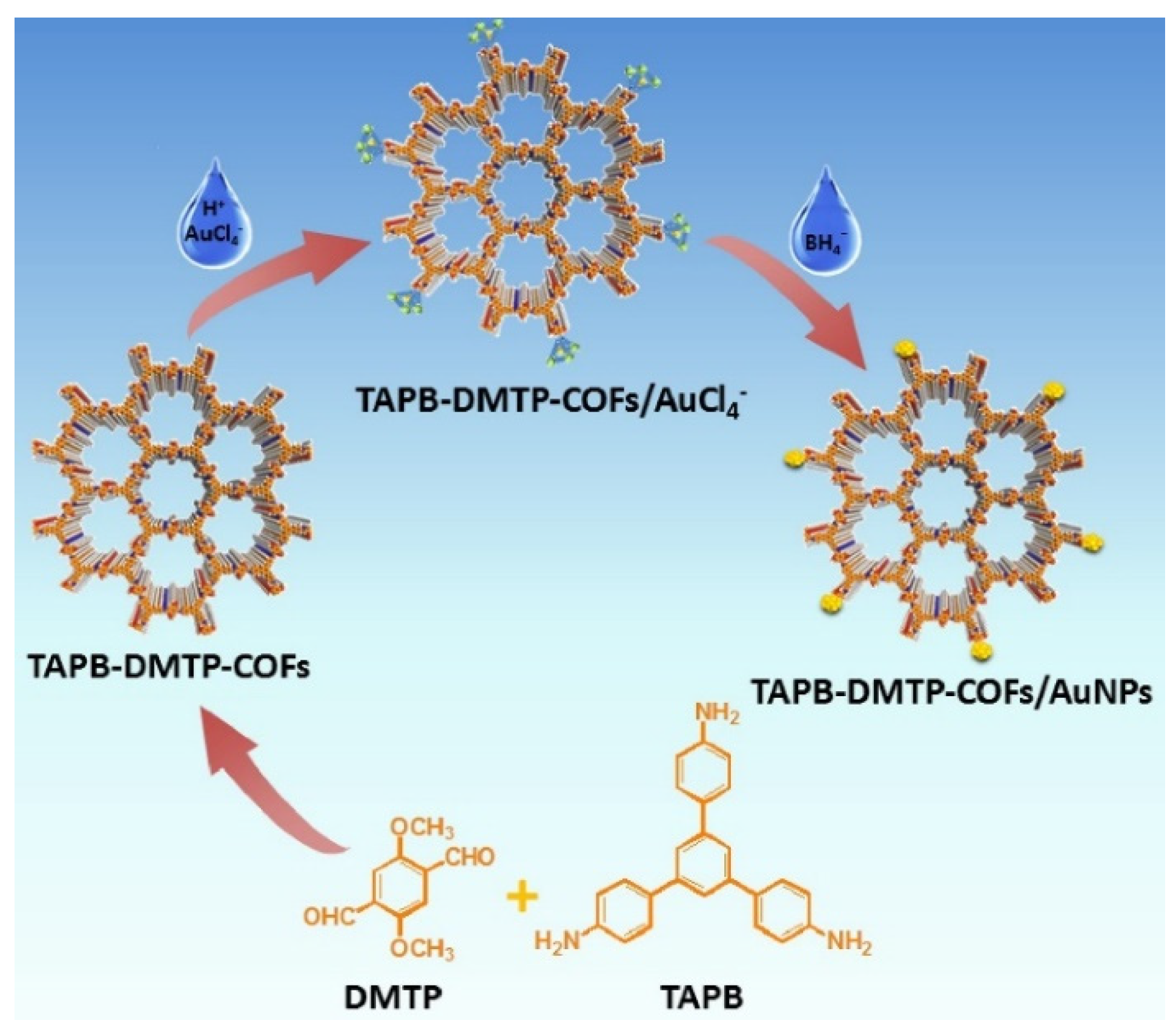

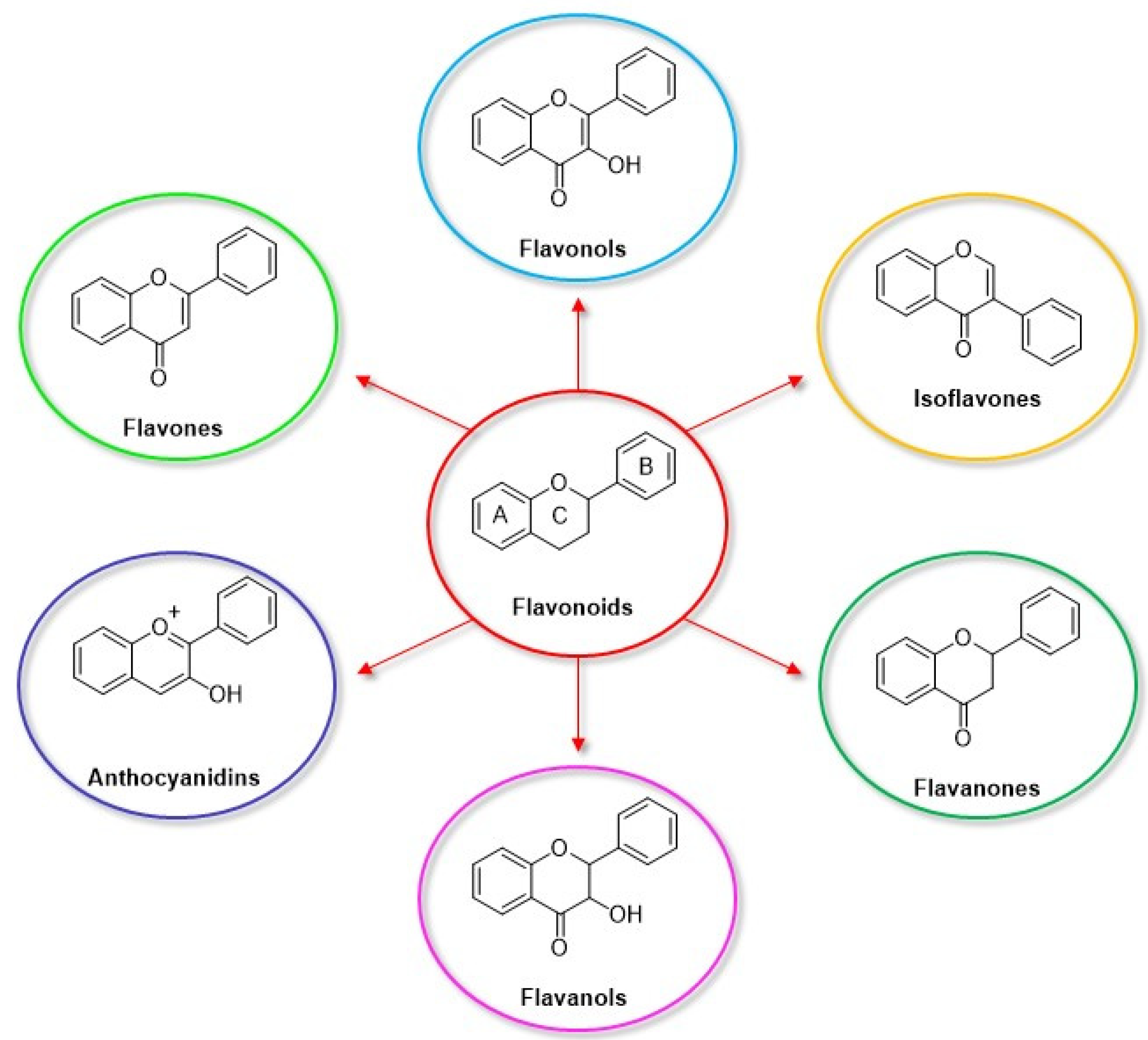



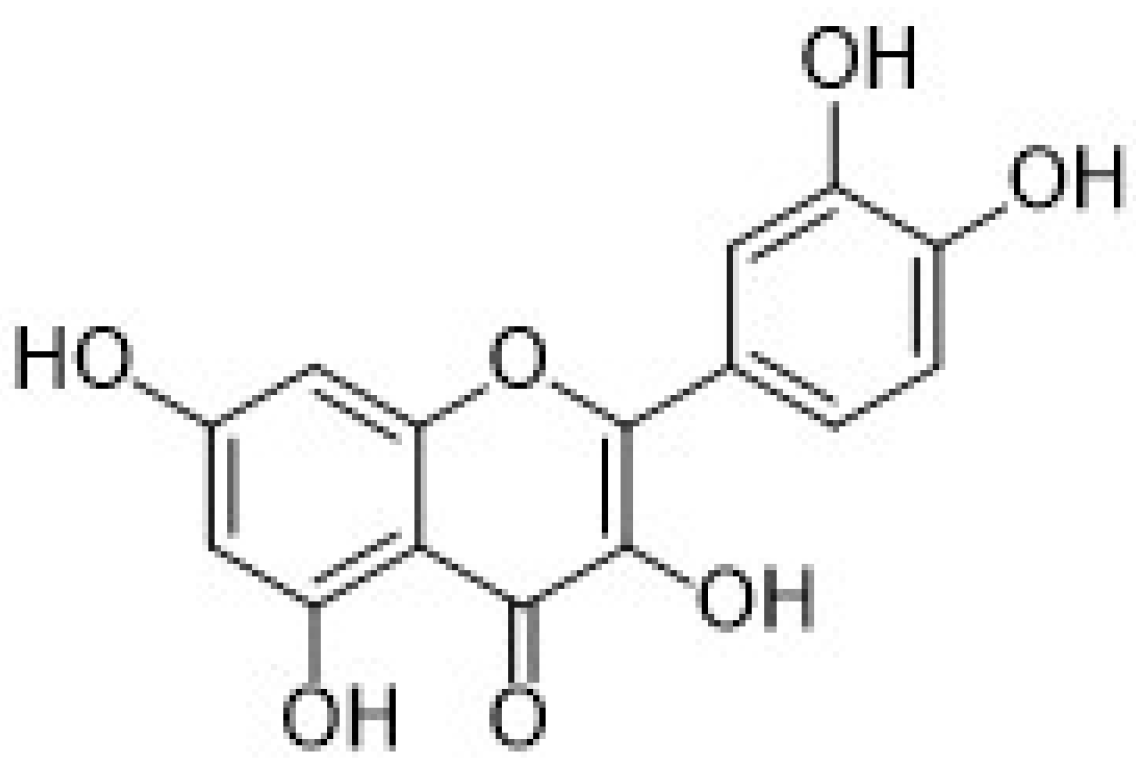

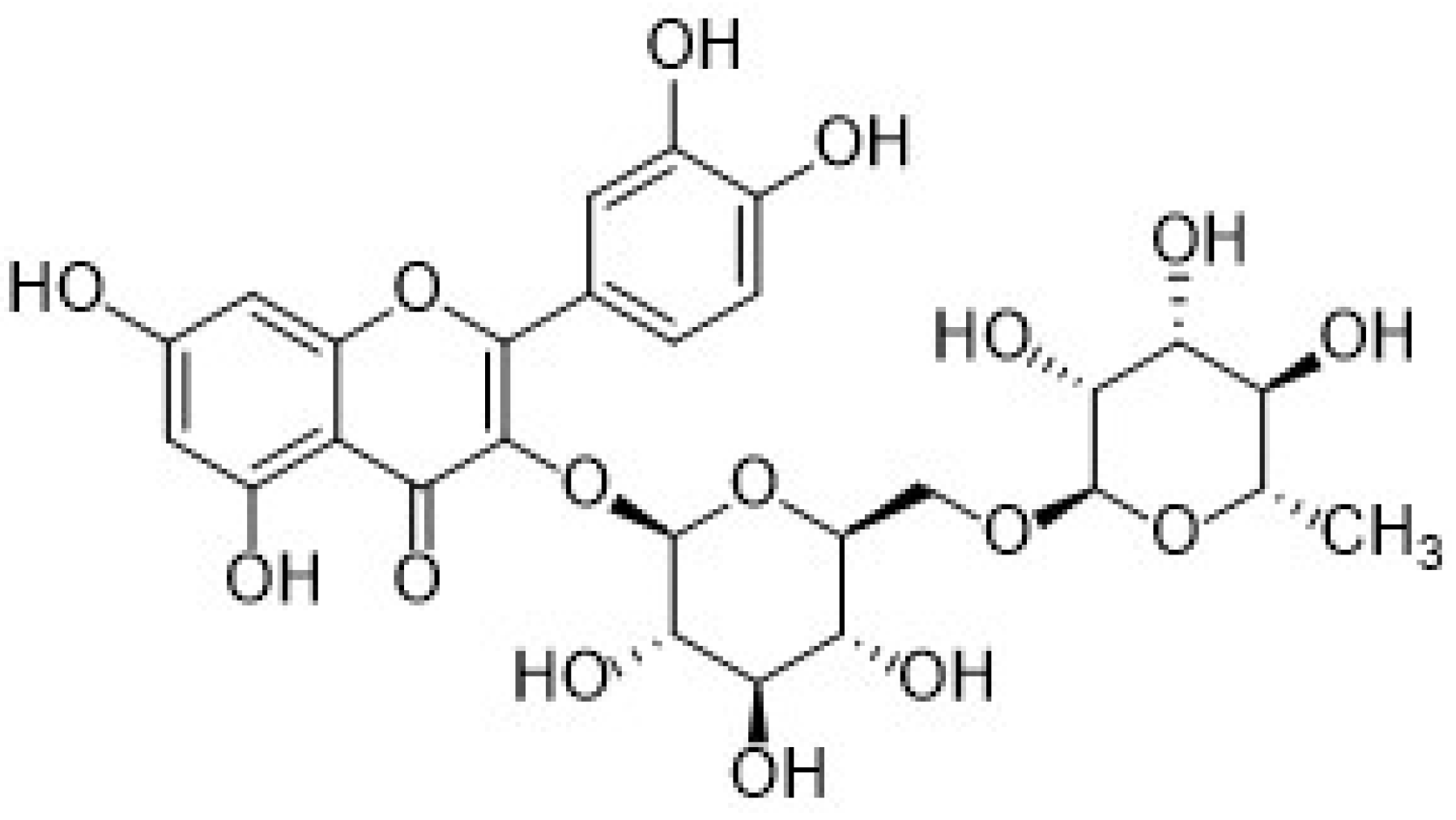

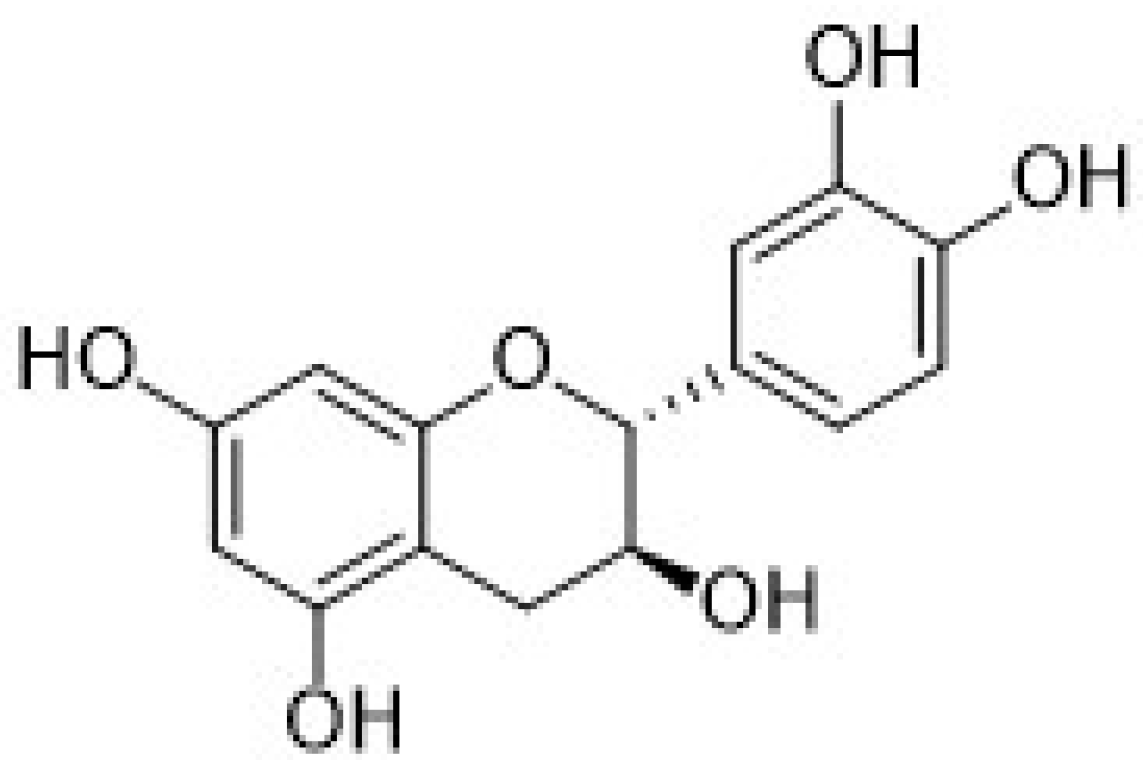

| Electrode | (Bio)sensor Format | Electrochemical Technique | Analyte/Sample | Linearity Range (μM) | LOD (μM) | Recovery (%) | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | Electrochemical sensor based on AuMCs/G nanocomposite | DPV | GA/black teas, urine | 0.05–8.00 | 0.0007 | 96–102.4 | HPLC | [72] |
| CPE | Electrochemical sensor based on ZrO2NPs/ChCl/ AuNPs nanocomposite | DPV | GA/green teas, urine, fruit juices | 0.22–55.00 | 0.025 | 97.9–103.8 | no | [73] |
| GCE | Electrochemical biosensor based on TYR immobilized by crosslinking on AuNPs coated with ESM | DPV | GA, CA, CH/black tea, red wine | GA 6–70 CA 5–65 CH 5–115 | GA 1.707 CA 0.752 CH 0.714 | no | HPLC | [74] |
| SPCE | Electrochemical sensor based on AuNPs prepared using Achantophora algae extracts as reducing agent | DPV | GA/Acanthophora algae extracts | no | no | no | no | [75] |
| AuE | Electrochemical sensor based on AuNPs/CHI nanocomposites | DPV | CA/red and white wines | 0.050–2000 | 0.025 | no | no | [80,81] |
| GCE | Electrochemical sensor based on PEDOT/AuNPs nanocomposite | CV | CA/fruit juices | 10–1000 | 4.24 | no | HPLC | [82] |
| GCE | Electrochemical sensor based on PEDOT-MeSH/YRFC/AuNPs | DPV | CA/human serum, urine | 0.02–320 | 0.014 | 97.4–105.0 | no | [83] |
| SPCE | Biosensor obtained by AuNPs electrodeposition, LAC immobilization, pyrrole electropolymerization | Amperometry | CA/propolis extracts | 1–250 | 0.83 | no | FC | [84] |
| AuNPs/SPCE | Biosensor obtained by TYR immobilization on AuNPs/SPCE | Amperometry | CA/beers | 2.5–12.50 | 2.3 | no | no | [85] |
| GCE | Electrochemical sensor obtained modifying a GCE with trimetallic PtAuRu NPs | DPV | CA | 7.9–16600 | 0.39 | no | no | [87] |
| SPCE | Electrochemical sensor based on g-C3N4/AuNPs nanocomposite | DPV/amperometry | CA/fruits, leaves, wine | DPV 0.0005–0.155 Amperometry 0.0025–1.025 | DPV 0.0001 Amperometry 0.0005 | 98.0–100.3 | no | [88] |
| SPCE | Electrochemical sensor based on WS2/catechin capped AuNPs/CB nanocomposite | DPV | CA/rapeseed oil, Kalanchoe crenata, apple puree, apple homogenized, apple juices | 0.3–200 | 0.1 | 86.0–108.0 | no | [89] |
| GCE | Electrochemical sensor based on GNs/AuNPs nanocomposite | DPV | CA/drug samples | 0.50–50 | 0.05 | 97.6–101.8 | no | [92] |
MWCNTs/SPCE | Electrochemical biosensor obtained by immobilizing TvL and depositing AuNPs simultaneously | Chronoamperometry | CA | 1–100 | 0.5 | no | no | [93] |
| GCE | Electrochemical sensor based on PdAu/PEDOT/rG nanocomposite | DPV | CA/red wine | 0.001–55 | 0.00037 | 98–104 | no | [94] |
| GCE | Electrochemical sensor based on Au/PEDOT/rG nanocomposite | DPV | CA/red wine | 0.01–46 | 0.004 | no | no | [95] |
| GCE | Electrochemical sensor based on AuPdNPs/GFs nanocomposite | DPV | CA/red wine | 0.03–938.97 | 0.006 | no | no | [96] |
| GCE | Electrochemical sensor based on Au-αFe2O3 NPs/rGO nanocomposite | DPV | CA/coffee | 19–1869 | 0.098 | no | no | [97] |
| GCE | Electrochemical sensor based on AuNPs/NGQDs nanocomposite | Amperometry | CA | 0.11–30.25 30.25–280.25 | 0.03 | no | no | [98] |
| GCE | Electrochemical sensor based on AuNPs-POM/MPC nanocomposite | DPV | CGA/pharmaceutical samples | 0.00228–3.24 | 0.00215 | no | Spectrophotometric analysis | [100] |
| GCE | Electrochemical sensor based on AuNPs doped COF composite | DPV | CGA/coffee, apple juice, honeysuckle | 0.010–40 | 0.0095 | 99.2–102.5 | HPLC | [103] |
| GCE | Electrochemical sensor based on AuNPs modified electrode | SWV | CGA/black and green teas | 0.0011–0.010 | 0.040 | 98–99 | no | [108] |
| AuNPs/G/SPCE | Electrochemical sensor using commercial SPCEs modified with AuNPs and G | CV | CGA/nutraceutical products | 0.1–1.20 | 0.062 | no | FT-IR | [109] |
| CNFs/AuNPs/SPCE | Electrochemical biosensor obtained immobilizing TYR on commercial SPCEs modified with AuNPs and CNFs | CV | FA/cosmetic products | 0.1–1.60 | 0.00289 | no | FT-IR | [114] |
| SPCE | Electrochemical sensor based on MIP/AuNPs/GO nanocomposite | DPV | FA/orange peels | 0.01–1 2–10 | 0.0031 | 99–103 | no | [115] |
| CPE | Electrochemical biosensor obtained immobilizing PER in AuNPs-BMIPF6- CTN composite | SWV | RA/pharmaceutical samples | 0.50–23.70 | 0.070 | 98–106.2 | no | [118] |
| Electrode | (Bio)Sensor Format | Electrochemical Technique | Analyte/Sample | Linearity Range (μM) | LOD (μM) | Recovery (%) | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| ITOE | Electrochemical sensor based on MIP/Au@Ag nanoshells nanocomposite | CV | RES/grape seed extracts | 2.0 × 10−5–9.0 × 10−3 | 7.1 × 10−6 | 96.7–106.3 | HPLC | [122] |
| GCE | Electrochemical sensor based on MIP/AuNPs/ GNs nanocomposite | CV | RES/red wine, grape skin | 0.01–10 | 0.0044 | 94.5–102.5 | HPLC | [123] |
| GCE | Electrochemical sensor based on MIP/AuNPs/ PANI | DPV | RES/red wine | 1.0–200 | 0.087 | 97.7–108 | HPLC | [124] |
| Electrode | (Bio)sensor Format | Electrochemical Technique | Analyte/Sample | Linearity Range (μM) | LOD (μM) | Recovery (%) | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | Electrochemical sensor based on BNNs/AuNPs nanocomposite | SWV | LUT/peanuts hull, Perilla | 5 × 10−6–0.0012 0.02–10 | 1.7 × 10−6 | 98.3–108.38 | HPLC | [132] |
| CILE | Electrochemical sensor based on AuNCs-modified CILE | DPV | LUT/drugs | 0.001–1 | 0.0004 | 95.0–96.7 | no | [134] |
| GCE | Electrochemical sensor based on AuNPs/MWCNTS/PEDOT nanohybrid | SWV | LUT/human serum | 1–100 100–15000 | 0.22 | 99.63–103.1 | no | [135] |
| GCE | Electrochemical sensor based on AuNPs/BPC/GCE | DPV | LUT/drugs and human urine | 0.15–1.8 1.8–10 | 0.07 | 97.00–105.10 (drugs) 98.5–106.17 (urine) | no | [136] |
| GCE | Electrochemical sensor based on AuNPs/SH-β-CD-GNs/GCE | DPV | LUT/human serum | 1.0 × 10−5–10 | 3.3x10−6 | 97.1–103.2 | no | [137] |
| CILE | Electrochemical sensor based on AuNPs/3DG-modified CILE | DPV | LUT/pharmaceutical products | 0.05–50 | 0.00759 | 95.28–103.77 | no | [138] |
| GCE | Electrochemical sensor based on AuNPs/GQDs/GCE | DPV | LUT/peanut hull | 0.01–10 | 0.001 | 98.8–101.4 | no | [139] |
| GCE | Electrochemical sensor based on AuNPs/enMWCNTs/GCE | CVAdSV | MYR/black and green teas, fruit juices | 0.050–40 | 0.0120 | 101–104 | no | [141] |
| CE | Electrochemical sensor based on AuNPs/CE | SWV | MYR/no real samples | 6.3–31.4 | 1.3 | no | no | [142] |
| ITOE | Electrochemical sensor based on AuNPs-mSnO2NS/ POM/ITO | Amperometry | MYR/fruit juices | 1–110 | 0.067 | 97.65–103.07 | no | [143] |
| GCE | Electrochemical sensor based on AuNPs/MWCNTs/GCE | SWV | QR,RT/fruit juices | 0.001–0.050 | 0.00033 | 96.3–99.4 | no | [146] |
| SPCE | Electrochemical sensor based on AuNPs/GEL/SPCE | DPV | QR/onion, apple | 0.02–34.5 | 0.0019 | 99.2–99.9 | no | [147] |
| PE | Electrochemical sensor based on AuNPs/CeO2 NPs@FGCM/PE | SWV | QR/apple and grape juices green tea, honeysuckle | 0.048–1.09 | 0.00037 | 97.8–102 | HPLC and spectrophotometry | [148] |
| GCE | Electrochemical sensor based on Fe3O4@SiO2/AuNPs/PANI/ GCE | DPV | QR/apple juice, tea, radish leaves, human serum and urine | 0.01–15 | 0.0038 | 95.6–101.1 | no | [151] |
| GCE | Electrochemical sensor based on Au-Co@NCNHP/GCE | DPV | QR/onion and drugs | 0.050–35 | 0.023 | 97.96–102.5 | HPLC | [153] |
| AuE | Electrochemical sensor based on hp-Au/AuE | Amperometry | QR/apple juice, green tea, onion, honeysuckle, pharmaceutical products | 0.02–100 | 0.0039 | 97.43–101.59 | no | [155] |
| NPG-AuE | Electrochemical sensor based on NPG-Au/AuE | DPV | QR/no real samples | 0.01–12 12–100 | 0.0011 | no | no | [156] |
| GCE | Electrochemical sensor based on AuNPs/GO/GCE | DPV | QR/pharmaceutical products | 1.0 × 10−6–10 × 10−5 | 3.0 × 10−6 | no | no | [157] |
| GCE | Electrochemical sensor based on AuNPs/GQDs/GCE | DPV | QR/human serum | 0.01–6 | 0.002 | 95–104.6 | no | [158] |
| GCE | Electrochemical sensor based on PB-rGO/TCD/AuNPs/GCE | DPV | QR/apple juice, red wine, honeysuckle | 0.005–0.4 | 0.00183 | 95–104.3 | no | [159] |
| GCE | Electrochemical sensor based on NH2-GQDs/AuNPs-β-CD/GCE | DPV | QR/honey, tea, honeysuckle, human serum | 0.001–0.21 | 0.285 × 10−3 | 97.5–106.2 | no | [160] |
| SPCE | Electrochemical sensor based on GQDs/AuNPs/SPCE | SWV | QR/human serum | 0.0001–1000 | 3.3 × 10−5 | 92.6–101.7 | no | [161] |
| CPE | Electrochemical biosensor based AuNPs-CD/LAC/CPE | SWV | RT/pharmaceutical products | 0.30–2.97 | 0.14 | 96.8–103.8 | Spectrophotometry | [163] |
| GCE | Electrochemical sensor based on AuNPs/HCNTs/GCE | Amperometry | RT/pharmaceutical products | 0.1–30 | 0.081 | 99.6–102.1 | no | [164] |
| CILE | Electrochemical sensor based on AuCNs/CILE | DPV | RT/pharmaceutical products | 0.004–700.0 | 0.00133 | 94.00–108.40 | no | [166] |
| GCE | Electrochemical biosensor based PPO/AuNPs/EDU-15/GCE | Amperometry | RT/black tea | 1.5–28 | 0.51 | 98.5–105.5 | no | [167] |
| GCE | Electrochemical sensor based on ErGO/AuNPs-MOFs/GCE | DPV | RT/pharmaceutical products | 0.007–0.14 0.14–0.4 | 0.00344 | 86.9–104.8 | no | [168] |
| GCE | Electrochemical sensor based on Au@ AuPtNPs/B-doped grahene/GCE | DPV | RT/pharmaceutical products | 0.002–4.0 | 2.84x10−4 | 97.23–101.65 | no | [169] |
| ANE | Electrochemical sensor based on AuNPs/G/ANE | DPV | RT/pharmaceutical products, human urine | 0.08–10 20–200 | 0.025 | 97.40–98.05 (pharmaceutical products) 97.20–103.60 (urine) | UV-vis | [170] |
| GCE | Electrochemical sensor based on Au-Ag NTs/NG/GCE | DPV | RT/pharmaceutical products | 0.1–420 | 0.015 | 97–106 | no | [171] |
| SPCE | Electrochemical sensor based on AuNPs/G/SPCE | SWV | RT/pharmaceutical products | 0.1–15 | 0.011 | 96.52–102.97 | UV-vis | [172] |
| SPCE | Electrochemical sensor based on MIP/AuNPs/G/SPCE | DPV | RT/pharmaceutical products | 0.04–60 | 0.014 | 98.0–104.7 | no | [173] |
| GCE | Electrochemical sensor based on MIP/AuNPs-MoS2-G/GCE | DPV | RT/gingko biloba, buckwheat tea, pharmaceutical products | 0.01–45 | 0.004 | 95.2–104.7 | no | [174] |
| GCE | Electrochemical sensor based on Cu2O-AuNPs/NG/GCE | DPV | RT/pharmaceutical products | 0.06–512.90 | 0.03 | 98.95–101.81 | HPLC | [177] |
| GCE | Electrochemical sensor based on NS-rGO/AuNPs/GCE | DPV | RT/pharmaceutical products | 0.0002–1.4 | 6.7 × 10−5 | 97.08–100.90 | no | [178] |
| Pt | Electrochemical biosensor based on TYR/AuNPs-PPY/Pt | CV | CAT/apple juices | 0.001–0.01 | 0.0012 | no | LC/MS/MS | [181] |
| GCE | Electrochemical biosensor based on TYR/AuNPs/GCE | DPV | CAT/teas | no | 7.7 | no | HPLC | [182] |
| AuE | Electrochemical sensor based on NG/Au@Pt NPs/AuE | DPV | CAT/teas | 0.1–45 | 0.00285 | 99.94–101.50 | no | [183] |
| Electrode | (Bio)sensor Format | Electrochemical Technique | Analyte/Sample | Linearity Range (μM) | LOD (μM) | Recovery (%) | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | Electrochemical sensor based on GCE modified with AuNPs | LSV | BHA, BHT, TBHQ/edible oils | BHA 0.55–8.32 BHT 0.9–10 TBHQ 1.2–16.8 | BHA 0.22 BHT 0.36 TBHQ 0.48 | BHA 93.7–105 BHT 92.3–97 TBHQ 91.6–103 | HPLC | [184] |
| EGP | Electrochemical sensor based on EGP modified with AuNPs and NiO NPs. | DPV | BHA/edible oils | 0.03–50 | 0.02 | 97.00–104.00 | no | [185] |
| SPCE | Electrochemical sensor based on SPCE modified with AuNPs and CHI, acting as MIP | DPV | BHA/chewing-gum, mayonnaise, potato chips | 0.055–111 | 0.0055 | no | Spectrophotometry | [186] |
| GCE | Electrochemical sensor based on a binary nanocomposite including AuNPs and ErGO | LSV | BHA, TBHQ/ edible oils | BHA 0.55–55.5 TBHQ 0.6–42 | BHA 0.23 TBHQ 0.30 | no | HPLC | [187] |
| GCE | Electrochemical sensor based on a nanocomposite including AuNPs, G and PVP as dispersing agent | LSV | BHA/ flour, soybean oil | 0.2–100 | 0.04 | 93–105 | no | [188] |
| GCE | Electrochemical biosensor using AuNPs, HRP and SAP NTs | LSV | BHA, PG/ olive and peanut oils, potato chips, cookies | BHA 1.7–277 PG 0.47–470 | BHA 0.26 PG 0.11 | 87.3–126.2 | HPLC | [189] |
| GCE | Electrochemical sensor using a nanocomposite including Au and SnO2 NPs, GNs, MWCNTs | DPV | TBHQ/edible oils, teas | 0.05–230 | 0.058 | 97.9–100.8 | HPLC | [190] |
| EGP | Electrochemical sensor based on EGP modified with AuNPs and MIP | DPV | TBHQ/edible oils | 0.08–100 | 0.07 | 95.80–102.3 | no | [191] |
| GCE | Electrochemical sensor based on GCE modified with AuPt bimetallic nanocrystals | DPV | TBHQ/canola oil, soybean oil | 0.35–625 | 0.075 | Soybean oil 99.2–102.1 Canola oil 98.4–102.5 | no | [192] |
| GCE | Electrochemical-based sensor based on GCE modified with Au-PdNPs, ErGO and MIP | DPV | TBHQ/edible oils | 3.0–361 | 0.28 | 99.4–108.5 | HPLC | [193] |
| GCE | Electrochemical-based sensor based on GCE modified with WC-AuNPs composite | SWV | TBHQ/soybean oil, blended oil, red wine | 0.005–0.075 | 0.0002 | Blended oil 88–97.3 Soybean oil 92–99 Red wine 94–97 | HPLC | [194] |
| GCE | Electrochemical-based sensor based on GCE modified with MIP/PtAuNPs-G/CNTs composite | Chronoamperometry | PG/vegetable oils | 0.070–10 | 0.0251 | 98.3–103.0 | no | [196] |
| GCE | Electrochemical-based sensor based on GCE modified with AuNPs/poly(p-ABSA) composite | DPV | PG/vegetable oils | 9–100 | 0.19 | 98.9–101.3 | no | [197] |
| GCE | Electrochemical sensor based on GCE modified with AuNPs and DDT as SAM | SWV | OG/ margarine, butter, and sunflower oil. | 0.20–1.20 | 0.0083 | 99.1–100.7 | Spectrophotometry | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrucci, R.; Bortolami, M.; Di Matteo, P.; Curulli, A. Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances. Nanomaterials 2022, 12, 959. https://doi.org/10.3390/nano12060959
Petrucci R, Bortolami M, Di Matteo P, Curulli A. Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances. Nanomaterials. 2022; 12(6):959. https://doi.org/10.3390/nano12060959
Chicago/Turabian StylePetrucci, Rita, Martina Bortolami, Paola Di Matteo, and Antonella Curulli. 2022. "Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances" Nanomaterials 12, no. 6: 959. https://doi.org/10.3390/nano12060959
APA StylePetrucci, R., Bortolami, M., Di Matteo, P., & Curulli, A. (2022). Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances. Nanomaterials, 12(6), 959. https://doi.org/10.3390/nano12060959