Construction of N-CDs and Calcein-Based Ratiometric Fluorescent Sensor for Rapid Detection of Arginine and Acetaminophen
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Experimental Instruments
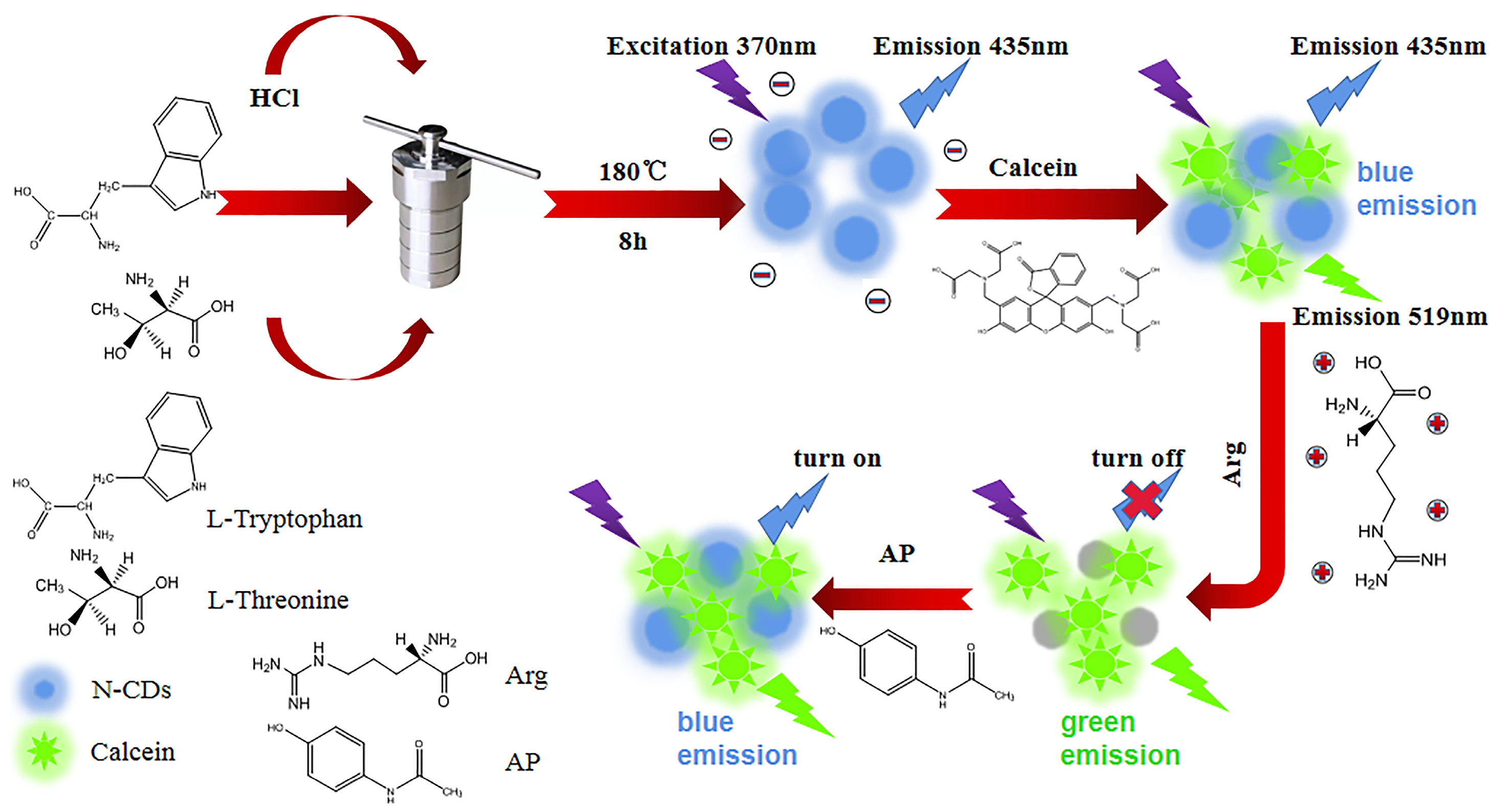
2.3. Synthesis of N-CDs
2.4. Quantum Yield of N-CDs
2.5. Construction of N-CD/Calcein Ratiometric Fluorescent Sensor
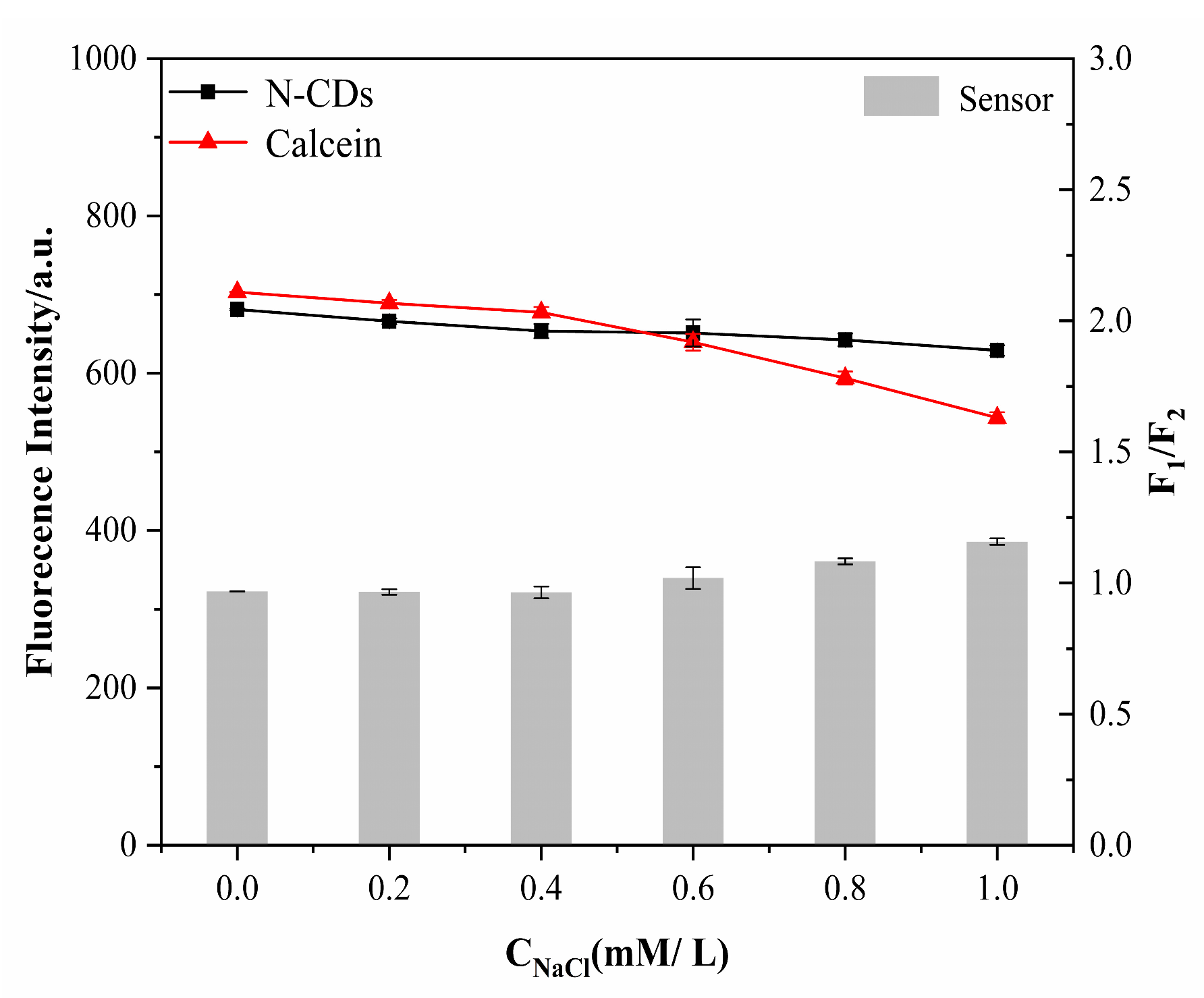
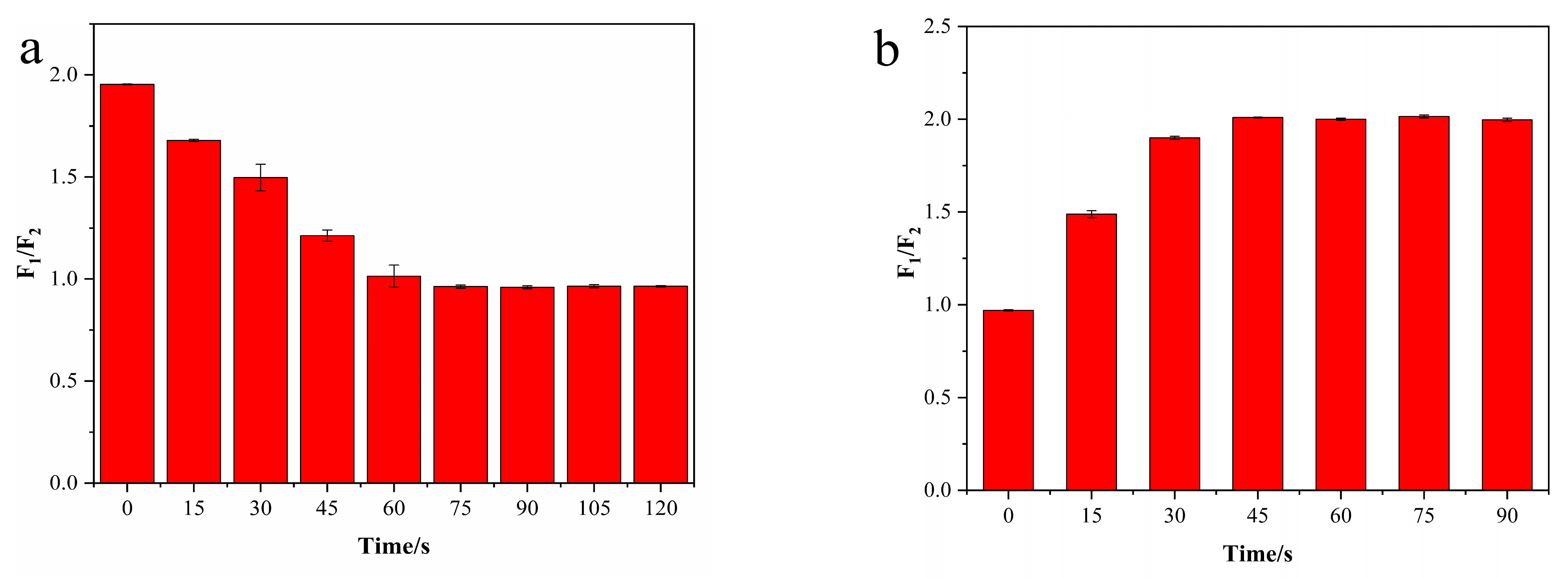
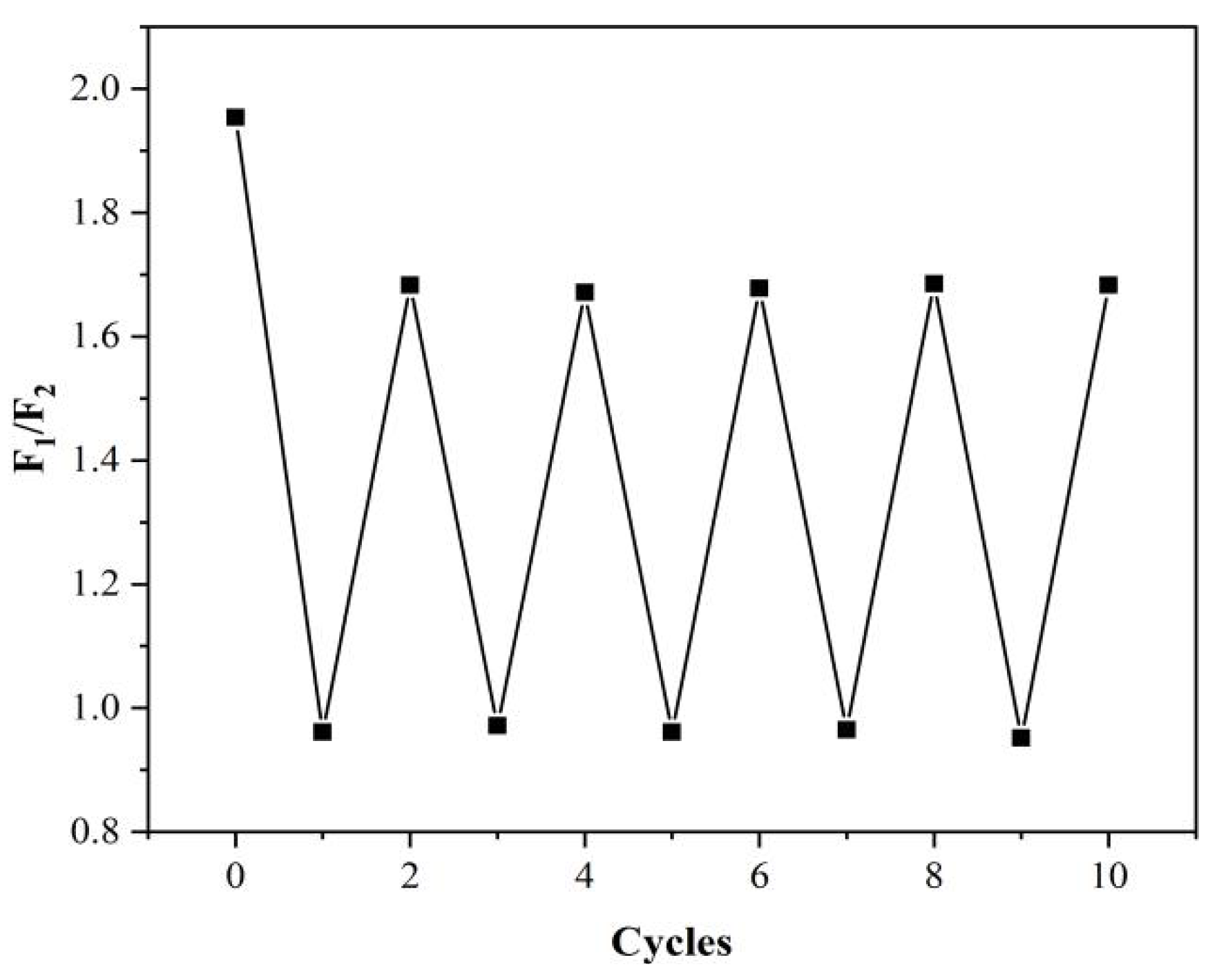
2.6. Stability Evaluation
2.7. Characteristics of N-CD/Calcein Sensor in Arg and AP Detection
3. Results and Discussion
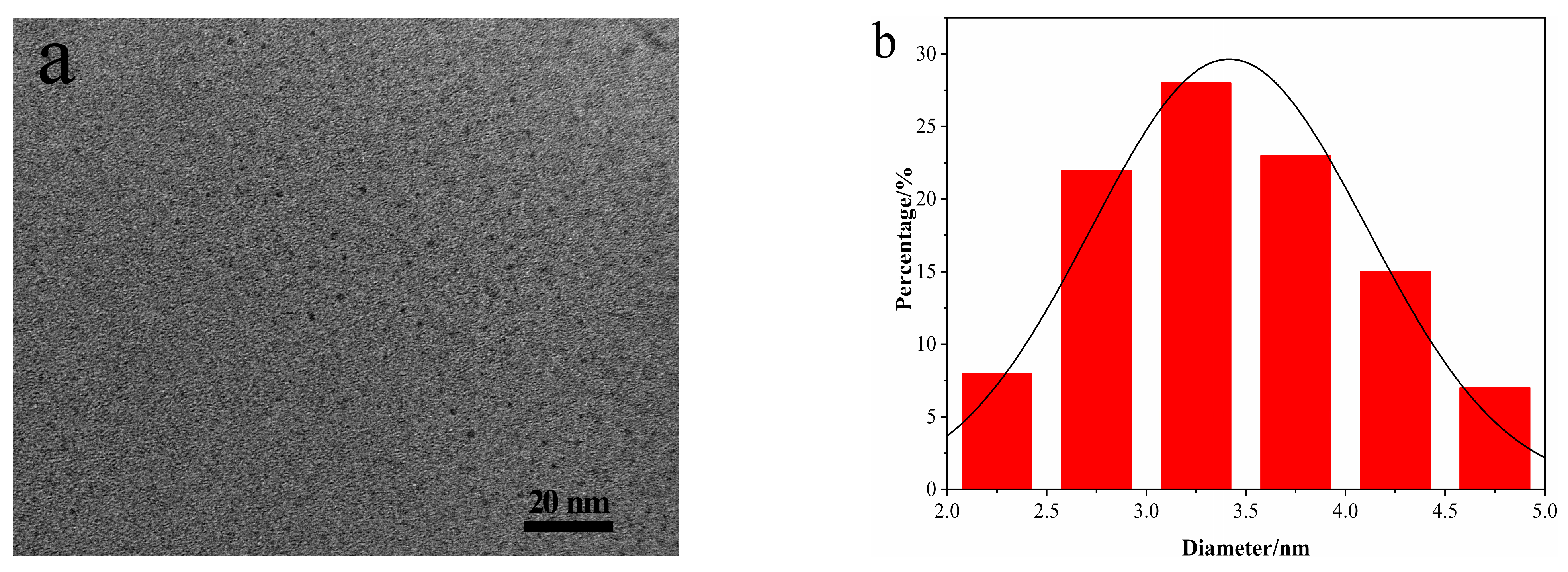
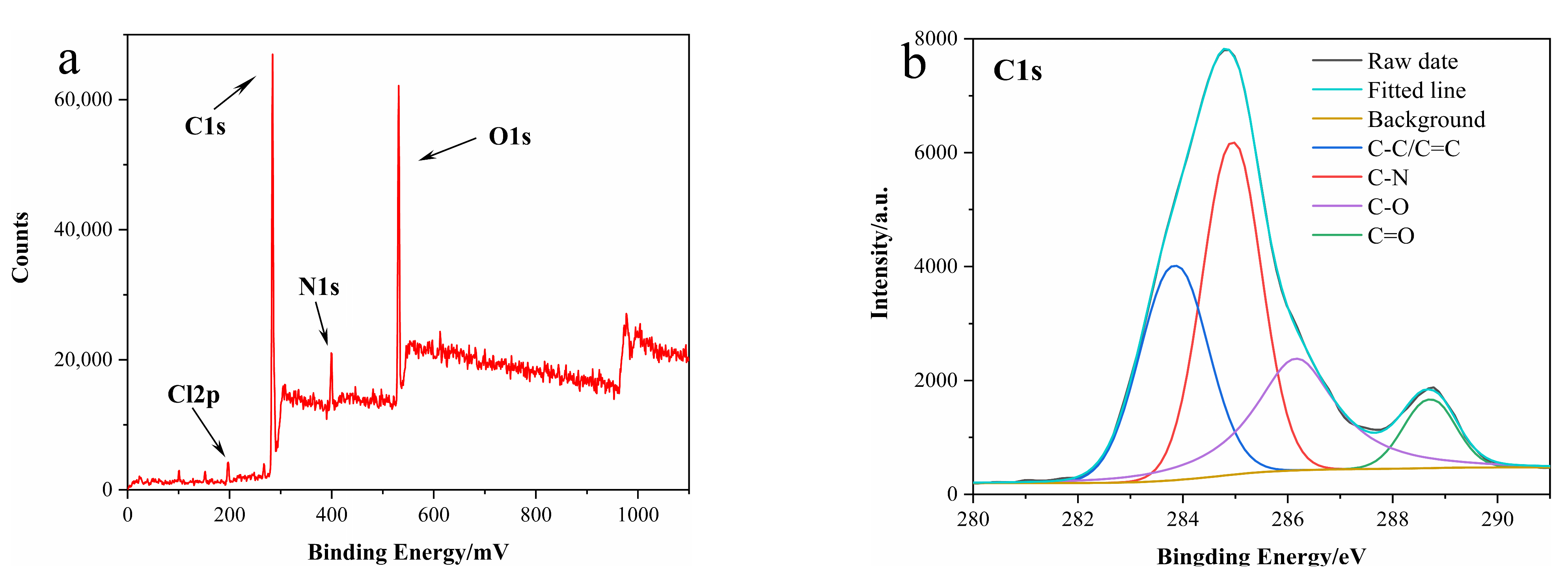
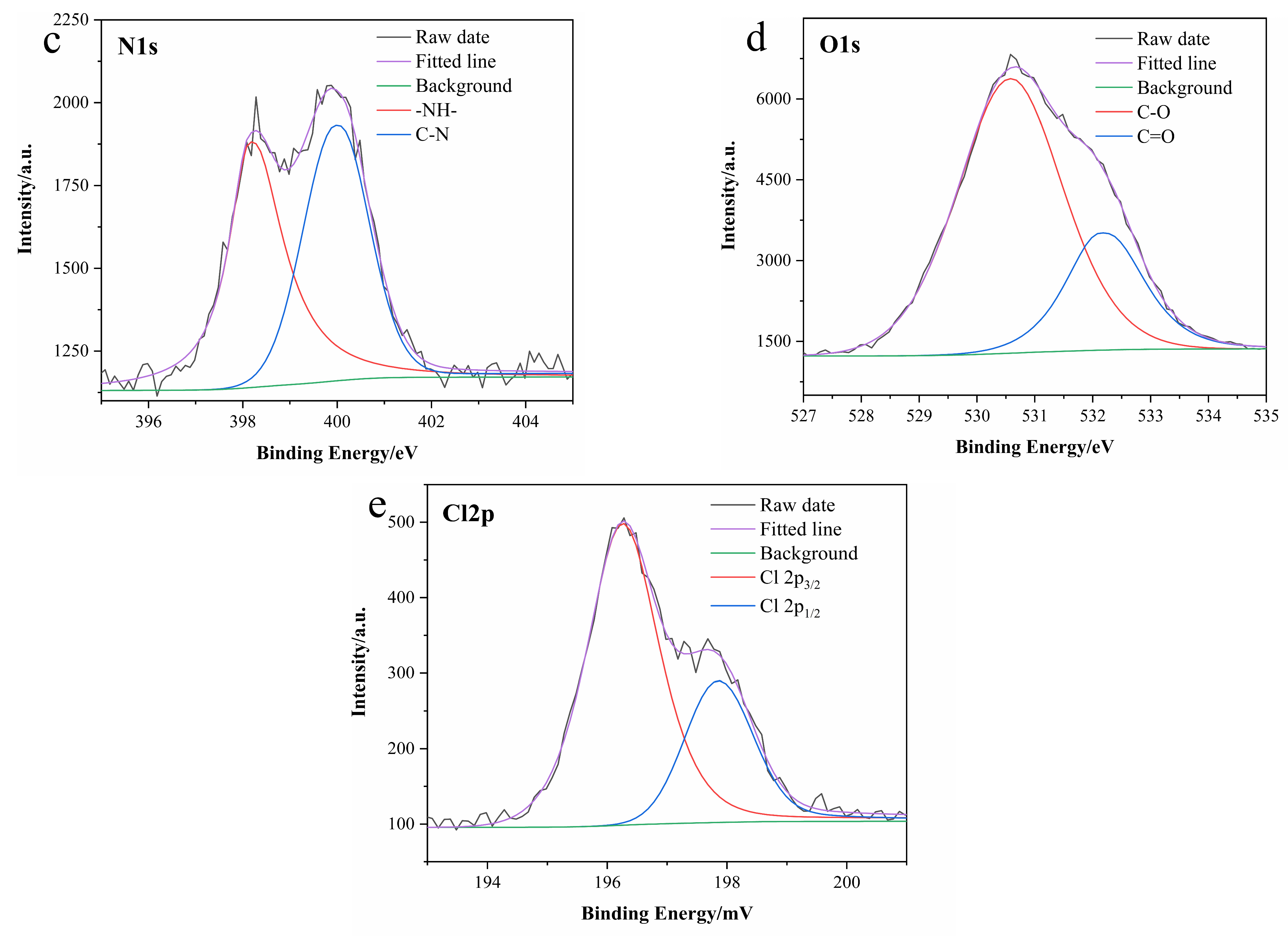
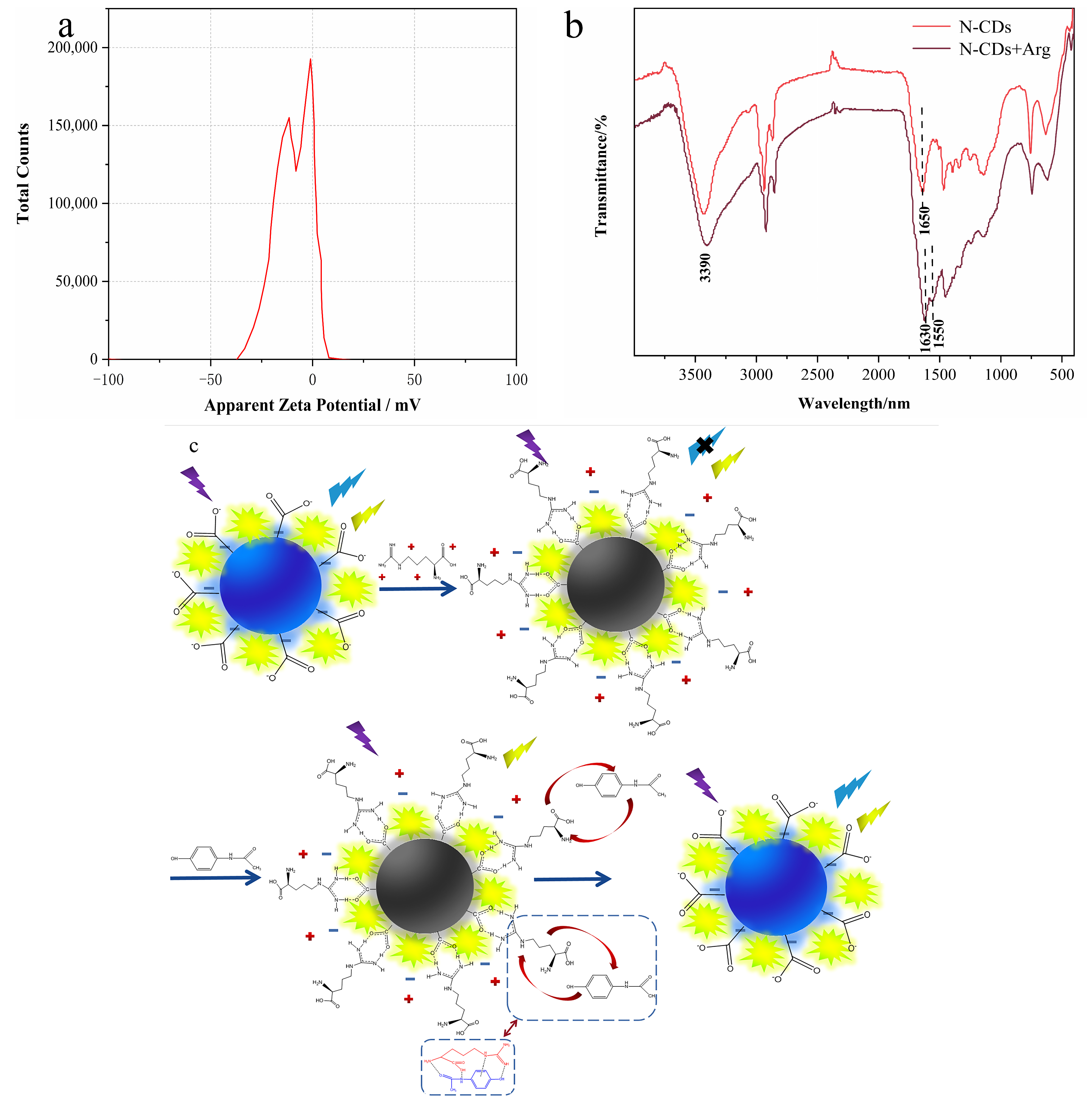
3.1. Characterization of N-CDs
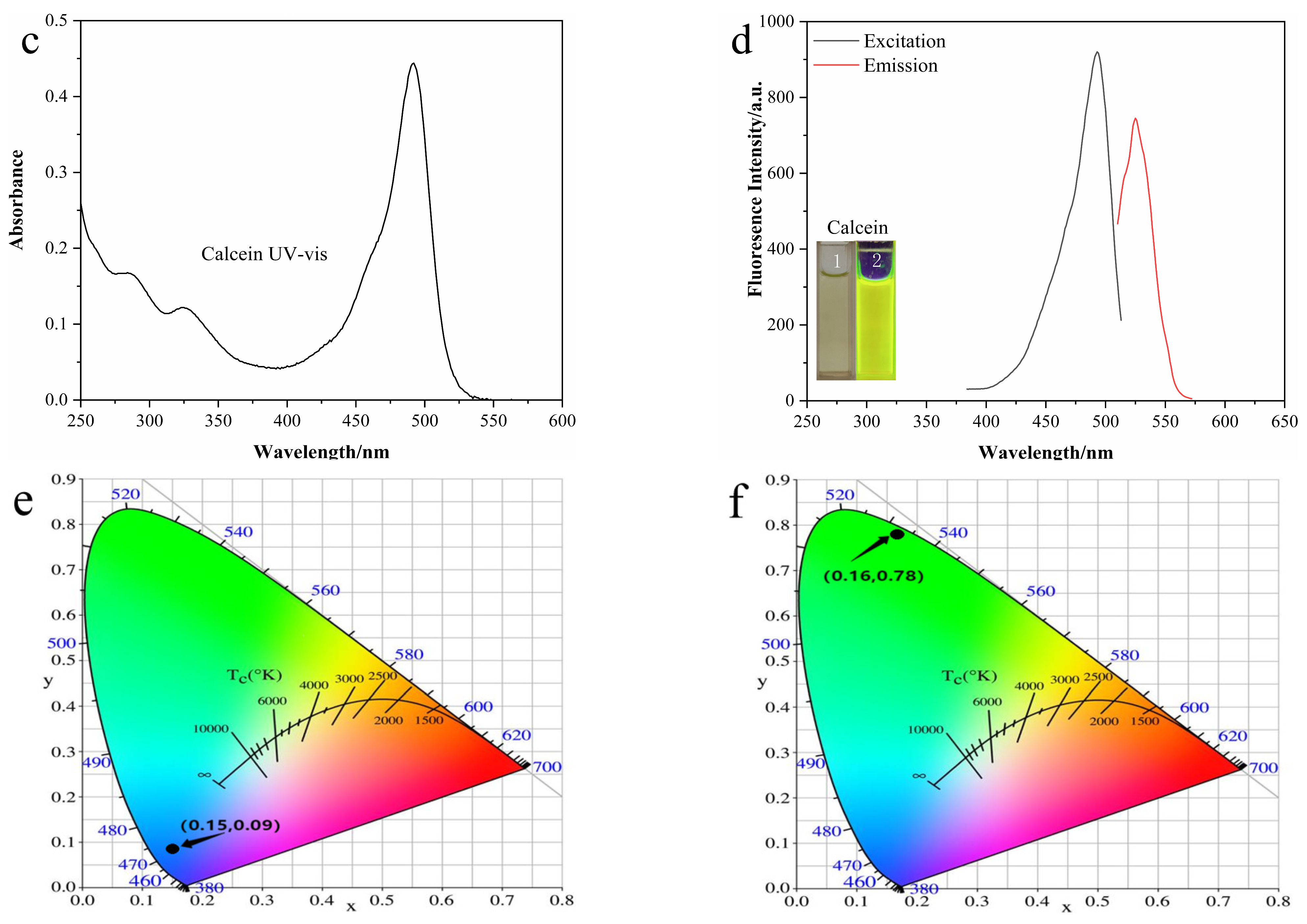
3.2. Optical Properties of N-CDs and Calcein
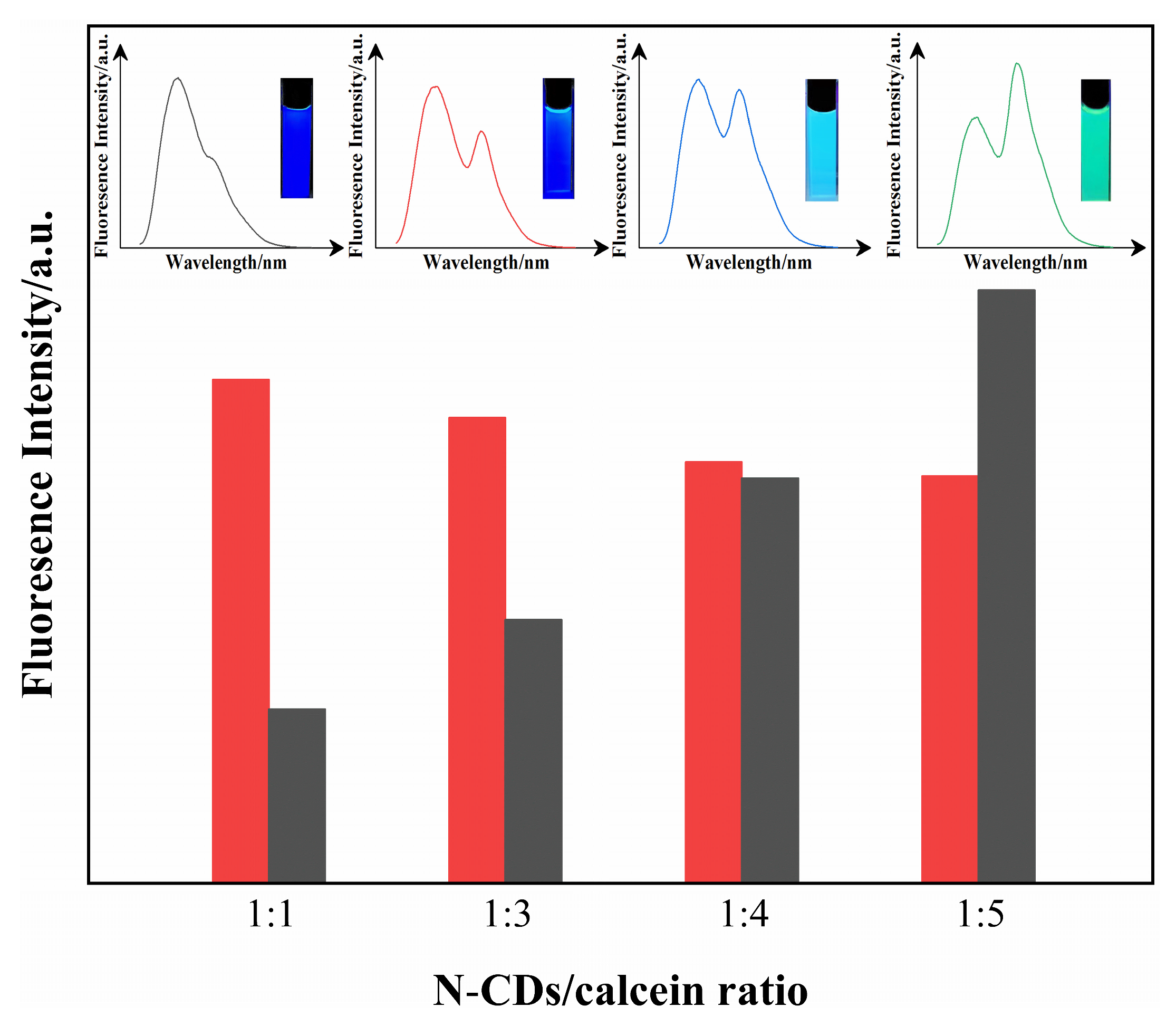
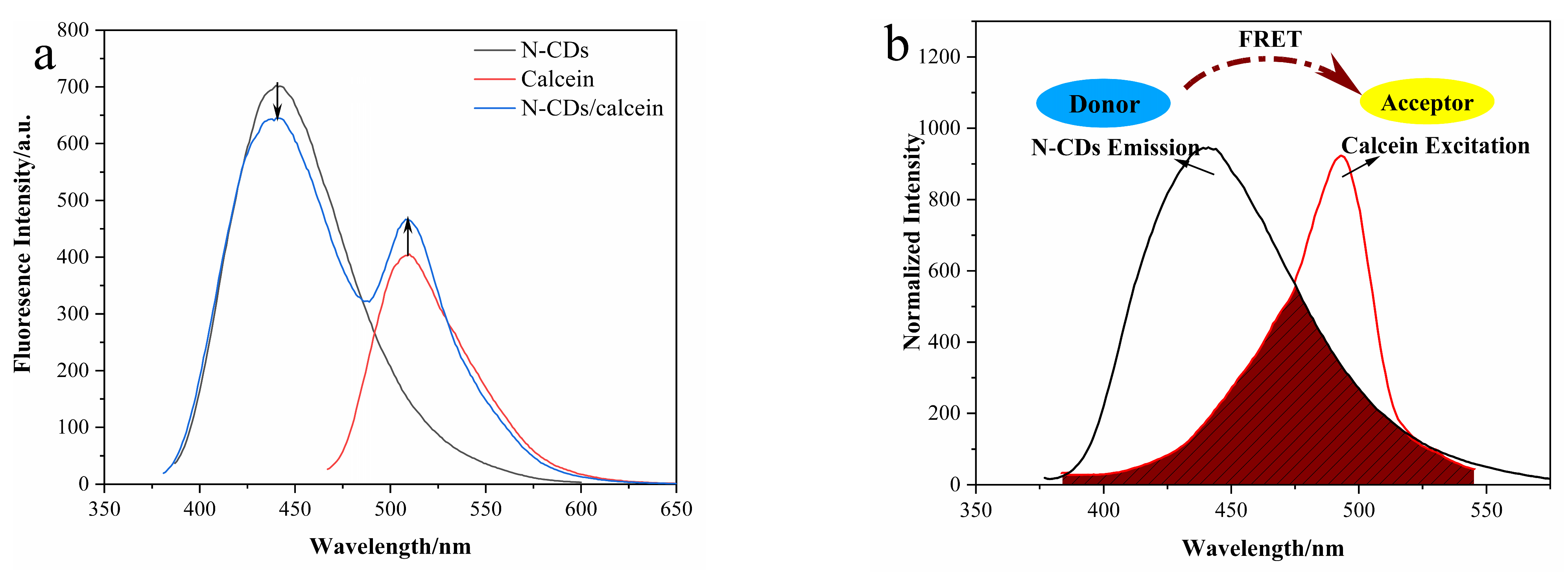
3.3. Construction of N-CD/Calcein Ratiometric Fluorescent Sensor
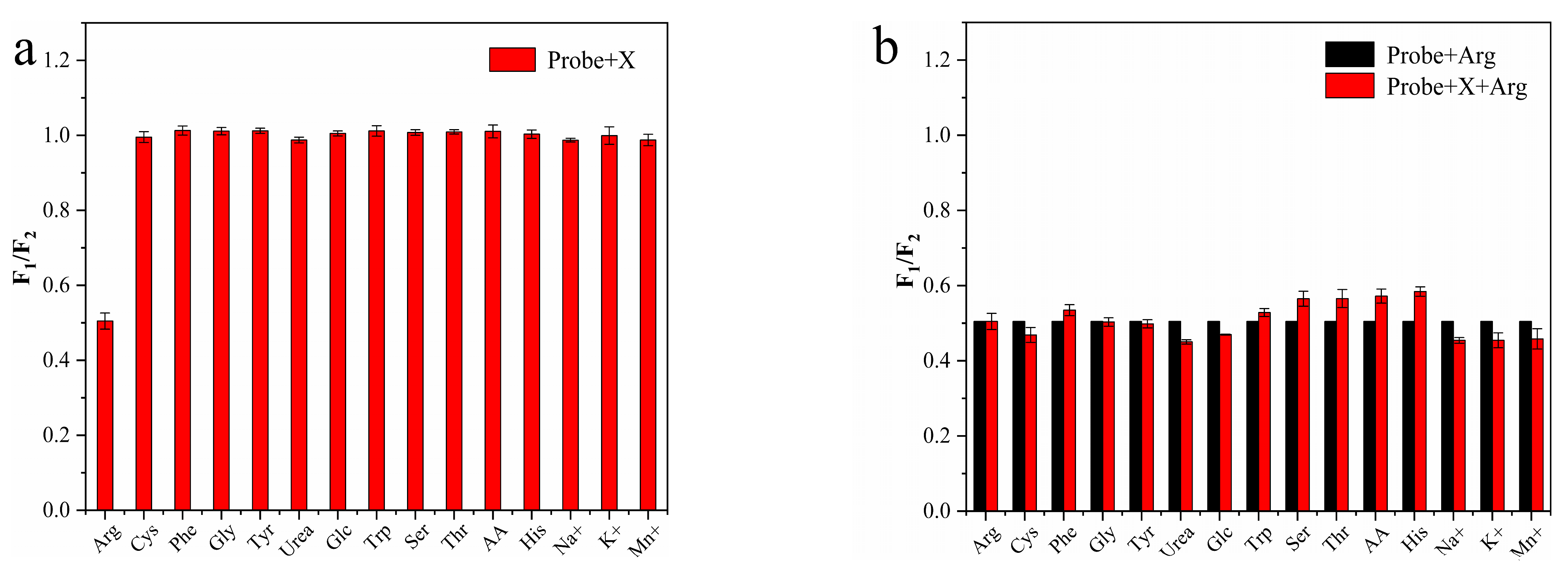
3.4. Characteristics of N-CD/Calcein Ratiometric Fluorescent Sensor
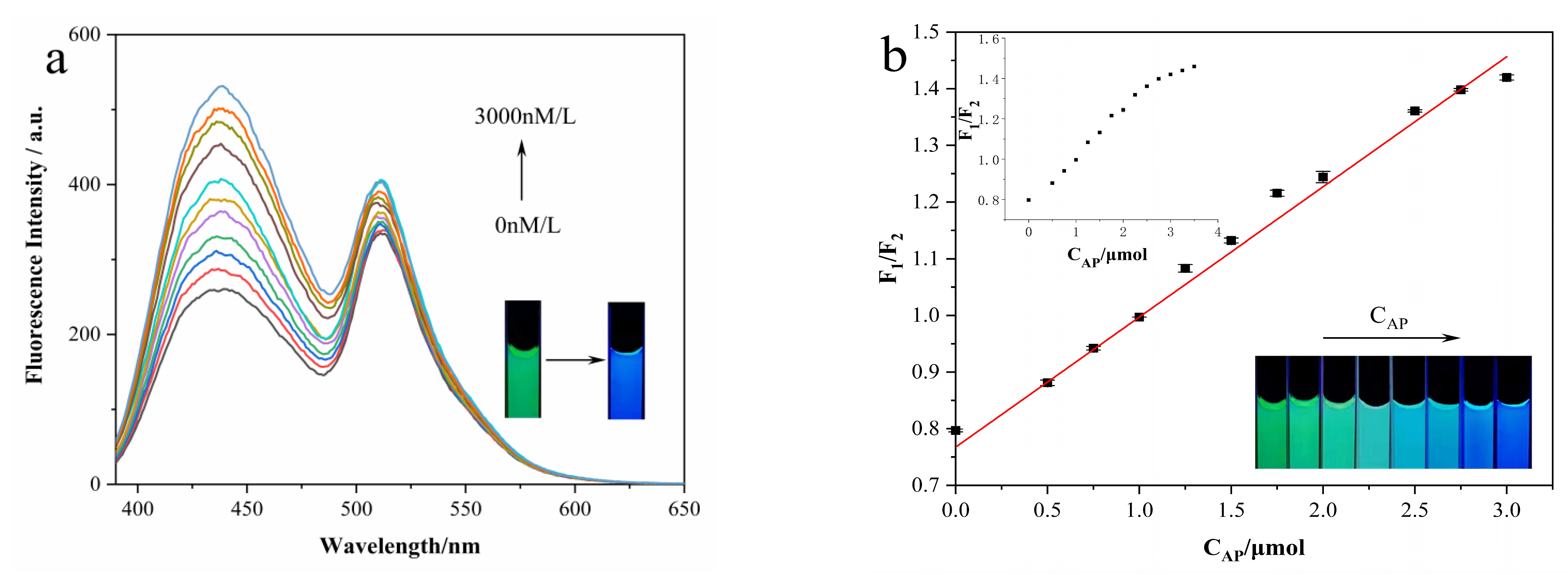
3.5. Ratiometric Fluorescence and Visual Detection of Arg and AP
3.6. Mechanism of the Ratiometric Sensing System
3.7. Detection of Arg and AP in Bovine Serum
3.8. Logic Gate Application and Reversibility of N-CD/Calcein Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marius, D.M.; Patrick, C. The role of L-arginine metabolism in neurocritical care patients. Neural Regen. Res. 2022, 17, 1446–1453. [Google Scholar]
- Navaei, A.H.; Shekerdemian, L.S.; Mohammad, M.A. Derangement of arginine and related amino acids in children undergoing surgery for congenital heart disease with cardiopulmonary bypass. Crit. Care Explor. 2020, 2, e0150. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Engelen, M.P.K.J.; Deutz, N.E.P. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Wagner, L.; Mahaney, J. Uremic levels of urea inhibit L-arginine transport in cultured endothelial cells. Am. J. Physiol.-Ren. Physiol. 2001, 280, F989–F995. [Google Scholar] [CrossRef]
- Ghorai, A.; Mondal, J.; Patra, G.K. A new Schiff base and its metal complex as colorimetric and fluorescent-colorimetric sensors for rapid detection of arginine. New J. Chem. 2016, 40, 7821–7830. [Google Scholar] [CrossRef]
- Bologna, K.; Cesana-Nigro, N.; Refardt, J. Effect of arginine on the hypothalamic-pituitary-adrenal axis in individuals with and without vasopressin deficiency. J. Clin. Endocrinol. Metab. 2020, 105, E2327–E2336. [Google Scholar] [CrossRef]
- Goumas, G.; Tentolouris, C.; Tousoulis, D. Therapeutic modification of the L-arginine-eNOS pathway in cardiovascular diseases. Atherosclerosis 2001, 154, 255–267. [Google Scholar] [CrossRef]
- Maarsingh, H.; Bossenga, B.E.; Bos, I.S.T. L-Arginine deficiency causes airway hyperresponsiveness after the late asthmatic reaction. Eur. Resp. J. 2009, 34, 91–199. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.; Bishop, A.E.; Polak, J.M. Pancreatic changes elicited by chronic administration of excess L-arginine. Exp. Mol. Pathol. 1994, 60, 71–87. [Google Scholar] [CrossRef]
- Vukosavljevic, N.; Jaron, D.; Barbee, K.A. Quantifying the L-arginine paradox in vivo. Microvasc. Res. 2005, 71, 48–54. [Google Scholar] [CrossRef]
- Savelieva, K.V.; Kamensky, A.A.; Ashmarin, I.P. The excess of alimentary L-arginine impairs place learning with food reinforcement in rats. Zhurnal Vyss. Nervn. Deyatelnosti Im I P Pavlov 1996, 46, 301–308. [Google Scholar]
- Huang, H.S.; Ma, M.C.; Chen, J. Chronic L-arginine administration increases oxidative and nitrosative stress in rat hyperoxaluric kidneys and excessive crystal deposition. Am. J. Physiol.-Ren. Physiol. 2007, 295, F388–F396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Q.; Jiang, L.; Tian, X. Rapid determination of acetaminophen and p-aminophenol in pharmaceutical formulations using miniaturized capillary electrophoresis with amperometric detection. Anal. Chim. Acta 2008, 606, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Prabakar, S.J.R.; Narayanan, S.S. Amperometric determination of paracetomol by a surface modified cobalt hexacyanoferrate graphite wax composite electrode. Talanta 2007, 72, 1818–1827. [Google Scholar] [CrossRef]
- Henderson, A.J.; Shaheen, S.O. Acetaminophen and asthma. Paediatr. Respir. Rev. 2012, 14, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Bruno, M.K. Contribution of acetaminophen-cysteine to acetaminophen nephrotoxicity in CD-1 mice: I. enhancement of acetaminophen nephrotoxicity by acetaminophen-cysteine. Toxicol. Appl. Pharmacol. 2004, 202, 151–159. [Google Scholar] [CrossRef]
- Heard, K.; Green, J. Acetylcysteine Therapy for acetaminophen poisoning. Curr. Pharm. Biotechnol. 2012, 13, 1917–1923. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Burton, P.J.; Blaschke, T.F. High-performance liquid chromatographic assay for the quantitation of L-arginine in human plasma. Anal. Chem. 1996, 68, 3520–3523. [Google Scholar] [CrossRef]
- Olson, D.L.; Lacey, M.E.; Webb, A.G. Nanoliter-volume H-1 NMR detection using periodic stopped-flow capillary electrophoresis. Anal. Chem. 1999, 71, 3070–3076. [Google Scholar] [CrossRef]
- Mira de Orduna, R. Quantitative determination of L-arginine by enzymatic end-point analysis. J. Agric. Food Chem. 2001, 49, 549–552. [Google Scholar] [CrossRef]
- Bahl, S.; Naqvi, S. Simple, rapid quantitative-determination of lysine and arginine by thin-layer chromatography. J. Agric. Food Chem. 1976, 24, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Bastone, A.; Diomede, L.; Parini, R. Determination of argininosuccinate lyase and arginase activities with an amino-acid analyzer. Anal. Biochem. 1990, 191, 384–389. [Google Scholar] [CrossRef]
- Kauffman, J.F.; Batykefer, L.M. Raman detected differential scanning calorimetry of polymorphic transformations in acetaminophen. J. Pharm. Biomed. Anal. 2008, 48, 1310–1315. [Google Scholar] [CrossRef]
- Heard, K.; Anderson, V. Serum Acetaminophen Protein Adduct Concentrations in Pediatric Emergency Department Patients. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.T.; Fan, Z.F. Application of magnesium ion doped carbon dots obtained via hydrothermal synthesis for arginine detection. New J. Chem. 2020, 44, 4842–4849. [Google Scholar] [CrossRef]
- Xu, J.L.; Cui, C.K.; Gong, T.Y. Ultrasonic-assisted synthesis of N-doped, multicolor carbon dots toward fluorescent inks, fluorescence sensors, and logic gate operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef]
- Chauhan, P.; Mundekkad, D.; Mukhenjee, A. Coconut carbon dots: Progressive large-scale synthesis, detailed biological activities and smart sensing aptitudes towards tyrosine. Nanomaterials 2022, 12, 162. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P. Microwave-synthesized polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef]
- Hou, L.; Chen, D.D.; Wang, R.T. Transformable honeycomb-like nanoassemblies of carbon dots for regulated multisite delivery and enhanced antitumor chemoimmunotherapy. Angew. Chem. Int. Ed. 2021, 60, 6581–6592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.B.; Sahu, A. Refractive-index tuning of highly fluorescent carbon dots. ACS Appl. Mater. Interfaces 2017, 9, 28930–28938. [Google Scholar] [CrossRef] [PubMed]
- Gedanken, A.; Kumar, V.B. A hydrothermal reaction of an aqueous solution of BSA yields highly fluorescent N doped C-dots used for imaging of live mammalian cells. J. Mater. Chem. B 2016, 4, 2913–2920. [Google Scholar]
- Mu, X.W.; Jiang, X. Sensitive ratiometric fluorescence probe based on chitosan carbon dots and calcein for Alkaline phosphatase detection and bioimaging in cancer cell. Anal. Chim. Acta 2021, 1188, 339163. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ren, X.; Sun, M.T. Carbon Dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Ramanarayanan, R.; Sindhu, S. Synthesis and characterisation of green luminescent carbon dots from guava leaf extract. Mater. Today Proc. 2020, 33, 2223–2227. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kumar, S. Target specific sptamer- induced self -assembly of fluorescent graphene quantum dots on palladium nanoparticles for sensitive detection of tetracycline in raw milk. Food. Chem. 2021, 346, 128893. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S. Immobilization of heteroatom-doped carbon dots onto nonpolar plastics for antifogging, antioxidant, and food monitoring application. Langmuir 2021, 37, 3508–3520. [Google Scholar] [CrossRef]
- Syed, R.A.; Masoomeh, S. Nanozymatic detection of thiocyanate through accelerating the growth of ultra-small gold nanoparticles/graphene quantum dots hybrids. Food Chem. 2022, 379, 132152. [Google Scholar]
- Bu, X.N.; Fu, Y.X.; Jiang, X.W. Self-assembly of DNA-templated copper nanoclusters and carbon dots for ratiometric fluorometric and visual determination of arginine and acetaminophen with a logic-gate operation. Microchim. Acta 2020, 187, 154. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, H.X. Synthesis of dual-emission fluorescence carbon quantum dots and their ratiometric fluorescent detection for arginine in 100% water solution. New J. Chem. 2019, 43, 13234–13239. [Google Scholar] [CrossRef]
- Gan, X.Q.; Angelina, E. Arginine-malate-based dual-emission carbon dots for uric acid determination in human serum with a miniaturized device. J. Mater. Sci. 2022, 57, 576–588. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, J.H.; Park, S.J. “Turn on” fluorescence sensor of glutathione based on inner filter effect of Co-doped carbon dot/gold nanoparticle composites. Nanomaterials 2022, 23, 190. [Google Scholar] [CrossRef] [PubMed]
- Molla, C.; Lee, H.; Ju, Y. Ratiometric fluorescence probe based on monochromaticdual-emission carbon nanodots with fluorescence cespectral change. Dyes Pigment. 2021, 197, 109883. [Google Scholar] [CrossRef]
- Xu, X.M.; Ren, D.D.; Chai, Y.Y. Dual-emission carbon dots-based fluorescent probe for ratiometric sensing of Fe(III) and pyrophosphate in biological samples. Sens. Actuators B Chem. 2019, 298, 126829. [Google Scholar] [CrossRef]
- Pang, S.; Liu, S.Y. Dual-emission carbon dots for ratiometric detection of Fe3+ ions and acid phosphatase. Anal. Chim. Acta 2020, 1105, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Lao, S.Y.; Ding, W.J. A novel ratiometric fluorescent probe for detection of iron ions and zinc ions based on dual-emission carbon dots. Sens. Actuators B Chem. 2018, 284, 186–192. [Google Scholar] [CrossRef]
- Huang, S.; Yao, J.D. One-step facile synthesis of nitrogen-doped carbon dots: A ratiometric fluorescent probes for evaluation of acetylcholinesterase activity and detection of organophosphorus pesticides in tap water and food. J. Agric. Food Chem. 2019, 67, 11244–11255. [Google Scholar] [CrossRef] [PubMed]
- Sudhan, N.; Subramani, K.; Karnan, M. Biomass-derived activated porous carbon from rice straw for a high-energy symmetric supercapacitor in aqueous and nonaqueous electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Srinivasan, K.; Subramanian, K.; Murugan, K. Sensitive fluorescence detection of mercury (II) in aqueous solution by the fluorescence quenching effect of MoS2 with DNA functionalized carbon dots. Analyst 2016, 141, 6344–6352. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Basu, H. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens. Actuators B Chem. 2015, 213, 434–443. [Google Scholar] [CrossRef]
- Xu, X.W.; Zhang, K.; Zhao, L. Aspirin-based carbon dots, a good biocompatibility of material applied for bioimaging and anti-inflammation. ACS Appl. Mater. Interfaces 2016, 8, 32706–32716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Sun, W.H.; Wu, P.Y. Highly photoluminescent carbon dots derived from egg white: Facile and green synthesis, photoluminescence properties, and multiple applications. ACS Sustain. Chem. Eng. 2015, 3, 1412–1418. [Google Scholar] [CrossRef]
- Papirer, E.; Lacroix, R.; Donnet, J.B. XPS study of the halogenation of carbon-black 2. chlorination. Carbon 1995, 33, 63–72. [Google Scholar] [CrossRef]
- Liu, B.S.; Gao, J. Fluorescence resonance energy transfer between acridine orange and rhodamine 6G and fluorescence quenching of rhodamine 6G reaction for the determination of proteins. Chin. J. Chem. 2005, 33, 546–548. [Google Scholar]
- Soldatkina, O.V.; Soldatkin, O.O.; Velychko, T.P. Conductometric biosensor for arginine determination in pharmaceutics. Bioelectrochemistry 2018, 124, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kaviya, S. Rapid naked eye detection of arginine by pomegranate peel extract stabilized gold nanoparticles. King Saud Univ. Sci. 2017, 31, 864–868. [Google Scholar] [CrossRef]
- Pu, W.; Zhao, H.W.; Huang, C.Z. Visual detection of arginine based on the unique guanidino group-induced aggregation of gold nanoparticles. Anal. Chim. Acta 2012, 764, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.S.; Luo, B.; Zhao, Y. Determination of Glutamine and Argininein Human Serum by Capillary Electrophoresis with UV Detection. Asian J. Org. Chem. 2012, 24, 565–567. [Google Scholar]
- Wei, M.; Lu, W.B.; Liu, G.Q. Ni2P nanosheets: A high catalytic activity platform for electrochemical detection of acetaminophen. Chin. J. Chem. 2021, 39, 1849–1854. [Google Scholar] [CrossRef]
- Liang, W.C.; Ren, H.L.; Li, Y.G. A robust electrochemical sensing based on bimetallic metal-organic framework mediated Mo2C for simultaneous determination of acetaminophen and isoniazid. Anal. Chim. Acta 2020, 1136, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.C.; Liu, L.L.; Li, Y.G. Nitrogen-rich porous carbon modified electrochemical sensor for the detection of acetaminophen. J. Electroanal. Chem. 2019, 855, 113496. [Google Scholar] [CrossRef]
- Liu, C.D.; Zhang, N.; Huang, X. Fabrication of a novel nanocomposite electrode with ZnO-MoO3 and biochar derived from mushroom biomaterials for the detection of acetaminophen in the presence of DA. Microchem. J. 2020, 161, 105719. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Yeom, S.; Oh, J.; Lee, E.J. Positive charge of arginine residues on histone H4 tail is required for maintenance of mating type in saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2018, 28, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Herrera, D.E.; Tirado-Guizar, A. Ratiometric arginine assay based on FRET between CdTe quantum dots and Cresyl violet. Microchim. Acta 2017, 184, 1997–2005. [Google Scholar] [CrossRef]







| Sensor | Linear Range | LOD | Ref. |
|---|---|---|---|
| Mg-CDs | 0.3–130 μM | 0.15 μM | [25] |
| DNA-CuNC/CDs | 0–100 μM | 0.35 μM | [38] |
| Conductometric biosensor | −2.5–500 μM | −2.5 μM | [54] |
| AuNPs | 1–6 μM | 10 μM | [55] |
| Citrate-capped AuNPs | 0.08–13.2 μM | 0.02 μM | [56] |
| Capillary | 30–1500 μM | 10 μM | [57] |
| N-CDs/calcein | 0.1–100 μM | 0.08 μM | This work |
| Sensor | Linear Range | LOD | Ref. |
|---|---|---|---|
| Ni2P nanosheets/GCE | 0.5~4500 μM | 0.11 μM | [58] |
| DNA-CuNC/CDs | 0–100 μM | 0.26 μM | [38] |
| Mo2C@BMZIFs/GCE | 0.1–300 μM | 0.03 μM | [59] |
| P-NC/GCE | 3–110 μM | 0.5 μM | [60] |
| ZnO-MoO3-C/GCE | 2.5–2000 μM | 1.14 μM | [61] |
| N-CDs/calcein/Arg | 0.03–3 μM | 0.02 μM | This Work |
| Sample | Found in Sample (μM) | Added (μM) | Measured (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Serum | 70.82 | 5 | 76.04 | 104.4 | 0.7 |
| 72.04 | 15 | 86.37 | 95.5 | 0.2 | |
| 70.91 | 25 | 96.07 | 100.6 | 0.4 |
| Sample | Found in Sample (μM) | Added (μM) | Measured (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Serum | 0 | 0.5 | 0.51 | 102.0 | 0.8 |
| 0 | 1.5 | 1.49 | 99.3 | 1.9 | |
| 0 | 2.5 | 2.49 | 99.6 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Li, Q.; Jing, J.; Jing, T.; Liu, C.; Qiu, L.; Sami, R.; Helal, M.; Ismail, K.A.; Aljahani, A.H. Construction of N-CDs and Calcein-Based Ratiometric Fluorescent Sensor for Rapid Detection of Arginine and Acetaminophen. Nanomaterials 2022, 12, 976. https://doi.org/10.3390/nano12060976
Qi H, Li Q, Jing J, Jing T, Liu C, Qiu L, Sami R, Helal M, Ismail KA, Aljahani AH. Construction of N-CDs and Calcein-Based Ratiometric Fluorescent Sensor for Rapid Detection of Arginine and Acetaminophen. Nanomaterials. 2022; 12(6):976. https://doi.org/10.3390/nano12060976
Chicago/Turabian StyleQi, Haiyan, Qiuying Li, Jing Jing, Tao Jing, Chuntong Liu, Lixin Qiu, Rokayya Sami, Mahmoud Helal, Khadiga Ahmed Ismail, and Amani H. Aljahani. 2022. "Construction of N-CDs and Calcein-Based Ratiometric Fluorescent Sensor for Rapid Detection of Arginine and Acetaminophen" Nanomaterials 12, no. 6: 976. https://doi.org/10.3390/nano12060976






