Wool-Based Carbon Fiber/MoS2 Composite Prepared by Low-Temperature Catalytic Hydrothermal Method and Its Application in the Field of Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of MoS2
2.1.2. Preparation of WCF and WCF–MoS2 Composites
2.2. Device Fabrication and Testing
2.3. Materials Characterization
3. Results and Discussion
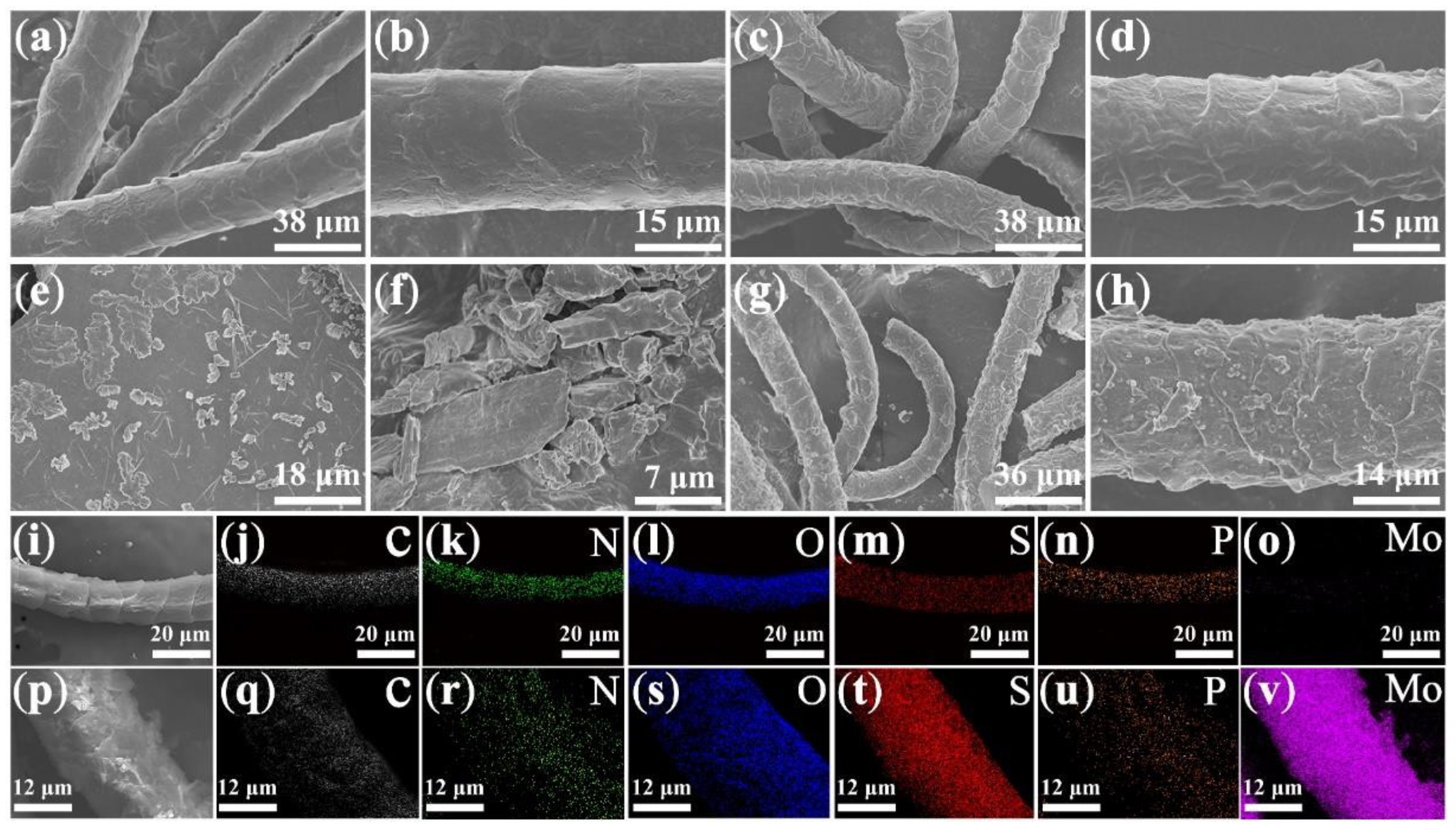
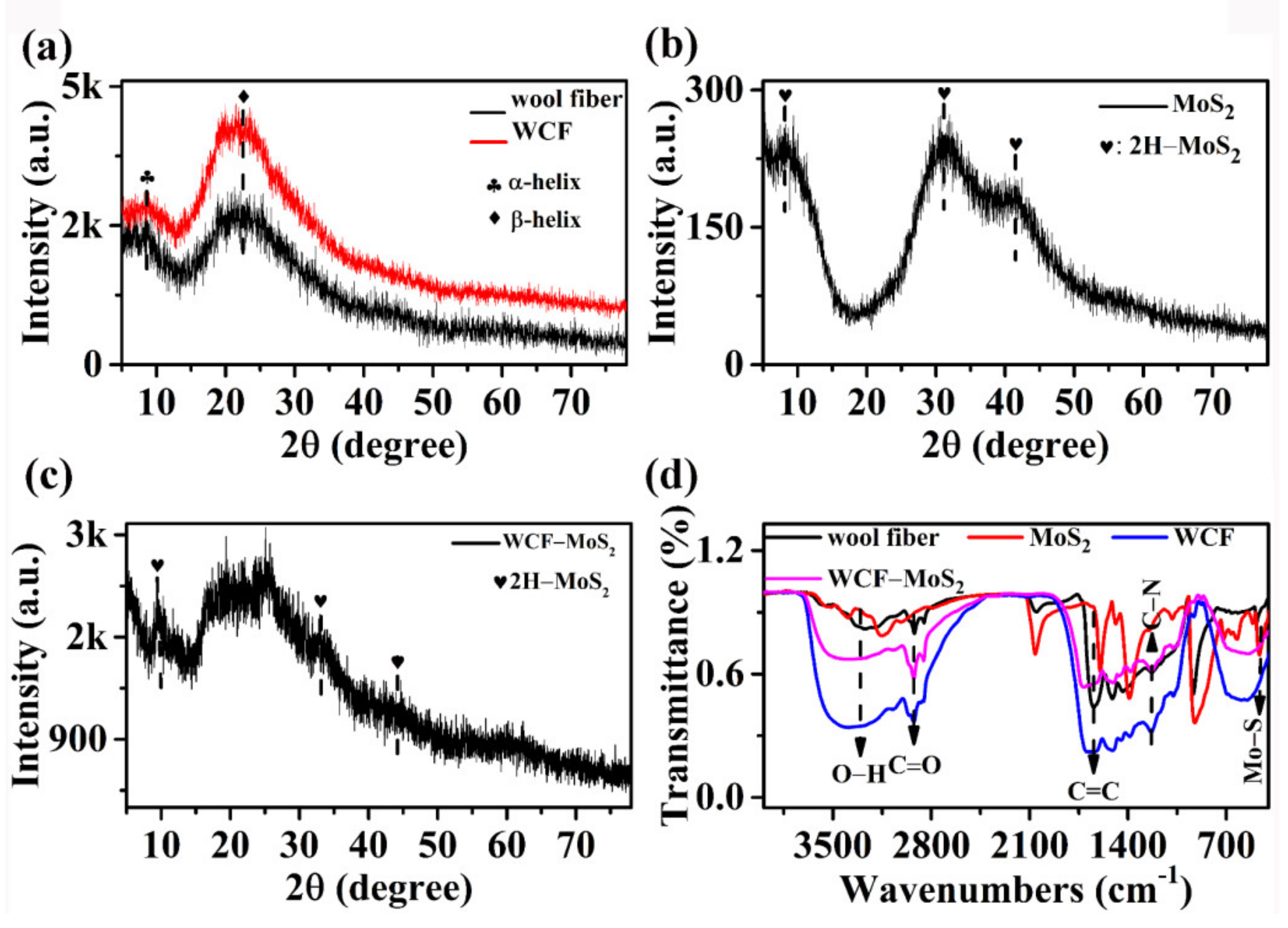
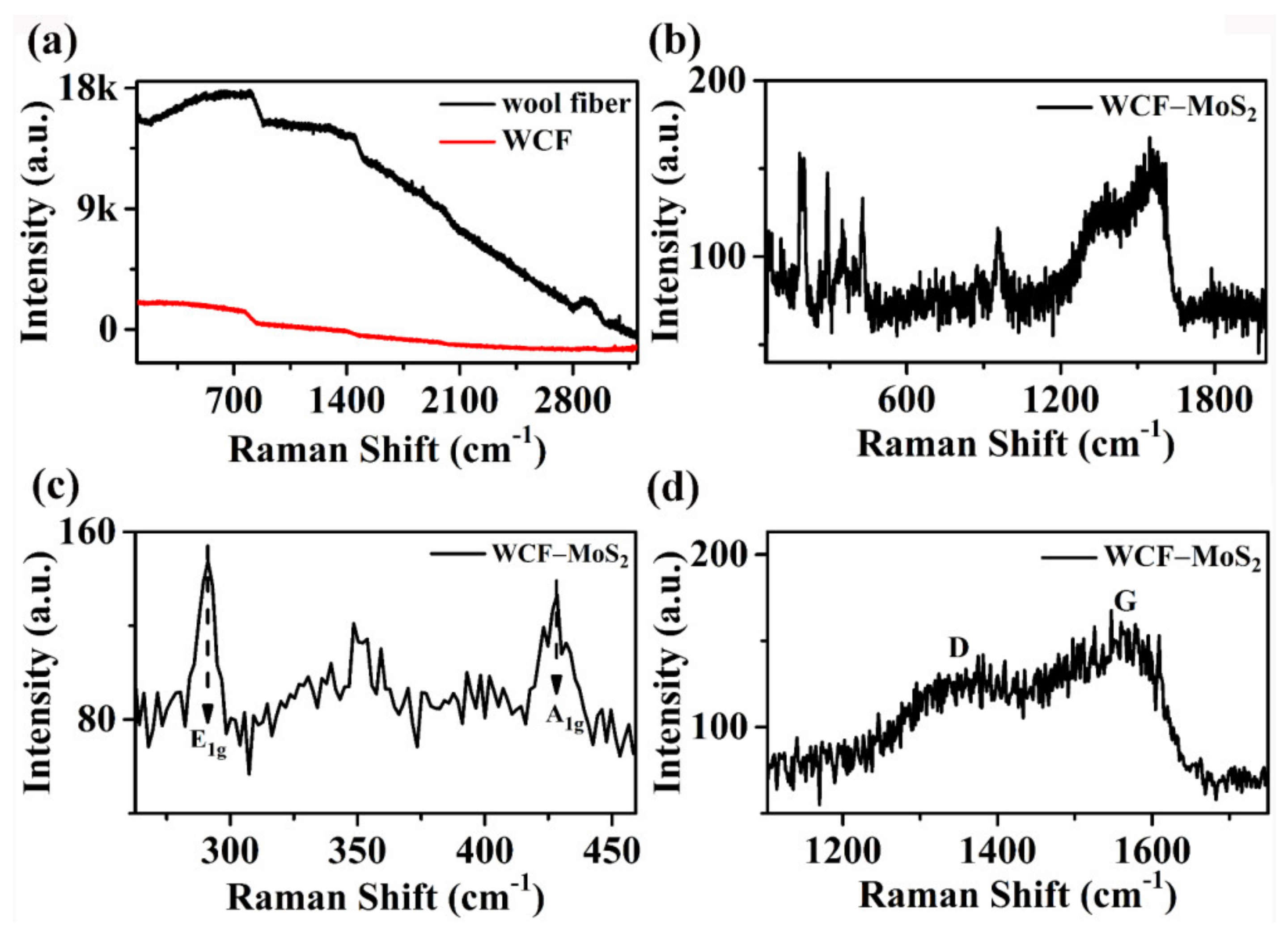
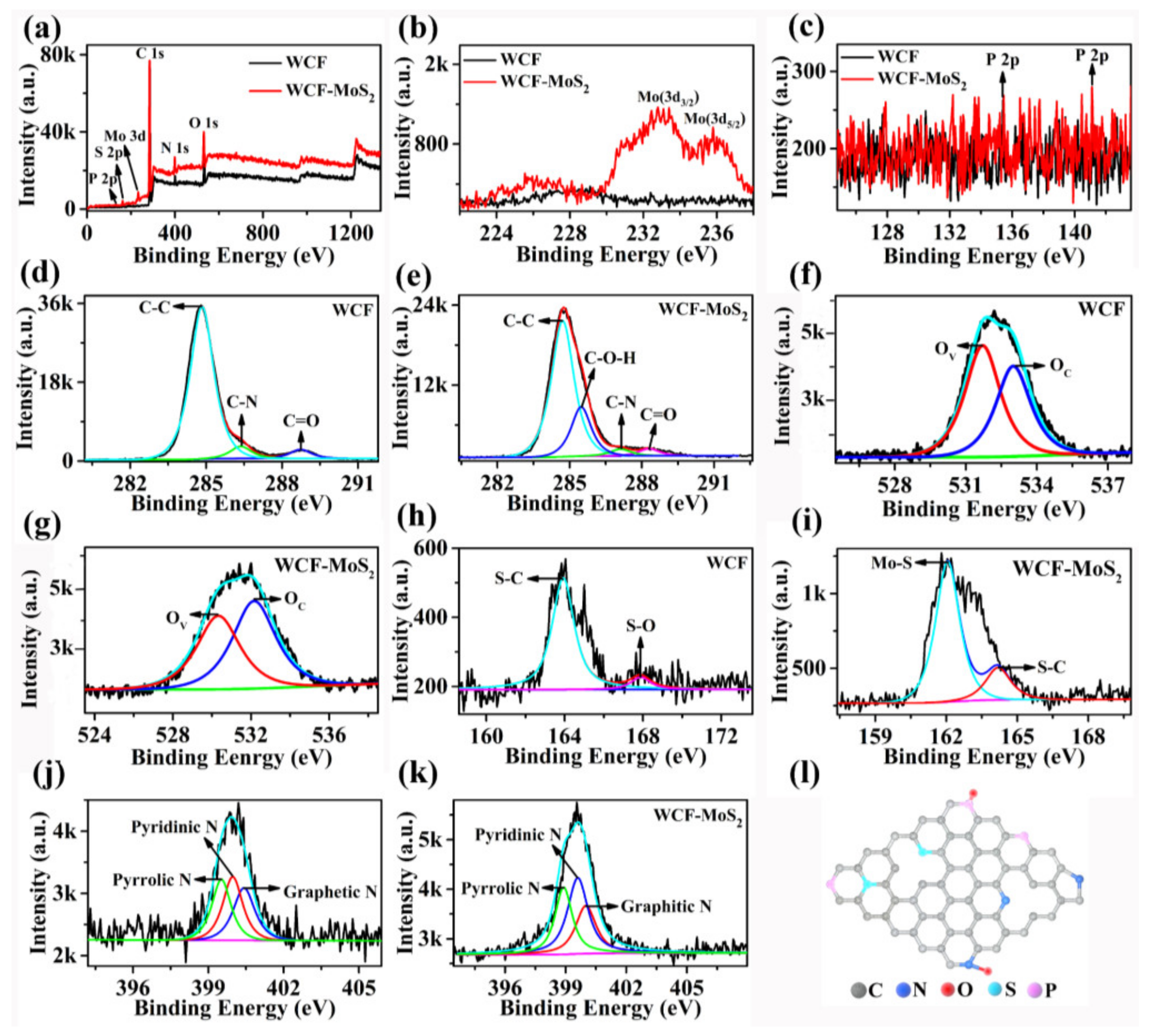
3.1. Structures and Morphologies of MoS2, WCF, and WCF–MoS2
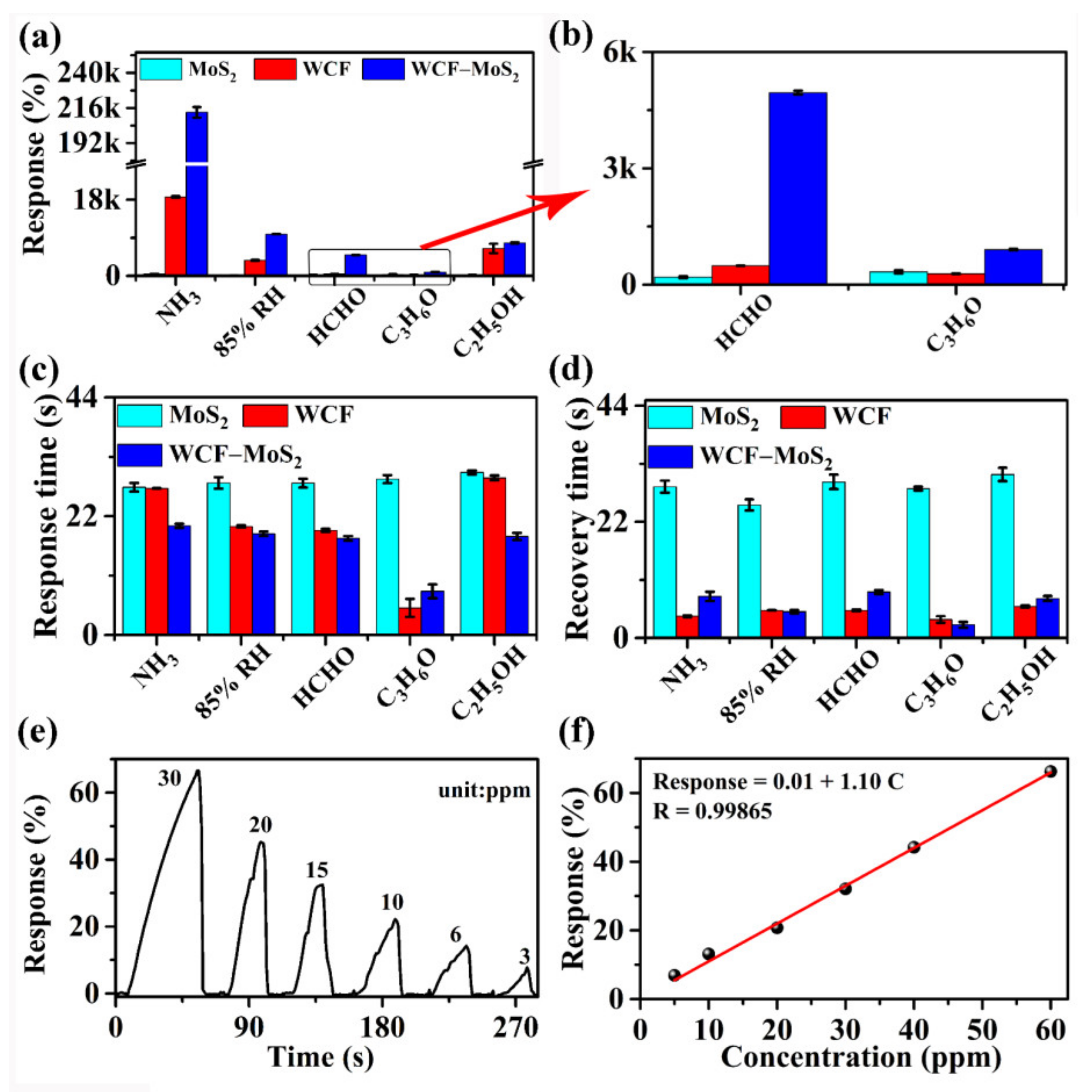
3.2. Sensing Performance of MoS2, WCF, and WCF–MoS2
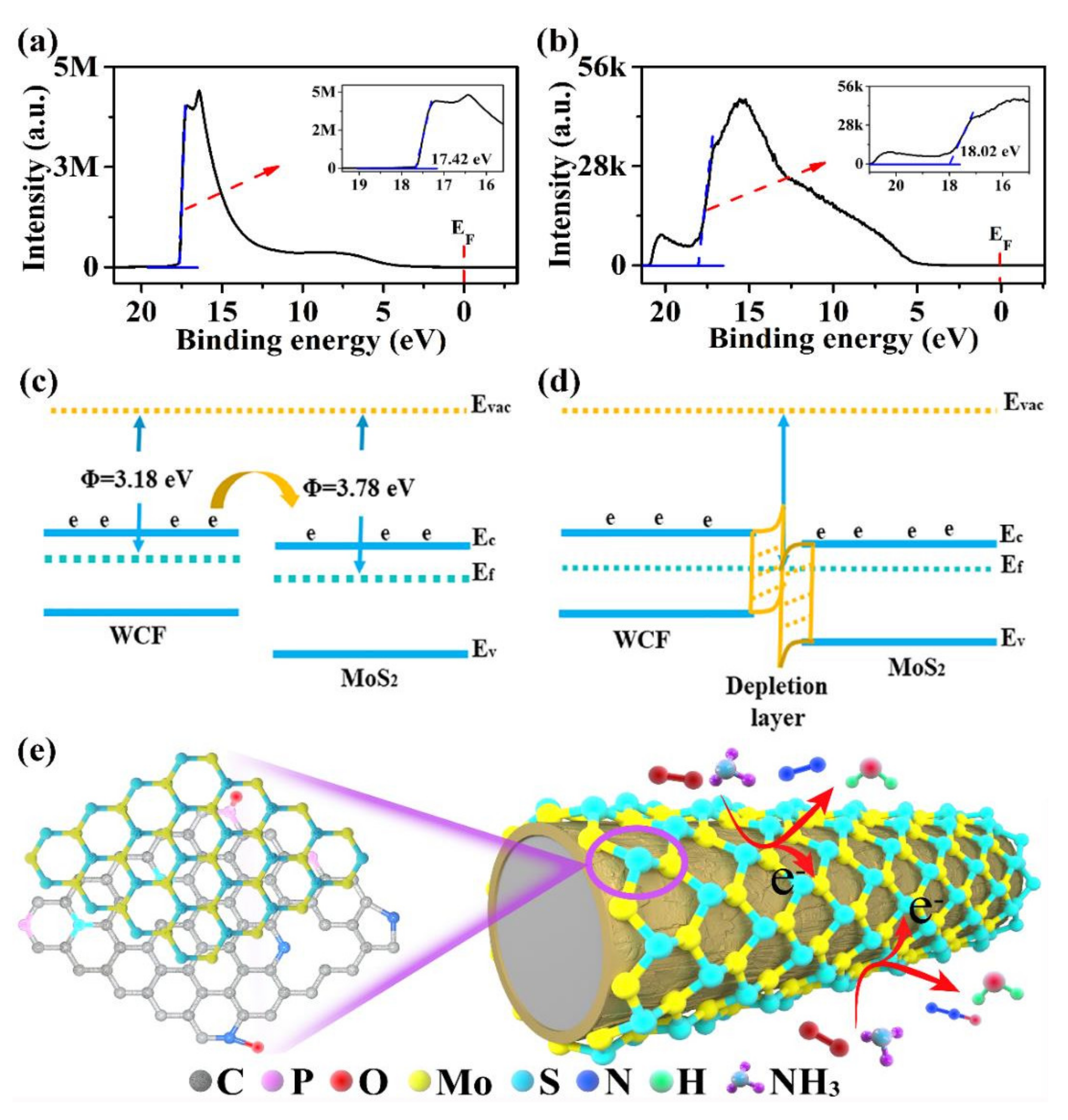
3.3. Possible Sensing Mechanisms of WCF–MoS2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schleussner, C.-F.; Rogelj, J.; Schaeffer, M.; Lissner, T.; Licker, R.; Fischer, E.M.; Knutti, R.; Levermann, A.; Frieler, K.; Hare, W. Science and policy characteristics of the Paris Agreement temperature goal. Nat. Clim. Chang。 2016, 6, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Holden, P.B.; Edwards, N.R.; Ridgwell, A.; Wilkinson, R.D.; Fraedrich, K.; Lunkeit, F.; Pollitt, H.E.; Mercure, J.-F.; Salas, P.; Lam, A.; et al. Climate–carbon cycle uncertainties and the Paris Agreement. Nat. Clim. Chang。 2018, 8, 609–613. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour。 Technol. 2019, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Porter, M.D.; Colosi, L.M. Is hydrothermal treatment coupled with carbon capture and storage an energy-producing negative emissions technology? Energy Convers. Manag. 2020, 203, 112252. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Cao, S.; Wu, Z.; Sun, Q.; Zhang, W.; Beysen, S.; Wang, S.; Shaymurat, T.; Zhang, M.; Duan, H. Gas sensing properties of cotton-based carbon fibers and ZnO/carbon fibers regulated by changing carbonization temperatures. Sens. Actuators B Chem. 2021, 337, 129818. [Google Scholar] [CrossRef]
- Alatzas, S.; Moustakas, K.; Malamis, D.; Vakalis, S. Biomass Potential from Agricultural Waste for Energetic Utilization in Greece. Energies 2019, 12, 1095. [Google Scholar] [CrossRef] [Green Version]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Le, Q.V.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Yi, Q.; Li, W.; Feng, J.; Xie, K. Carbon cycle in advanced coal chemical engineering. Chem. Soc. Rev. 2015, 44, 5409–5445. [Google Scholar] [CrossRef]
- Song, B.; Zhang, M.; Teng, Y.; Zhang, X.; Deng, Z.; Huo, L.; Gao, S. Highly selective ppb-level H2S sensor for spendable detection of exhaled biomarker and pork freshness at low temperature: Mesoporous SnO2 hierarchical architectures derived from waste scallion root. Sens. Actuators B Chem. 2020, 307, 127662. [Google Scholar] [CrossRef]
- Liu, W.; Tian, K.; He, Y.; Jiang, H.; Yu, H. High-Yield Harvest of Nanofibers/Mesoporous Carbon Composite by Pyrolysis of Waste Biomass and Its Application for High Durability Electrochemical Energy Storage. Environ. Sci. Technol. 2014, 48, 13951–13959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, F.; Zhu, S.; Lin, Y.; Meng, Z.; Yu, R.; Liu, X. Meso-Reconstruction of Wool Keratin 3D “Molecular Springs” for Tunable Ultra-Sensitive and Highly Recovery Strain Sensors. Small 2020, 16, 2000128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, N.; Wang, Q.; Wang, P.; Yuan, J.; Shen, J.; Fan, X. Disulfide bond reconstruction: A novel approach for grafting of thiolated chitosan onto wool. Carbohydr. Polym. 2019, 203, 369–377. [Google Scholar] [CrossRef]
- Macdermid-Watts, K.; Pradhan, R.; Dutta, A. Catalytic Hydrothermal Carbonization Treatment of Biomass for Enhanced Activated Carbon: A Review. Waste Biomass Valoriz. 2021, 12, 2171–2186. [Google Scholar] [CrossRef]
- Hu, X.; Nango, K.; Bao, L.; Li, T.; Hasan, M.D.M.; Li, C. High Yields of Solid Carbonaceous Materials from Biomass. Green Chem. 2019, 21, 1128–1140. [Google Scholar] [CrossRef]
- Snyder, B.F. Costs of biomass pyrolysis as a negative emission technology: A case study. Int. J. Energy Res. 2019, 43, 1232–1244. [Google Scholar] [CrossRef]
- Momodu, D.; Okafor, C.; Manyala, N.; Bello, A.; Zebazekana, M.G.; Ntsoenzok, E. Transformation of Plant Biomass Waste into Resourceful Activated Carbon Nanostructures for Mixed-Assembly Type Electrochemical Capacitors. Waste Biomass Valoriz. 2019, 10, 1741–1753. [Google Scholar] [CrossRef]
- Bi, H.; Yin, Z.; Cao, X.; Xie, X.; Tan, C.; Huang, X.; Chen, B.; Chen, F.; Yang, Q.; Bu, X.; et al. Carbon Fiber Aerogel Made from Raw Cotton: A Novel, Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Liu, W.; Yang, L.; Zhang, B.; Wang, L.; Ji, G.; Xu, Z. Biomass-Derived Porous Carbon-Based Nanostructures for Microwave Absorption. Nano-Micro Lett. 2019, 11, 77–93. [Google Scholar] [CrossRef] [Green Version]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S.; Tian, N.; Yan, F.; You, C.; Yang, Y. Carbon foams: 3D porous carbon materials holding immense potential. J. Mater. Chem. A 2020, 8, 23699–23723. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Yu, H. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Thompson, E.; Danks, A.E.; Bourgeois, L.; Schnepp, Z. Iron-catalyzed graphitization of biomass. Green Chem. 2015, 17, 551–556. [Google Scholar] [CrossRef]
- Shao, L.; Wu, Z.; Duan, H.; Talgar, S. Discriminative and rapid detection of ozone realized by sensor array of Zn2+ doping tailored MoS2 ultrathin nanosheets. Sens. Actuators B Chem. 2018, 258, 937–946. [Google Scholar] [CrossRef]
- He, M.; Lei, J.; Zhou, C.; Shi, H.; Sun, X.; Gao, B. Growth of vertical MoS2 nanosheets on carbon materials by chemical vapor deposition: Influence of substrates. Mater. Res. Express 2019, 6, 1150c1. [Google Scholar] [CrossRef]
- Livneh, T.; Sterer, E. Resonant Raman scattering at exciton states tuned by pressure and temperature in 2H-MoS2. Phys. Rev. B 2010, 81, 229–235. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Wang, X.; Zhang, X. Fabrication and performance of wool keratin/functionalized graphene oxide composite fibers. Mater. Today Sustain. 2019, 3–4, 100006. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Rasheed, A.; Ahmad, S. Preparation and characterization of wool fiber reinforced nonwoven alginate hydrogel for wound dressing. Cellulose 2021, 28, 7941–7951. [Google Scholar] [CrossRef]
- Pandey, S.; Fosso-Kankeu, E.; Spiro, M.J.; Waanders, F.; Kumar, N.; Ray, S.S.; Kim, J.; Kang, M. Equilibrium, kinetic, and thermodynamic studies of lead ion adsorption from mine wastewater onto MoS2-clinoptilolite composite. Mater. Today Chem. 2020, 18, 100376. [Google Scholar] [CrossRef]
- Alavijeh, M.S.; Maghsoudpour, A.; Khayat, M.; Rad, I.; Hatamie, S. Cobalt ferrite decoration of molybdenum disulfide nanosheets; development of a nanocomposite-mediated hyperthermia method. J. Mech. Sci. Technol. 2021, 35, 1319–1325. [Google Scholar] [CrossRef]
- Ferreira, E.H.; Moutinho, M.V.O.; Stavale, F.; Lucchese, M.M.; Capaz, R.B.; Achete, C.A.; Jorio, A. Evolution of the Raman spectra from single-, few-, and many-layer graphene with increasing disorder. Phys. Rev. 2010, 82, 125429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Han, W.; Wu, J.; Milana, S.; Lu, Y.; Li, Q.; Ferrari, A.C.; Tan, P. Raman spectroscopy of shear and Layer Breathing Modes in Multilayer MoS2. Phys. Rev. B 2013, 87, 1504–1509. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Hsu, Y.-T.; Chang, Y.; Chiu, C.; Hsu, C.-L.; Hsu, W.-T.; Chang, W.; He, H.; Li, L. Band gap-tunable molybdenum sulfide selenide monolayer alloy. Small 2014, 10, 2589–2594. [Google Scholar] [CrossRef]
- Cao, P.; Peng, J.; Liu, S.; Cui, Y.; Hu, Y.; Chen, B.; Li, J.; Zhai, M. Tuning the Composition and Structure of Amorphous Molybdenum Sulfide/Carbon Black Nanocomposites by Radiation Technique for Highly Efficient Hydrogen Evolution. Sci. Rep. 2017, 7, 16048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Wu, Z.; Duan, H.; Jia, D. Detection of Triacetone Triperoxide (TATP) Precursors with an Array of Sensors Based on MoS2/RGO Composites. Sensors 2019, 19, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Wu, Z.; Zhang, M.; Qin, Z.; Cao, S.; Zhong, F.; Li, S.; Duan, H.; Zhang, J. Improved Gas-Sensitive Properties by a Heterojunction of Hollow Porous Carbon Microtubes Derived from Sycamore Fibers. ACS Sustain. Chem. Eng. 2021, 9, 14345–14352. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Cheng, X.; Zhang, X.; Guo, C.; Xu, Y.; Gao, S.; Major, Z.; Zhao, H.; Huo, L. Fast detection of NO2 by porous SnO2 nanotoast sensor at low temperature. J. Hazard. Mater. 2021, 419, 126414. [Google Scholar] [CrossRef]
- Wu, X.; Qian, C.; Wu, H.; Xu, L.; Bu, L.; Piao, Y.; Diao, G.; Chen, M. Gestated Uniform Yolk-Shell Sn@N-Doped Hollow Mesoporous Carbon Spheres with Buffer Space for Boosting Lithium Storage Performance. Chem. Commun. 2020, 56, 7629–7632. [Google Scholar] [CrossRef]
- Wu, H.; Xia, L.; Ren, J.; Zheng, Q.; Xu, C.; Lin, D. A high-efficiency N/P co-doped graphene/CNT@porous carbon hybrid matrix as cathode host for high performance lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 20458–20472. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Gopalakrishnan, K.; Govindaraj, A. Synthesis, properties and applications of graphene doped with boron, nitrogen and other elements. Nanotoday 2014, 9, 324–343. [Google Scholar] [CrossRef]
- Haridas, V.; Sukhananazerin, A.; Sneha, J.M.; Pullithadathil, B.; Narayanan, B. α-Fe2O3 loaded less-defective graphene sheets as chemiresistive gas sensor for selective sensing of NH3. Appl. Surf. Sci. 2020, 517, 146158. [Google Scholar] [CrossRef]
- Su, P.-G.; Yang, L. NH3 gas sensor based on Pd/SnO2/RGO ternary composite operated at room-temperature. Sens. Actuators B Chem. 2016, 223, 202–208. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hu, N.; Wang, Y.; Zhang, Y.; Zhou, Z. Ammonia gas sensors based on chemically reduced graphene oxide sheets self-assembled on Au electrodes. Nanoscale Res. Lett. 2014, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinbumrung, A.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Optical and ammonia-sensing properties of SnO2 nanoparticles synthesized using a 900 W microwave. Jpn. J. Appl. Phys. 2016, 55, 085001. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Li, Z.; Su, H.; Alkurd, N.R.; Zhou, W.; Wang, L.; Du, B.; Tang, Y.; Ao, D.; et al. Surface acoustic wave ammonia sensor based on ZnO/SiO2 composite film. J. Hazard. Mater. 2015, 285, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Fu, T. CuS-doped CuO nanoparticles sensor for detection of H2S and NH3 at room temperature. Electrochim. Acta 2013, 112, 230–235. [Google Scholar] [CrossRef]
- Tohidi, S.; Parhizkar, M.; Bidadi, H.; Mohammad-Rezaei, R. High-performance chemiresistor-type NH3 gas sensor based on three-dimensional reduced graphene oxide/polyaniline hybrid. Nanotechnology 2020, 31, 415501. [Google Scholar] [CrossRef]
- Wang, X.; Gu, D.; Li, X.; Lin, S.; Zhao, S.; Rumyantseva, M.N.; Gaskov, A.M. Reduced graphene oxide hybridized with WS2 nanoflakes based heterojunctions for selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2019, 282, 290–299. [Google Scholar] [CrossRef]
- Garg, N.; Kumar, M.; Kumari, N.; Deep, A.; Sharma, A. Chemoresistive Room-Temperature Sensing of Ammonia Using Zeolite Imidazole Framework and Reduced Graphene Oxide (ZIF-67/rGO) Composite. ACS Omega 2020, 5, 27492–27501. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, C.; Zu, B.; Li, Y.; Dou, X. Contactless and Rapid Discrimination of Improvised Explosives Realized by Mn2+ Doping Tailored ZnS Nanocrystals. Adv. Funct. Mater. 2016, 26, 4578–4586. [Google Scholar] [CrossRef]
- Liu, X.; He, J.; Tang, D.; Liu, Q.; Wen, J.; Yu, W.; Lu, Y.; Zhu, D.; Liu, W.; Cao, P.; et al. Band alignment of atomic layer deposited high-k Al2O3/multilayer MoS2 interface determined by X-ray photoelectron spectroscopy. J. Alloys Compd. 2015, 650, 502–507. [Google Scholar] [CrossRef]
- Tao, J.; Yu, X.; Liu, Q.; Liu, G.; Tang, H. Internal electric field induced S–scheme heterojunction MoS2/CoAl LDH for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 585, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, M.; Liu, L.; Ruan, X.; Yan, J.; Zhao, W.; Yun, J.; Wang, Y.; Qin, S.; Zhang, T. Novel SnO2@ZnO hierarchical nanostructures for highly sensitive and selective NO2 gas sensing. Sens. Actuators B Chem. 2018, 257, 714–727. [Google Scholar] [CrossRef]

| Materials | Concentration (ppm) | Temperature (°C) | Response (%) | Response Time (s) | Recovery Time (s) | Limit of Detection (LoD; ppb) | Reference |
|---|---|---|---|---|---|---|---|
| Py-rGO | 50 | 200 | - | 134 | 310 | - | [44] |
| SnO2 | 1055 | 350 | - | 9 | 37 | - | [45] |
| SnO2/Pd/RGO | 5 | RT | 7.6 | 25,200 | 3000 | - | [43] |
| ZnO/SiO2 | 50 | RT | - | - | - | - | [46] |
| CuS | - | - | - | 55 | 43 | - | [47] |
| 3D RGO/PANI | 50 | RT | 11 | 370 | 675 | - | [48] |
| α-Fe2O3/graphene | 10 | 250 | 13.5 | 152 | 648 | - | [42] |
| rGO-WS2 | 10 | 33.5 | 121 | 60 | 300 | - | [49] |
| ZIF-67/rGO | 50 | RT | 477 | 46.4 | 66.5 | 74 | [50] |
| CMTs | 50 | RT | 362 | 16 | 4 | 63 | [37] |
| ZnO/CMTs | 2091 | 14 | 4 | 9 | |||
| WCF-MoS2 | 3 | RT | 8 | 16 | 5 | 19.1 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Wu, Z.; Qin, Z.; Chen, F.; Lv, C.; Zhang, M.; Shaymurat, T.; Duan, H. Wool-Based Carbon Fiber/MoS2 Composite Prepared by Low-Temperature Catalytic Hydrothermal Method and Its Application in the Field of Gas Sensors. Nanomaterials 2022, 12, 1105. https://doi.org/10.3390/nano12071105
Xia Y, Wu Z, Qin Z, Chen F, Lv C, Zhang M, Shaymurat T, Duan H. Wool-Based Carbon Fiber/MoS2 Composite Prepared by Low-Temperature Catalytic Hydrothermal Method and Its Application in the Field of Gas Sensors. Nanomaterials. 2022; 12(7):1105. https://doi.org/10.3390/nano12071105
Chicago/Turabian StyleXia, Yidan, Zhaofeng Wu, Zhangjie Qin, Fengjuan Chen, Changwu Lv, Min Zhang, Talgar Shaymurat, and Haiming Duan. 2022. "Wool-Based Carbon Fiber/MoS2 Composite Prepared by Low-Temperature Catalytic Hydrothermal Method and Its Application in the Field of Gas Sensors" Nanomaterials 12, no. 7: 1105. https://doi.org/10.3390/nano12071105






