Improvement of Plant Responses by Nanobiofertilizer: A Step towards Sustainable Agriculture
Abstract
:1. Introduction
2. Constituents of Nanobiofertilizer
2.1. Nanoparticles
2.1.1. Silicon Nanoparticles (SiNPs)
2.1.2. Zinc Nanoparticles (ZnNPs)
2.1.3. Copper Nanoparticles (CuNPs)
2.1.4. Iron Nanoparticles (FeNPs)
2.1.5. Silver Nanoparticles (AgNPs)
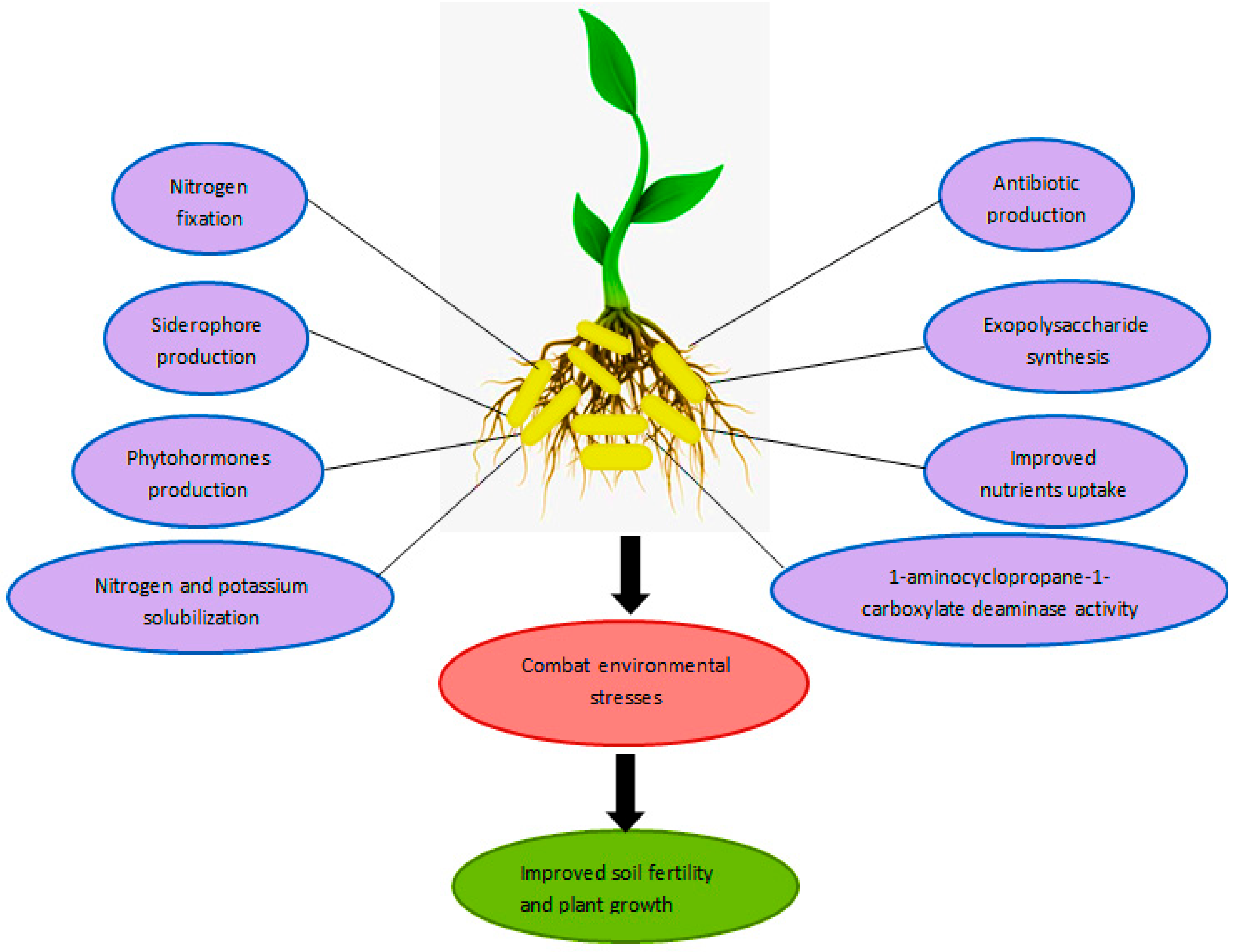
2.2. Biofertilizer
3. Formulation of Nanobiofertilizer
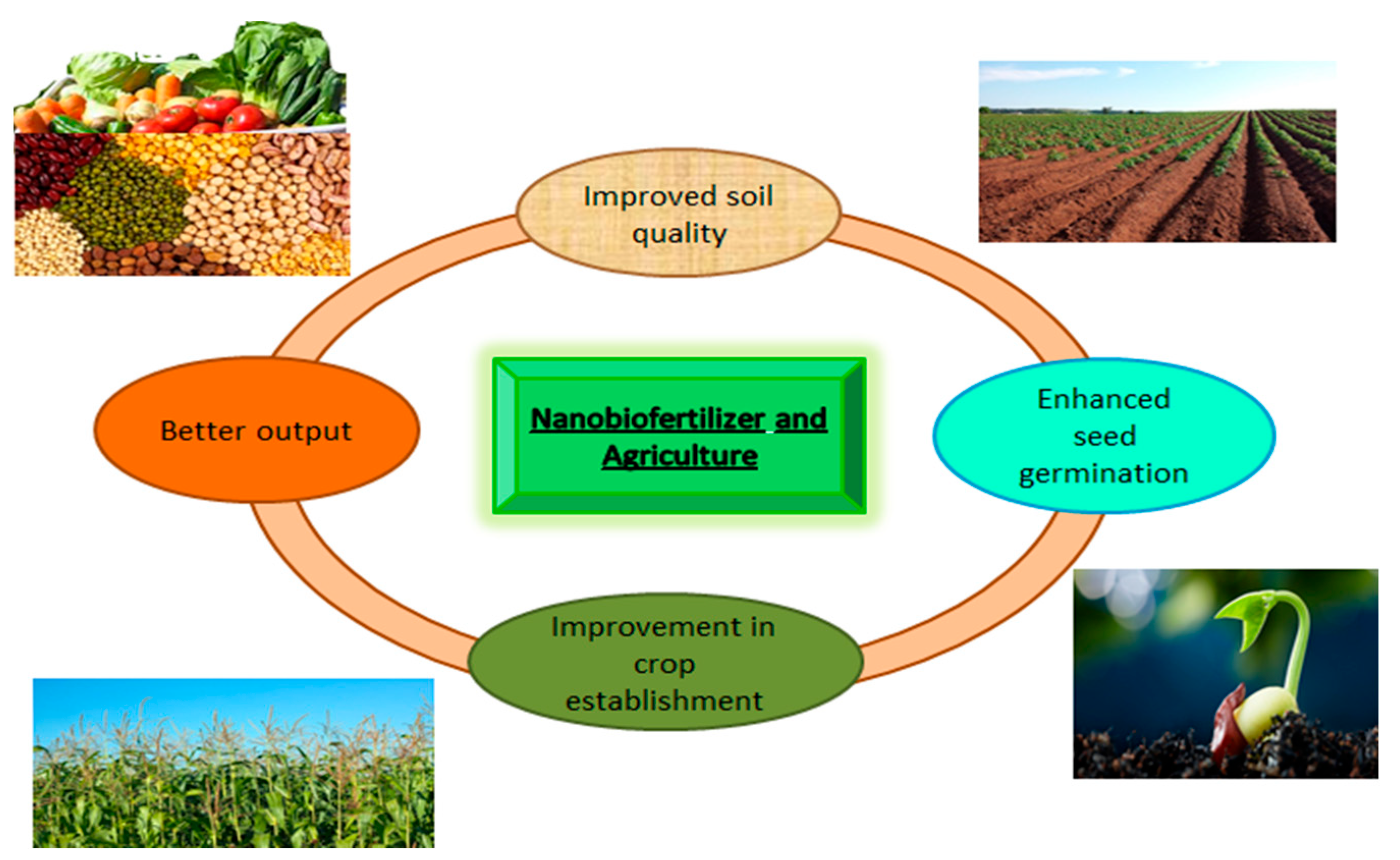
4. Nanobiofertilizer for Improving Soil Fertility
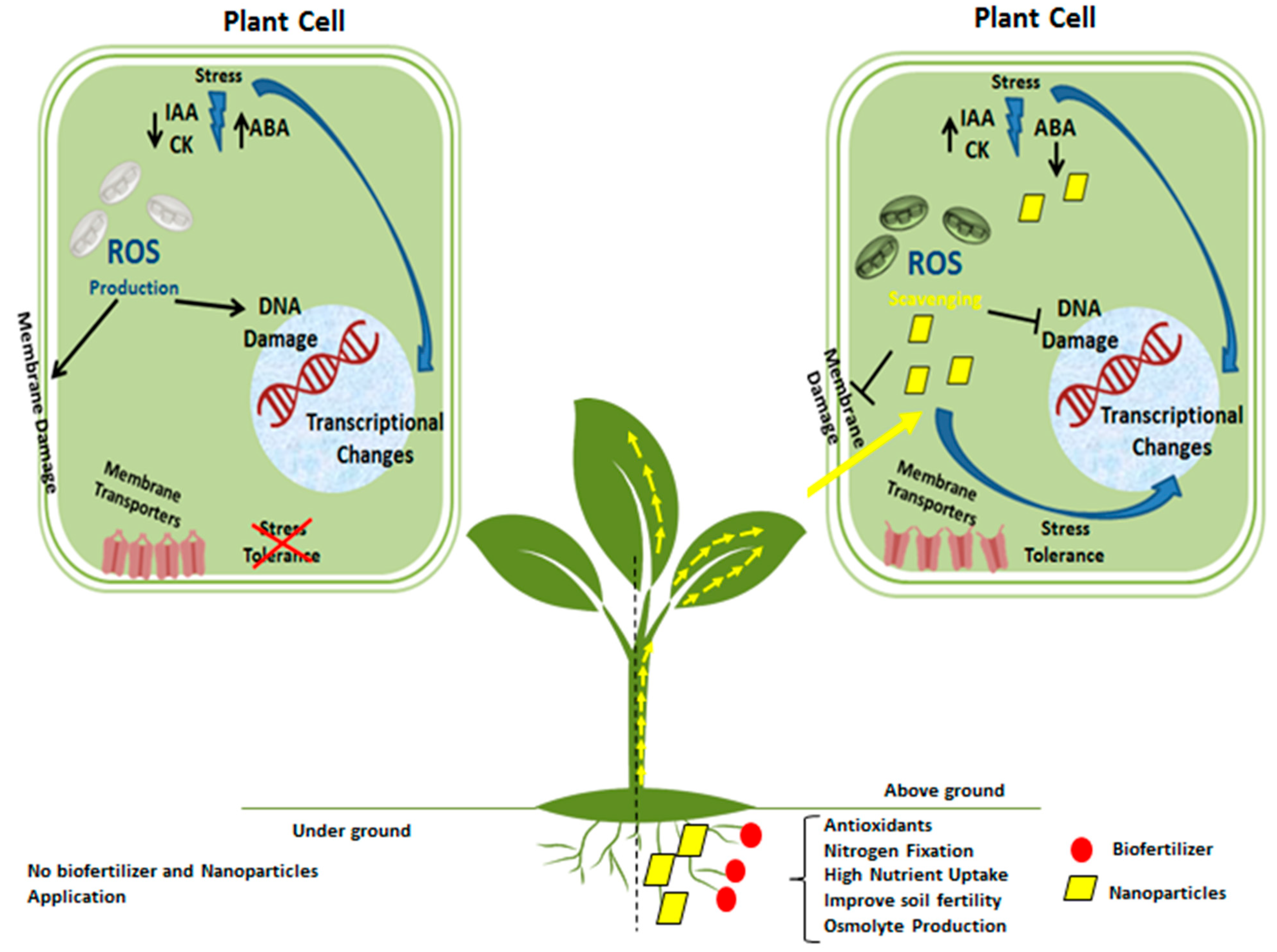
5. Plants’ Responses to Nanobiofertilizer
6. Current Scenario of Nanobiofertilizer Research and Applications
7. Constraints and Future Perspectives of Nanobiofertilizer
- (1)
- There is a need to use advanced equipment and protocols to formulate nanobiofertilizer with good quality, a longer shelf life, a low cost, and ease of use.
- (2)
- Detailed investigation of nanobiofertilizers should be carried out to evaluate their effects on human health.
- (3)
- A multifunctional nanobiofertilizer should be developed, which will be adequate for several crops.
- (4)
- An economic evaluation of nanobiofertilizer should be conducted. The nanobiofertilizer should be tested for its compatibility in various types of soil and environments.
- (5)
- Industries should collaborate to perform large-scale production, and field trials should be carried out.
- (6)
- There is a need to develop awareness among farmers about the side effects of chemical fertilizer and how nanobiofertilizer will reduce the cost and provide long-lasting effects.
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qaim, M. Role of new plant breeding technologies for food security and sustainable agricultural development. Appl. Econ. Perspect. Policy 2020, 42, 129–150. [Google Scholar] [CrossRef]
- Čermelj, A.M.; Golob, A.; Vogel-Mikuš, K.; Germ, M. Silicon mitigates negative impacts of drought and UV-b radiation in plants. Plants 2022, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Mumivand, H.; Shayganfar, A.; Tsaniklidis, G.; Bistgani, Z.E.; Fanourakis, D.; Nicola, S. Pheno-morphological and essential oil composition responses to UVA radiation and protectants: A case study in three Thymus species. Horticulturae 2022, 8, 31. [Google Scholar] [CrossRef]
- Mason, O.U.; Hazen, T.; Borglin, S.; Chain, P.; Dubinsky, E.; Fortney, J.L.; Han, J.; Holman, H.-Y.N.; Hultman, J.; Lamendella, R.; et al. Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 2012, 6, 1715–1727. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Ostadi, A.; Javanmard, A.; Amani Machiani, M.; Morshedloo, M.R.; Nouraein, M.; Rasouli, F.; Maggi, F. Effect of different fertilizer sources and harvesting time on the growth characteristics, nutrient uptakes, essential oil productivity and composition of Mentha x piperita L. Ind. Crops Prod. 2020, 148, 112290. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Fanourakis, D.; Aliniaeifard, S.; Kotsiras, A.; Delis, C.; Tsaniklidis, G. Leaf age-dependent effects of boron toxicity in two Cucumis melo varieties. Agronomy 2021, 11, 759. [Google Scholar] [CrossRef]
- Zhou, W.; Ma, Q.; Wu, L.; Hu, R.; Jones, D.L.; Chadwick, D.R.; Jiang, Y.; Wu, Y.; Xia, X.; Yang, L.; et al. The effect of organic manure or green manure incorporation with reductions in chemical fertilizer on yield-scaled N2O emissions in a citrus orchard. Agric. Ecosyst. Environ. 2022, 326, 107806. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Senapati, N.; Halford, N.G.; Semenov, M.A. Vulnerability of European wheat to extreme heat and drought around flowering under future climate. Environ. Res. Lett. 2021, 16, 024052. [Google Scholar] [CrossRef]
- Du, C.; Abdullah, J.J.; Greetham, D.; Fu, D.; Yu, M.; Ren, L.; Li, S.; Lu, D. Valorization of food waste into biofertiliser and its field application. J. Clean. Prod. 2018, 187, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Wang, P.; Wang, X.; Zou, M.; Liu, C.; Hao, J. Microalgae as biofertilizer in modern agriculture. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 397–411. [Google Scholar] [CrossRef]
- El-Bassi, L.; Ibn Ferjani, A.; Jeguirim, M.; Bennici, S.; Jellali, S.; Akrout, H.; Thevenin, N.; Ruidavets, L.; Muller, A.; Limousy, L. Production of a biofertilizer from exhausted grape marc waste: Agronomic and environmental impact on plant growth. Biomass-Convers. Biorefinery 2020, 1–14. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, H. Nano-biofertilizers: Harnessing dual benefits of nano-nutrient and bio-fertilizers for enhanced nutrient use efficiency and sustainable productivity. In Nanoscience for Sustainable Agriculture; Springer: Cham, Switzerland, 2019; pp. 51–73. ISBN 9783319978529. [Google Scholar] [CrossRef]
- Alen’Kina, S.A.; Nikitina, V.E. Stimulating effect from lectins of associative bacteria of the genus Azospirillum on the germination and morphometric characteristics of spring wheat sprouts in simulated abiotic stress. Russ. J. Plant Physiol. 2021, 68, 315–321. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant. 2021, 172, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef]
- Igiehon, O.N.; Babalola, O.O. Rhizobium and mycorrhizal fungal species improved soybean yield under drought stress conditions. Curr. Microbiol. 2021, 78, 1615–1627. [Google Scholar] [CrossRef]
- Eliaspour, S.; Sharifi, R.S.; Shirkhani, A.; Farzaneh, S. Effects of biofertilizers and iron nano-oxide on maize yield and physiological properties under optimal irrigation and drought stress conditions. Food Sci. Nutr. 2020, 8, 5985–5998. [Google Scholar] [CrossRef]
- Pudake, R.N.; Chauhan, N.; Kole, C. Nanoscience for Sustainable Agriculture; Pudake, R.N., Chauhan, N., Kole, C., Eds.; Springer: Cham, Switzerland, 2019; ISBN 9783319978529. [Google Scholar]
- Timmusk, S.; Seisenbaeva, G.; Behers, L. Titania (TiO2) nano-particles enhance the performance of growth-promoting rhizobacteria. Sci. Rep. 2018, 8, 617. [Google Scholar] [CrossRef]
- Sonali, J.M.I.; Kavitha, R.; Kumar, P.S.; Rajagopal, R.; Gayathri, K.V.; Ghfar, A.A.; Govindaraju, S. Application of a novel nanocomposite containing micro-nutrient solubilizing bacterial strains and CeO2 nanocomposite as bio-fertilizer. Chemosphere 2022, 286, 131800. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 1–34. [Google Scholar] [CrossRef]
- Fatima, A.; Singh, S.; Prasad, S.M. Interaction between copperoxide nanoparticles and plants: Uptake, accumulation and phytotoxicity. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2020; pp. 143–161. [Google Scholar] [CrossRef]
- Baazaoui, N.; Sghaier-Hammami, B.; Hammami, S.B.M.; Khefacha, R.; Chaari, S.; Elleuch, L.; Messaoud, M.; Abdelly, C. A handbook guide to better use of nanoparticles in plants. Commun. Soil Sci. Plant Anal. 2021, 52, 287–321. [Google Scholar] [CrossRef]
- Anjum, S.; Anjum, I.; Hano, C.; Kousar, S. Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Adv. 2019, 9, 40404–40423. [Google Scholar] [CrossRef] [Green Version]
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Caubet, M.; Cornu, S.; Saby, N.P.A.; Meunier, J.-D. Agriculture increases the bioavailability of silicon, a beneficial element for crop, in temperate soils. Sci. Rep. 2020, 10, 19999. [Google Scholar] [CrossRef]
- Meunier, J.-D.; Sandhya, K.; Prakash, N.B.; Borschneck, D.; Dussouillez, P. pH as a proxy for estimating plant-available Si? A case study in rice fields in Karnataka (South India). Plant Soil 2018, 432, 143–155. [Google Scholar] [CrossRef]
- McGinnity, P. Silicon and Its Role in Crop Production. 2015. Available online: http://planttuff.com/wp-content/uploads/2015/12/silicon-agriculture-iiterature-rvw-1.pdf (accessed on 10 March 2022).
- Greger, M.; Landberg, T.; Vaculík, M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Réthoré, E.; Yvin, J.-C.; Hosseini, S.A. The regulatory role of silicon in mitigating plant nutritional stresses. Plants 2020, 9, 1779. [Google Scholar] [CrossRef]
- Han, Y.-Q.; Wen, J.-H.; Peng, Z.-P.; Zhang, D.-Y.; Hou, M.-L. Effects of silicon amendment on the occurrence of rice insect pests and diseases in a field test. J. Integr. Agric. 2018, 17, 2172–2181. [Google Scholar] [CrossRef]
- Helaly, M.N.; El-Hoseiny, H.; El-Sheery, N.I.; Rastogi, A.; Kalaji, H.M. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol. Biochem. 2017, 118, 31–44. [Google Scholar] [CrossRef]
- Snehal, S.; Lohani, P. Silica nano-particles: Its green synthesis and importance in agriculture. J. Pharmacogn. Phytochem. 2018, 7, 3383–3393. [Google Scholar]
- Jabeen, N.; Maqbool, Q.; Sajjad, S.; Minhas, A.; Younas, U.; Anwaar, S.; Nazar, M.; Kausar, R.; Hussain, S.Z. Biosynthesis and characterisation of nano-silica as potential system for carrying streptomycin at nano-scale drug delivery. IET Nanobiotechnol. 2017, 11, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Sanati, A.M.; Maleki, M. Production of silica nanoparticles from rice husk as agricultural waste by environmental friendly technique. Environ. Stud. Persian Gulf 2015, 2, 56–65. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front. Environ. Sci. 2016, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.; Chauhan, D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; Rehman, M.Z.U.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2020, 27, 4958–4968. [Google Scholar] [CrossRef]
- Kalteh, M.; Taj Alipour, Z.; Ashraf, S.; Aliabadi, M.M.; Nosratabadi, A.F. Effect of silica nano-particles on basil (Ocimum basili-cum) under salinity stress. J. Chem. Health Risks 2014, 4, 49–55. [Google Scholar]
- Danish Toor, M.; Adnan, M.; Javed, M.S.; Habibah, U.E.; Arshad, A.; Mughees, M.; Ahmad, R.; Danish, M. Foliar application of Zn: Best way to mitigate drought stress in plants. A review. Int. J. Appl. Res. 2020, 6, 16–20. [Google Scholar]
- Liu, D.-Y.; Liu, Y.-M.; Zhang, W.; Chen, X.-P.; Zou, C.-Q. Zinc uptake, translocation, and remobilization in winter wheat as affected by soil application of Zn fertilizer. Front. Plant Sci. 2019, 10, 426. [Google Scholar] [CrossRef]
- Rafiq, M.; Ali, A.; Malik, M.A.; Hussain, M. Effect of fertilizer levels and plant densities on yield and protein contents of autumn planted maize. Pak. J. Agric. Sci. 2010, 47, 201–208. [Google Scholar]
- Ma, D.; Sun, D.; Wang, C.; Ding, H.; Qin, H.; Hou, J.; Huang, X.; Xie, Y.; Guo, T. Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front. Plant Sci. 2017, 8, 860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, K.S.; Rahman, A.U.; Tajuddin; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Boruah, F.; Parween, N. Synthesis and characterization of ZnO nano-particles using leaf extract of Camellia sinesis and evaluation of their antimicrobial efficacy. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 444–450. [Google Scholar]
- Lawre, S.; Laware, S.L.; Raskar, S. Influence of zinc oxide nanoparticles on growth, flowering and seed productivity in onion metal oxide nanomaterials view project influence of zinc and iron nano-prtilces on chickpea: Nodulation and growth view project flowering and seed productivity in onion. Int. J. Curr. Microbiol. Sci. 2014, 3, 874–881. [Google Scholar]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Akhavan Hezaveh, T.; Rahmani, F.; Alipour, H.; Pourakbar, L. Effects of foliar application of ZnO nanoparticles on secondary metabolite and micro-elements of camelina (Camelina sativa L.) under salinity stress. J. Stress Physiol. Biochem. 2020, 16, 54–69. [Google Scholar]
- Talebi, R.; Drostkar, E.; Kanouni, H. Foliar application of Fe, Zn and NPK nano-fertilizers on seed yield and morphological traits in chickpea under rainfed condition Article Citation: Foliar application of Fe, Zn and NPK nano-fertilizers on seed yield and morphological traits in chickpea under rainfed condition. J. Resour. Ecol. 2016, 4, 221–228. [Google Scholar]
- Semida, W.; Abdelkhalik, A.; Mohamed, G.; El-Mageed, T.A.; El-Mageed, S.A.; Rady, M.; Ali, E. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Lopez-Lima, D.; Mtz-Enriquez, A.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The bifunctional role of copper nano-particles in tomato: Effective treatment for Fusarium wilt and plant growth promoter. Sci. Hortic. 2021, 277, 109810. [Google Scholar] [CrossRef]
- Priyanka, N.; Geetha, N.; Ghorbanpour, M.; Venkatachalam, P. Role of engineered zinc and copper oxide nanoparticles in promoting plant growth and yield: Present status and future prospects. In Advances in Phytonanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 183–201. [Google Scholar] [CrossRef]
- Lafmejani, Z.N.; Jafari, A.A.; Moradi, P.; Moghadam, A.L. Impact of foliar application of copper sulphate and copper nanoparticles on some morpho-physiological traits and essential oil composition of peppermint (Mentha piperita L.). Herba Pol. 2018, 64, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- Nagar, N.; Devra, V. Green synthesis and characterization of copper nano-particles using Azadirachta indica leaves. Mater. Chem. Phys. 2018, 213, 44–51. [Google Scholar] [CrossRef]
- Akl, M.A.; Amer, M.W. Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. Chem. Int. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Dave, V.; Tak, K.; Sadhu, V.; Reddy, K.R. Green synthesis and characterization of copper nano-particles by Tinospora cardifolia to produce nature-friendly copper nano-coated fabric and their antimicrobial evaluation. J. Microbiol. Methods 2019, 160, 107–116. [Google Scholar] [CrossRef]
- Chung, I.; Rahuman, A.A.; Marimuthu, S.; Kirthi, A.V.; Anbarasan, K.; Padmini, P.; Rajakumar, G. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp. Ther. Med. 2017, 14, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Momeni, S.S.; Sajadi, S.M. Green synthesis of copper nanoparticles using Plantago asiatica leaf extract and their application for the cyanation of aldehydes using K4Fe(CN)6. J. Colloid Interface Sci. 2017, 506, 471–477. [Google Scholar] [CrossRef]
- Abbasifar, A.; Shahrabadi, F.; ValizadehkKaji, B. Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J. Plant Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef] [Green Version]
- Pourjamshid, S.A. Study the effect of iron, zinc and manganese foliar application on morphological and agronomic traits of bread wheat (Chamran cultivar) under different irrigation regimes. Environ. Stress. Crop Sci. 2021, 14, 109–118. [Google Scholar] [CrossRef]
- Vaghar, M.S.; Sayfzadeh, S.; Zakerin, H.R.; Kobraee, S.; Valadabadi, S.A. Foliar application of iron, zinc, and manganese nano-chelates improves physiological indicators and soybean yield under water deficit stress. J. Plant Nutr. 2020, 43, 2740–2756. [Google Scholar] [CrossRef]
- Mohamadipoor, R.; Mahboub Khomami, A. Effect of application of iron fertilizers in two methods “foliar and soil application” on growth characteristics of Spathyphyllum illusion. Eur. J. Exp. Biol. 2013, 3, 241–245. [Google Scholar]
- Herlekar, M.; Barve, S.; Kumar, R. Plant-mediated green synthesis of iron nanoparticles. J. Nanoparticles 2014, 2014, 140614. [Google Scholar] [CrossRef] [Green Version]
- Ebrahiminezhad, A.; Zare-Hoseinabadi, A.; Sarmah, A.K.; Taghizadeh, S.; Ghasemi, Y.; Berenjian, A. Plant-mediated synthesis and applications of iron nanoparticles. Mol. Biotechnol. 2017, 60, 154–168. [Google Scholar] [CrossRef] [PubMed]
- El-Desouky, H.S.; Islam, K.R.; Bergefurd, B.; Gao, G.; Harker, T.; Abd-El-Dayem, H.; Ismail, F.; Mady, M.; Zewail, R.M.Y. Nano iron fertilization significantly increases tomato yield by increasing plants’ vegetable growth and photosynthetic efficiency. J. Plant Nutr. 2021, 44, 1649–1663. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Yasmeen, F.; Razzaq, A.; Iqbal, M.N.; Jhanzab, H.M. Effect of silver, copper and iron nanoparticles on wheat germination. Int. J. Biosci. 2015, 6, 112–117. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; Rehman, M.Z.U.; Hussain, A.; Hussain, K.; Chatha, S.A.S.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef]
- Öktem, M.; Keleş, Y. The role of silver ions in the regulation of the senescence process in Triticum aestivum. Turk. J. Biol. 2018, 42, 517–526. [Google Scholar] [CrossRef]
- Kumari, M.; Pandey, S.; Bhattacharya, A.; Mishra, A.; Nautiyal, C. Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum. Plant Physiol. Biochem. 2017, 121, 216–225. [Google Scholar] [CrossRef]
- Mahendran, D.; Geetha, N.; Venkatachalam, P. Role of silver nitrate and silver nano-particles on tissue culture medium and enhanced the plant growth and development. In In Vitro Plant Breeding towards Novel Agronomic Traits: Biotic and Abiotic Stress Tolerance; Springer: Singapore, 2019; pp. 59–74. ISBN 9789813298248. [Google Scholar]
- Ahmed, R.H.; Mustafa, D.E. Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int. Nano Lett. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Soliman, M.; Qari, S.H.; Abu-Elsaoud, A.; El-Esawi, M.; Alhaithloul, H.; Elkelish, A. Rapid green synthesis of silver nanoparticles from blue gum augment growth and performance of maize, fenugreek, and onion by modulating plants cellular antioxidant machinery and genes expression. Acta Physiol. Plant. 2020, 42, 148. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Siril, E.A.; Muthukrishnan, S. Role of biogenic silver nanoparticles on hyperhydricity reversion in Dianthus chinensis L. an in vitro model culture. J. Plant Growth Regul. 2021, 41, 23–39. [Google Scholar] [CrossRef]
- Mustafa, G.; Hasan, M.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Komatsu, S. A comparative proteomic analysis of engineered and bio synthesized silver nanoparticles on soybean seedlings. J. Proteom. 2020, 224, 103833. [Google Scholar] [CrossRef]
- Mahawar, H.; Prasanna, R.; Gogoi, R.; Singh, S.B.; Chawla, G.; Kumar, A. Synergistic effects of silver nanoparticles augmented Calothrix elenkinii for enhanced biocontrol efficacy against Alternaria blight challenged tomato plants. 3 Biotech 2020, 10, 102. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Nejad, A.R.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and nanographene oxide improves rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, 1–15. [Google Scholar] [CrossRef]
- Nejatzadeh, F. Effect of silver nanoparticles on salt tolerance of Satureja hortensis l. during in vitro and in vivo germination tests. Heliyon 2021, 7, e05981. [Google Scholar] [CrossRef]
- Bastami, A.; Amirnia, R.; Sayyed, R.; Enshasy, H. The effect of mycorrhizal fungi and organic fertilizers on quantitative and qualitative traits of two important Satureja species. Agronomy 2021, 11, 1285. [Google Scholar] [CrossRef]
- Almutairi, Z.M. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics 2016, 9, 106–114. [Google Scholar]
- Abdel-Haliem, M.; Hegazy, H.S.; Hassan, N.S.; Naguib, D. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Cheng, B.; Chen, F.; Wang, C.; Liu, X.; Yue, L.; Cao, X.; Wang, Z.; Xing, B. The molecular mechanisms of silica nanomaterials enhancing the rice (Oryza sativa L.) resistance to planthoppers (Nilaparvata lugens Stal). Sci. Total Environ. 2021, 767, 144967. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, S.; Iranbakhsh, A.; Shamili, M.; Ardebili, Z.O. Copper nanoparticles mediated physiological changes and transcriptional variations in microRNA159 (miR159) and mevalonate kinase (MVK) in pepper; potential benefits and phytotoxicity assessment. J. Environ. Chem. Eng. 2021, 9, 106151. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef]
- Baniebrahim, S.; Pishkar, L.; Iranbakhsh, A.; Talei, D.; Barzin, G. Physiological and molecular responses of black cumin (Nigella sativa L.) seedlings to silver nanoparticles. J. Plant Nutr. 2021, 44, 2885–2896. [Google Scholar] [CrossRef]
- Kaveh, R.; Li, Y.-S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. Pollut. Res. 2014, 21, 8858–8869. [Google Scholar] [CrossRef]
- Noori, A.; Donnelly, T.; Colbert, J.; Cai, W.; Newman, L.A.; White, J.C. Exposure of tomato (Lycopersicon esculentum) to silver nanoparticles and silver nitrate: Physiological and molecular response. Int. J. Phytoremediation 2019, 22, 40–51. [Google Scholar] [CrossRef]
- Kohan-Baghkheirati, E.; Geisler-Lee, J. Gene expression, protein function and pathways of arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 2015, 5, 436–467. [Google Scholar] [CrossRef]
- Vafa, Z.; Sohrabi, Y.; Sayyed, R.; Suriani, N.L.; Datta, R. Effects of the combinations of rhizobacteria, mycorrhizae, and seaweed, and supplementary irrigation on growth and yield in wheat cultivars. Plants 2021, 10, 811. [Google Scholar] [CrossRef]
- Malusà, E.; Pinzari, F.; Canfora, L. Efficacy of biofertilizers: Challenges to improve crop production. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 17–40. [Google Scholar] [CrossRef]
- Sagar, A.; Rai, S.; Ilyas, N.; Sayyed, R.Z.; Al-Turki, A.I.; El Enshasy, H.A.; Simarmata, T. Halotolerant rhizobacteria for salinity-stress mitigation: Diversity, mechanisms and molecular approaches. Sustainability 2022, 14, 490. [Google Scholar] [CrossRef]
- Enrico, J.M.; Piccinetti, C.F.; Barraco, M.R.; Agosti, M.B.; Eclesia, R.P.; Salvagiotti, F. Biological nitrogen fixation in field pea and vetch: Response to inoculation and residual effect on maize in the Pampean region. Eur. J. Agron. 2020, 115, 126016. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Choudhury, A.R.; Roy, S.K.; Choi, J.; Sayyed, R.Z.; Sa, T. Biomolecular painstaking utilization and assimilation of phosphorus under indigent stage in agricultural crops. In Antioxidants in Plant-Microbre Interaction; Springer: Singapore, 2021; pp. 565–588. [Google Scholar] [CrossRef]
- Baba, Z.A.; Hamid, B.; Sheikh, T.A.; Alotaibi, S.H.; El Enshasy, H.A.; Ansari, M.J.; Zuan, A.T.K.; Sayyed, R.Z. Psychrotolerant Mesorhizobium sp. isolated from temperate and cold desert regions solubilizes potassium and produces multiple plant growth promoting metabolites. Molecules 2021, 26, 5758. [Google Scholar] [CrossRef] [PubMed]
- Kusale, S.; Attar, Y.; Sayyed, R.; Malek, R.; Ilyas, N.; Suriani, N.; Khan, N.; El Enshasy, H. Production of plant beneficial and antioxidants metabolites by Klebsiellavariicola under salinity stress. Molecules 2021, 26, 1894. [Google Scholar] [CrossRef] [PubMed]
- Kusale, S.; Attar, Y.; Sayyed, R.; El Enshasy, H.; Hanapi, S.; Ilyas, N.; Elgorban, A.; Bahkali, A.; Marraiki, N. Inoculation of Klebsiella variicola alleviated salt stress and improved growth and nutrients in wheat and maize. Agronomy 2021, 11, 927. [Google Scholar] [CrossRef]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.; Dailin, D.; El Enshasy, H.; Suriani, N.L.; Herlambang, S. Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability 2021, 13, 5394. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.; Baba, Z.; Sheikh, T.; Reddy, M.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Fasusi, O.; Cruz, C.; Babalola, O. Agricultural sustainability: Microbial biofertilizers in rhizosphere management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
- Sumbul, A.; Ansari, R.A.; Rizvi, R.; Mahmood, I. Azotobacter: A potential bio-fertilizer for soil and plant health management. Saudi J. Biol. Sci. 2020, 27, 3634–3640. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Panichikkal, J.; Puthiyattil, N.; Raveendran, A.; Nair, R.A.; Krishnankutty, R.E. Application of encapsulated Bacillus licheniformis supplemented with chitosan nanoparticles and rice starch for the control of Sclerotium rolfsii in Capsicum annuum (L.) seedlings. Curr. Microbiol. 2021, 78, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Rise, R.; Moradi-Pour, M. The effect of Bacillus subtilis Vru1 encapsulated in alginate-bentonite coating enriched with titanium nanoparticles against Rhizoctonia solani on bean. Int. J. Biol. Macromol. 2020, 152, 1089–1097. [Google Scholar] [CrossRef]
- Panichikkal, J.; Prathap, G.; Nair, R.A.; Krishnankutty, R.E. Evaluation of plant probiotic performance of Pseudomonas sp. encapsulated in alginate supplemented with salicylic acid and zinc oxide nano-particles. Int. J. Biol. Macromol. 2021, 166, 138–143. [Google Scholar] [CrossRef]
- Singh, P.; Ghosh, D.; Manyapu, V.; Yadav, M.; Majumder, S. Synergistic impact of iron (iii) oxide nano-particles and organic waste on growth and development of Solanum lycopersicum plants: New paradigm in nanobiofertilizer. Plant Arch. 2019, 19, 339–344. [Google Scholar]
- Janmohammadi, M.; Navid, A.; Segherloo, A.E.; Sabaghnia, N. Impact of nano-chelated micronutrients and biological fertilizers on growth performance and grain yield of maize under deficit irrigation condition. Biologija 2016, 62, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, D.M.; Satapathy, K.C.; Panda, B. Biofertilizers and nanofertilizers for sustainable agriculture: Phycoprospects and challenges. Sci. Total Environ. 2022, 803, 149990. [Google Scholar] [CrossRef]
- Sharma, A.; Patel, S.; Menghani, E. Synthesis, application and prospects of nano-biofertilizers: A reappraisal. J. Phytol. Res. 2021, 34, 79–85. [Google Scholar]
- Shafiei-Masouleh, S.-S. Use of magnetic nano-chitosan as bio-fertilizer to reduce production period in three cyclamen cultivars. J. Soil Sci. Plant Nutr. 2021, 22, 281–293. [Google Scholar] [CrossRef]
- Ali, S.S.; Darwesh, O.M.; Kornaros, M.; Al-Tohamy, R.; Manni, A.; El-Shanshoury, A.E.-R.R.; Metwally, M.A.; Elsamahy, T.; Sun, J. Nano-biofertilizers: Synthesis, advantages, and applications. In Biofertilizers; Woodhead Publishing: Cambridge, MA, USA, 2021; pp. 359–370. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the vulnerable cretan endemic Verbascum arcturus L. (scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Vedamurthy, A.B.; Bhattacharya, S.; Das, A.; Shruthi, S.D. Exploring nanomaterials with rhizobacteria in current agricultural scenario. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 487–503. [Google Scholar]
- Shcherbakova, E.N.; Shcherbakov, A.V.; Andronov, E.; Gonchar, L.N.; Kalenska, S.; Chebotar, V.K. Combined pre-seed treatment with microbial inoculants and Mo nanoparticles changes composition of root exudates and rhizosphere microbiome structure of chickpea (Cicer arietinum L.) plants. Symbiosis 2017, 73, 57–69. [Google Scholar] [CrossRef]
- Bibi, F.; Ilyas, N.; Arshad, A.; Khalid, A.; Saeed, M.; Ansar, S.; Batley, J. Formulation and efficacy testing of bio-organic fertilizer produced through solid-state fermentation of agro-waste by Burkholderia cenocepacia. Chemosphere 2022, 291, 132762. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N. Role of nanosilicab to boost the activities of metabolites in Triticum aestivum facing drought stress. Plant soil. 2022. [Google Scholar] [CrossRef]
- Sharifi, R.S.; Khalilzadeh, R.; Pirzad, A.; Anwar, S. Effects of biofertilizers and nano zinc-iron oxide on yield and physicochemical properties of wheat under water deficit conditions. Commun. Soil Sci. Plant Anal. 2020, 51, 2511–2524. [Google Scholar] [CrossRef]
- Morsy, N.M.; Shams, A.S.; Abdel-Salam, M.A. Zinc foliar spray on snap beans using nano-Zn with N-soil application using mineral, organic and biofertilizer. Middle East J. Agric. Res. 2017, 6, 1301–1312. [Google Scholar]
- Davod, T.; Reza, Z.; Azghandi Ali, V.; Mehrdad, C. Effects of nanosilver and nitroxin biofertilizer on yield and yield components of potato minitubers. Int. J. Agric. Biol. 2011, 13, 986–990. [Google Scholar]
- Ghooshchi, F. Influence of titanium and bio-fertilizers on some agronomic and physiological attributes of triticale exposed to cadmium stress. Glob. NEST J. 2017, 19, 458–463. [Google Scholar]
- Mir, S.; Sirousmehr, A.; Shirmohammadi, E. Effect of nano and biological fertilizers on carbohydrate and chlorophyll content of forage sorghum (Speedfeed hybrid). Int. J. Biosci. 2015, 6, 157–164. [Google Scholar] [CrossRef]
- Baragaño, D.; Forján, R.; Fernandez-Perez, B.; Ayala, J.; Afif, E.; Gallego, J.L.R. Application of biochar, compost and ZVI nanoparticles for the remediation of As, Cu, Pb and Zn polluted soil. Environ. Sci. Pollut. Res. 2020, 27, 33681–33691. [Google Scholar] [CrossRef]
- Najafi, S.; Nazari Nasi, H.; Tuncturk, R.; Tuncturk, M.; Sayyed, R.Z.; Amirnia, R. Biofertilizer Application Enhances Drought Stress Tolerance and Alters the Antioxidant Enzymes in Medicinal Pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 2021, 7, 588. [Google Scholar] [CrossRef]
- Daryabeigi Zand, A.; Tabrizi, A.M.; Heir, A.V. The influence of association of plant growth-promoting rhizobacteria and zero-valent iron nano-particles on removal of antimony from soil by Trifolium repens. Environ. Sci. Pollut. Res. 2020, 27, 42815–42829. [Google Scholar] [CrossRef] [PubMed]
- Khati, P.; Parul; Bhatt, P.; Nisha; Kumar, R.; Sharma, A. Effect of nanozeolite and plant growth promoting rhizobacteria on maize. 3 Biotech 2018, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Rizwan, M.; Ali, S.; Adrees, M.; Qayyum, M.F. Effect of composted organic amendments and zinc oxide nano-particles on growth and cadmium accumulation by wheat; a life cycle study. Environ. Sci. Pollut. Res. 2020, 27, 23926–23936. [Google Scholar] [CrossRef] [PubMed]
- Farnia, A.; Omidi, M.; Farnia. Effect of nano-zinc chelate and nano-biofertilizer on yield and yield components of maize (Zea mays L.), under water stress condition. Indian J. Nat Sci. 2015, 5, 4614. [Google Scholar]
- Shukla, S.K.; Kumar, R.; Mishra, R.K.; Pandey, A.; Pathak, A.; Zaidi, M.; Srivastava, S.K.; Dikshit, A. Prediction and validation of gold nanoparticles (GNPs) on plant growth promoting rhizobacteria (PGPR): A step toward development of nano-biofertilizers. Nanotechnol. Rev. 2015, 4, 439–448. [Google Scholar] [CrossRef]
- Ahmed, B.; Syed, A.; Rizvi, A.; Shahid, M.; Bahkali, A.H.; Khan, M.S.; Musarrat, J. Impact of metal-oxide nanoparticles on growth, physiology and yield of tomato (Solanum lycopersicum L.) modulated by Azotobacter salinestris strain ASM. Environ. Pollut. 2021, 269, 116218. [Google Scholar] [CrossRef]
- Shah, A.A.; Aslam, S.; Akbar, M.; Ahmad, A.; Khan, W.U.; Yasin, N.A.; Ali, B.; Rizwan, M.; Ali, S. Combined effect of Bacillus fortis IAGS 223 and zinc oxide nanoparticles to alleviate cadmium phytotoxicity in Cucumis melo. Plant Physiol. Biochem. 2021, 158, 1–12. [Google Scholar] [CrossRef]
- Hafez, E.; Osman, H.; Gowayed, S.; Okasha, S.; Omara, A.; Sami, R.; El-Monem, A.A.; El-Razek, U.A. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Mushtaq, T.; Shah, A.; Akram, W.; Yasin, N.A. Synergistic ameliorative effect of iron oxide nanoparticles and Bacillus subtilis S4 against arsenic toxicity in Cucurbita moschata: Polyamines, antioxidants, and physiochemical studies. Int. J. Phytoremediat. 2020, 22, 1408–1419. [Google Scholar] [CrossRef]
- Yasmin, H.; Mazher, J.; Azmat, A.; Nosheen, A.; Naz, R.; Hassan, M.N.; Noureldeen, A.; Ahmad, P. Combined application of zinc oxide nanoparticles and biofertilizer to induce salt resistance in safflower by regulating ion homeostasis and antioxidant defence responses. Ecotoxicol. Environ. Saf. 2021, 218, 112262. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Al Jabri, A.B.; Jassim, R.A.H.; Jabar, A.K. The effect of nano nitrogen and bio-fertilizer types on npk concentration in soil and okra plant. Plant Arch. 2020, 20, 4031–4037. [Google Scholar]
- Faridvand, S.; Amirnia, R.; Tajbakhsh, M.; El Enshasy, H.A.; Sayyed, R.Z. The effect of foliar application of magnetic water and nano-fertilizers on phytochemical and yield characteristics of fennel. Horticulturae 2021, 7, 475. [Google Scholar] [CrossRef]
- Fadaki, S.H.; Mehdi Nezhad, N.; Mohammad Pour, R. Effects of nano and nano bio-fertilizer on physiological, biochemical characteristics and yield of roselle under drought stress. J. Crops Improv. 2018, 20, 45–66. [Google Scholar]
- Jahangir, S.; Javed, K.; Bano, A. Nanoparticles and plant growth promoting rhizobacteria (PGPR) modulate the physiology of onion plant under salt stress. Pak. J. Bot. 2020, 52, 1473–1480. [Google Scholar] [CrossRef]
- Chaudhary, P.; Khati, P.; Chaudhary, A.; Maithani, D.; Kumar, G.; Sharma, A. Cultivable and metagenomic approach to study the combined impact of nanogypsum and Pseudomonas taiwanensis on maize plant health and its rhizospheric microbiome. PLoS ONE 2021, 16, e0250574. [Google Scholar] [CrossRef]
- Agri, U.; Chaudhary, P.; Sharma, A. In vitro compatibility evaluation of agriusable nanochitosan on beneficial plant growth-promoting rhizobacteria and maize plant. Natl. Acad. Sci. Lett. 2021, 44, 555–559. [Google Scholar] [CrossRef]
- Gahoi, P.; Omar, R.A.; Verma, N.; Gupta, G.S. Rhizobacteria and Acylated homoserine lactone-based nanobiofertilizer to improve growth and pathogen defense in Cicer arietinum and Triticum aestivum Plants. ACS Agric. Sci. Technol. 2021, 1, 240–252. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.; Ahmed, N.; Skalicky, M.; Danish, S.; Fahad, S.; Hassan, F.; Hassan, M.; Brestic, M.; EL Sabagh, A.; et al. Biofertilizer-based zinc application enhances maize growth, gas exchange attributes, and yield in zinc-deficient soil. Agriculture 2021, 11, 310. [Google Scholar] [CrossRef]
- Boroumand, N.; Behbahani, M.; Dini, G. Combined effects of phosphate solubilizing bacteria and nanosilica on the growth of land cress plant. J. Soil Sci. Plant Nutr. 2020, 20, 232–243. [Google Scholar] [CrossRef]
- Baddour, A.G.; Attia, R.H. Effect of foliar application with nano-micronutrients and organic fertilization on snap bean under sandy soil condition. J. Soil Sci. Agric. Eng. 2021, 12, 25–32. [Google Scholar] [CrossRef]
- Al-Burki, H.A.; Al-Ajeel, S.A. Effect of bio-fertilizer and nanoscale elements on root nodules formation and their chemical content of two Phaseolus vulgaris l varieties. Plant Arch. 2021, 21, 1476–1480. [Google Scholar] [CrossRef]
- Rasouli, H.; Popović-Djordjević, J.; Sayyed, R.Z.; Zarayneh, S.; Jafari, M.; Fazeli-Nasab, B. Nanoparticles: A new threat to crop plants and soil rhizobia? In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2020; pp. 201–214. [Google Scholar] [CrossRef]
- Bhari, R.; Kaur, M.; Singh, R.S. Chicken feather waste hydrolysate as a superior biofertilizer in agroindustry. Curr. Microbiol. 2021, 78, 2212–2230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of nano-particles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]

| Nanoparticles | Plants | Stress | Effect on Genes Expression | References |
|---|---|---|---|---|
| Silicon nanoparticles | Solanum lycopersicum | Salinity | Upregulation of NCED3, TAS14, CRK1, and AREB genes Downregulation of MAPK3, DDF2, MPAK2, ERF3, APX2, and RBOH1 genes | [87] |
| Oryza sativa | Salinity | Upregulation of LSi1 and LSi2 genes | [88] | |
| Oryza sativa | Biotic stress | Upregulation of A2WZ30, B8AP99, A2YNH4, B8AZZ8, Fo2, B8B. B8A9F5, B8BF84. B8BHM9, and 2XRR2 genes | [89] | |
| Copper nanoparticles | Piper nigrum | - | Upregulation of miR159 Downregulation of MVK gene | [90] |
| Iron nanoparticles | Oryza sativa | Cd and drought stress | Downregulation of OsLCT1, OsHMA3, and OsHMA2 genes | [91] |
| Silver nanoparticles | Nigella sativa | - | Upregulation of PAL and CHS genes | [92] |
| Arabidopsis thaliana | - | Upregulation of 286 genes Downregulation of 81 genes | [93] | |
| Arabidopsis thaliana | - | Upregulation of PCS, GS, GR, and GSTU12 genes | [94] | |
| Solanum lycopersicum | - | Upregulation of PAL and EIX genes | [95] | |
| Arabidopsis thaliana | - | Upregulation of 438 genes | [96] |
| Nanobiofertilizer | Plants | Conditions | Plants Responses | References |
|---|---|---|---|---|
| Nano-chelated B and Zn + Biofertilizer | Zea mays | Drought stress |
| [125] |
| Oxide NPs of Fe-Zn + Biofertilizer (Azospirillum, Pseudomonas and, Azotobacter) | Triticum aestivum | Stress and nonstress conditions |
| [126] |
| ZnNPs + Biofertilizer (Rhizobium) + Organic fertilizer | Phaseolus vulgaris L. | Normal |
| [127] |
| AgNPs + Biofertilizer (Nitroxin) | Solanum tuberosum L. | Normal |
| [128] |
| TiNPs + Biofertilizer (Azospirillum brasilense, A. caulinodans and, Azotobacter chroococcum) | Triticum secale | Cadmium stress |
| [129] |
| Nano-fertilizer + Biofertilizer (Azetobacter) | Sorghum bicolor (L.) Moench. | Normal |
| [130] |
| Zero-valen FeNPs + Biofertilizer (compost and biochar) | Brassica juncea L. | Heavy metal stress |
| [131] |
| Acylated homoserine coated Fe-carbon nanofibres + Biofertilizer (Panebacillus polymyxa) | Cicer aretianum L. and T. aestivum | Biotic stress |
| [132] |
| Zero valent FeNPs + Biofertilizer (Pseudomonas fluorescens) | Trifolium repens | Antimony stress |
| [133] |
| Nanozeolite + Biofertilizer (Bacillus spp.) | Z. mays | Normal |
| [134] |
| ZnO NPs + biofertilizer (composted biochar farmyard manure) | T. aestivum | Cd stress |
| [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, N.; Ilyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.Z.; Mashwani, Z.-u.-R.; Poczai, P. Improvement of Plant Responses by Nanobiofertilizer: A Step towards Sustainable Agriculture. Nanomaterials 2022, 12, 965. https://doi.org/10.3390/nano12060965
Akhtar N, Ilyas N, Meraj TA, Pour-Aboughadareh A, Sayyed RZ, Mashwani Z-u-R, Poczai P. Improvement of Plant Responses by Nanobiofertilizer: A Step towards Sustainable Agriculture. Nanomaterials. 2022; 12(6):965. https://doi.org/10.3390/nano12060965
Chicago/Turabian StyleAkhtar, Nosheen, Noshin Ilyas, Tehseen Ahmad Meraj, Alireza Pour-Aboughadareh, R. Z. Sayyed, Zia-ur-Rehman Mashwani, and Peter Poczai. 2022. "Improvement of Plant Responses by Nanobiofertilizer: A Step towards Sustainable Agriculture" Nanomaterials 12, no. 6: 965. https://doi.org/10.3390/nano12060965







