Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review
Abstract
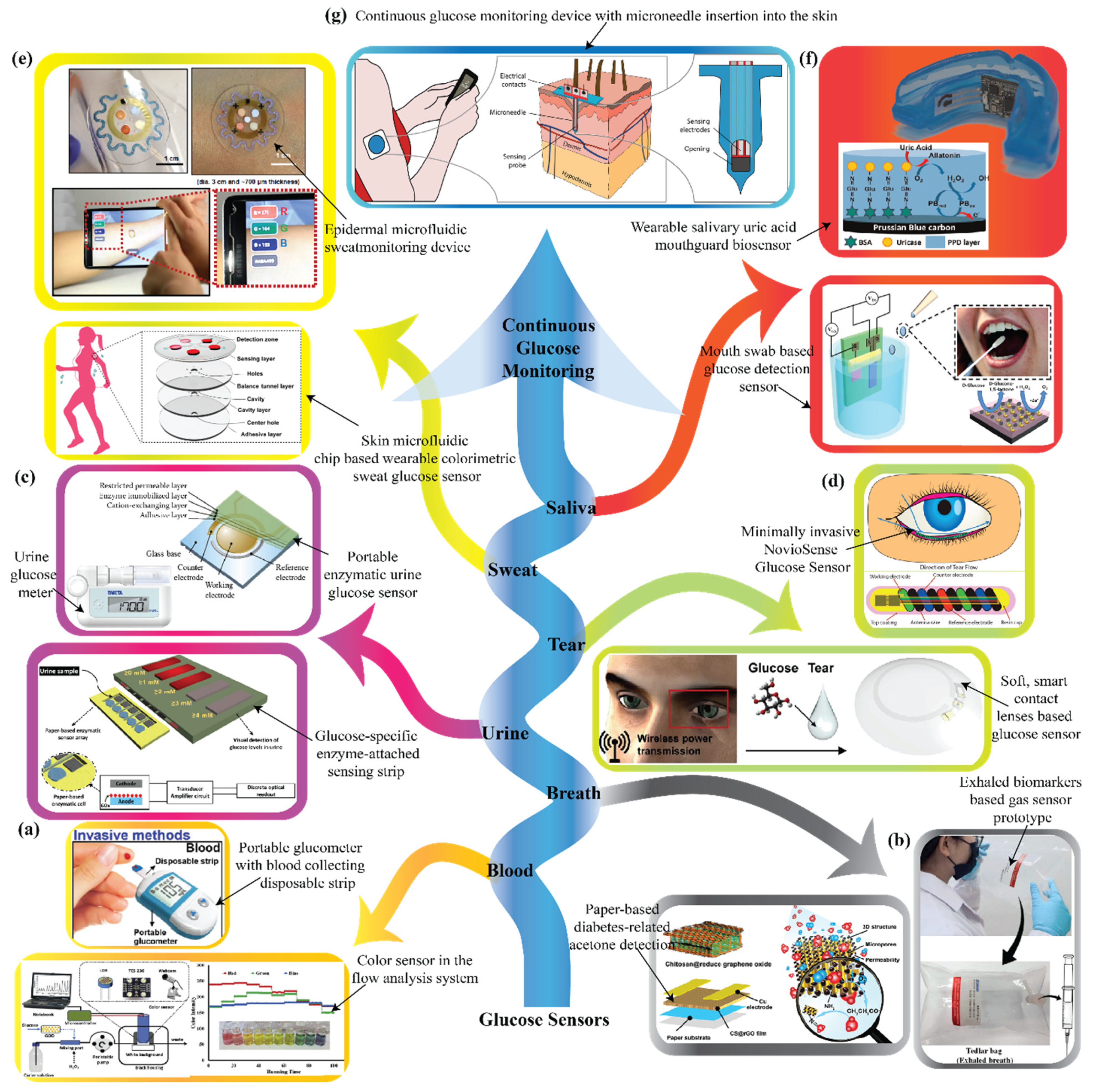
:1. Introduction
2. Recent Developments in Various Biological Fluid-Based Glucose Sensors
2.1. Saliva-Based Glucose Sensors
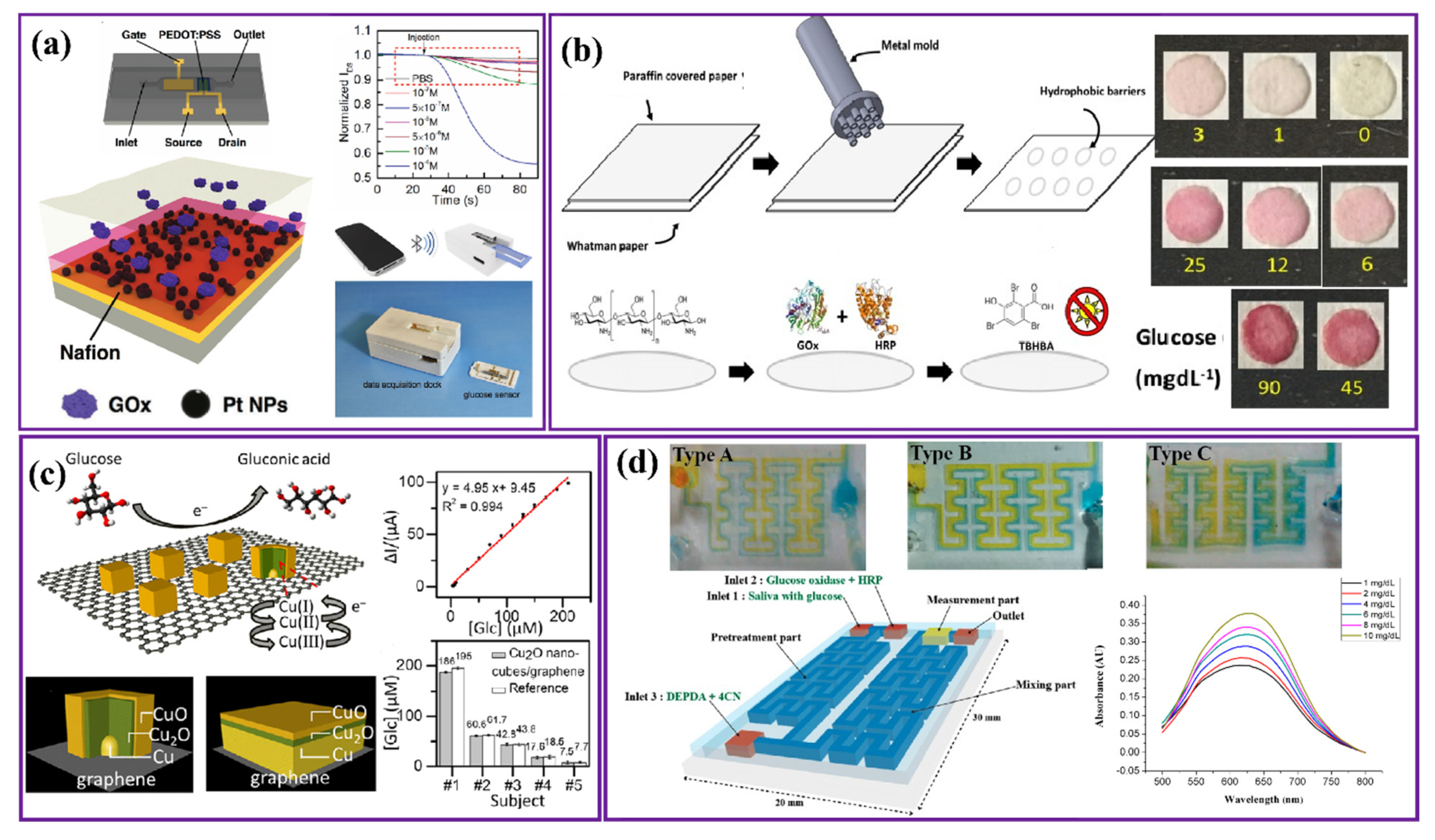
2.2. Tear-Based Glucose Sensors
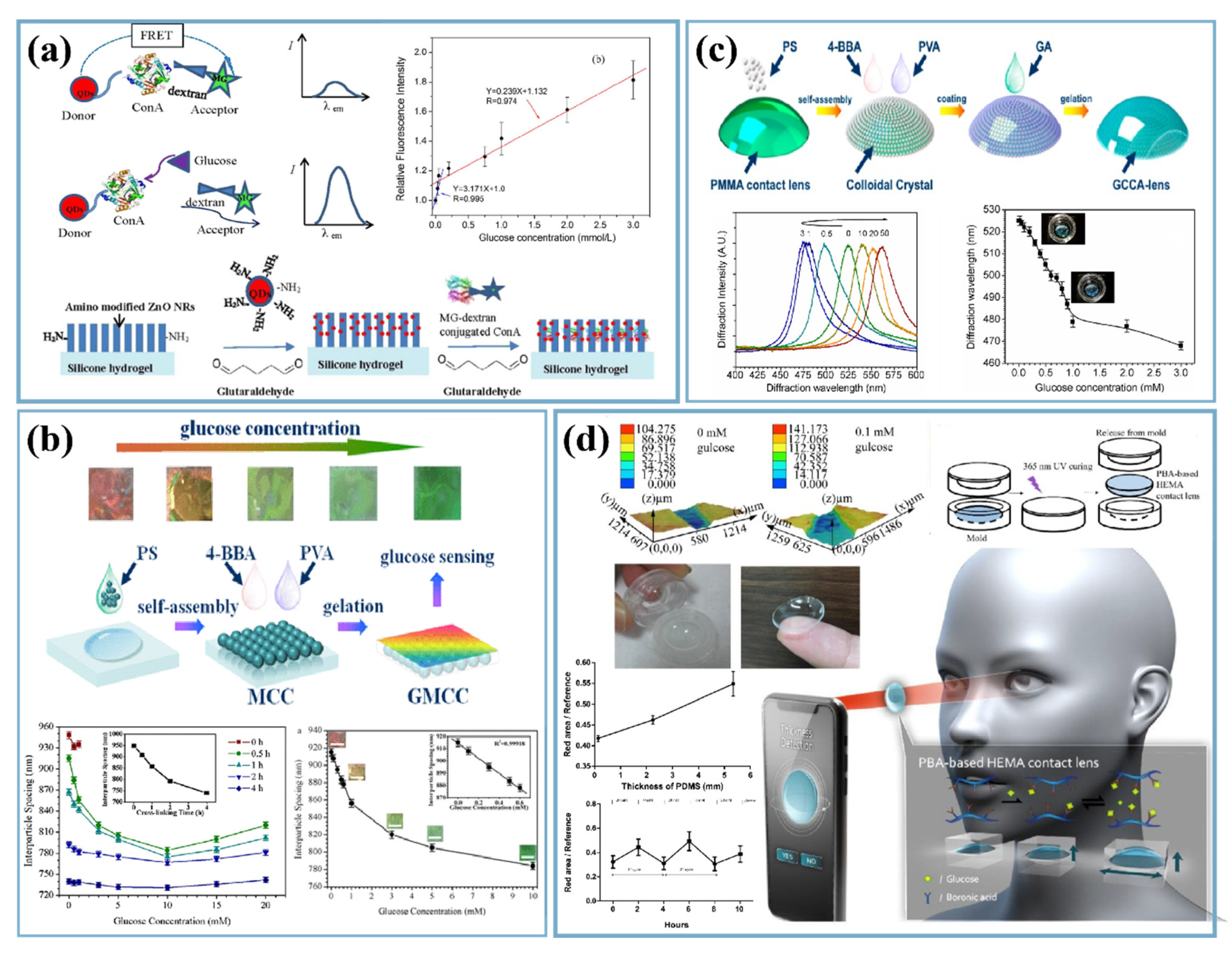
2.3. Urine-Based Glucose Sensors

2.4. Sweat- and Interstitial Fluid-Based Glucose Sensors
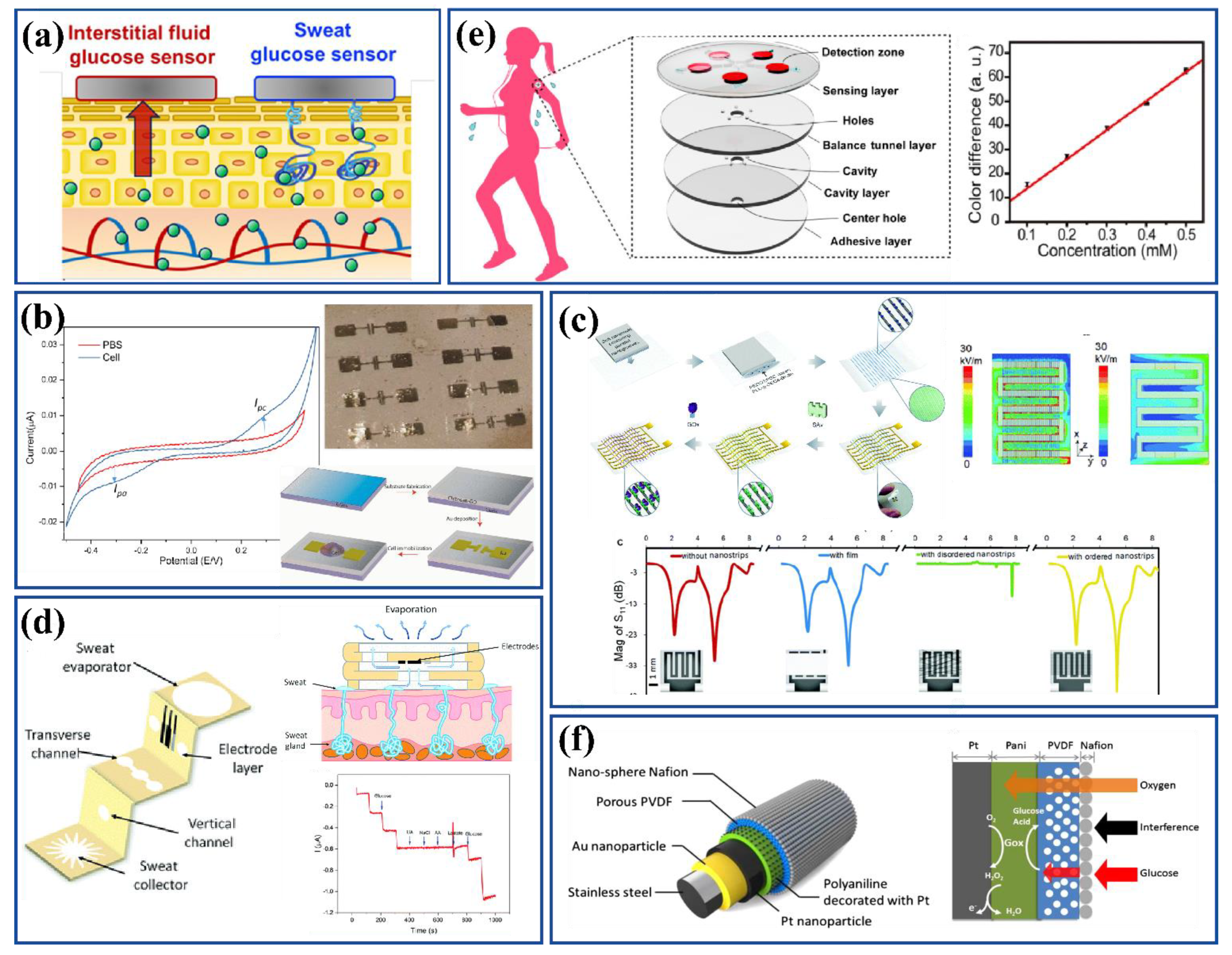
2.5. Breath-Based Glucose Sensors

2.6. Blood-Based Glucose Sensors
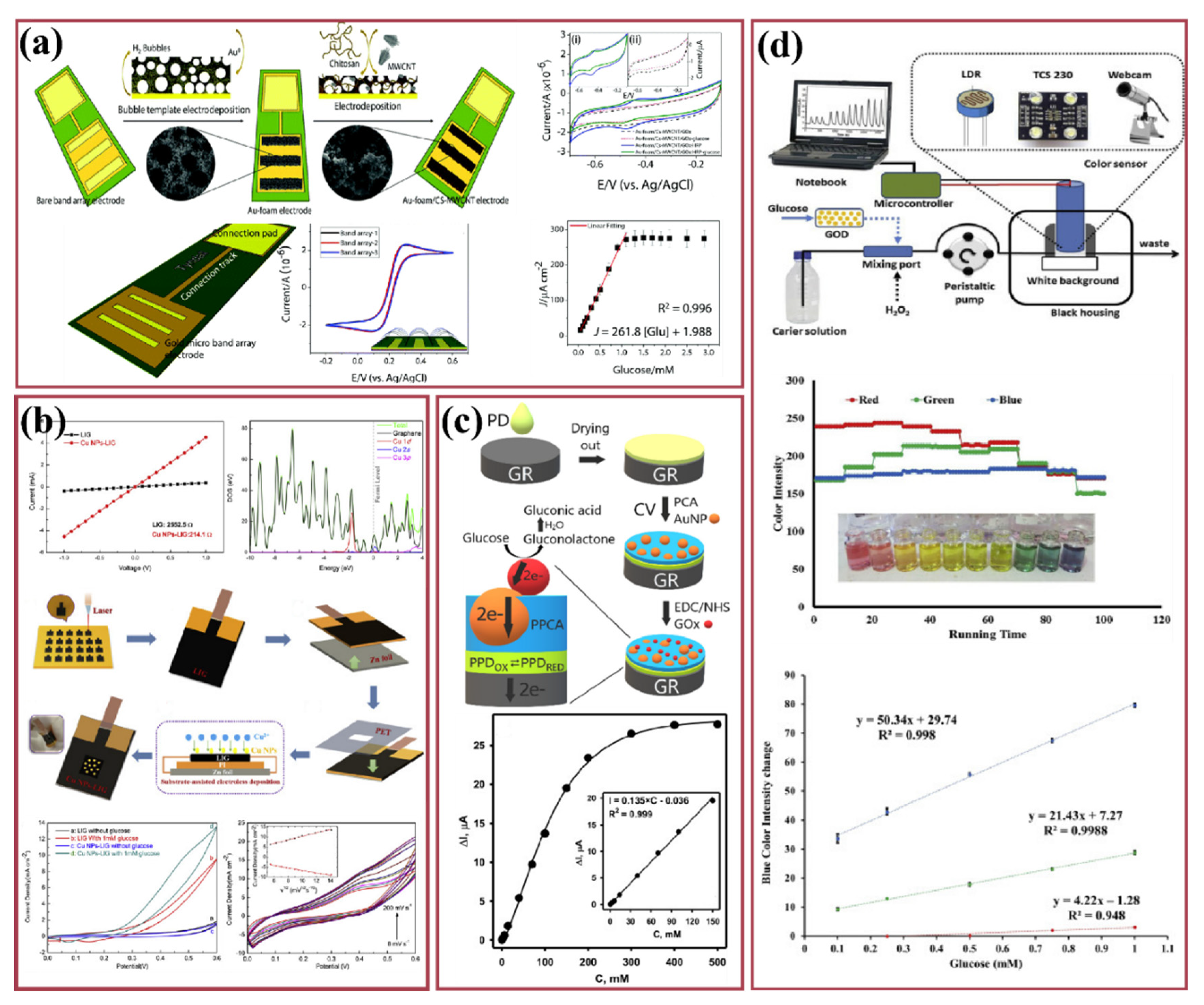
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2020. Available online: https://www.who.int/health-topics/diabetes (accessed on 25 April 2021).
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- GHDx. Global Burden of Disease Collaborative Network. Available online: http://ghdx.healthdata.org/organizations/global-burden-disease-collaborative-network (accessed on 25 April 2021).
- Market Data Forecast. Glucose Monitoring Device Market Size, Share, Growth—2020 to 2025—COVID-19 Impact. Available online: http://www.marketdataforecast.com/ (accessed on 21 May 2021).
- Cleveland Clinic. Hyperglycemia: Causes, Symptoms, Treatments & Prevention. Available online: https://my.clevelandclinic.org/health/diseases/9815-hyperglycemia-high-blood-sugar (accessed on 31 January 2022).
- Healthline. The Effects of Low Blood Sugar on Your Body. 15 August 2018. Available online: https://www.healthline.com/health/low-blood-sugar-effects-on-body (accessed on 31 January 2022).
- Zhao, R.; Lu, Z.; Yang, J.; Zhang, L.; Li, Y.; Zhang, X. Drug Delivery System in the Treatment of Diabetes Mellitus. Front. Bioeng. Biotechnol. 2020, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCowen, K.; Smith, R. Diabetes Mellitus: Classification and Chemical Pathology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 2, pp. 17–24. [Google Scholar]
- Zhao, M.; Leung, P.S. Revisiting the use of biological fluids for noninvasive glucose detection. Future Med. Chem. 2020, 12, 645–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foxdal, P.; Bergqvist, Y.; Eckerbom, S.; Sandhagen, B. Improving Lactate Analysis with the YSI 2300 Gl: Hemolyzing Blood Samples Makes Results Comparable with Those for Deproteinized Whole Blood. Clin. Chem. 1992, 38, 2110–2114. [Google Scholar] [CrossRef]
- Stork, A.D.M.; Kemperman, H.; Erkelens, D.W.; Veneman, T.F. Comparison of the accuracy of the hemocue glucose analyzer with the Yellow Springs Instrument glucose oxidase analyzer, particularly in hypoglycemia. Eur. J. Endocrinol. 2005, 153, 275–281. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Wu, P.; Shao, Q.; Hu, Y.; Jin, J.; Yin, Y.; Zhang, H.; Cai, C. Direct electrochemistry of glucose oxidase assembled on graphene and application to glucose detection. Electrochim. Acta 2010, 55, 8606–8614. [Google Scholar] [CrossRef]
- Hu, S.; Lu, Q.; Xu, Y. Chapter 17—Biosensors based on direct electron transfer of protein. In Electrochemical Sensors, Biosensors and their Biomedical Applications; Zhang, X., Ju, H., Wang, J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 531–581. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, X.; Heinig, N.F.; Thomas, J.P.; Zhang, L.; Leung, K.T. Nonenzymatic Saliva-Range Glucose Sensing Using Electrodeposited Cuprous Oxide Nanocubes on a Graphene Strip. ACS Appl. Nano Mater. 2021, 4, 4790–4799. [Google Scholar] [CrossRef]
- Hassan, M.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-L.; Feng, J.-J.; Cai, L.-Y.; Fang, Q.-X.; Chen, J.-R.; Wang, A.-J. Facile synthesis of monodisperse porous Cu2O nanospheres on reduced graphene oxide for non-enzymatic amperometric glucose sensing. Electrochim. Acta 2014, 115, 103–108. [Google Scholar] [CrossRef]
- Casella, I.G.; Destradis, A.; Desimoni, E. Colloidal gold supported onto glassy carbon substrates as an amperometric sensor for carbohydrates in flow injection and liquid chromatography. Analyst 1996, 121, 249–254. [Google Scholar] [CrossRef]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef]
- Oliver, N.S.; Toumazou, C.; Cass, A.E.G.; Johnston, D.G. Glucose sensors: A review of current and emerging technology. Diabet. Med. 2009, 26, 197–210. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jackman, J.A.; Park, J.H.; Tan, E.-L.; Cho, N.-J. A flexible, ultra-sensitive chemical sensor with 3D biomimetic templating for diabetes-related acetone detection. J. Mater. Chem. B 2017, 5, 4019–4024. [Google Scholar] [CrossRef]
- Xing, X.; Du, L.; Feng, D.; Wang, C.; Yao, M.; Huang, X.; Zhang, S.; Yang, D. Individual gas sensor detecting dual exhaled biomarkers via a temperature modulated n/p semiconducting transition. J. Mater. Chem. A 2020, 8, 26004–26012. [Google Scholar] [CrossRef]
- Ito, N.; Miyashita, M.; Ikeda, S. 1—Portable urine glucose sensor. In Chemical, Gas, and Biosensors for Internet of Things and Related Applications; Mitsubayashi, K., Niwa, O., Ueno, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–12. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Tahernia, M.; Choi, S. An Equipment-Free, Paper-Based Electrochemical Sensor for Visual Monitoring of Glucose Levels in Urine. SLAS Technol. Transl. Life Sci. Innov. 2019, 24, 499–505. [Google Scholar] [CrossRef]
- Kownacka, A.E.; Vegelyte, D.; Joosse, M.; Anton, N.; Toebes, B.J.; Lauko, J.; Buzzacchera, I.; Lipinska, K.; Wilson, D.A.; Geelhoed-Duijvestijn, N.; et al. Clinical Evidence for Use of a Noninvasive Biosensor for Tear Glucose as an Alternative to Painful Finger-Prick for Diabetes Management Utilizing a Biopolymer Coating. Biomacromolecules 2018, 19, 4504–4511. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, J.; Kim, S.-Y.; Cheong, W.H.; Jang, J.; Park, Y.-G.; Na, K.; Kim, Y.-T.; Heo, J.H.; Lee, C.Y.; et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixao, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Ribet, F.; Stemme, G.; Roxhed, N. Real-time intradermal continuous glucose monitoring using a minimally invasive microneedle-based system. Biomed. Microdevices 2018, 20, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allied Market Research. Continuous Glucose Monitoring Systems Market Growth Analysis—2027. Available online: https://www.alliedmarketresearch.com/global-continuous-glucose-monitoring-systems-market (accessed on 18 May 2021).
- Fatoni, A.; Aziz, A.N.; Anggraeni, M.D. Low-cost and real-time color detector developments for glucose biosensor. Sens. Bio-Sens. Res. 2020, 28, 100325. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Y.; Su, L.; Zhao, D.; Zhao, L.; Zhang, X. Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose. Anal. Chem. 2019, 91, 14803–14807. [Google Scholar] [CrossRef] [Green Version]
- The Hong Kong Polytechnic University. Biological Sensor Can Detect Glucose Levels in Saliva More Accurately and Cost-Efficiently Than Blood Test. Available online: https://phys.org/news/2017-05-biological-sensor-glucose-saliva-accurately.html (accessed on 28 June 2021).
- Cheah, J.S.; Wong, A.F. A rapid and simple blood sugar determination using the Ames reflectance meter and Dextrostix system: A preliminary report. Singap. Med. J. 1974, 15, 51–52. [Google Scholar]
- OpenWetWare. Glucose Sensors. Available online: https://openwetware.org/wiki/Glucose_Sensors (accessed on 18 May 2021).
- Lipani, L.; Dupont, B.G.R.; Doungmene, F.; Marken, F.; Tyrrell, R.M.; Guy, R.H.; Ilie, A. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 2018, 13, 504–511. [Google Scholar] [CrossRef]
- Fathi, E.; Mesbah-Namin, S.A.; Farahzadi, R. Biomarkers in Medicine: An Overview. Br. J. Med. Med. Res. 2013, 4, 1701–1718. [Google Scholar] [CrossRef]
- Sun, M.; Pei, X.; Xin, T.; Liu, J.; Ma, C.; Cao, M.; Zhou, M. A Flexible Microfluidic Chip-Based Universal Fully Integrated Nanoelectronic System with Point-of-Care Raw Sweat, Tears, or Saliva Glucose Monitoring for Potential Noninvasive Glucose Management. Anal. Chem. 2022, 94, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Pin, K.Y.; Reddy, V.S.; Jayathilaka, W.A.D.M.; Ji, D.; Serrano-García, W.; Bhargava, S.K.; Ramakrishna, S.; Chinnappan, A. Micro/nanofiber-based noninvasive devices for health monitoring diagnosis and rehabilitation. Appl. Phys. Rev. 2020, 7, 041309. [Google Scholar] [CrossRef]
- Xu, T.; Jin, W.; Wang, Z.; Cheng, H.; Huang, X.; Guo, X.; Ying, Y.; Wu, Y.; Wang, F.; Wen, Y.; et al. Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials 2018, 8, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, M.T.; Gupta, S.; Sunitha, J.; Dawar, G.; Sinha, N.; Rallan, N.S. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J. Oral Maxillofac. Pathol. 2017, 21, 334–339. [Google Scholar] [CrossRef]
- Gug, I.T.; Tertis, M.; Hosu, O.; Cristea, C. Salivary biomarkers detection: Analytical and immunological methods overview. TrAC Trends Anal. Chem. 2019, 113, 301–316. [Google Scholar] [CrossRef]
- Torné-Morató, H.; Donati, P.; Pompa, P.P. Nanoplasmonic Strip Test for Salivary Glucose Monitoring. Nanomaterials 2021, 12, 105. [Google Scholar] [CrossRef]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.W.; Yan, F. Flexible Organic Electrochemical Transistors for Highly Selective Enzyme Biosensors and Used for Saliva Testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef]
- Elkington, D.; Wasson, M.; Belcher, W.; Dastoor, P.C.; Zhou, X. Printable organic thin film transistors for glucose detection incorporating inkjet-printing of the enzyme recognition element. Appl. Phys. Lett. 2015, 106, 263301. [Google Scholar] [CrossRef]
- Ji, X.; Lau, H.Y.; Ren, X.; Peng, B.; Zhai, P.; Feng, S.-P.; Chan, P.K.L. Highly Sensitive Metabolite Biosensor Based on Organic Electrochemical Transistor Integrated with Microfluidic Channel and Poly(N-vinyl-2-pyrrolidone)-Capped Platinum Nanoparticles. Adv. Mater. Technol. 2016, 1, 1600042. [Google Scholar] [CrossRef] [Green Version]
- Gualandi, I.; Tessarolo, M.; Mariani, F.; Arcangeli, D.; Possanzini, L.; Tonelli, D.; Fraboni, B.; Scavetta, E. Layered Double Hydroxide-Modified Organic Electrochemical Transistor for Glucose and Lactate Biosensing. Sensors 2020, 20, 3453. [Google Scholar] [CrossRef]
- Shende, P.; Sahu, P. Enzyme bioconjugated PAMAM dendrimers for estimation of glucose in saliva. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 469–475. [Google Scholar] [CrossRef]
- Soni, A.; Jha, S.K. Smartphone based non-invasive salivary glucose biosensor. Anal. Chim. Acta 2017, 996, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Santana-Jiménez, L.A.; Márquez-Lucero, A.; Osuna, V.; Estrada-Moreno, I.; Dominguez, R.B. Naked-Eye Detection of Glucose in Saliva with Bienzymatic Paper-Based Sensor. Sensors 2018, 18, 1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, D.G.; Jung, D.; Kong, S.H. A Lab-on-a-Chip-Based Non-Invasive Optical Sensor for Measuring Glucose in Saliva. Sensors 2017, 17, 2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyankov, G.; Eftimov, T.; Malinowski, N.; Belina, E.; Kisov, H.; Mikulic, P.; Bock, W. A highly efficient biosensor based on MAPLE deposited hemoglobin on LPGs around phase matching turning point. Opt. Laser Technol. 2020, 123, 105907. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Lu, Y.; Sheng, K.; Liu, W.; Chen, C.; Li, Y.; Dong, B.; Song, H. Engineered IrO2@NiO Core–Shell Nanowires for Sensitive Non-enzymatic Detection of Trace Glucose in Saliva. Anal. Chem. 2016, 88, 12346–12353. [Google Scholar] [CrossRef]
- Anderson, K.; Poulter, B.; Dudgeon, J.; Li, S.-E.; Ma, X. A Highly Sensitive Nonenzymatic Glucose Biosensor Based on the Regulatory Effect of Glucose on Electrochemical Behaviors of Colloidal Silver Nanoparticles on MoS2. Sensors 2017, 17, 1807. [Google Scholar] [CrossRef] [Green Version]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Coyle, V.E.; Kandjani, A.; Field, M.R.; Hartley, P.; Chen, M.; Sabri, Y.M.; Bhargava, S.K. Co3O4 needles on Au honeycomb as a non-invasive electrochemical biosensor for glucose in saliva. Biosens. Bioelectron. 2019, 141, 111479. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Wang, M.L. Noninvasive glucose monitoring using saliva nano-biosensor. Sens. Bio-Sens. Res. 2015, 4, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Sha, R.; Durai, L.; Badhulika, S. Facile in-situ preparation of few-layered reduced graphene oxide—Niobium pentoxide composite for non-enzymatic glucose monitoring. In Proceedings of the 2018 4th IEEE International Conference on Emerging Electronics (ICEE), Bangalore, India, 16–19 December 2018; pp. 1–4. [Google Scholar]
- Nirala, N.R.; Tiwari, M.; Prakash, R. A nanoporous palladium(II) bridged coordination polymer acting as a peroxidase mimic in a method for visual detection of glucose in tear and saliva. Mikrochim. Acta 2018, 185, 245. [Google Scholar] [CrossRef]
- Fakhri, N.; Salehnia, F.; Beigi, S.M.; Aghabalazadeh, S.; Hosseini, M.; Ganjali, M.R. Enhanced peroxidase-like activity of platinum nanoparticles decorated on nickel- and nitrogen-doped graphene nanotubes: Colorimetric detection of glucose. Mikrochim. Acta 2019, 186, 385. [Google Scholar] [CrossRef] [PubMed]
- Beigi, S.M.; Mesgari, F.; Hosseini, M.; Aghazadeh, M.; Ganjali, M.R.; Beigi, S. An enhancement of luminol chemiluminescence by cobalt hydroxide decorated porous graphene and its application in glucose analysis. Anal. Methods 2019, 11, 1346–1352. [Google Scholar] [CrossRef]
- Yu, L.; Yang, Z.; An, M. Lab on the eye: A review of tear-based wearable devices for medical use and health management. Biosci. Trends 2019, 13, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Pieragostino, D.; D’Alessandro, M.; Di Ioia, M.; Di Ilio, C.; Sacchetta, P.; Del Boccio, P. Unraveling the molecular repertoire of tears as a source of biomarkers: Beyond ocular diseases. Proteom.—Clin. Appl. 2014, 9, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.K.; Sarin, G.S. Tear glucose levels in normal people and in diabetic patients. Br. J. Ophthalmol. 1980, 64, 693–695. [Google Scholar] [CrossRef] [Green Version]
- Kudo, H.; Sawada, T.; Kazawa, E.; Yoshida, H.; Iwasaki, Y.; Mitsubayashi, K. A flexible and wearable glucose sensor based on functional polymers with Soft-MEMS techniques. Biosens. Bioelectron. 2006, 22, 558–562. [Google Scholar] [CrossRef]
- Peng, B.; Lu, J.; Balijepalli, A.S.; Major, T.C.; Cohan, B.E.; Meyerhoff, M.E. Evaluation of enzyme-based tear glucose electrochemical sensors over a wide range of blood glucose concentrations. Biosens. Bioelectron. 2013, 49, 204–209. [Google Scholar] [CrossRef]
- La Belle, J.T.; Adams, A.; Lin, C.-E.; Engelschall, E.; Pratt, B.; Cook, C.B. Self-monitoring of tear glucose: The development of a tear based glucose sensor as an alternative to self-monitoring of blood glucose. Chem. Commun. 2016, 52, 9197–9204. [Google Scholar] [CrossRef]
- Chen, L.; Tse, W.H.; Chen, Y.; McDonald, M.; Melling, J.; Zhang, J. Nanostructured biosensor for detecting glucose in tear by applying fluorescence resonance energy transfer quenching mechanism. Biosens. Bioelectron. 2017, 91, 393–399. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, T.; Kong, W.; Zhang, Z.; Qu, H.; Chen, W.; Wang, Y.; Luo, L.; Zheng, L. ZIF-67 derived porous Co3O4 hollow nanopolyhedron functionalized solution-gated graphene transistors for simultaneous detection of glucose and uric acid in tears. Biosens. Bioelectron. 2018, 101, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dong, Z.-Q.; Shen, J.-H.; Chen, H.-W.; Zhu, Y.-H.; Zhu, Z.-G. 2D Photonic Crystal Hydrogel Sensor for Tear Glucose Monitoring. ACS Omega 2018, 3, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, C. Hydrogel-Based Colloidal Photonic Crystal Devices for Glucose Sensing. Polymers 2020, 12, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, J.-L.; Chen, C.; Shen, J.-H.; Zhao, X.-L.; Qian, S.-H.; Zhu, Z.-G. A Gelated Colloidal Crystal Attached Lens for Noninvasive Continuous Monitoring of Tear Glucose. Polymers 2017, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-R.; Hung, C.-C.; Chiu, H.-Y.; Chang, B.-H.; Li, B.-R.; Cheng, S.-J.; Yang, J.-W.; Lin, S.-F.; Chen, G.-Y. Noninvasive Glucose Monitoring with a Contact Lens and Smartphone. Sensors 2018, 18, 3208. [Google Scholar] [CrossRef] [Green Version]
- Zou, R.; Shan, S.; Huang, L.; Chen, Z.; Lawson, T.; Lin, M.; Yan, L.; Liu, Y. High-Performance Intraocular Biosensors from Chitosan-Functionalized Nitrogen-Containing Graphene for the Detection of Glucose. ACS Biomater. Sci. Eng. 2020, 6, 673–679. [Google Scholar] [CrossRef]
- Cowart, S.L.; Stachura, M.E. Glucosuria. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. Available online: http://www.ncbi.nlm.nih.gov/books/NBK245/ (accessed on 9 March 2021).
- Scullion, M.G.; Krauss, T.F.; Di Falco, A. Slotted Photonic Crystal Sensors. Sensors 2013, 13, 3675–3710. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Ji, W.; Chu, S.; Qian, S.; Wang, F.; Masson, J.-F.; Han, X.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643. [Google Scholar] [CrossRef]
- Zhu, J.; Du, H.-F.; Zhang, Q.; Zhao, J.; Weng, G.-J.; Li, J.-J.; Zhao, J.-W. SERS detection of glucose using graphene-oxide-wrapped gold nanobones with silver coating. J. Mater. Chem. C 2019, 7, 3322–3334. [Google Scholar] [CrossRef]
- Aidinis, K.; Goudarzi, K.; Esmaeili, A.H. Optical sensor based on two-dimensional photonic crystals for measuring glucose in urine. Opt. Eng. 2020, 59, 057104. [Google Scholar] [CrossRef]
- Swain, K.P.; Palai, G.; Prasad, M.V.S.V.; Sahoo, J.; Moharana, J.K. Realization of accurate urine-glucose sensor using triangular photonic crystal structure. In Proceedings of the 2016 International Conference on Signal Processing, Communication, Power and Embedded System (SCOPES), Paralakhemundi, India, 3–5 October 2016; pp. 1021–1024. [Google Scholar]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef] [PubMed]
- Inan, H.; Poyraz, M.; Inci, F.; Lifson, M.A.; Baday, M.; Cunningham, B.T.; Demirci, U. Photonic crystals: Emerging biosensors and their promise for point-of-care applications. Chem. Soc. Rev. 2017, 46, 366–388. [Google Scholar] [CrossRef] [PubMed]
- Cytiva. Surface Plasmon Resonance. Available online: https://www.cytivalifesciences.com/en/us/solutions/protein-research/knowledge-center/surface-plasmon-resonance/surface-plasmon-resonance (accessed on 9 June 2021).
- Pilot, R.; Signorini, R.; Fabris, L. Surface-enhanced raman spectroscopy: Principles, substrates, and applications. In Metal Nanoparticles and Clusters: Advances in Synthesis, Properties and Applications; Deepak, F.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 89–164. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, Y.; Zhang, W.; Ye, S.; Zhang, H.; Xie, F.; Gong, L.; Wei, Z.; Jin, H.; Chen, J. Highly sensitive detection of glucose: A quantitative approach employing nanorods assembled plasmonic substrate. Talanta 2017, 165, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Hussain, F.; Evans, N.; Rolinski, O.J.; Birch, D. Fluorescence-based glucose sensors. Biosens. Bioelectron. 2005, 20, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Ren, Y.; Zhang, H.; Ma, Y.; Niu, X.; Chen, X. One-step synthesis of nitrogen, boron co-doped fluorescent carbon nanoparticles for glucose detection. J. Biol. Chem. Lumin. 2017, 32, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, T.; Romey, M.A.; Zhu, P.C.; Holody, M.Z.; Shinkai, S. A Study of Boronic Acid Based Fluorescent Glucose Sensors. J. Fluoresc. 2004, 14, 499–512. [Google Scholar] [CrossRef]
- Go, A.; Kim, H.T.; Park, Y.J.; Park, S.R.; Lee, M.-H. Fabrication of Repeatedly Usable Pt-Electrode Chip Coated With Solidified Glucose Oxidase and Ascorbate Oxidase for the Quantification of Glucose in Urine. IEEE Sens. Lett. 2019, 3, 1. [Google Scholar] [CrossRef]
- Pezhhan, H.; Akhond, M.; Shamsipur, M. A novel nanoplatform encapsulating glucose oxidase for spectrophotometric biosensing of hydrogen peroxide and glucose. Anal. Methods 2020, 12, 345–357. [Google Scholar] [CrossRef]
- Karim, N.; Anderson, S.R.; Singh, S.; Ramanathan, R.; Bansal, V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens. Bioelectron. 2018, 110, 8–15. [Google Scholar] [CrossRef]
- Yang, C.; Feng, W.; Li, Y.; Tian, X.; Zhou, Z.; Lu, L.; Nie, Y. A promising method for diabetes early diagnosis via sensitive detection of urine glucose by Fe Pd/rGO. Dye. Pigment. 2019, 164, 20–26. [Google Scholar] [CrossRef]
- Toghill, K.; Compton, R. Electrochemical Non-Enzymatic Glucose Sensors: A Perspective and an Evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Sun, Y.; Li, Y.; Wang, N.; Xu, Q.Q.; Xu, L.; Lin, M. Copper-based Metal-organic Framework for Non-enzymatic Electrochemical Detection of Glucose. Electroanalysis 2018, 30, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Savariraj, A.D.; Vinoth, V.; Mangalaraja, R.; Arun, T.; Contreras, D.; Akbari-Fakhrabadi, A.; Valdés, H.; Banat, F. Microwave-assisted synthesis of localized surface plasmon resonance enhanced bismuth selenide (Bi2Se3) layers for non-enzymatic glucose sensing. J. Electroanal. Chem. 2020, 856, 113629. [Google Scholar] [CrossRef]
- Janyasupab, M. Development of non-enzymatic N-doped graphene supported cobalt/iron amperometric based sensor for glucose detection in urine. In Proceedings of the 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences—IECBES, Kuching, Malaysia, 3–6 December 2018; pp. 577–582. [Google Scholar]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bhide, A.; Muthukumar, S.; Saini, A.; Prasad, S. Simultaneous lancet-free monitoring of alcohol and glucose from low-volumes of perspired human sweat. Sci. Rep. 2018, 8, 6507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Lu, W.; Yuan, Q.; Zheng, Y.; Yao, B. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta 2019, 198, 86–92. [Google Scholar] [CrossRef]
- Luo, X.; Guo, L.; Liu, Y.; Shi, W.; Gai, W.; Cui, Y. Wearable Tape-Based Smart Biosensing Systems for Lactate and Glucose. IEEE Sens. J. 2020, 20, 3757–3765. [Google Scholar] [CrossRef]
- Karyakin, A.A. Prussian Blue and Its Analogues: Electrochemistry and Analytical Applications. Electroanalysis 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Zahed, A.; Barman, S.C.; Das, P.S.; Yoon, H.S.; Yoon, S.H.; Park, J.Y. Highly flexible and conductive poly (3, 4-ethylene dioxythiophene)-poly (styrene sulfonate) anchored 3-dimensional porous graphene network-based electrochemical biosensor for glucose and pH detection in human perspiration. Biosens. Bioelectron. 2020, 160, 112220. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.; Xie, Y.; Qian, C.; Ma, W.; Wang, L.; Song, Y. Ratiometric electrochemical glucose sensor based on electroactive Schiff base polymers. Sens. Actuators B Chem. 2019, 285, 264–270. [Google Scholar] [CrossRef]
- Kafi, A.; Paul, A.; Vilouras, A.; Dahiya, R. Chitosan-graphene oxide based ultra-thin conformable sensing patch for cell-health monitoring. In Proceedings of the 2018 IEEE Sensors Applications Symposium (SAS), Seoul, Korea, 12–14 March 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Kafi, A.; Paul, A.; Vilouras, A.; Hosseini, E.S.; Dahiya, R.S. Chitosan-Graphene Oxide-Based Ultra-Thin and Flexible Sensor for Diabetic Wound Monitoring. IEEE Sens. J. 2020, 20, 6794–6801. [Google Scholar] [CrossRef] [Green Version]
- Xue, Q.; Li, Z.; Wang, Q.; Pan, W.; Chang, Y.; Duan, X. Nanostrip flexible microwave enzymatic biosensor for noninvasive epidermal glucose sensing. Nanoscale Horizons 2020, 5, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Dautta, M.; Alshetaiwi, M.; Escobar, J.; Tseng, P. Passive and wireless, implantable glucose sensing with phenylboronic acid hydrogel-interlayer RF resonators. Biosens. Bioelectron. 2020, 151, 112004. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Bariya, M.; Kivimäki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.; Lin, Y.; et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Liang, B.; Tu, T.; Wei, J.; Fang, L.; Ye, X. Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv. 2019, 9, 5674–5681. [Google Scholar] [CrossRef] [Green Version]
- Dardano, P.; Rea, I.; De Stefano, L. Microneedles-based electrochemical sensors: New tools for advanced biosensing. Curr. Opin. Electrochem. 2019, 17, 121–127. [Google Scholar] [CrossRef]
- Latif, U.; Dickert, F.L.; Blach, R.; Feucht, H. Biocompatible Membranes and Coatings for Glucose Sensor. J. Chem. Soc. Pak. 2013, 35, 17–22. [Google Scholar]
- Vallejo-Heligon, S.G.; Brown, N.L.; Reichert, W.M.; Klitzman, B. Porous, Dexamethasone-loaded polyurethane coatings extend performance window of implantable glucose sensors in vivo. Acta Biomater. 2016, 30, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Burugapalli, K.; Wijesuriya, S.; Far, M.Y.; Song, W.; Moussy, F.; Zheng, Y.; Ma, Y.; Wu, Z.; Li, K. Electrospun polyurethane-core and gelatin-shell coaxial fibre coatings for miniature implantable biosensors. Biofabrication 2013, 6, 015002. [Google Scholar] [CrossRef] [Green Version]
- Poulos, N.G.; Hall, J.R.; Leopold, M.C. Functional Layer-By-Layer Design of Xerogel-Based First-Generation Amperometric Glucose Biosensors. Langmuir 2015, 31, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Ribet, F.; Stemme, G.; Roxhed, N. Ultra-miniaturization of a planar amperometric sensor targeting continuous intradermal glucose monitoring. Biosens. Bioelectron. 2017, 90, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, C.; Chen, W.; Chen, Y.; Zhang, J.X. PVDF-Nafion nanomembranes coated microneedles for in vivo transcutaneous implantable glucose sensing. Biosens. Bioelectron. 2015, 74, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive Microneedle-based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.B.; Lee, W.-C.; Cho, C.-H.; Park, D.-S.; Cho, S.J.; Shim, Y.-B. Continuous glucose monitoring using a microneedle array sensor coupled with a wireless signal transmitter. Sens. Actuators B Chem. 2019, 281, 14–21. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, H.; Park, J.; Kim, D.; Park, J. Skin-attachable and implantable polymer microneedle biosensor for continuous glucose monitoring. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 404–407. [Google Scholar]
- Nyein, H.Y.Y.; Bariya, M.; Tran, B.; Ahn, C.H.; Brown, B.J.; Ji, W.; Davis, N.; Javey, A. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 2021, 12, 1823. [Google Scholar] [CrossRef]
- Tang, W.; Yin, L.; Sempionatto, J.R.; Moon, J.; Teymourian, H.; Wang, J. Touch-Based Stressless Cortisol Sensing. Adv. Mater. 2021, 33, 2008465. [Google Scholar] [CrossRef]
- Yin, L.; Moon, J.-M.; Sempionatto, J.R.; Lin, M.; Cao, M.; Trifonov, A.; Zhang, F.; Lou, Z.; Jeong, J.-M.; Lee, S.-J.; et al. A passive perspiration biofuel cell: High energy return on investment. Joule 2021, 5, 1888–1904. [Google Scholar] [CrossRef]
- Lin, S.; Wang, B.; Zhao, Y.; Shih, R.; Cheng, X.; Yu, W.; Hojaiji, H.; Lin, H.; Hoffman, C.; Ly, D.; et al. Natural Perspiration Sampling and in Situ Electrochemical Analysis with Hydrogel Micropatches for User-Identifiable and Wireless Chemo/Biosensing. ACS Sens. 2019, 5, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Nagamine, K.; Mano, T.; Nomura, A.; Ichimura, Y.; Izawa, R.; Furusawa, H.; Matsui, H.; Kumaki, D.; Tokito, S. Noninvasive Sweat-Lactate Biosensor Emplsoying a Hydrogel-Based Touch Pad. Sci. Rep. 2019, 9, 10102. [Google Scholar] [CrossRef] [Green Version]
- Sempionatto, J.R.; Moon, J.-M.; Wang, J. Touch-Based Fingertip Blood-Free Reliable Glucose Monitoring: Personalized Data Processing for Predicting Blood Glucose Concentrations. ACS Sens. 2021, 6, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Wang, J.; Gao, N.; He, H.; Zou, K.; Ma, M.; Zhou, Y.; Cai, Z.; Chang, G.; He, Y. A gold electrode modified with a gold-graphene oxide nanocomposite for non-enzymatic sensing of glucose at near-neutral pH values. Mikrochim. Acta 2019, 186, 722. [Google Scholar] [CrossRef]
- Roberts, K.; Jaffe, A.; Verge, C.; Thomas, P.S. Noninvasive Monitoring of Glucose Levels: Is Exhaled Breath the Answer? J. Diabetes Sci. Technol. 2012, 6, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Weatherspoon, D.; Burgess, L. Why Does my Breath Smell Like Acetone? 2019. Available online: https://www.medicalnewstoday.com/articles/319683 (accessed on 25 April 2021).
- Saasa, V.; Beukes, M.; Lemmer, Y.; Mwakikunga, B. Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus. Diagnostics 2019, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.; Walton, C.; Hoashi, S.; Evans, M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J. Breath Res. 2009, 3, 046004. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Sheng, K.; Zhou, X.; Dong, B.; Lu, G.; Song, H. A highly sensitive and moisture-resistant gas sensor for diabetes diagnosis with Pt@In2O3 nanowires and a molecular sieve for protection. NPG Asia Mater. 2018, 10, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Zhang, D.; Zhang, L.; Lu, G. Non-invasive blood glucose monitoring for diabetics by means of breath signal analysis. Sens. Actuators B Chem. 2012, 173, 106–113. [Google Scholar] [CrossRef]
- Lekha, S.; Suchetha, M. Non-invasive diabetes detection and classification using breath analysis. In Proceedings of the IEEE International Conference on Communications and Signal Processing, Melmaruvathur, India, 2–4 April 2015; pp. 955–958. [Google Scholar]
- Patil, A.; Kad, A.; Kharat, S. Non-Invasive Method for Diabetes Detection using CNN and SVM Classifier. Int. J. Sci. Res. Eng. Dev. 2020, 3, 9–13. [Google Scholar]
- Thati, A.; Biswas, A.; Chowdhury, S.R.; Sau, T.K. Breath acetone-based non-invasive detection of blood glucose levels. Int. J. Smart Sens. Intell. Syst. 2015, 8, 1244–1260. [Google Scholar] [CrossRef] [Green Version]
- Tankasala, D.; Ng, G.P.; Smith, M.S.; Bendell, J.R.; Linnes, J.C. Selective Collection and Condensation of Exhaled Breath for Glucose Detection. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 3890–3893. [Google Scholar] [CrossRef]
- Tankasala, D.; Linnes, J.C. Noninvasive glucose detection in exhaled breath condensate. Transl. Res. 2019, 213, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Joshi, R.; Vishnu, G.K.A.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 024001. [Google Scholar] [CrossRef] [PubMed]
- Shokrekhodaei, M.; Quinones, S. Review of Non-Invasive Glucose Sensing Techniques: Optical, Electrical and Breath Acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Mbi, A.; Shepherd, M. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: Exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, X.; Yin, H.; Wang, Z.; Jiang, C.; Liu, W.; Chen, Z.; Yuan, Y.; Li, Y.; Wang, C. Study of breath acetone and its correlations with blood glucose and blood beta-hydroxybutyrate using an animal model with lab-developed type 1 diabetic rats. RSC Adv. 2015, 5, 71002–71010. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, M.; Wang, Z.; Chen, Z.; Zhao, X.; Yuan, Y.; Li, Y.; Wang, C. A portable real-time ringdown breath acetone analyzer: Toward potential diabetic screening and management. Sensors 2016, 16, 1199. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jiang, L.; Zhao, L.; Liu, F.; You, R.; Yang, Z.; He, J.; Liu, T.; Zhang, C.; Wang, C.; et al. Stabilized zirconia-based acetone sensor utilizing Fe2TiO5-TiO2 sensing electrode for noninvasive diagnosis of diabetics. Sens. Actuators B Chem. 2020, 321, 128489. [Google Scholar] [CrossRef]
- Rydosz, A. A Negative Correlation Between Blood Glucose and Acetone Measured in Healthy and Type 1 Diabetes Mellitus Patient Breath. J. Diabetes Sci. Technol. 2015, 9, 881–884. [Google Scholar] [CrossRef]
- Andrews, B.T.E.; Denzer, W.; Hancock, G.; Lunn, A.D.; Peverall, R.; Ritchie, G.A.D.; Williams, K. Measurement of breath acetone in patients referred for an oral glucose tolerance test. J. Breath Res. 2018, 12, 036015. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Z.; Yuan, Y.; Chen, Z.; Zhao, X.; Li, Y.; Wang, C. Continuous monitoring of breath acetone, blood glucose and blood ketone in 20 type 1 diabetic outpatients over 30 days. J. Anal. Bioanal. Tech. 2017, 8, 1000386. [Google Scholar]
- Blood: Components, Functions, Groups, and Disorders. 25 August. 2017. Available online: https://www.medicalnewstoday.com/articles/196001 (accessed on 18 June 2021).
- Sharma, P.; Panchal, A.; Yadav, N.; Narang, J. Analytical techniques for the detection of glycated haemoglobin underlining the sensors. Int. J. Biol. Macromol. 2020, 155, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Thalmayer, A.S.; Zeising, S.; Fischer, G.; Lübke, M. Commercial and Scientific Solutions for Blood Glucose Monitoring—A Review. Sensors 2022, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Juska, V.B.; Pemble, M.E. A dual-enzyme, micro-band array biosensor based on the electrodeposition of carbon nanotubes embedded in chitosan and nanostructured Au-foams on microfabricated gold band electrodes. Analyst 2020, 145, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Cete, S.; Ozyurt, M.; Yildirim, E.; Akin, D. A novel biosensor with the use of polypyrrole–poly(sodium-4-styrenesulphonate) as a dopant in the determination of glucose. Chem. Pap. 2020, 74, 799–808. [Google Scholar] [CrossRef]
- Márquez, A.; Jimenez-Jorquera, C.; Domínguez, C.; Berbel, X.M. Electrodepositable alginate membranes for enzymatic sensors: An amperometric glucose biosensor for whole blood analysis. Biosens. Bioelectron. 2017, 97, 136–142. [Google Scholar] [CrossRef]
- Cánovas, R.; Parrilla, M.; Blondeau, P.; Andrade, F.J. A novel wireless paper-based potentiometric platform for monitoring glucose in blood. Lab Chip 2017, 17, 2500–2507. [Google Scholar] [CrossRef] [Green Version]
- Cánovas, R.; Blondeau, P.; Andrade, F.J. Modulating the mixed potential for developing biosensors: Direct potentiometric determination of glucose in whole, undiluted blood. Biosens. Bioelectron. 2020, 163, 112302. [Google Scholar] [CrossRef]
- Qasemi, S.; Ghaemy, M. Highly sensitive and strongly fluorescent gum tragacanth based superabsorbent hydrogel as a new biosensor for glucose optical detection. J. Mater. Chem. C 2020, 8, 4148–4156. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Silva, P.R.; Lima, A.P.; Rocha, D.P.; Oliveira, T.C.; do Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Richter, E.M.; Muñoz, R.A. 3D-Printed graphene/polylactic acid electrode for bioanalysis: Biosensing of glucose and simultaneous determination of uric acid and nitrite in biological fluids. Sens. Actuators B Chem. 2020, 307, 127621. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Azharudeen, A.M.; Karthiga, R.; Rajarajan, M.; Suganthi, A. Fabrication, characterization of polyaniline intercalated NiO nanocomposites and application in the development of non-enzymatic glucose biosensor. Arab. J. Chem. 2020, 13, 4053–4064. [Google Scholar] [CrossRef]
- Balasubramanian, P.; He, S.-B.; Deng, H.-H.; Peng, H.-P.; Chen, W. Defects engineered 2D ultrathin cobalt hydroxide nanosheets as highly efficient electrocatalyst for non-enzymatic electrochemical sensing of glucose and l-cysteine. Sens. Actuators B Chem. 2020, 320, 128374. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Glumbokaite, L.; Ramanaviciene, A.; Ramanavicius, A. Reagent-less amperometric glucose biosensor based on nanobiocomposite consisting of poly(1,10-phenanthroline-5,6-dione), poly(pyrrole-2-carboxylic acid), gold nanoparticles and glucose oxidase. Microchem. J. 2020, 154, 104665. [Google Scholar] [CrossRef]


| Glucose Sensors | Method of Detection | Biological Fluid Used | Linear Detection Range | Limit of Detection | References |
|---|---|---|---|---|---|
| CuO/PCL@PPy/ITO | Electrochemistry | Saliva | 2 µM–6 mM | 0.8 µM | [44] |
| Co3O4 needles on Au honeycomb | Electrochemistry | Saliva | 20–100 µM | 20 µM | [60] |
| rGO modified Nb2O5 | Electrochemistry | Tears, Urine, Saliva | 1–10 mM | 1 mM | [62] |
| Nanoporous palladium(II) bridged coordination polymer | Colorimetry | Tears Saliva | 0–47 nM | 61 nM 91 nM | [63] |
| Pt/Ni@NGT paper based device | Colorimetry | Tears, Saliva | 0.1–50 mM | 1 pM | [64] |
| PG/Co(OH)2 | Chemiluminescence | Tears, Saliva | 3.0 × 10−9–4.0 × 10−5 mol L−1 | 6.4 × 10−10 mol L−1 | [65] |
| Flexible OECTs- GOx-GO/PANI/Nafion-graphene/Pt | Electrochemistry | Saliva | 0.1 µM–1 mM | 0.01 µM | [48] |
| IrO2@ NiO nanowires | Electrochemistry | Saliva | 0.5 μM–2.5 mM | 0.31 µM | [57] |
| PEDOT:PSS with Ni/Al LDH | Electrochemistry | Saliva | 0.1–8.0 mM | 0.02 mM | [51] |
| AgNPs/MoS2 | Electrochemistry | Saliva, Sweat | 0.1–1000 µM | 0.03 µM | [58] |
| Hb deposited LPG | Electrochemistry | Saliva | 0.05–2 mmol/L | 0.1 mmol/L | [56] |
| Glucose Sensors | Method of Detection | Biological Fluid Used | Linear Detection Range | Limit of Detection | References |
|---|---|---|---|---|---|
| rGO modified Nb2O5 | Electrochemistry | Tears, Urine, Saliva | 1–10 mM | 1 mM | [62] |
| Nanoporous palladium(II) bridged coordination polymer | Colorimetry | Tear Saliva | 0–47 nM | 61 nM 91 nM | [63] |
| Pt/Ni@NGT paper- based device | Colorimetry | Tears, Saliva | 0.1–50 mM | 1 pM | [64] |
| PG/Co(OH)2 | Chemiluminescence | Tears, Saliva | 3.0 × 10−9–4.0 × 10−5 mol L−1 | 6.4 × 10−10 mol L−1 | [65] |
| CdSe/ZnS donor, malachite green dextran acceptor on ZnO nanorods-silicon hydrogel lens | Fluorescence resonance energy transfer | Tears | 0.03–3 mmol/L | 0.03 mmol/L | [72] |
| GOx-CHIT/Co3O4 /Au | Electrochemistry | Tears | 0–100 nM | 100 nM | [73] |
| Pt/Ir wire with selective layer of Nafion and 1,3-diaminobenzene | Electrochemical/colorimetry | Tears | 0–40 mM | 1 µM | [75] |
| PS-GCCA on RGP contact lens | Colorimetry/diffraction spectrometry | Tears | 0–50 mM | 0.05 mM | [76] |
| PS-MCC | Colorimetry/Diffraction spectroscopy | Tears, Blood | 0–20 mM | 20 mM | [74] |
| COOH chitosan- functionalized NG | Electrochemistry | Tears | 0–12 mM | 9.5 µM | [78] |
| Glucose Sensors | Method of Detection | Biological Fluid Used | Linear Detection Range | Limit of Detection | References |
|---|---|---|---|---|---|
| rGO modified Nb2O5 | Electrochemistry | Tears, Urine, Saliva | 1–10 mM | 1 mM | [62] |
| PMBA@Au/optical fiber with AET/AuNPs | SPR | Urine | 8 × 10−8–5 × 10−2 M | 0.8 µM | [81] |
| GO decorated AuNBs@Ag | Raman spectroscopy/SPR | Urine | 0.01–10−4 µM | 80 nM | [82] |
| 2D Triangular photonic crystal structure | Photonic band gap | Urine | 0 gm/dL to 10 gm/dL | [84] | |
| N,B doped CNPs | Fluorescence | Urine | 0–900 µM | 1.8 µM | [91] |
| Fe3O4–Fe(OH)3@GOx–polyDA | Colorimetry/UV Spectroscopy | Urine | 5–500 µM | 3 µM | [94] |
| Ag+NPs @ cotton fabric | Electrochemistry | Urine | 100–2000 µM | 80 µM | [95] |
| Fe-Pd/rGO | Colorimetry/UV spectrometry | Urine | 0–200 µM | 1.76 µM | [96] |
| Cu-MOF | Electrochemistry | Urine | 0.06 μM–5 mM | 10.5 nM | [98] |
| Bi2Fe3-few layers modified GC electrodes | Electrochemistry | Urine | 10–100 µM | 6.1 µM | [99] |
| CoFe@N-Graphene | Electrochemistry | Urine | 0–3.25 mM | 37.7 µM | [100] |
| Glucose Sensors | Method of Detection | Biological Fluid Used | Linear Detection Range | Limit of Detection | References |
|---|---|---|---|---|---|
| 5-layered microfluidic chip | Colorimetry | Sweat | 0.1–0.5 mM | 0.03 mM | [36] |
| AgNPs/MoS2 | Electrochemistry | Saliva, Sweat | 0.1–1000 µM | 0.03 µM | [58] |
| PET-based Au electrodes | Electrochemistry | Sweat | 0.02–1.11 mM | 2.7 µM | [104] |
| Carbon graphite ink on adhesive tapes | Electrochemistry | Sweat | 0.48–2.59 mM | 0.80 µM | [105] |
| PEDOT:PSS/LIG | Electrochemistry | Sweat | 10 μM–9.2 mM | 3 µM | [107] |
| SBPthi nanosheets | Electrochemistry | Sweat | 0.82 μM–4.0 mM | 0.27 µM | [108] |
| 3D-PMED | Electrochemistry | Sweat | 0–1.9 mM | 5 µM | [114] |
| PVDF/Nafion/PtNPs/PANI nanofiber needle electrodes | Electrochemistry | Interstitial fluid | 0–20 mM | [121] | |
| Au/Au-multiwalled carbon nanotubes (MWCNTs)/ poly-methylene blue (pMB)/FADGDH electrodes | Electrochemistry | Interstitial fluid | 0.05–5 mM | 7 μM | [122] |
| COC-PPY polymer needle/Au/pTCA-GOx/Nafion | Electrochemistry | Interstitial fluid | 0.05–20.0 mM | 19.4 (±0.62) μA | [123] |
| Hard-PDMS / PI / Au/rGO/GOx/ Nafion electrodes | Electrochemistry | Interstitial fluid | 0–30 mM | 0.198 µM | [124] |
| Au/GO/AuNPs | Electrochemistry | Sweat | 0.05–42 mM | 12 µM | [133] |
| Glucose Sensors | Method of Detection | Biological Fluid Used | Linear Detection Range | Limit of Detection | References |
|---|---|---|---|---|---|
| Chitosan cryogel beads with TCS230 | Colorimetry | Diluted blood | 0.1–2.5 mM | 0.14 mM | [35] |
| PS-MCC | Colorimetry/Diffraction spectroscopy | Tears, Blood | 0–20 mM | [80] | |
| Au foam@ CNT-modified Chitosan | Electrochemistry | Blood serum | 0.05–1.1 mM | 0.025 mM | [157] |
| Polypyrrole–poly (sodium- 4 -styrenesulphonate) film | Electrochemistry | Blood | 1 × 10−8–1 × 10−3 M | [158] | |
| Calcium alginate hydrogel membrane | Electrochemistry | Whole blood | 2–12 mM | 126 µM | [159] |
| Nafion/Aquivion-coated Pt electrodes | Potentiometric Electrochemistry | Whole blood | 0.3–3 mM | [160] | |
| GT/AA/MBA/FlA-DA/ CdTe quantum dots-Hydrogel | Fluorescence | Whole blood | 0.1–10 µM | 0.1 µM | [162] |
| G-PLA | Electrochemistry | Blood | 0.5–250 μmol L−1 | 15 μmol L−1 | [163] |
| NiO/PANI | Electrochemistry | Blood serum | 0–100 µM | 0.19 µM | [165] |
| VCo-Co(OH)2 nanosheets | Electrochemistry | Blood serum | 0.4 μM–8.23 mM | 295 nM | [166] |
| PD/PPCA/AuNPs/Graphite electrode | Electrochemistry | Blood serum | 0.2–150 mM | 80 µM | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, V.S.; Agarwal, B.; Ye, Z.; Zhang, C.; Roy, K.; Chinnappan, A.; Narayan, R.J.; Ramakrishna, S.; Ghosh, R. Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review. Nanomaterials 2022, 12, 1082. https://doi.org/10.3390/nano12071082
Reddy VS, Agarwal B, Ye Z, Zhang C, Roy K, Chinnappan A, Narayan RJ, Ramakrishna S, Ghosh R. Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review. Nanomaterials. 2022; 12(7):1082. https://doi.org/10.3390/nano12071082
Chicago/Turabian StyleReddy, Vundrala Sumedha, Bhawana Agarwal, Zhen Ye, Chuanqi Zhang, Kallol Roy, Amutha Chinnappan, Roger J. Narayan, Seeram Ramakrishna, and Rituparna Ghosh. 2022. "Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review" Nanomaterials 12, no. 7: 1082. https://doi.org/10.3390/nano12071082
APA StyleReddy, V. S., Agarwal, B., Ye, Z., Zhang, C., Roy, K., Chinnappan, A., Narayan, R. J., Ramakrishna, S., & Ghosh, R. (2022). Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review. Nanomaterials, 12(7), 1082. https://doi.org/10.3390/nano12071082









