Green Silver Nanoparticles Synthesized from Taverniera couneifolia Elicits Effective Anti-Diabetic Effect in Alloxan-Induced Diabetic Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Plant Collection and Extraction
2.3. Investiagtions of Phytoconstituents
2.4. Green Synthesis of AgNPs
2.5. Physicochemical Characterizations of AgNPs
2.6. In Vivo Induction of Diabetes
2.7. In-Vivo Acute Toxicity
2.8. Experimental Treatments in Animals and Ethics
2.9. Oral Glucose Tolerance Test
2.10. Serology of Various Treated Groups
2.11. Urine Analysis
2.12. Statistical Analysis
3. Results
3.1. Qualitative Assesment of Bioactive Metabolites
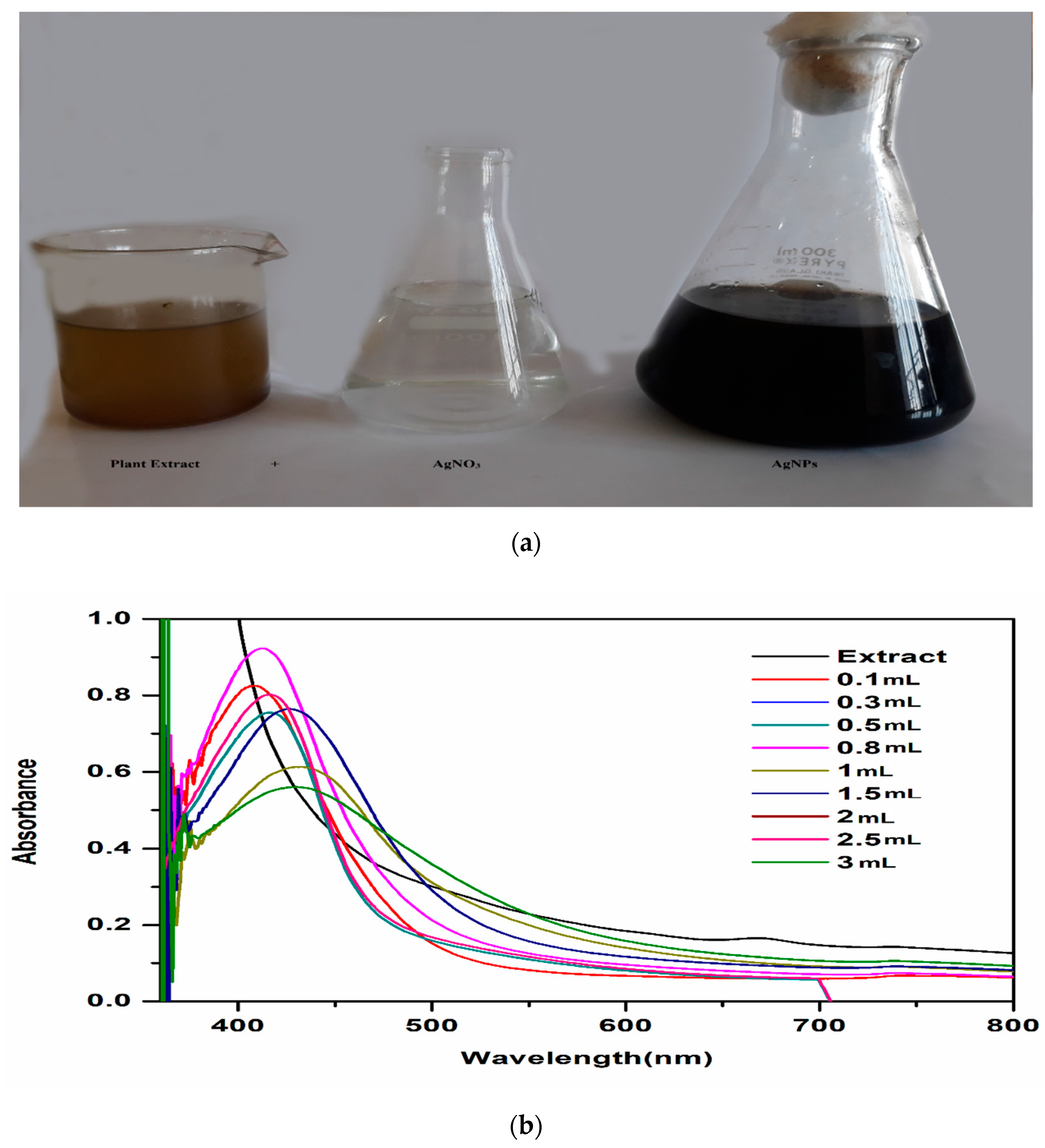
3.2. Green Synthesis of AgNPs
3.3. Physicochemical Characteristics
3.3.1. UV-Vis Analysis
3.3.2. FTIR Analysis
3.3.3. XRD Analysis
3.3.4. SEM Analysis
3.3.5. EDX Analysis
3.4. In Vivo Effects of T. couneifolia-Mediated AgNPs on Diabetes
3.4.1. Phytogenic AgNPs Does Not Cause Acute Toxicity
3.4.2. Phytogenic AgNPs Lower High Blood Glucose Levels
3.4.3. Serological and Urine Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Li, W.; Li, X.; Zhang, M.; Chen, L.; Zheng, Y.; Sun, G.; Ruan, C. Antidiabetic effects of malonyl ginsenosides from Panax ginseng on type 2 diabetic rats induced by high-fat diet and streptozotocin. J. Ethnopharmacol. 2013, 145, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Karthick, V.; Kumar, V.G.; Dhas, T.S.; Singaravelu, G.; Sadiq, A.M.; Govindaraju, K. Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats—An in vivo approach. Colloids Surf. B Biointerfaces 2014, 122, 505–511. [Google Scholar] [CrossRef]
- Lautamaki, R.; Airaksinen, K.E.J.; Seppanen, M.; Toikka, J.; Luotolahti, M.; Ball, E.; Borra, R.; Harkonen, R.; Iozzo, P.; Stewart, M.; et al. Rosiglitazone improves myocardial glucose uptake in patients with type 2 diabetes and coronary artery disease: A 16-week randomized, double-blind, placebo-controlled study. Diabetes 2005, 54, 2787–2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tornio, A.; Niemi, M.; Neuvonen, P.J.; Backman, J.T. Drug interactions with oral antidiabetic agents: Pharmacokinetic mechanisms and clinical implications. Trends Pharmacol. Sci. 2012, 33, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Kumar, V.G.; Karthick, V.; Dhas, T.S.; Potluri, S.L. Hydrothermal synthesis of hydroxyapatite plates prepared using low molecular weight heparin (LMWH). Colloids Surf. B Biointerfaces 2013, 111, 764–768. [Google Scholar] [CrossRef]
- Wright Jr, E.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Christopher, J.R. Type 2 Diabetes-a Matter of B cell Life and Death. Science 2005, 307, 380–384. [Google Scholar]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Modi, P. Diabetes beyond insulin: Review of new drugs for treatment of diabetes mellitus. Curr. Drug Discov. Technol. 2007, 4, 39–47. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. The role of oxidative stress in diabetic complications. Curr. Opin. Endocrinol. Diabetes Obes. 1996, 3, 277–284. [Google Scholar] [CrossRef]
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Liu, X. Silver nanoparticles—The real “silver bullet” in clinical medicine? Medchemcomm 2010, 1, 125–131. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, 43. [Google Scholar]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Smitha, S.L.; Nissamudeen, K.M.; Philip, D.; Gopchandran, K.G. Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 186–190. [Google Scholar] [CrossRef]
- Bae, C.H.; Nam, S.H.; Park, S.M. Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl. Surf. Sci. 2002, 197, 628–634. [Google Scholar] [CrossRef]
- Basavaraja, S.; Balaji, S.D.; Lagashetty, A.; Rajasab, A.H.; Venkataraman, A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. Green synthesis of silver nanoparticles using Cycas leaf. Int. J. Green Nanotechnol. Phys. Chem. 2010, 1, P110–P117. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Nasir, E. Flora of West Pakistan. No. 100, Papilionaceae; University of Karachi: Karachi, Pakistan, 1977. [Google Scholar]
- Nisar Ul Haq, M.; Wazir, S.M.; Ullah, F.; Khan, R.A.; Shah, M.S.; Khatak, A. Phytochemical and biological evaluation of defatted seeds of Jatropha curcas. Sains Malays. 2016, 45, 1435–1442. [Google Scholar]
- Harborne, A.J. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Jain, V.C.; Shah, D.P.; Sonani, N.G.; Dhakara, S.; Patel, N.M. Pharmacognostical and preliminary phytochemical investigation of Lawsonia inermis L. leaf. Rom. J. Biol.-Plant Biol 2010, 55, 127–133. [Google Scholar]
- Khan, N.A.; Niaz, A.; Zaman, M.I.; Khan, F.A.; Nisar-ul-haq, M.; Tariq, M. Sensitive and selective colorimetric detection of Pb2+ by silver nanoparticles synthesized from Aconitum violaceum plant leaf extract. Mater. Res. Bull. 2018, 102, 330–336. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunghez, I.R.; Patrascu, M.E.B.; Badea, N.; Doncea, S.M.; Popescu, A.; Ion, R.M. Antioxidant silver nanoparticles green synthesized using ornamental plants. J. Optoelectron. Adv. Mater. 2012, 14, 1016–1022. [Google Scholar]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Mansour, H.A.; Newairy, A.-S.A.; Yousef, M.I.; Sheweita, S.A. Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology 2002, 170, 221–228. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Mohammad, F. Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319145020. [Google Scholar]
- Suresh, S.N.; Nagarajan, N. Antimicrobial activity and preliminary phytochemical analysis of Begonia malabarica Lam. J. Pure Appl. Microbiol. 2009, 3, 801–803. [Google Scholar]
- Gonzalez-Guevara, J.L.; Gonzalez-Lavaut, J.A.; Pino-Rodriguez, S.; Garcia-Torres, M.; Carballo-Gonzalez, M.T.; Echemendia-Arana, O.A.; Molina-Torres, J.; Prieto-Gonzalez, S. Phytochemical screening and in vitro antiherpetic activity of four Erythtroxylum species. Acta Farm. Bonaer 2004, 23, 506–509. [Google Scholar]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf. B Biointerfaces 2009, 68, 55–60. [Google Scholar] [CrossRef]
- Anyasor, G.N.; Ogunwenmo, O.; Oyelana, O.A.; Akpofunure, B.E. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl. (Costaceae). Afr. J. Biotechnol. 2010, 9, 4880–4884. [Google Scholar]
- Igbinosa, O.O.; Igbinosa, E.O.; Aiyegoro, O.A. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn). Afr. J. Pharm. Pharmacol. 2009, 3, 58–62. [Google Scholar]
- Thitilertdecha, N.; Teerawutgulrag, A.; Rakariyatham, N. Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT-Food Sci. Technol. 2008, 41, 2029–2035. [Google Scholar] [CrossRef]
- Lee, C.P.-D.; Hsiu, S.-L.; Hou, Y.-C. Flavonoids in herbs: Biological fates and potential interactions with xenobiotics. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Vadlapudi, V.; Kaladhar, D. Antimicrobial study of plant extracts of Datura metel L. against some important disease causing pathogens. Asian Pac. J. Trop. Dis. 2012, 2, S94–S97. [Google Scholar] [CrossRef]
- Akharaiyi, F.C. Antibacterial, phytochemical and antioxidant activities of Datura metel. Int. J. PharmTech Res. 2011, 3, 478–483. [Google Scholar]
- Ayoola, G.A.; Coker, H.A.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar]
- Alabri, T.H.A.; Al Musalami, A.H.S.; Hossain, M.A.; Weli, A.M.; Al-Riyami, Q. Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud Univ. 2014, 26, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Camacho-López, M.A.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Padalia, H.; Moteriya, P.; Chanda, S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Hudlikar, M.; Joglekar, S.; Dhaygude, M.; Kodam, K. Green synthesis of TiO2 nanoparticles by using aqueous extract of Jatropha curcas L. latex. Mater. Lett. 2012, 75, 196–199. [Google Scholar] [CrossRef]
- Singhal, G.; Bhavesh, R.; Kasariya, K.; Sharma, A.R.; Singh, R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanopart. Res. 2011, 13, 2981–2988. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Janjua, M.R.S.A.; Iqbal, M.A.; Rashid, U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Roopan, S.M.; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Pant, G.; Nayak, N.; Prasuna, R.G. Enhancement of antidandruff activity of shampoo by biosynthesized silver nanoparticles from Solanum trilobatum plant leaf. Appl. Nanosci. 2013, 3, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Vivek, R.; Thangam, R.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process. Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- Zaheer, Z. Bio-conjugated silver nanoparticles: From Ocimum sanctum and role of cetyltrimethyl ammonium bromide. Colloids Surf. B Biointerfaces 2013, 108, 90–94. [Google Scholar] [CrossRef]
- Kalakotla, S.; Jayarambabu, N.; Mohan, G.K.; Mydin, R.B.S.M.N.; Gupta, V.R. A novel pharmacological approach of herbal mediated cerium oxide and silver nanoparticles with improved biomedical activity in comparison with Lawsonia inermis. Colloids Surf. B Biointerfaces 2019, 174, 199–206. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Bonjar, G.H.S.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally safe biosynthesis of gold nanoparticles using plant water extracts. Nanomaterials 2021, 11, 2033. [Google Scholar] [CrossRef]
- Heydari, M.; Yousefi, A.R.; Nikfarjam, N.; Rahdar, A.; Kyzas, G.Z.; Bilal, M. Plant-based nanoparticles prepared from protein containing tribenuron-methyl: Fabrication, characterization, and application. Chem. Biol. Technol. Agric. 2021, 8, 53. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Sevastyanov, D.A.; Zelenikhin, P.V.; Padnya, P.L.; Evtugyn, V.G.; Osin, Y.N.; Stoikov, I.I. Nanoparticles based on the zwitterionic pillar[5]arene and Ag+: Synthesis, self-assembly and cytotoxicity in the human lung cancer cell line A549. Beilstein J. Nanotechnol. 2020, 11, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The Role of Calix [n] arenes and Pillar [n] arenes in the Design of Silver Nanoparticles: Self-Assembly and Application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charbgoo, F.; Ahmad, M.B.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malapermal, V.; Botha, I.; Krishna, S.B.N.; Mbatha, J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1294–1305. [Google Scholar] [CrossRef]
- Gylling, H.; Miettinen, T.A. Cholesterol absorption, synthesis, and LDL metabolism in NIDDM. Diabetes Care 1997, 20, 90–95. [Google Scholar] [CrossRef]
- Attanayake, A.P.; Jayatilaka, K.A.P.W.; Pathirana, C.; Mudduwa, L.K.B. Study of antihyperglycaemic activity of medicinal plant extracts in alloxan induced diabetic rats. Anc. Sci. Life 2013, 32, 193. [Google Scholar] [CrossRef] [Green Version]
- Ragini, V.; KVSRG, P.; Bharathi, K. Antidiabetic and antioxidant activity of Shorea tumbuggaia Rox. Int. J. Innov. Pharm. Res. 2011, 2, 113–121. [Google Scholar]
- Fröde, T.S.; Medeiros, Y.S. Animal models to test drugs with potential antidiabetic activity. J. Ethnopharmacol. 2008, 115, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Swanston-Flatt, S.K.; Day, C.; Bailey, C.J.; Flatt, P.R. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 1990, 33, 462–464. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Ironkwe, A.; Iroaganachi, M.A. Effect of Livingstone potato (Plectranthus esculenthus NE Br) on diabetes and its complications in streptozotocin induced diabetes in rats. Diabetes Metab. J. 2014, 38, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Shaik, A.H.; Rasool, S.N.; Reddy, A.V.K.; Kareem, M.A.; Krushna, G.S.; Devi, K.L. Cardioprotective effect of HPLC standardized ethanolic extract of Terminalia pallida fruits against isoproterenol-induced myocardial infarction in albino rats. J. Ethnopharmacol. 2012, 141, 33–40. [Google Scholar] [CrossRef]
- Shanmugasundaram, E.R.B.; Rajeswari, G.; Baskaran, K.; Kumar, B.R.R.; Shanmugasundaram, K.R.; Ahmath, B.K. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J. Ethnopharmacol. 1990, 30, 281–294. [Google Scholar] [CrossRef]
- Shanmugam, S.; Bhavani, P. Studies on the comparison of phytochemical constituents and antimicrobial activity of Curcuma longa varieties. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 573–581. [Google Scholar]
- Kumar, C.M.K.; Yugandhar, P.; Savithramma, N. Biological synthesis of silver nanoparticles from Adansonia digitata L. fruit pulp extract, characterization, and its antimicrobial properties. J. Intercult. Ethnopharmacol. 2016, 5, 79. [Google Scholar] [CrossRef] [PubMed]










| Tests | Present (+) | Absent (-) |
|---|---|---|
| Coumarins | - | |
| Terpenoids | + | |
| Amino acids | + | |
| Carbohydrates | + | |
| Sterols | - | |
| Quinones | + | |
| Phenols | + | |
| Alkaloids | + | |
| Flavonoids | + | |
| Tannins | + | |
| Glycosides | + | |
| Proteins | - | |
| Saponins | - |
| LEU | URO | PRO | PH | SG | BIL | GLU | |
|---|---|---|---|---|---|---|---|
| Group1–Control | - | - | - | 5 | 1 | - | - |
| Group2–DM | 14 | 1.5 | 30 ± 0.3 | 9 | 1.55 | 1.09 | 255 ± 15 |
| Group3–DM control | - | 1 | 28 ± 0.4 | 4 | 1 | 1 | - |
| Group4–T Extract | - | 0.9 | 15 | 5.3 | 1.33 | 1.06 | - |
| Group5–T NPs | - | 1.1 | 14 ± 0.15 | 4.8 | 1.12 | 1.02 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ul Haq, M.N.; Shah, G.M.; Menaa, F.; Khan, R.A.; Althobaiti, N.A.; Albalawi, A.E.; Alkreathy, H.M. Green Silver Nanoparticles Synthesized from Taverniera couneifolia Elicits Effective Anti-Diabetic Effect in Alloxan-Induced Diabetic Wistar Rats. Nanomaterials 2022, 12, 1035. https://doi.org/10.3390/nano12071035
Ul Haq MN, Shah GM, Menaa F, Khan RA, Althobaiti NA, Albalawi AE, Alkreathy HM. Green Silver Nanoparticles Synthesized from Taverniera couneifolia Elicits Effective Anti-Diabetic Effect in Alloxan-Induced Diabetic Wistar Rats. Nanomaterials. 2022; 12(7):1035. https://doi.org/10.3390/nano12071035
Chicago/Turabian StyleUl Haq, Muhammad Nisar, Ghulam Mujtaba Shah, Farid Menaa, Rahmat Ali Khan, Norah A. Althobaiti, Aishah E. Albalawi, and Huda Mohammed Alkreathy. 2022. "Green Silver Nanoparticles Synthesized from Taverniera couneifolia Elicits Effective Anti-Diabetic Effect in Alloxan-Induced Diabetic Wistar Rats" Nanomaterials 12, no. 7: 1035. https://doi.org/10.3390/nano12071035








