A Green and Facile Microvia Filling Method via Printing and Sintering of Cu-Ag Core-Shell Nano-Microparticles
Abstract
:1. Introduction
2. Experiments
2.1. Materials Preparation
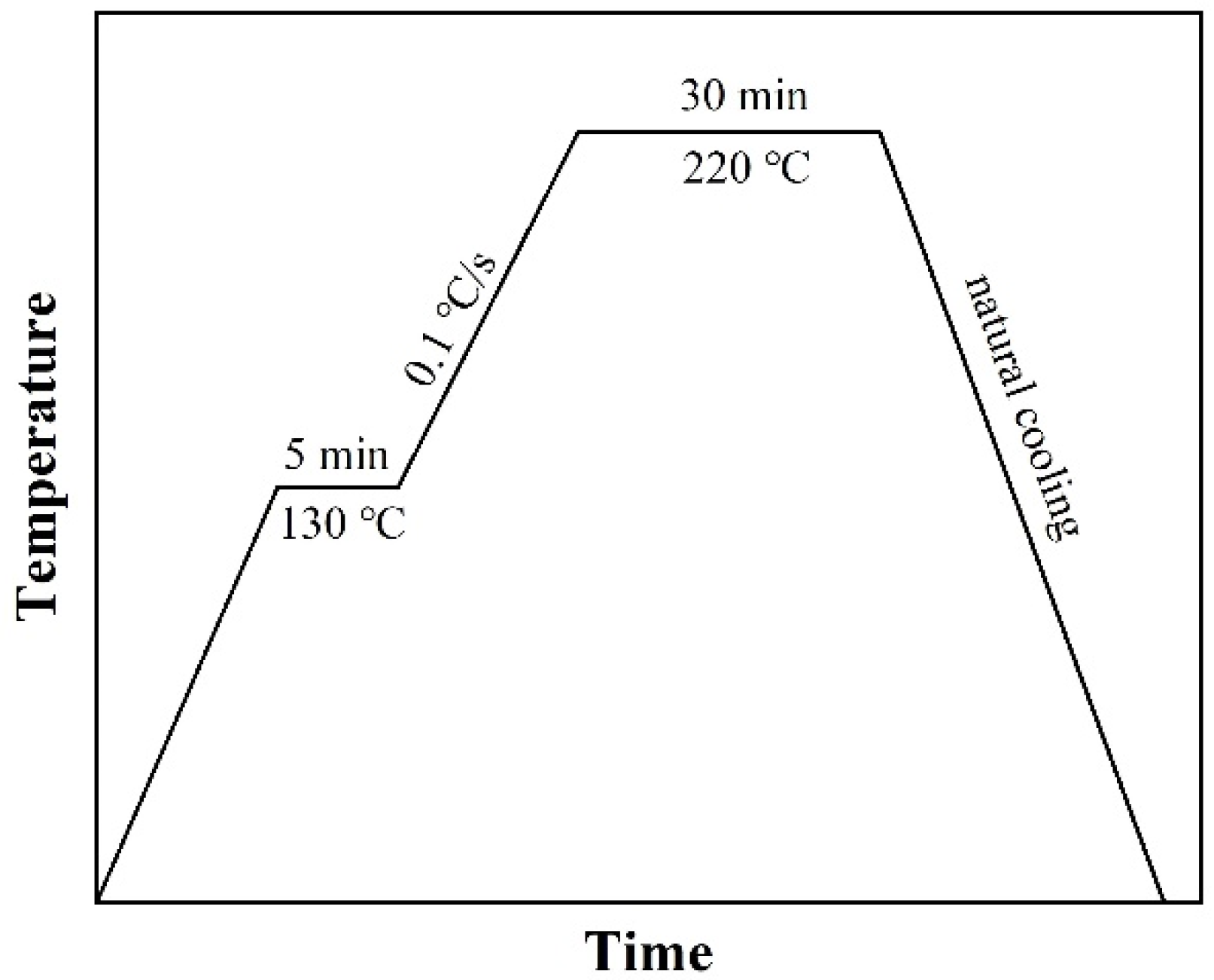
2.2. Filling Method
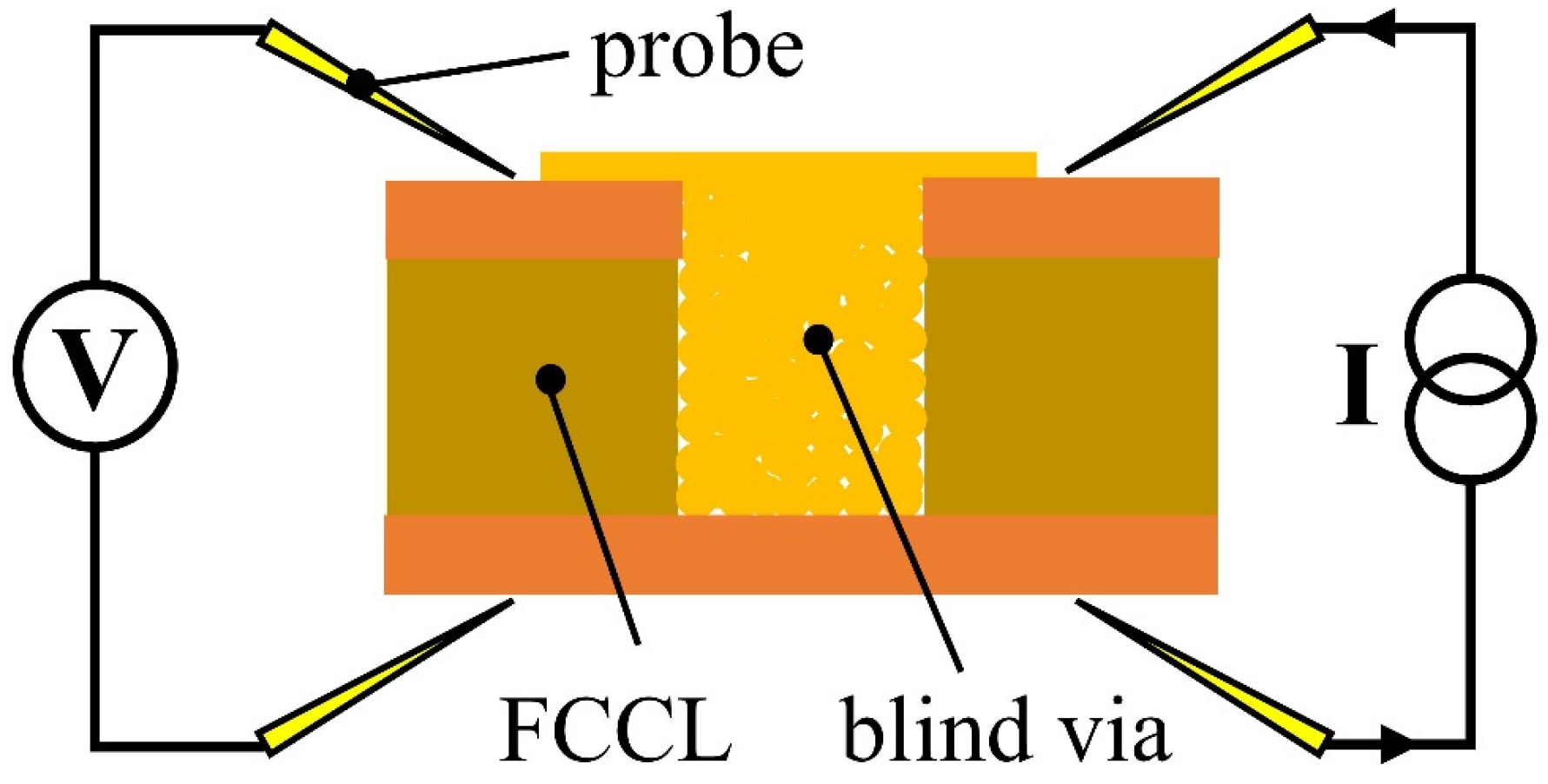
2.3. Characterization
3. Results and Discussions
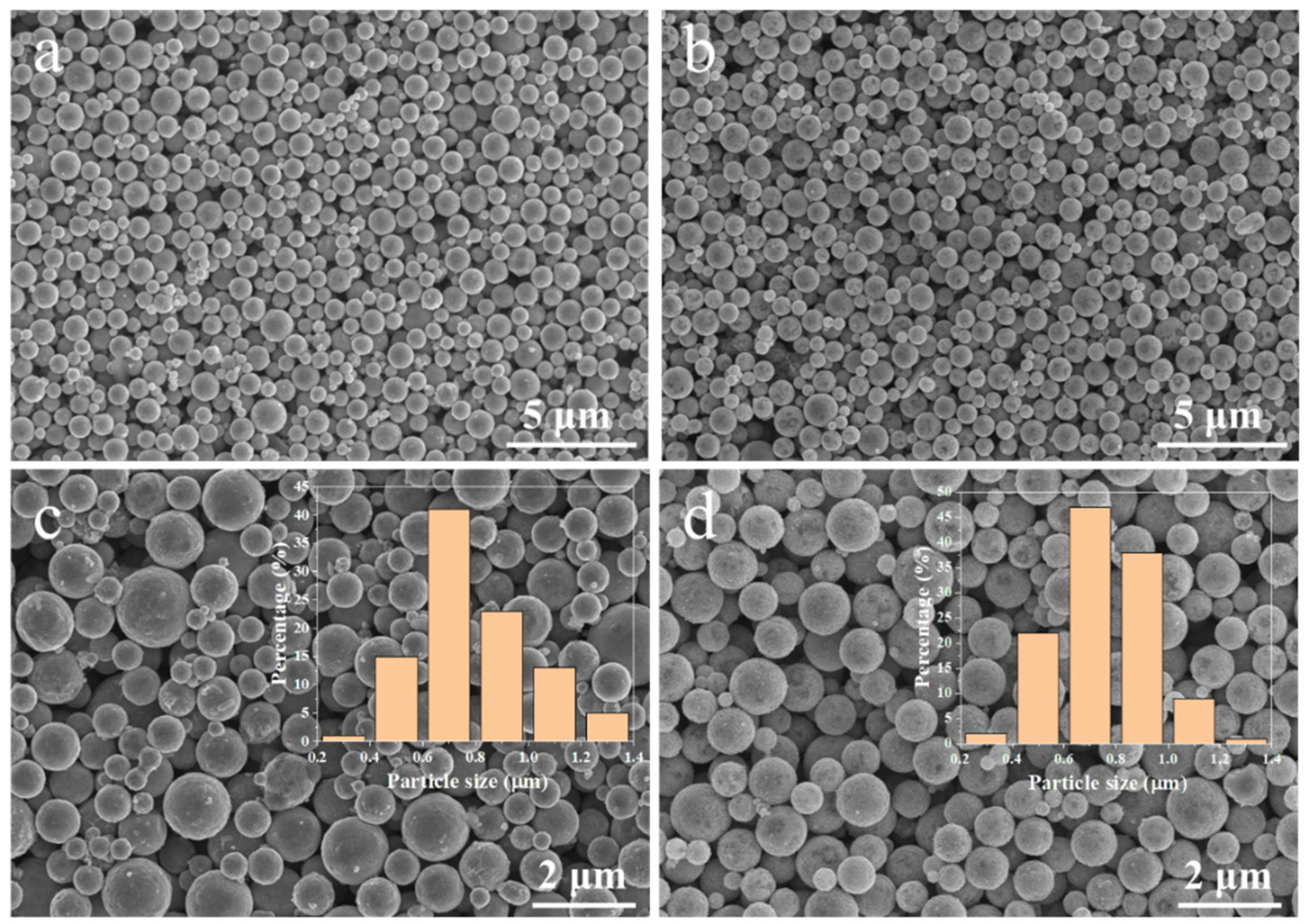
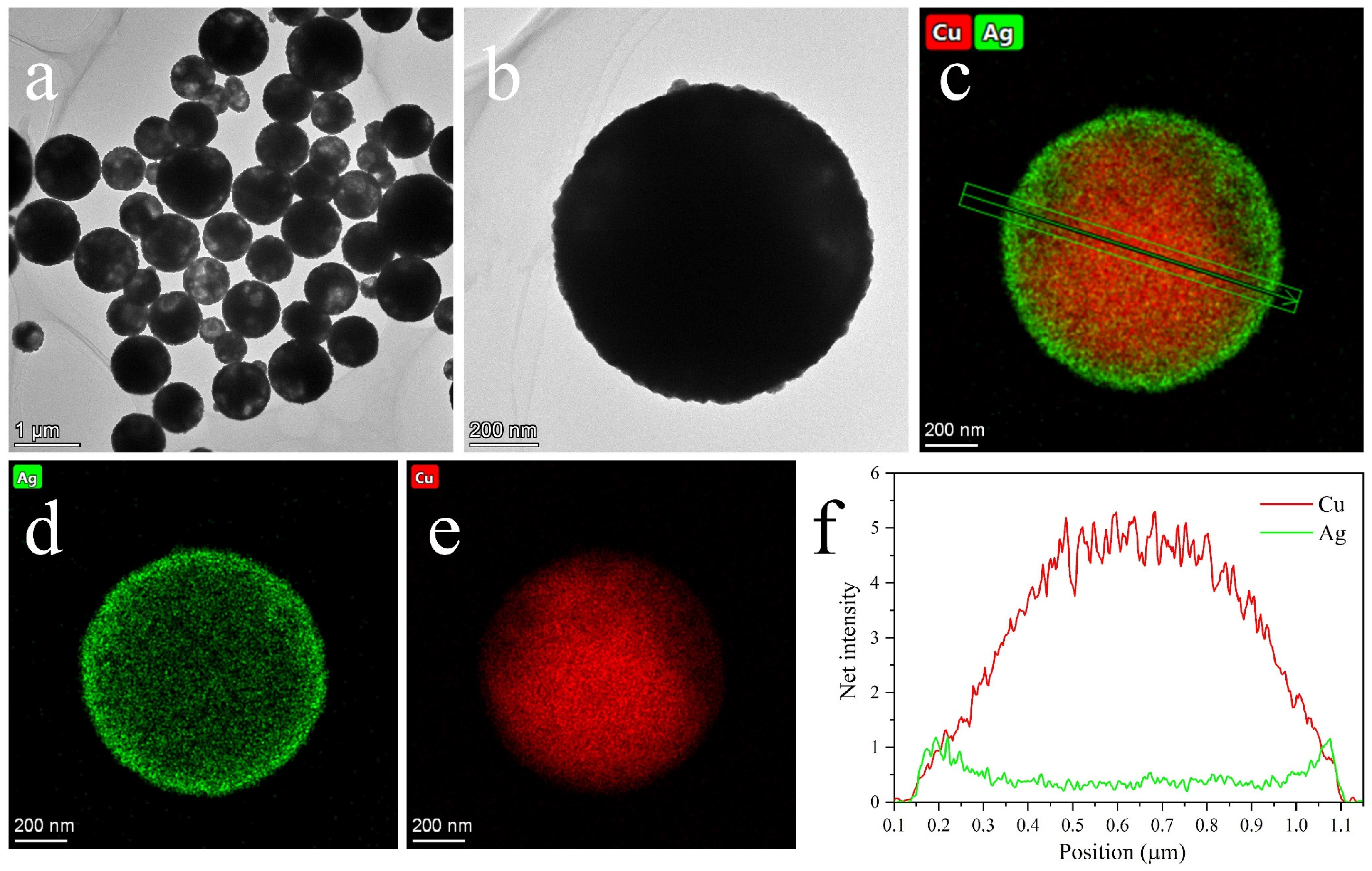
3.1. Micro-Structures of Cu@Ag NMPs
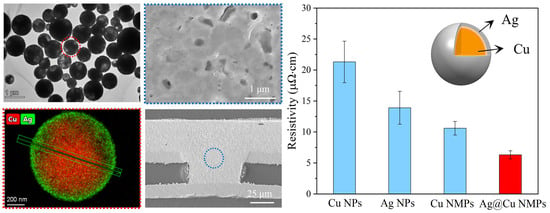
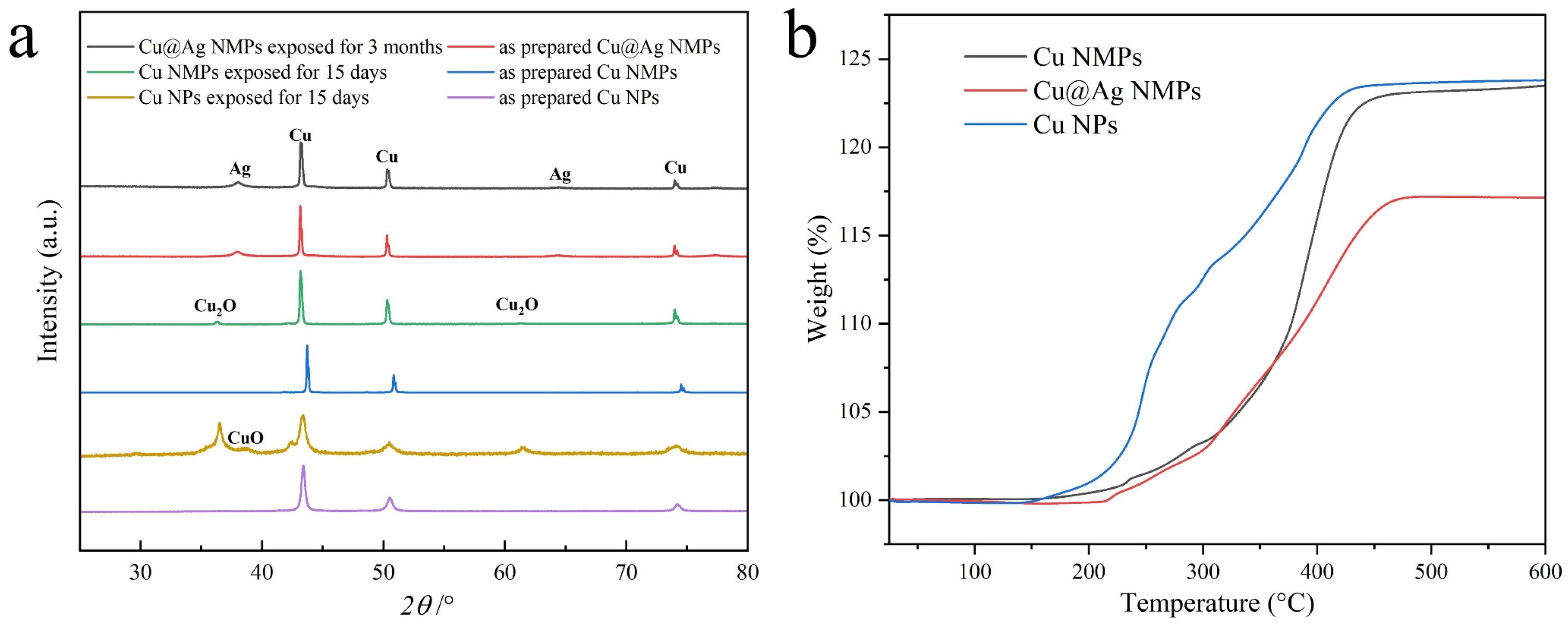
3.2. Oxidation Resistance of Cu@Ag NMPs
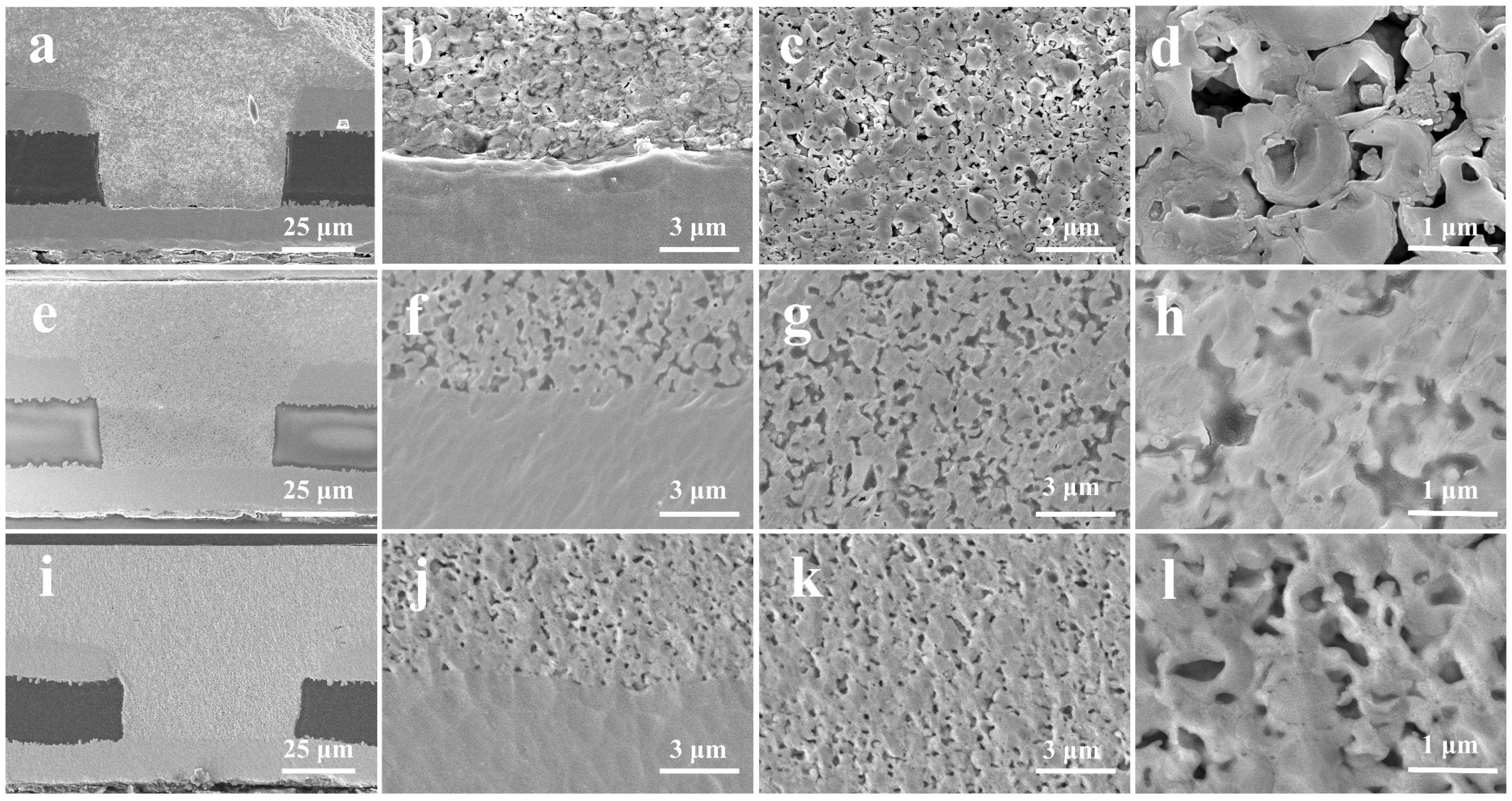
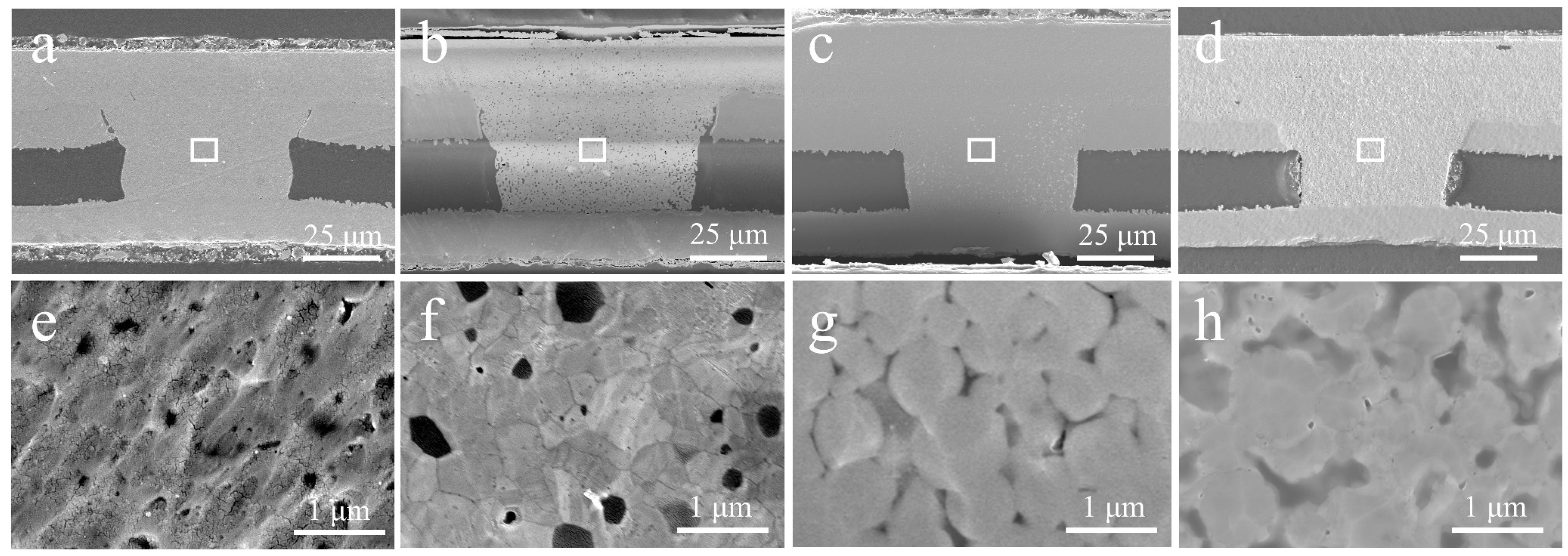
3.3. Characterization of Microvia Filling Performance
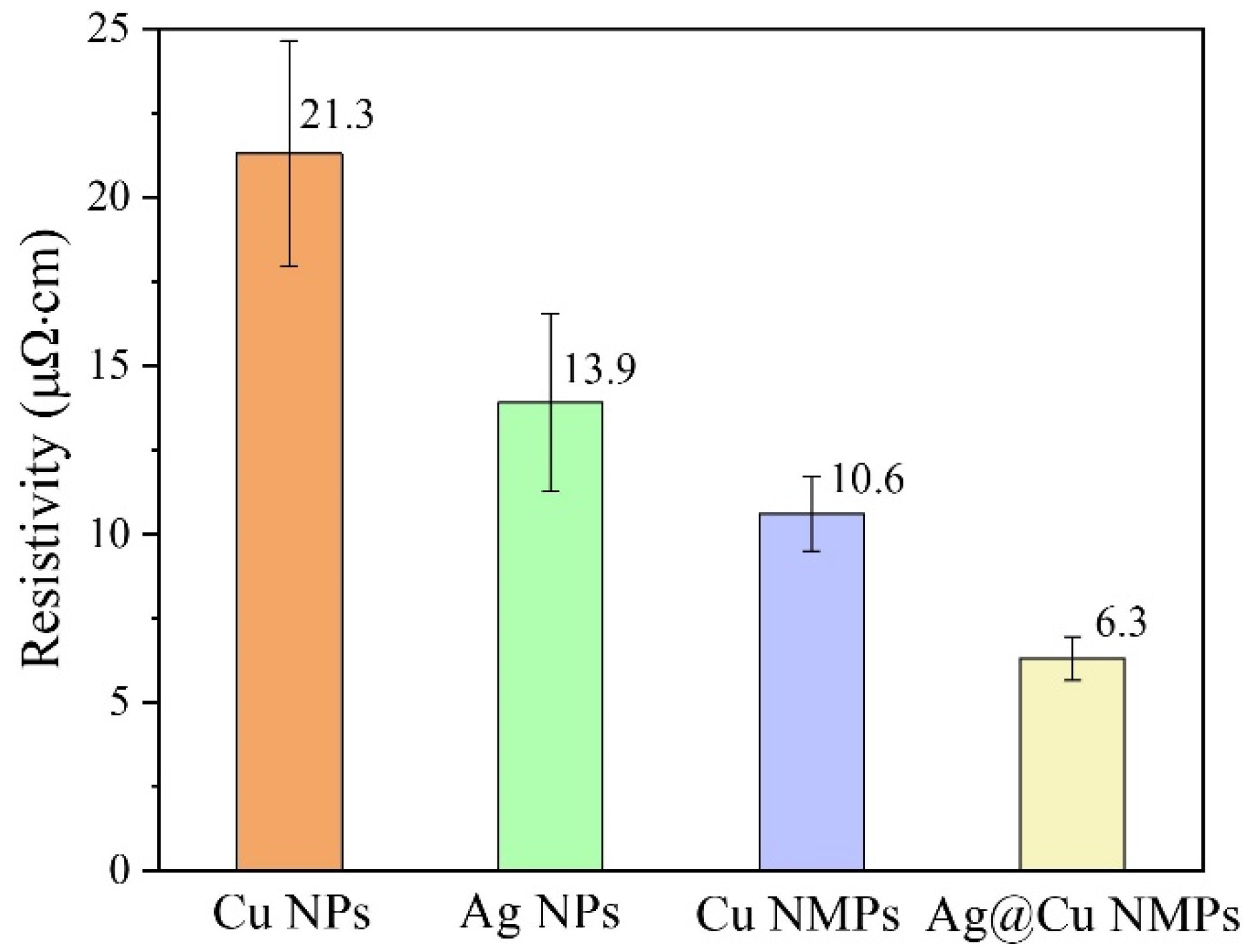
3.4. Advantage of Cu@Ag NMPs in Microvia Filling
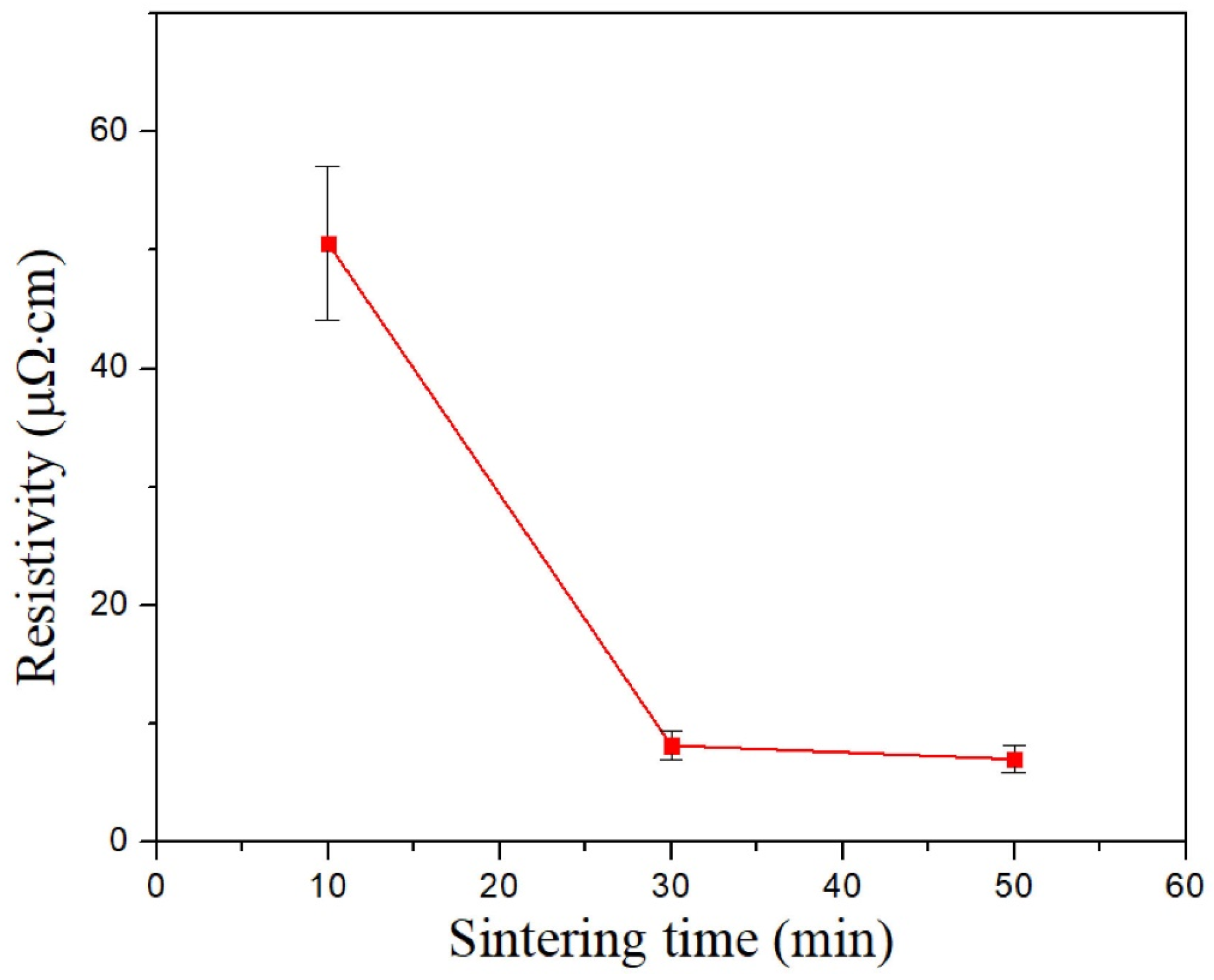
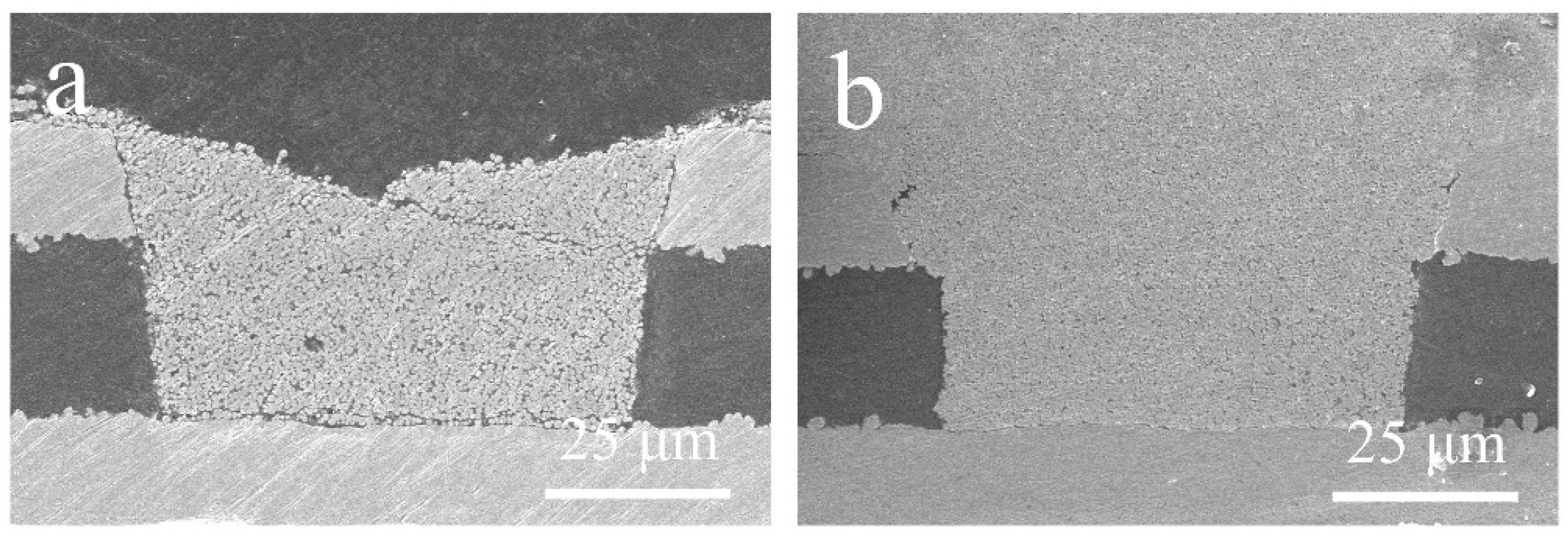
3.5. Effect of Dense Refill Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, L.; Wang, S.; Wang, C.; Chen, G.; Chen, Y.; He, W.; Tan, Z. Improved Uniformity of Conformal Through-Hole Copper Electrodeposition by Revision of Plating Cell Configuration. J. Electrochem. Soc. 2015, 162, D575–D583. [Google Scholar] [CrossRef]
- Chuang, H.-C.; Lai, W.-H.; Sanchez, J. An investigation of supercritical-CO2copper electroplating parameters for application in TSV chips. J. Micromech. Microeng. 2014, 25, 015004. [Google Scholar] [CrossRef]
- Khorramdel, B.; Mäntysalo, M. Fabrication and electrical characterization of partially metallized vias fabricated by inkjet. J. Micromech. Microeng. 2016, 26, 045017. [Google Scholar] [CrossRef]
- Kang, B.J.; Oh, J.H. Geometrical characterization of inkjet-printed conductive lines of nanosilver suspensions on a polymer substrate. Thin Solid Film. 2010, 518, 2890–2896. [Google Scholar] [CrossRef]
- Tao, P.; Chen, Y.; Cai, W.; Meng, Z. Effect of Copper Sulfate and Sulfuric Acid on Blind Hole Filling of HDI Circuit Boards by Electroplating. Materials 2020, 14, 85. [Google Scholar] [CrossRef]
- Dow, W.-P.; Yen, M.-Y.; Liao, S.-Z.; Chiu, Y.-D.; Huang, H.-C. Filling mechanism in microvia metallization by copper electroplating. Electrochim. Acta 2008, 53, 8228–8237. [Google Scholar] [CrossRef]
- Chu, J.-H.; Joo, S.-J.; Kim, H.-S. Development of a via-hole connection process via intense pulsed light sintering with Cu micro/Ag nano-hybrid ink for a multi-layered flexible printed circuit board. Thin Solid Film. 2019, 680, 1–11. [Google Scholar] [CrossRef]
- Overmeyer, L.; Olsen, E.; Hoffmann, G.-A. Additive manufacturing of copper vertical interconnect accesses by laser processing. CIRP Ann. 2021, 70, 163–166. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, H.Q.; Zhang, X.Y.; Liu, L.; Peng, P.; Zou, G.S.; Zhou, Y.N. Preparation of nanoparticle and nanowire mixed pastes and their low temperature sintering. J. Alloys Compd. 2017, 690, 86–94. [Google Scholar] [CrossRef]
- Min, H.; Lee, B.; Jeong, S.; Lee, M. Fabrication of 10 µm-scale conductive Cu patterns by selective laser sintering of Cu complex ink. Opt. Laser Technol. 2017, 88, 128–133. [Google Scholar] [CrossRef]
- Hong, S.; Yeo, J.; Kim, G.; Kim, D.; Lee, H.; Kwon, J.; Lee, H.; Lee, P.; Ko, S.H. Nonvacuum, Maskless Fabrication of a Flexible Metal Grid Transparent Conductor by Low-Temperature Selective Laser Sintering of Nanoparticle Ink. ACS Nano 2013, 7, 5024–5031. [Google Scholar] [PubMed]
- Back, S.; Kang, B. Low-cost optical fabrication of flexible copper electrode via laser-induced reductive sintering and adhesive transfer. Opt. Lasers Eng. 2018, 101, 78–84. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Yang, S.; Li, Y.; Huang, J. Multiscale simulation study of laser sintering of inkjet-printed silver nanoparticle inks. Int. J. Heat Mass Transf. 2020, 159, 120110. [Google Scholar] [CrossRef]
- Kang, B.; Han, S.; Kim, J.; Ko, S.; Yang, M. One-Step Fabrication of Copper Electrode by Laser-Induced Direct Local Reduction and Agglomeration of Copper Oxide Nanoparticle. J. Phys. Chem. C 2011, 115, 23664–23670. [Google Scholar]
- Yang, G.; Xu, G.; Li, Q.; Zeng, Y.; Zhang, Y.; Hao, M.; Cui, C. Understanding the sintering and heat dissipation behaviours of Cu nanoparticles during low-temperature selective laser sintering process on flexible substrates. J. Phys. D Appl. Phys. 2021, 54, 375304. [Google Scholar]
- Hsiao, C.-H.; Kung, W.-T.; Song, J.-M.; Chang, J.-Y.; Chang, T.-C. Development of Cu-Ag pastes for high temperature sustainable bonding. Mater. Sci. Eng. A 2017, 684, 500–509. [Google Scholar] [CrossRef]
- Kisiel, R.; Borecki, J.; Kozioł, G.; Felba, J. Conductive adhesives for through holes and blind vias metallization. Microelectron. Reliab. 2005, 45, 1935–1940. [Google Scholar] [CrossRef]
- Cho, C.-L.; Kao, H.-L.; Chang, L.-C.; Wu, Y.-H.; Chiu, H.-C. Inkjet-printed vertical interconnects for ultrathin system-on-package technology. Surf. Coat. Technol. 2019, 359, 85–89. [Google Scholar] [CrossRef]
- Ham, Y.-H.; Kim, D.-P.; Park, K.-S.; Jeong, Y.-S.; Yun, H.-J.; Baek, K.-H.; Kwon, K.-H.; Lee, K.; Do, L.-M. Dual etch processes of via and metal paste filling for through silicon via process. Thin Solid Film. 2011, 519, 6727–6731. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, P.; Lin, W.; Liu, Q.; Chen, Z.; Cao, J.; Yang, G.; Cui, C. Synergy effect of mixed sintering accelerator on the deoxidation and sintering property improvement of Cu nanoparticles at low temperature. Appl. Phys. A 2021, 127, 783. [Google Scholar] [CrossRef]
- Liu, P.; He, W.-Q.; Lu, A.-X. Preparation of low-temperature sintered high conductivity inks based on nanosilver self-assembled on surface of graphene. J. Cent. South. Univ. 2019, 26, 2953–2960. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Ji, H.; Li, M. Highly Conductive Cu–Cu Joint Formation by Low-Temperature Sintering of Formic Acid-Treated Cu Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 33289–33298. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, H.; Li, M.; Li, Y.; Chen, S.; Bao, B.; Song, Y. Rayleigh Instability-Assisted Satellite Droplets Elimination in Inkjet Printing. ACS Appl. Mater. Interfaces 2017, 9, 41521–41528. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Bao, H.; Li, X.; Jin, S.; Gu, Z. Research on electrical conductive adhesives filled with mixed filler. Int. J. Adhes. Adhes. 2014, 48, 159–163. [Google Scholar] [CrossRef]
- Iwai, T.; Sakai, T.; Mizutani, D.; Sakuyama, S.; Iida, K.; Inaba, T.; Fujisaki, H.; Tamura, A.; Miyazawa, Y. Glass Multilayer Package Substrate using Conductive Paste Via Connection. In Proceedings of the 2018 IEEE CPMT Symposium Japan (ICSJ), Kyoto, Japan, 19–21 November 2018; pp. 105–108. [Google Scholar]
- Iwai, T.; Sakai, T.; Mizutani, D.; Sakuyama, S.; Iida, K.; Inaba, T.; Fujisaki, H.; Miyazawa, Y. A Novel Inorganic Substrate by Three Dimensionally Stacked Glass Core Technology. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 29 May–1 June 2018; pp. 1987–1992. [Google Scholar]
- Iwai, T.; Sakai, T.; Mizutani, D.; Sakuyama, S.; Iida, K.; Inaba, T.; Fujisaki, H.; Tamura, A.; Miyazawa, Y. Multilayer Glass Substrate with High Density Via Structure for All Inorganic Multi-chip Module. In Proceedings of the 2019 IEEE 69th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2019; pp. 1952–1957. [Google Scholar]
- Bach, H.L.; Yu, Z.; Letz, S.; Bayer, C.F.; Waltrich, U.; Schletz, A.; Maerz, M. Vias in DBC Substrates for Embedded Power Modules. In Proceedings of the CIPS 2018 10th International Conference on Integrated Power Electronics Systems, Stuttgart, Germany, 20–22 March 2018; pp. 1–5. [Google Scholar]
- Li, J.; Zhou, G.; Wang, J.; Hong, Y.; He, W.; Wang, S.; Wang, C.; Chen, Y.; Yang, W.; Ma, C.; et al. Nickel-nanoparticles-assisted direct copper-electroplating on polythiophene conductive polymers for PCB dielectric holes. J. Taiwan Inst. Chem. Eng. 2019, 100, 262–268. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, D.; Zou, G.; Liu, L.; Zhou, Y.N. Preparation of Oxidation-Resistant Ag-Cu Alloy Nanoparticles by Polyol Method for Electronic Packaging. J. Electron. Mater. 2018, 48, 1286–1293. [Google Scholar] [CrossRef]
- Chee, S.-S.; Lee, J.-H. Preparation and oxidation behavior of Ag-coated Cu nanoparticles less than 20 nm in size. J. Mater. Chem. C 2014, 2, 5372–5381. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Zhou, J.; Chen, Z.; Liao, X.; Wang, X.; Shi, B. Lightweight and high-performance electromagnetic radiation shielding composites based on a surface coating of Cu@Ag nanoflakes on a leather matrix. J. Mater. Chem. C 2016, 4, 914–920. [Google Scholar] [CrossRef]
- Li, C.; Gong, X.; Tang, L.; Zhang, K.; Luo, J.; Ling, L.; Pu, J.; Li, T.; Li, M.; Yao, Y. Electrical property enhancement of electrically conductive adhesives through Ag-coated-Cu surface treatment by terephthalaldehyde and iodine. J. Mater. Chem. C 2015, 3, 6178–6184. [Google Scholar] [CrossRef]
- Jenkins, S. CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press, Chemical Engineering: Boca Raton, FL, USA, 2012; p. 9. Available online: https://books.google.nl/books/about/CRC_Handbook_of_Chemistry_and_Physics_93.html?id=-BzP7Rkl7WkC&redir_esc=y (accessed on 9 February 2022).
- Osowiecki, W.T.; Ye, X.C.; Satish, P.; Bustillo, K.C.; Clark, E.L.; Alivisatos, A.P. Tailoring Morphology of Cu-Ag Nanocrescents and Core-Shell Nanocrystals Guided by a Thermodynamic Model. J. Am. Chem. Soc. 2018, 140, 8569–8577. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.F.; Xu, W.; Zhang, T.; Shi, H.B.; Wang, T. Room temperature sintering of Cu-Ag core-shell nanoparticles conductive inks for printed electronics. Chem. Eng. J. 2019, 364, 310–319. [Google Scholar] [CrossRef]
- Lee, C.; Kim, N.R.; Koo, J.; Lee, Y.J.; Lee, H.M. Cu-Ag core–shell nanoparticles with enhanced oxidation stability for printed electronics. Nanotechnology 2015, 26, 455601. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zou, Q.; Wang, P.; Lai, H.; Lai, T.; Zeng, X.; Li, Z.; Luo, J.; Zhang, Y.; Cui, C. Towards understanding the facile synthesis of well-covered Cu-Ag core-shell nanoparticles from a complexing model. J. Alloys Compd. 2021, 874, 159900. [Google Scholar]
- Wu, Y.N.; Zhou, M.M.; Zhang, B.R.; Wu, B.Z.; Li, J.; Qiao, J.L.; Guan, X.H.; Li, F.T. Amino acid assisted templating synthesis of hierarchical zeolitic imidazolate framework-8 for efficient arsenate removal. Nanoscale 2014, 6, 1105–1112. [Google Scholar]
- Yang, T.H.; Guo, Z.L.; Fu, Y.M.; Cheng, Y.T.; Song, Y.F.; Wu, P.W. A low temperature inkjet printing and filling process for low resistive silver TSV fabrication in a SU-8 substrate. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 749–752. [Google Scholar]
- Yang, G.; Lai, H.; Lin, W.; Tong, J.; Peng, Z.; Cao, J.; Luo, J.; Zhang, Y.; Cui, C. Approaching the structure-property relationship of sintered metal nano/microparticles from the perspective of the agglomerate size effect. Powder Technol. 2022, 399, 117254. [Google Scholar]
- Quack, N.; Sadie, J.; Subramanian, V.; Wu, M.C. Through Silicon Vias and thermocompression bonding using inkjet-printed gold nanoparticles for heterogeneous MEMS integration. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 834–837. [Google Scholar]
- Das, R.; Egitto, F.; Markovich, V. Nano-and micro-filled conducting adhesives for z-axis interconnections: New direction for high-speed, high-density, organic microelectronics packaging. Circuit World 2008, 34, 3–12. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Shi, T.; Cheng, C.; Fan, J.; Cheng, S.; Li, T.; Liao, G.; Tang, Z. Depressing of CuCu bonding temperature by composting Cu nanoparticle paste with Ag nanoparticles. J. Alloys Compd. 2017, 709, 700–707. [Google Scholar] [CrossRef]
- Konrad, K.; Szalapak, J.; Małgorzata, J.; Anna, M.; Elżbieta, Z.; Jakub, K.; Teodorczyk, M. Influence of nanoparticles content in silver paste on mechanical and electrical properties of LTJT joints. Adv. Powder Technol. 2015, 26, 907–913. [Google Scholar] [CrossRef]
- Yokoyama, S.; Motomiya, K.; Takahashi, H.; Tohji, K. Green synthesis of Cu micro/nanoparticles for low-resistivity Cu thin films using ascorbic acid in aqueous solution. J. Mater. Chem. C 2016, 4, 7494–7500. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Luo, S.; Lai, T.; Lai, H.; Luo, B.; Li, Z.; Zhang, Y.; Cui, C. A Green and Facile Microvia Filling Method via Printing and Sintering of Cu-Ag Core-Shell Nano-Microparticles. Nanomaterials 2022, 12, 1063. https://doi.org/10.3390/nano12071063
Yang G, Luo S, Lai T, Lai H, Luo B, Li Z, Zhang Y, Cui C. A Green and Facile Microvia Filling Method via Printing and Sintering of Cu-Ag Core-Shell Nano-Microparticles. Nanomaterials. 2022; 12(7):1063. https://doi.org/10.3390/nano12071063
Chicago/Turabian StyleYang, Guannan, Shaogen Luo, Tao Lai, Haiqi Lai, Bo Luo, Zebo Li, Yu Zhang, and Chengqiang Cui. 2022. "A Green and Facile Microvia Filling Method via Printing and Sintering of Cu-Ag Core-Shell Nano-Microparticles" Nanomaterials 12, no. 7: 1063. https://doi.org/10.3390/nano12071063
APA StyleYang, G., Luo, S., Lai, T., Lai, H., Luo, B., Li, Z., Zhang, Y., & Cui, C. (2022). A Green and Facile Microvia Filling Method via Printing and Sintering of Cu-Ag Core-Shell Nano-Microparticles. Nanomaterials, 12(7), 1063. https://doi.org/10.3390/nano12071063