MOF-Derived Cu@N-C Catalyst for 1,3-Dipolar Cycloaddition Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Material and Methods
2.2. Synthesis of Cu(im)2
2.3. Synthesis of Cu@N-C(x) (X Represents Different Pyrolysis Temperature)
2.4. General Procedure for the Cycloaddition Reaction
2.5. Recycling of Cu@N-C(600) Catalyst
2.6. Metal Leaching Test of Cu@N-C(600) Catalyst
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Becker, M.L. “Click” reactions: A versatile toolbox for the synthesis of peptide-conjugates. Chem. Soc. Rev. 2014, 43, 7013–7039. [Google Scholar] [CrossRef]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click chemistry in materials science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef] [Green Version]
- Lal, K.; Yadav, P. Recent advancements in 1,4-disubstituted 1H-1,2,3-triazoles as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2018, 18, 21–37. [Google Scholar] [CrossRef]
- Lin, S.; Sharma, A. Recent advances in the synthesis and synthetic applications of 1,2,3-triazoles. Chem. Heterocycl. Compd. 2018, 54, 314–316. [Google Scholar] [CrossRef]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: Review. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef]
- Recnik, L.-M.; Mindt, T.L.; Recnik, L.-M.; Kandioller, W.; Mindt, T.L.; Recnik, L.-M.; Mindt, T.L. 1,4-Disubstituted 1,2,3-triazoles as amide bond surrogates for the stabilisation of linear peptides with biological activity. Molecules 2020, 25, 3576. [Google Scholar] [CrossRef]
- Sahu, A.; Sahu, P.; Agrawal, R. A recent review on drug modification using 1,2,3-triazole. Curr. Chem. Biol. 2020, 14, 71–87. [Google Scholar] [CrossRef]
- Slavova, K.I.; Todorov, L.T.; Kostova, I.P.; Belskaya, N.P.; Palafox, M.A. Developments in the application of 1,2,3-triazoles in cancer treatment. Recent Pat. Anticancer Drug Discov. 2020, 15, 92–112. [Google Scholar] [CrossRef] [PubMed]
- Agouram, N.; Mestafa El Hadrami, E.; Bentama, A. 1,2,3-Triazoles as biomimetics in peptide science. Molecules 2021, 26, 2937. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Ding, S. 1,2,3-Triazole-based sequence-defined oligomers and polymers. Poly. Chem. 2021, 12, 2668–2688. [Google Scholar] [CrossRef]
- Da, S.M.; Forezi, L.; Lima, C.G.S.; Amaral, A.A.P.; Ferreira, P.G.; de Souza, M.C.B.V.; Cunha, A.C.; da Silva, D.C.F.; Ferreira, V.F. Bioactive 1,2,3-triazoles: An account on their synthesis, structural diversity and biological applications. Chem. Rec. 2021, 21, 2782–2807. [Google Scholar]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef]
- Forezi, D.S.M.L.; Cardoso, F.C.M.; Gonzaga, T.G.D.; da Silva, D.C.F.; Ferreira, V.F. Alternative routes to the click method for the synthesis of 1,2,3-triazoles, an important heterocycle in medicinal chemistry. Curr. Top. Med. Chem. 2018, 18, 1428–1453. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpliess, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Noriega, S.; Leyva, E.; Moctezuma, E.; Flores, L.; Loredo-Carrillo, S. Recent catalysts used in the synthesis of 1,4-disubstituted 1,2,3-triazoles by heterogeneous and homogeneous methods. Curr. Org. Chem. 2020, 24, 536–549. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Sangwan, J.; Luxmi, R.; Kumar, K.; Pahwa, A. Synthetic routes for 1,4-disubstituted 1,2,3-triazoles: A review. Curr. Org. Chem. 2019, 23, 860–900. [Google Scholar] [CrossRef]
- Meldal, M.; Tornoe, C.W. Cu-catalyzed azide-aldyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xu, G.; Du, Z.; Fu, Y. Cu(BTC)-MOF catalyzed multicomponent reaction to construct 1,4-disubstituted-1,2,3-triazoles. Polyhedron 2018, 151, 515–519. [Google Scholar] [CrossRef]
- Luz, I.; Llabrés i Xamena, F.X.; Corma, A. Bridging homogeneous and heterogeneous catalysis with MOFs: “Click” reactions with Cu-MOF catalysts. J. Catal. 2010, 276, 134–140. [Google Scholar] [CrossRef]
- Li, P.; Regati, S.; Huang, H.; Arman, H.D.; Zhao, J.C.G.; Chen, B. A metal–organic framework as a highly efficient and reusable catalyst for the solvent-free 1,3-dipolar cycloaddition of organic azides to alkynes. Inorg. Chem. Front. 2015, 2, 42–46. [Google Scholar] [CrossRef]
- Mansano Willig, J.C.; Granetto, G.; Reginato, D.; Dutra, F.R.; Poruczinski, É.F.; de Oliveira, I.M.; Stefani, H.A.; de Campos, S.D.; de Campos, É.A.; Manarin, F.; et al. A comparative study between Cu(INA)2-MOF and [Cu(INA)2(H2O)4] complex for a click reaction and the Biginelli reaction under solvent-free conditions. RSC Adv. 2020, 10, 3407–3415. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-H.; Wang, J.-C.; Yang, S.; Li, Y.-A.; Dong, Y.-B. CuI@UiO-67-IM: A MOF-based bifunctional composite triphase-transfer catalyst for sequential one-pot azide–alkyne cycloaddition in water. Inorg. Chem. 2017, 56, 8341–8347. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [Green Version]
- Marpaung, F.; Kim, M.; Khan, J.H.; Konstantinov, K.; Yamauchi, Y.; Hossain, M.S.A.; Na, J.; Kim, J. Metal–organic framework (MOF)-derived nanoporous carbon materials. Chem. Asian J. 2019, 14, 1331–1343. [Google Scholar] [CrossRef]
- Han, A.; Wang, B.; Kumar, A.; Qin, Y.; Jin, J.; Wang, X.; Yang, C.; Dong, B.; Jia, Y.; Liu, J.; et al. Recent advances for MOF-derived carbon-supported single-atom catalysts. Small Methods 2019, 3, 1800471. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, Y. Research progress on prepartion of MOF-derived porous carbon materials through pyrolysis. Chin. Sci. Bull. 2018, 63, 2246–2263. (In Chinese) [Google Scholar]
- Cao, X.; Tan, C.; Sindoro, M.; Zhang, H. Hybrid micro-/nano-structures derived from metal–organic frameworks: Preparation and applications in energy storage and conversion. Chem. Soc. Rev. 2017, 46, 2660–2677. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Chen, Y.; Li, S.; Zhang, S.; Feng, X.; Zhou, J.; Wang, B. Metal-organic frameworks derived porous carbons: Syntheses, porosity and gas sorption properties. Chin. J. Chem. 2016, 34, 157–174. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energ. Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF-derived nanoporous carbons and their promising applications. J. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal organic frameworks for energy storage and conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Hu, L.; Li, W.; Wang, L.; Wang, B. Turning metal-organic frameworks into efficient single-atom catalysts via pyrolysis with a focus on oxygen reduction reaction catalysts. EnergyChem 2021, 3, 100056. [Google Scholar] [CrossRef]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.G.; Shieh, F.-K.; Wu, K.C.W. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coordin. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, R.; Jiao, L.; Jiang, H.-L. Metal–organic framework-derived porous materials for catalysis. Coordin. Chem. Rev. 2018, 362, 1–23. [Google Scholar] [CrossRef]
- Yamane, I.; Sato, K.; Otomo, R.; Yanase, T.; Miura, A.; Nagahama, T.; Kamiya, Y.; Shimada, T. Ultrahigh-pressure preparation and catalytic activity of MOF-derived Cu nanoparticles. Nanomaterials 2021, 11, 1040. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Muthuchamy, N.; Yoon, C.; Joo, S.H.; Park, K.H. MOF-derived Cu@Cu2O nanocatalyst for oxygen reduction reaction and cycloaddition reaction. Nanomaterials 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Zhang, J.; Liu, P.; Xie, J.; Dai, B. 2-Pyrrolecarbaldiminato-Cu(II) complex catalyzed three-component 1,3-dipolar cycloaddition for 1,4-disubstituted 1,2,3-triazoles synthesis in water at room temperature. RSC Adv. 2015, 5, 6661–6665. [Google Scholar] [CrossRef]
- Hao, C.; Zhou, C.; Xie, J.; Zhang, J.; Liu, P.; Dai, B. An Efficient Copper-catalyzed one-pot synthesis of 1-aryl-1,2,3-triazoles from arylboronic acids in water under mild conditions. Chin. J. Chem. 2015, 33, 1317–1320. [Google Scholar] [CrossRef]
- Masciocchi, N.; Bruni, S.; Cariati, E.; Cariati, F.; Galli, S.; Sironi, A. Extended polymorphism in copper(II) imidazolate polymers: a spectroscopic and XRPD structural study. Inorg. Chem. 2001, 40, 5897–5905. [Google Scholar] [CrossRef]
- Luz, I.; Corma, A.; Llabrés i Xamena, F.X. Cu-MOFs as active, selective and reusable catalysts for oxidative C–O bond coupling reactions by direct C–H activation of formamides, aldehydes and ethers. Catal. Sci. Technol. 2014, 4, 1829–1836. [Google Scholar] [CrossRef]
- Karahan, Ö.; Biçer, E.; Taşdemir, A.; Yürüm, A.; Gürsel, S.A. Development of efficient copper-based MOF-derived catalysts for the reduction of aromatic nitro compounds. Eur. J. Inorg. Chem. 2018, 2018, 1073–1079. [Google Scholar] [CrossRef]
- Bonyasi, R.; Gholinejad, M.; Saadati, F.; Nájera, C. Copper ferrite nanoparticle modified starch as a highly recoverable catalyst for room temperature click chemistry: Multicomponent synthesis of 1,2,3-triazoles in water. New J. Chem. 2018, 42, 3078–3086. [Google Scholar] [CrossRef] [Green Version]
- Khalili, D.; Kavoosi, L.; Khalafi-Nezhad, A. Copper aluminate spinel in click chemistry: An efficient heterogeneous nanocatalyst for the highly regioselective synthesis of triazoles in water. Synlett 2019, 30, 2136–2142. [Google Scholar] [CrossRef]
- Hasanpour, Z.; Maleki, A.; Hosseini, M.; Gorgannezhad, L.; Nejadshafiee, V.; Ramazani, A.; Haririan, I.; Shafiee, A.; Khoobi, M. Efficient multicomponent synthesis of 1,2,3-triazoles catalyzed by Cu(II) supported on PEI[Rate]Fe3O4 MNPs in a water/PEG300 system. Turk. J. Chem. 2017, 41, 294–307. [Google Scholar] [CrossRef]
- Karimi Zarchi, M.A.; Nazem, F. One-pot three-component synthesis of 1,4-disubstituted 1H-1,2,3-triazoles using green and recyclable cross-linked poly(4-vinylpyridine)-supported copper sulfate/sodium ascorbate in water/t-BuOH system. J. Iran. Chem. Soc. 2014, 11, 1731–1742. [Google Scholar] [CrossRef]
- Haito, A.; Yamaguchi, M.; Chatani, N. Ru3(CO)12-catalyzed carbonylation of C−H bonds by triazole-directed C−H activation. Asian J. Org. Chem. 2018, 7, 1315–1318. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, L. An efficient regioselective copper-catalyzed approach to the synthesis of 1,2,3-triazoles from N-tosylhydrazones and azides. Tetrahedron Lett. 2016, 57, 5132–5134. [Google Scholar] [CrossRef]
- Dehbanipour, Z.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I. Copper(II) bisthiazole complex immobilized on silica nanoparticles: Preparation, characterization and its application as a highly efficient catalyst for click synthesis of 1,2,3-triazoles. Polyhedron 2017, 138, 21–30. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Si, J.; Yue, C.; Xia, C.; Li, F. Truncated concave octahedral Cu2O nanocrystals with {hkk} high-index facets for enhanced activity and stability in heterogeneous catalytic azide–alkyne cycloaddition. Green Chem. 2018, 20, 832–837. [Google Scholar] [CrossRef]
- Pérez, J.M.; Cano, R.; Ramón, D.J. Multicomponent azide–alkyne cycloaddition catalyzed by impregnated bimetallic nickel and copper on magnetite. RSC Adv. 2014, 4, 23943–23951. [Google Scholar] [CrossRef] [Green Version]
- Larionov, V.A.; Stashneva, A.R.; Titov, A.A.; Lisov, A.A.; Medvedev, M.G.; Smol’yakov, A.F.; Tsedilin, A.M.; Shubina, E.S.; Maleev, V.I. Mechanistic study in azide–alkyne cycloaddition (CuAAC) catalyzed by bifunctional trinuclear copper(I) pyrazolate complex: Shift in rate-determining step. J. Catal. 2020, 390, 37–45. [Google Scholar] [CrossRef]
- De Angelis, S.; Franco, M.; Trimini, A.; Gonzalez, A.; Sainz, R.; Degennaro, L.; Romanazzi, G.; Carlucci, C.; Petrelli, V.; de la Esperanza, A.; et al. A study of graphene-based copper catalysts: Copper(I) nanoplatelets for batch and continuous-flow applications. Chem. Asian J. 2019, 14, 3011–3018. [Google Scholar] [CrossRef]
- Rajabi, M.; Albadi, J.; Momeni, A. Click synthesis of 1,4-disubstituted-1,2,3-triazoles catalyzed by melamine-supported CuO nanoparticles as an efficient recyclable catalyst in water. Res. Chem. Intermediat. 2020, 46, 3879–3889. [Google Scholar] [CrossRef]
- Ötvös, S.B.; Georgiádes, Á.; Ádok-Sipiczki, M.; Mészáros, R.; Pálinkó, I.; Sipos, P.; Fülöp, F. A layered double hydroxide, a synthetically useful heterogeneous catalyst for azide−alkyne cycloadditions in a continuous-flow reactor. Appl. Catal. A: Gen. 2015, 501, 63–73. [Google Scholar] [CrossRef] [Green Version]

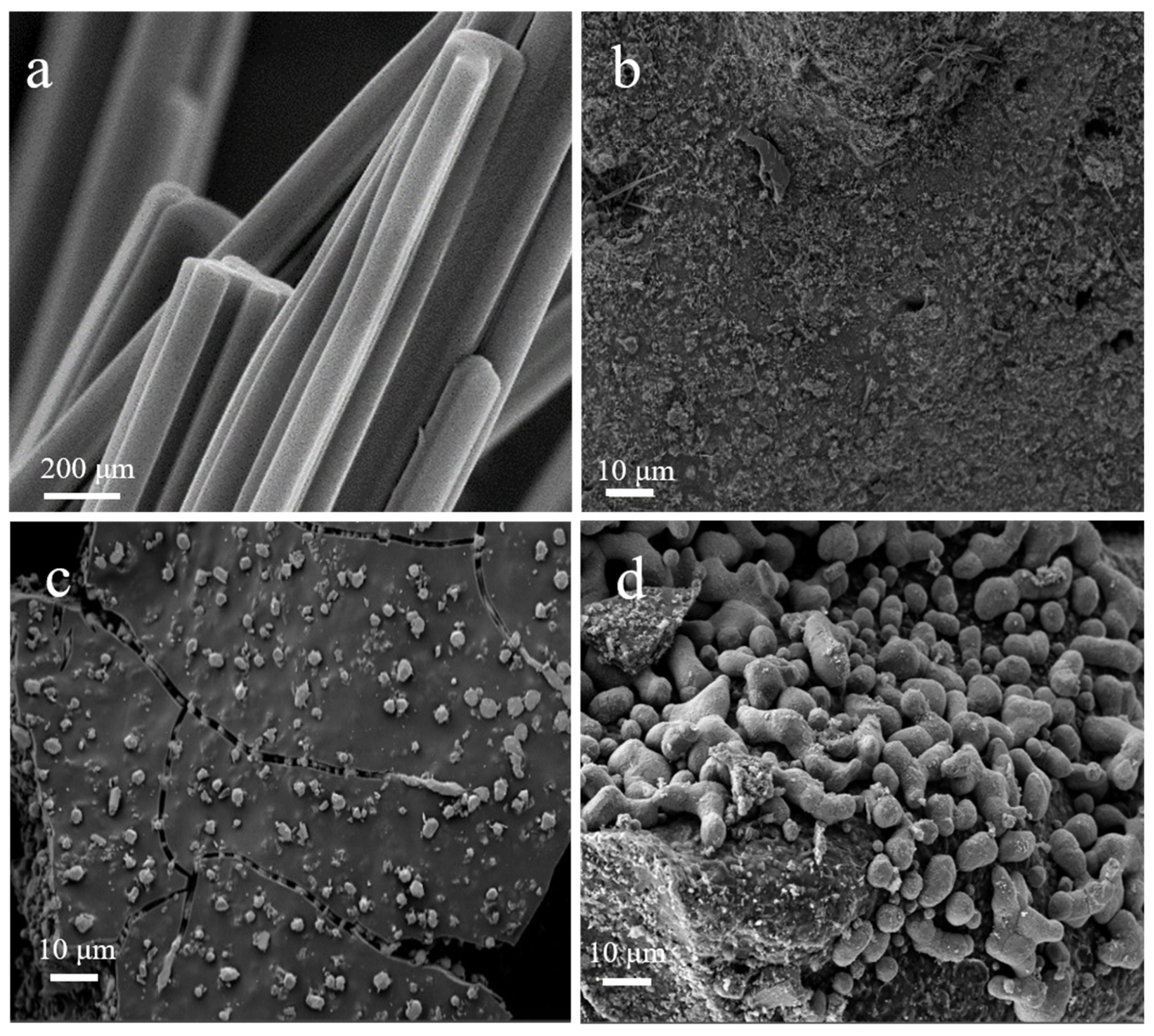
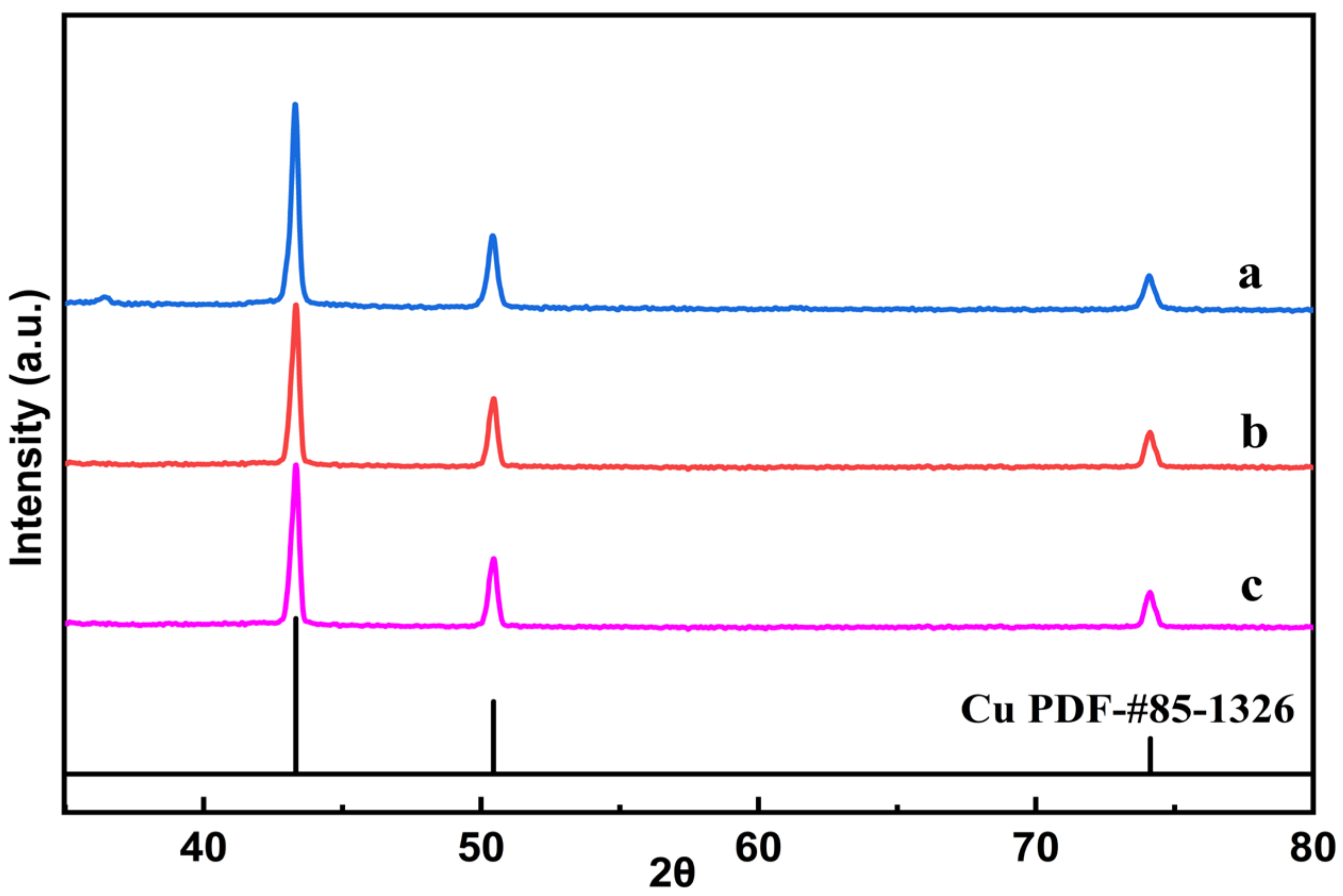


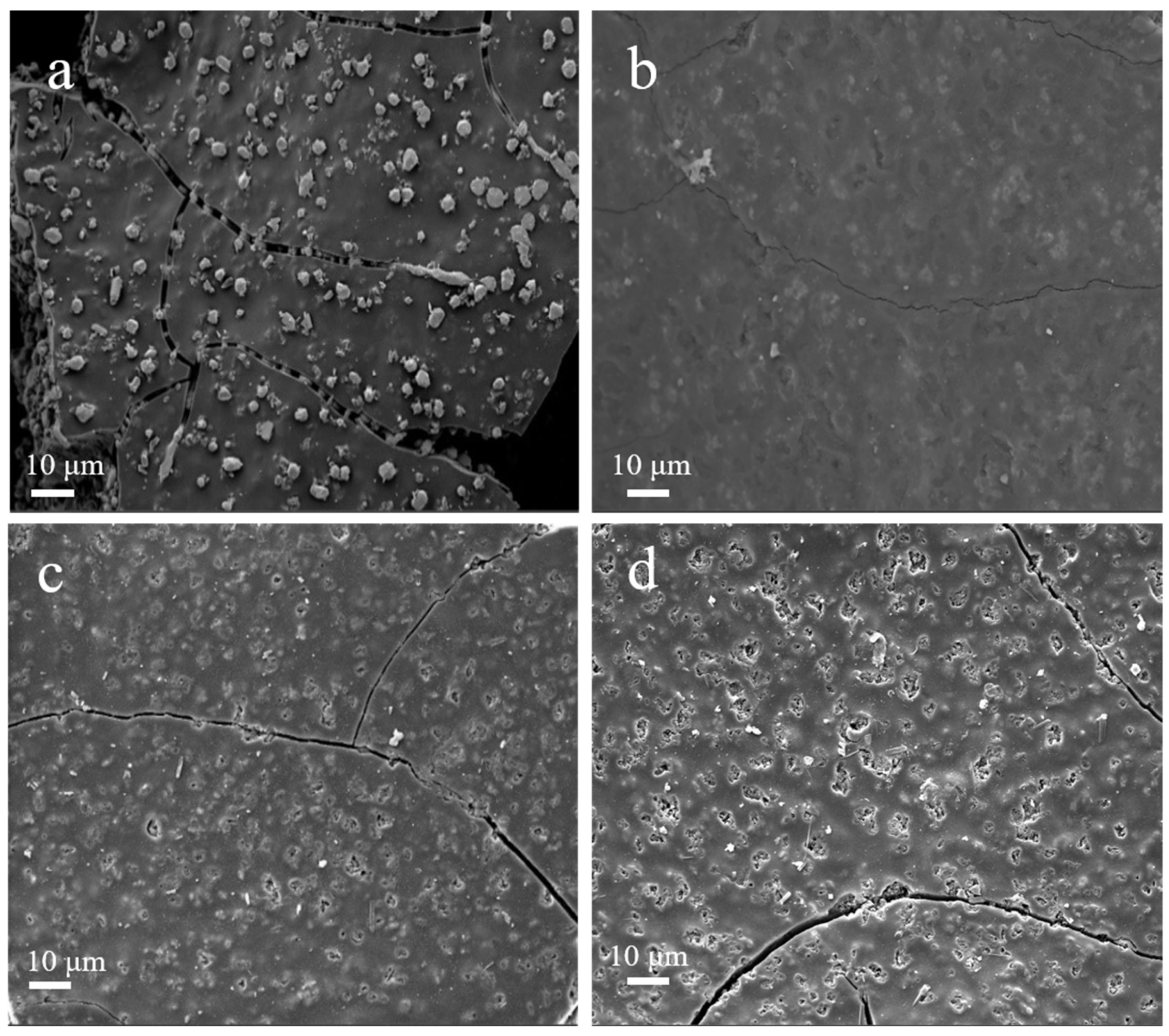
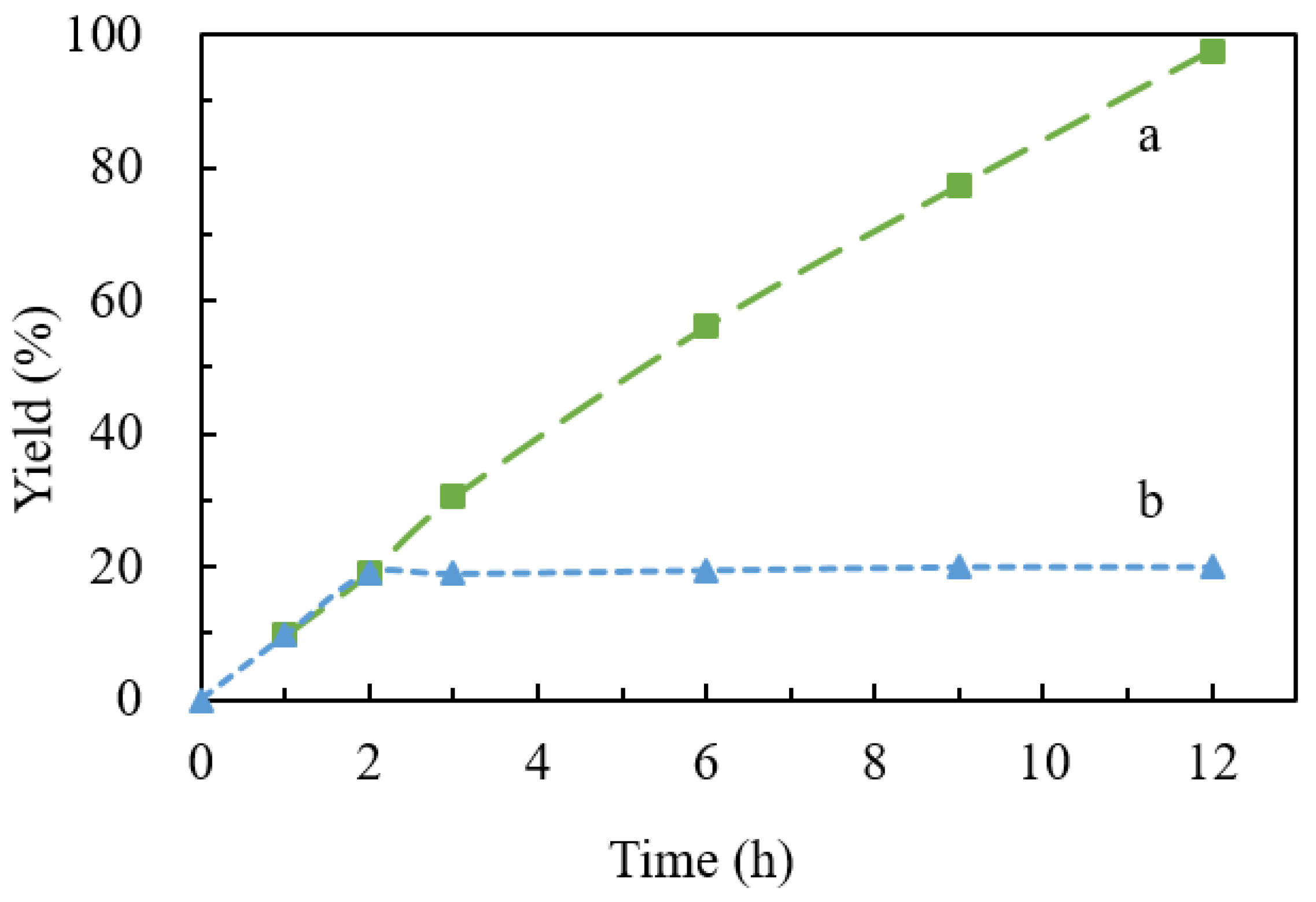
| Sample | Cu Content (%) 1 | C Content (%) 2 | N Content (%) 2 | H Content (%) 2 |
|---|---|---|---|---|
| Cu(im)2 | 32.14 3 | 36.45 3 | 28.34 3 | 3.06 3 |
| Cu(im)2 | 29.20 | 35.49 | 27.89 | 2.70 |
| Cu@N-C(400) | 39.03 | 22.76 | 16.29 | 1.30 |
| Cu@N-C(600) | 48.85 | 23.98 | 14.48 | 0.86 |
| Cu@N-C(800) | 73.73 | 12.58 | 1.31 | 0.24 |
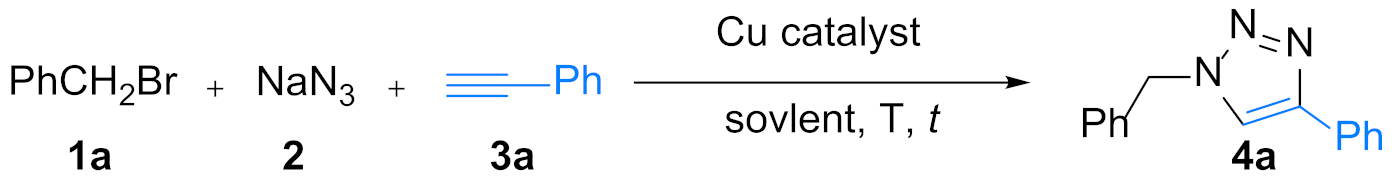
| Entry | Catalyst | Solvent (v/v) | T (°C) | Yield (%) 2 |
|---|---|---|---|---|
| 1 | Cu(im)2 | H2O | 50 | 20 |
| 2 | - | H2O | 50 | trace |
| 3 | Cu@N-C(400) | H2O | 50 | 83 |
| 4 | Cu@N-C(600) | H2O | 50 | 94 |
| 5 | Cu@N-C(800) | H2O | 50 | 78 |
| 6 | Cu@N-C(600) | EtOH | 50 | 90 |
| 7 | Cu@N-C(600) | i-PrOH | 50 | 68 |
| 8 | Cu@N-C(600) | t-BuOH | 50 | 7 |
| 9 | Cu@N-C(600) | EtOH/H2O (3/1) | 50 | 96 |
| 10 | Cu@N-C(600) | EtOH/H2O (1/3) | 50 | 88 |
| 11 | Cu@N-C(600) | EtOH/H2O (1/1) | 50 | 40 |
| 12 | Cu@N-C(600) | i-PrOH/H2O (3/1) | 50 | 92 |
| 13 | Cu@N-C(600) | i-PrOH/H2O (1/3) | 50 | 86 |
| 14 | Cu@N-C(600) | i-PrOH/H2O (1/1) | 50 | 74 |
| 15 | Cu@N-C(600) | t-BuOH/H2O (3/1) | 50 | 98 |
| 16 | Cu@N-C(600) | t-BuOH/H2O (1/3) | 50 | 45 |
| 17 | Cu@N-C(600) | t-BuOH/H2O (1/1) | 50 | 55 |
| 18 | Cu@N-C(600) | t-BuOH/H2O (3/1) | 50 | 80 3 |
| 19 | Cu@N-C(600) | t-BuOH/H2O (3/1) | 40 | 56 |
| 20 | Cu@N-C(600) | t-BuOH/H2O (3/1) | 25 | 50 |
| 21 | CuSO4 + NaAsc | t-BuOH/H2O (3/1) | 50 | 88 4 |

| Scope of alkynes(14 examples) | Scope of benzyl halides(19 examples) | ||
|---|---|---|---|
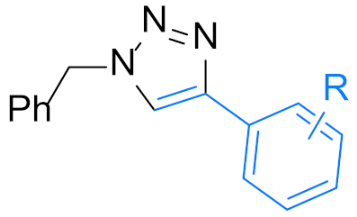 | 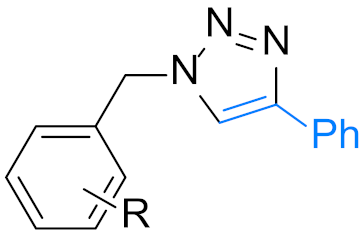 | ||
| 4a: R=H | 12 h, 98% | 4n: R = 4-Me | 12 h, 86% |
| 24 h, 90% (X=Cl) | 4o: R = 3,5-di-Me | 12 h, 88% | |
| 4b: R = 4-Me | 12 h, 90% | 4p: R = 3-OMe | 24 h, 88% |
| 4c: R = 4-Et | 24 h, 93% | 4q: R = 3,5-di-OMe | 24 h, 82% |
| 4d: R = 4-OMe | 12 h, 90% | 4r: R = 4-t-Bu | 14 h, 87% |
| 4e: R = 3-Me | 12 h, 92% 2 | 4s: R = 4-F | 12 h, 80% |
| 4f: R = 2-Me | 12 h, 90% | 24 h, 94% (X=Cl) | |
| 4g: R = 4-F | 12 h, 97% | 4t: R = 4-Cl | 24 h, 72% |
| 4h: R = 4-Cl | 24 h, 91% | 4u: R = 4-Br | 24 h, 70% |
| 4i: R = 4-Br | 24 h, 90% | 4v: R = 4-NO2 | 12 h, 85% 2 |
| 4j: R = 3-F | 12 h, 93% 2 | 4w: R = 4-CF3 | 15 h, 90% |
| 4k: R = 3-Br | 24 h, 91% 2 | 4x: R = 2-F | 24 h, 94% |
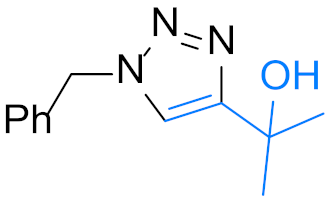 |  | 4y: R = 2-Cl | 24 h, 95% |
| 4z: R = 2,5-di-F | 24 h, 82% | ||
| 4aa: R = 3-F | 16 h, 90% | ||
| 4ab: R = 3-Cl | 24 h, 94% | ||
| 4ac: R = 3-Br | 15 h, 90% 2 | ||
| 4l, 12 h, 97% 2 | 4m, 18 h, 66% 2 | 4ad: R = 3,4-di-Cl | 12 h, 76% |
| 4ae: R = 3-Cl, 4-F | 24 h, 94% | ||
| Sample | Cu Leaching (%) 1 |
|---|---|
| First run | 1.7 |
| Second run | 0.5 |
| Third run | 0.4 |
| Fourth run | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, X.; Gong, S.; Xie, J. MOF-Derived Cu@N-C Catalyst for 1,3-Dipolar Cycloaddition Reaction. Nanomaterials 2022, 12, 1070. https://doi.org/10.3390/nano12071070
Wang Z, Zhou X, Gong S, Xie J. MOF-Derived Cu@N-C Catalyst for 1,3-Dipolar Cycloaddition Reaction. Nanomaterials. 2022; 12(7):1070. https://doi.org/10.3390/nano12071070
Chicago/Turabian StyleWang, Zhuangzhuang, Xuehao Zhou, Shaofeng Gong, and Jianwei Xie. 2022. "MOF-Derived Cu@N-C Catalyst for 1,3-Dipolar Cycloaddition Reaction" Nanomaterials 12, no. 7: 1070. https://doi.org/10.3390/nano12071070







