Preparation of Hollow Niobium Oxide Nanospheres with Enhanced Catalytic Activity for Oxidative Desulfurization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Catalyst
2.2.1. Preparation of 3D Mesoporous Carbon
- First, 70 mg of lysine was dissolved in 69.3 g of deionized water. Subsequently, 5.4 g of tetraethyl orthosilicate was added to the lysine solution and then transferred to a water bath and stirred at 60 °C for 24 h. The stirring speed was maintained at 500 rpm.
- A total of 10 g of the above sol was added to 40 g of water. Subsequently, 10.4 g of tetraethyl orthosilicate was added to the lysine solution and then transferred to a water bath and stirred at 60 °C for 48 h. The stirring speed was maintained at 500 rpm.
- The obtained silica nanosphere sols were poured into a Petri dish and dried in an oven at 70 °C for 12 h to allow the spheres to settle and remove water. The obtained solids were calcined in air at 550 °C for 6 h to remove the organic components and to obtain silica templates.
- Histidine (0.75~2.0 g) and silica nanospheres (1 g) were mixed evenly by grinding. The mixture was subsequently transferred to a tube furnace and directly heated to 900 °C for 3 h under a nitrogen atmosphere.
- The calcined solid was ground into powder and dispersed in potassium hydroxide solution (70 mL, 6 mol/L). Then, the mixed solution was transferred to a 100 mL Teflon-lined autoclave and heated to 180 °C for 48 h. The mixed solution was naturally cooled to room temperature and filtered, washed with deionized water until the filtrate was nearly neutral and then dried at 60 °C for 12 h. Finally, 3D mesoporous carbon was obtained, denoted as 3DmC-900.
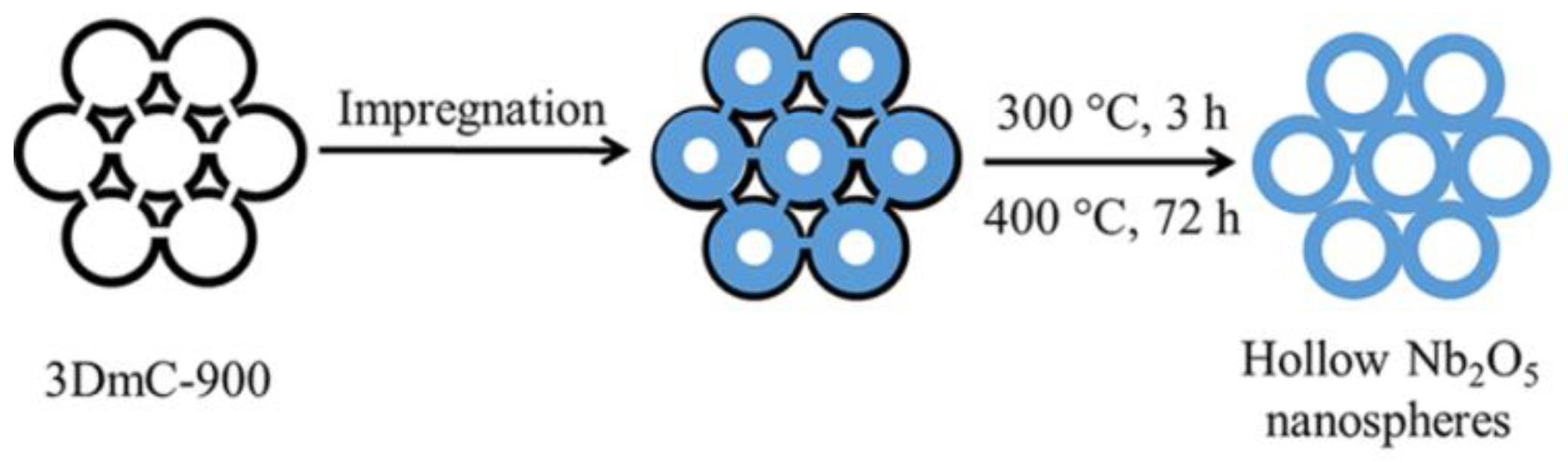
2.2.2. Preparation of Hollow Niobium Oxide Nanospheres
- First, 0.1 g of 3DmC-900 was added to an excess of niobium pentachloride ethanol solution (0.75 g/mL), stirred until a uniform mixture was obtained and then allowed to stand for 12 h at room temperature.
- The mixed solution was filtered under vacuum to remove excess precursors, and then the obtained solid powder was dried in a vacuum drying oven for 3 h.
- The dried solid powder was calcined at 300 °C under a nitrogen atmosphere for 3 h to convert niobium pentachloride into niobium oxide and then calcined at 400 °C under an air atmosphere for 72 h to remove the carbon template. Finally, the hollow niobium oxide nanospheres were obtained.
2.3. Characterization of Materials
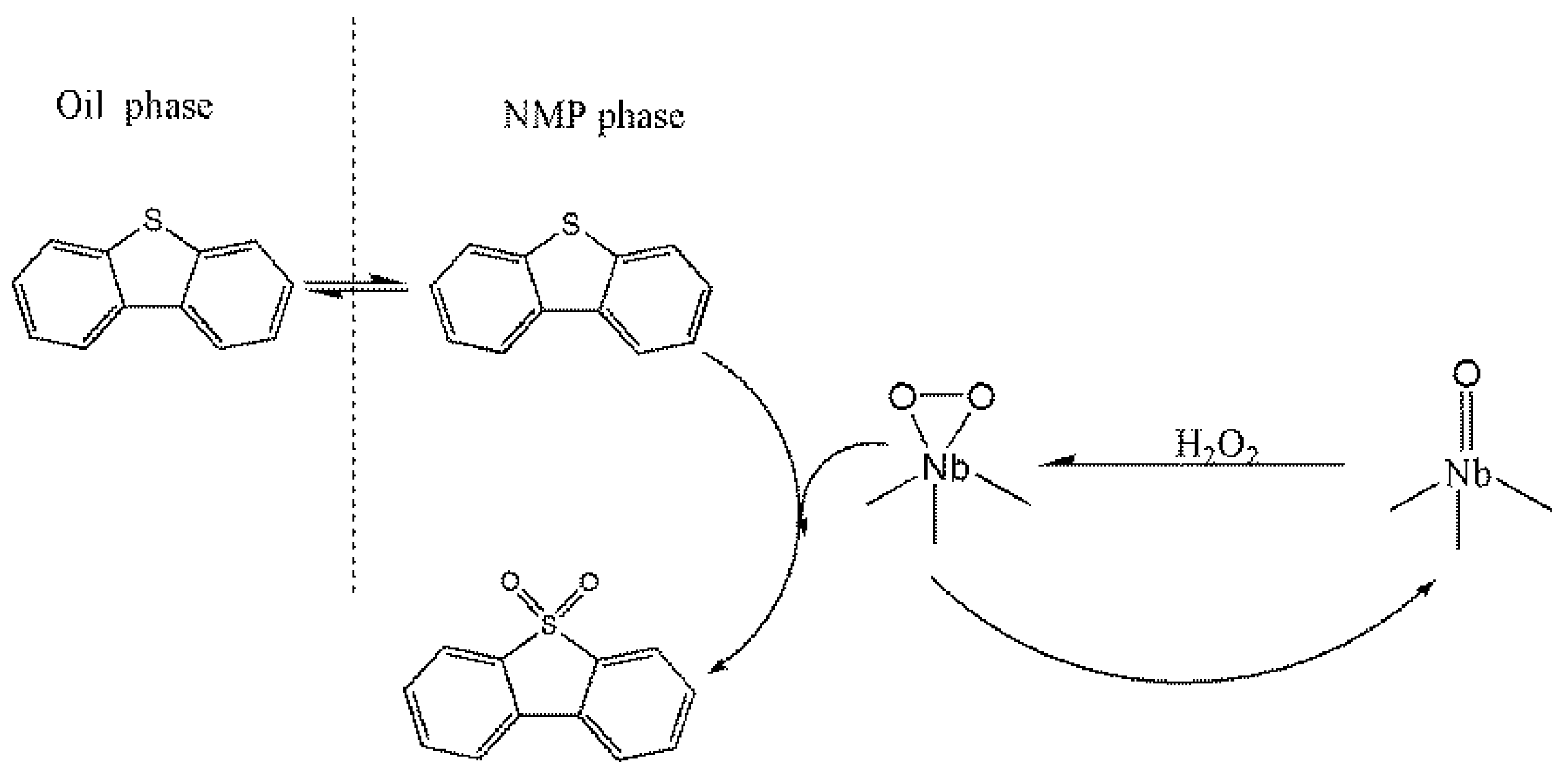
2.4. Catalytic Oxidation Desulfurization Experiments
3. Results and Discussion
3.1. Morphology and Structure of the Catalysts
3.1.1. 3D Mesoporous Carbon
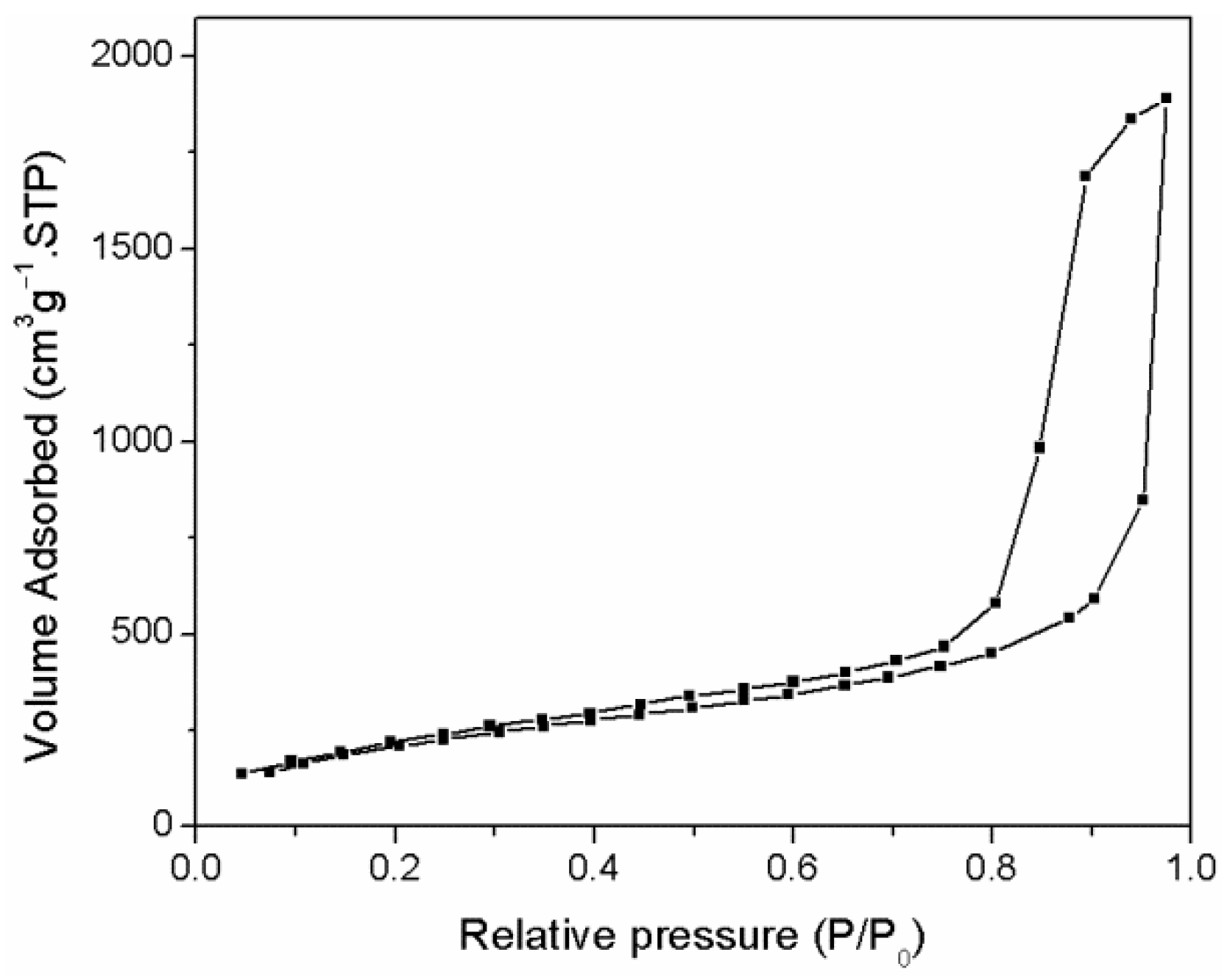
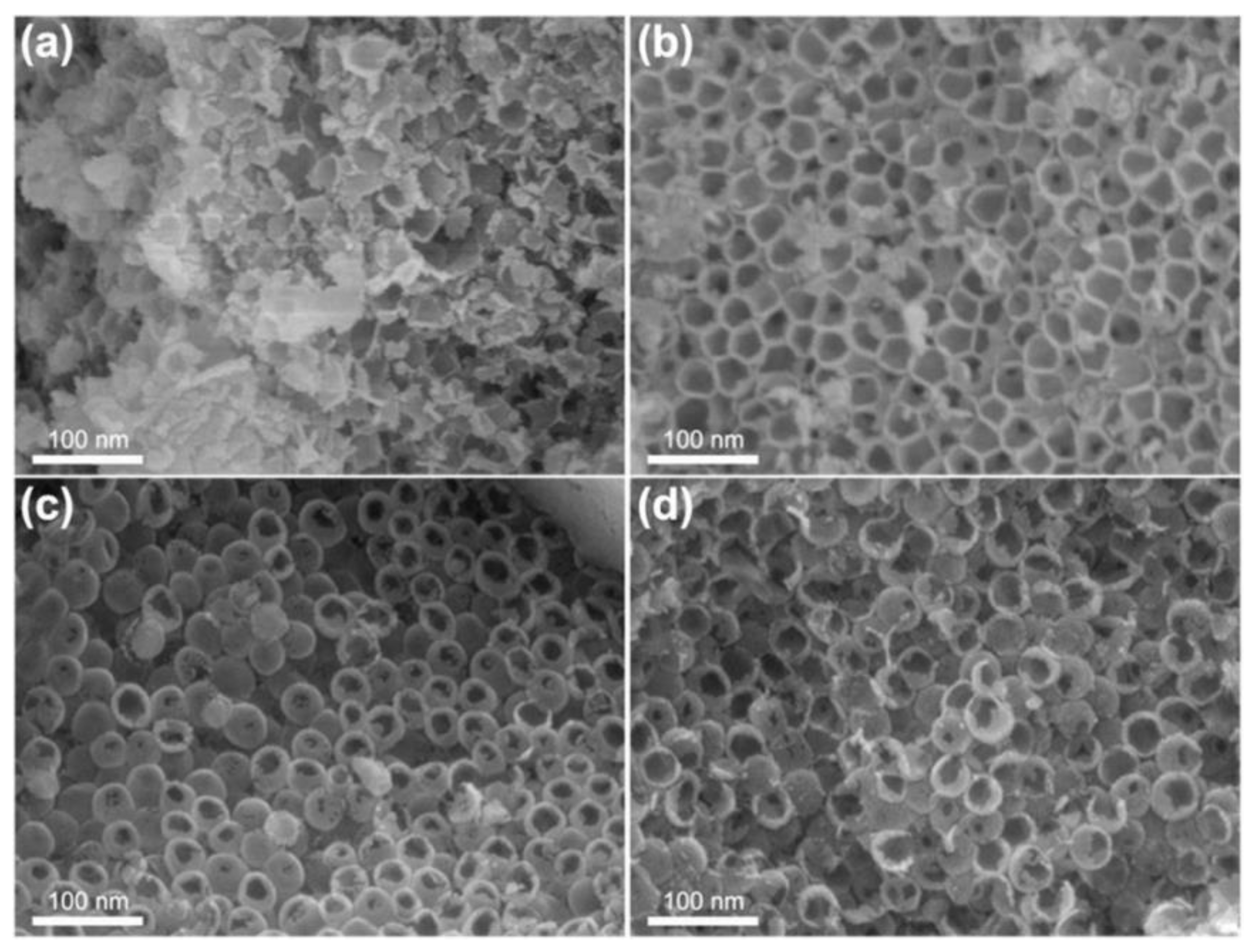
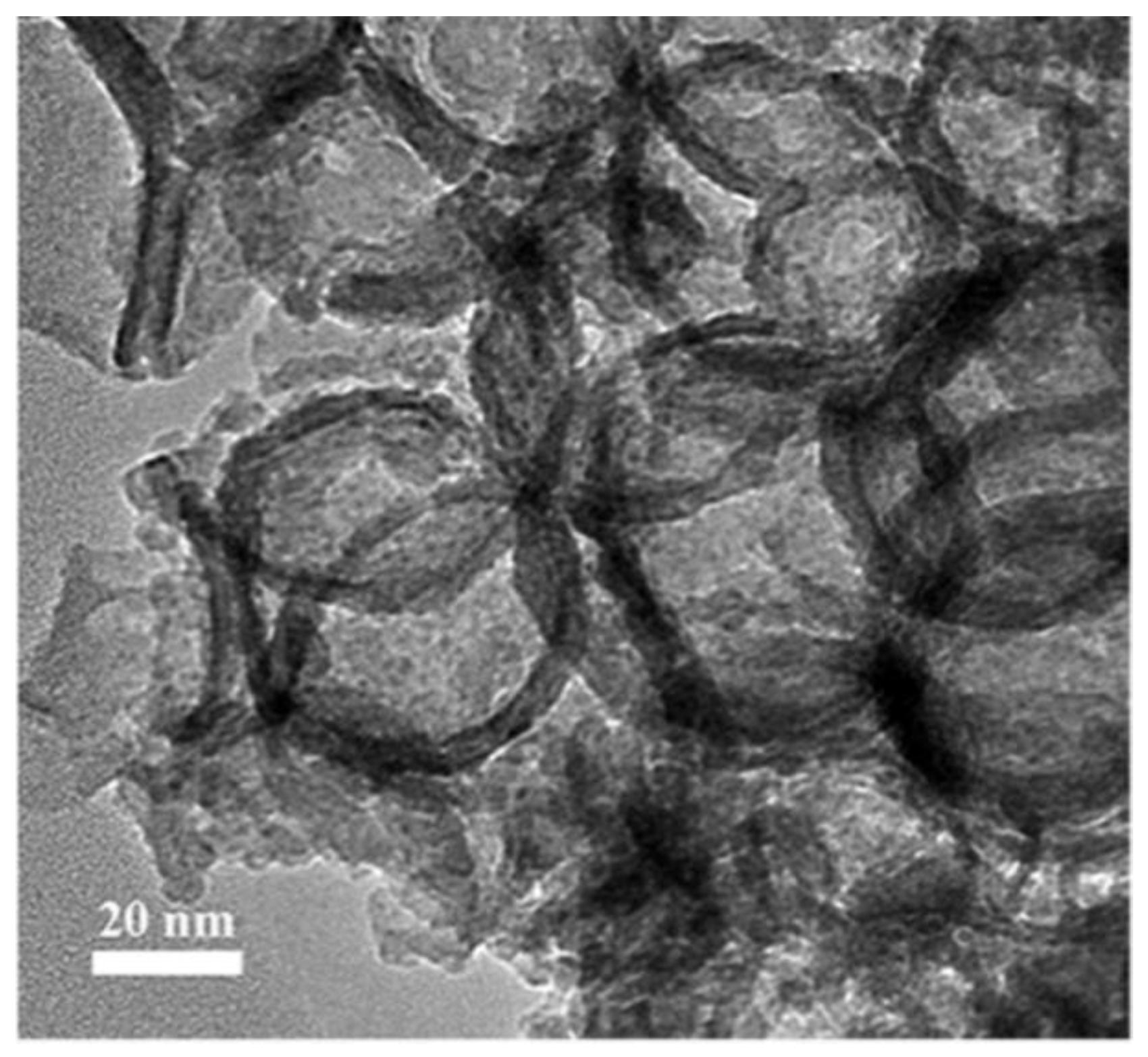
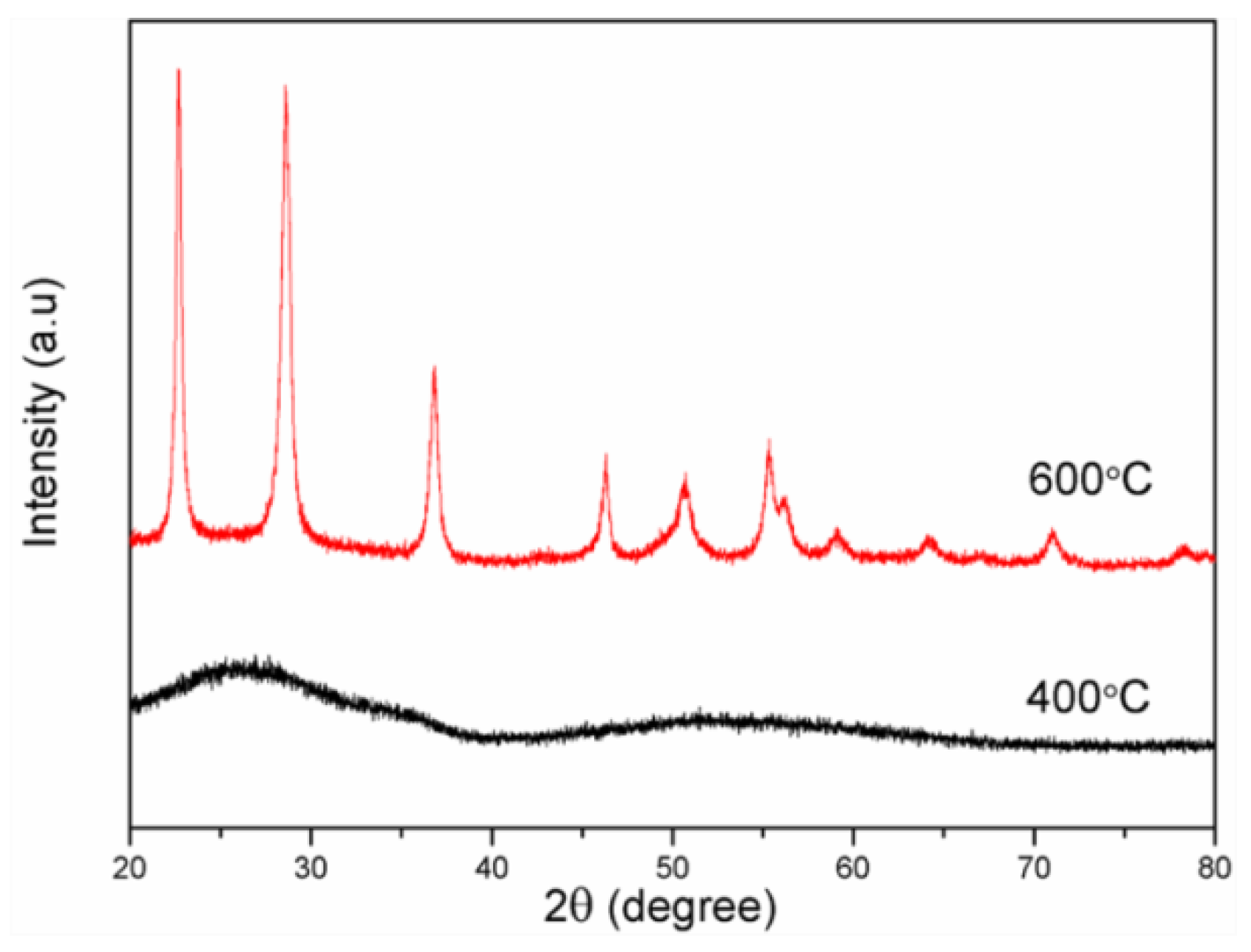
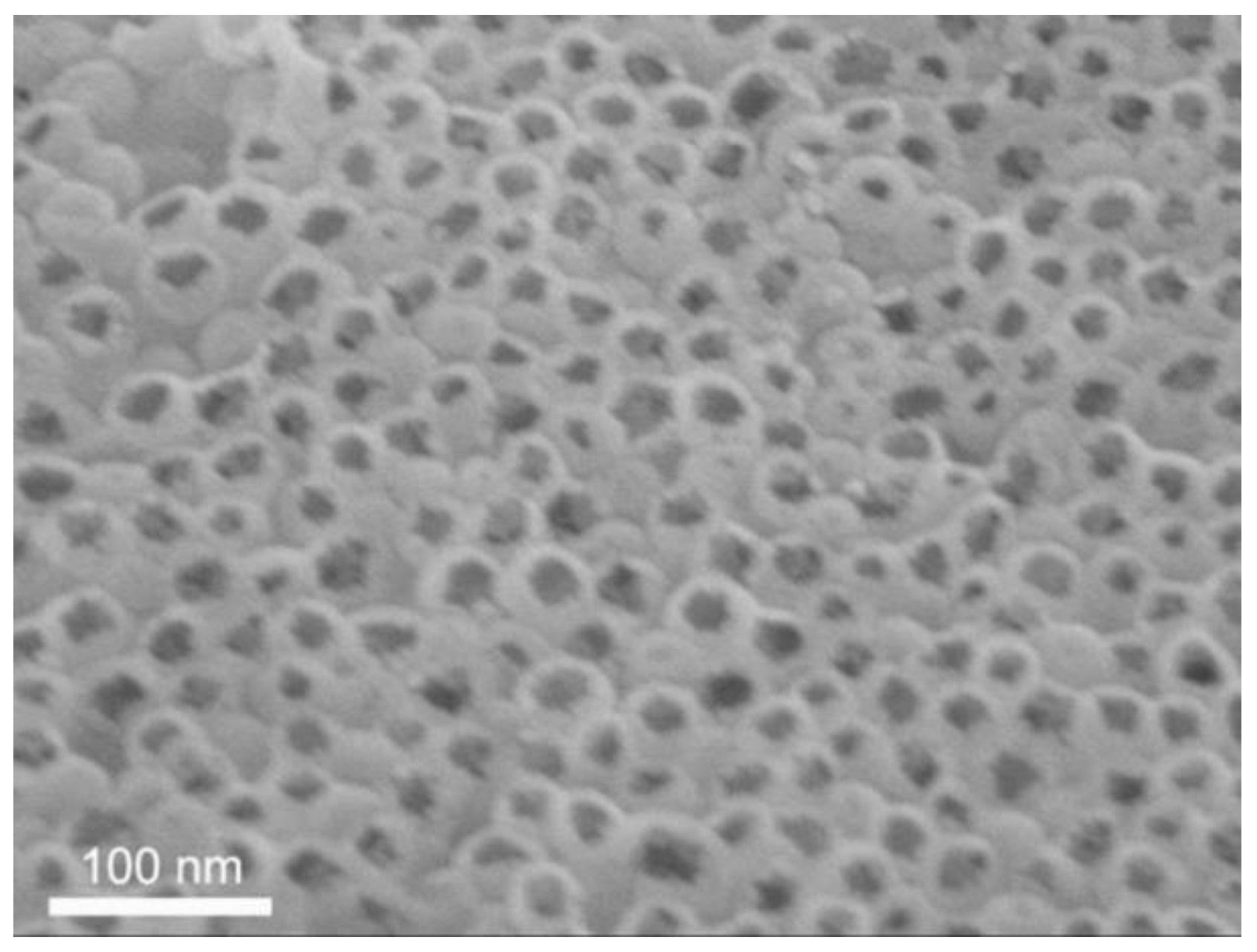
3.1.2. Hollow Nb2O5 Nanospheres
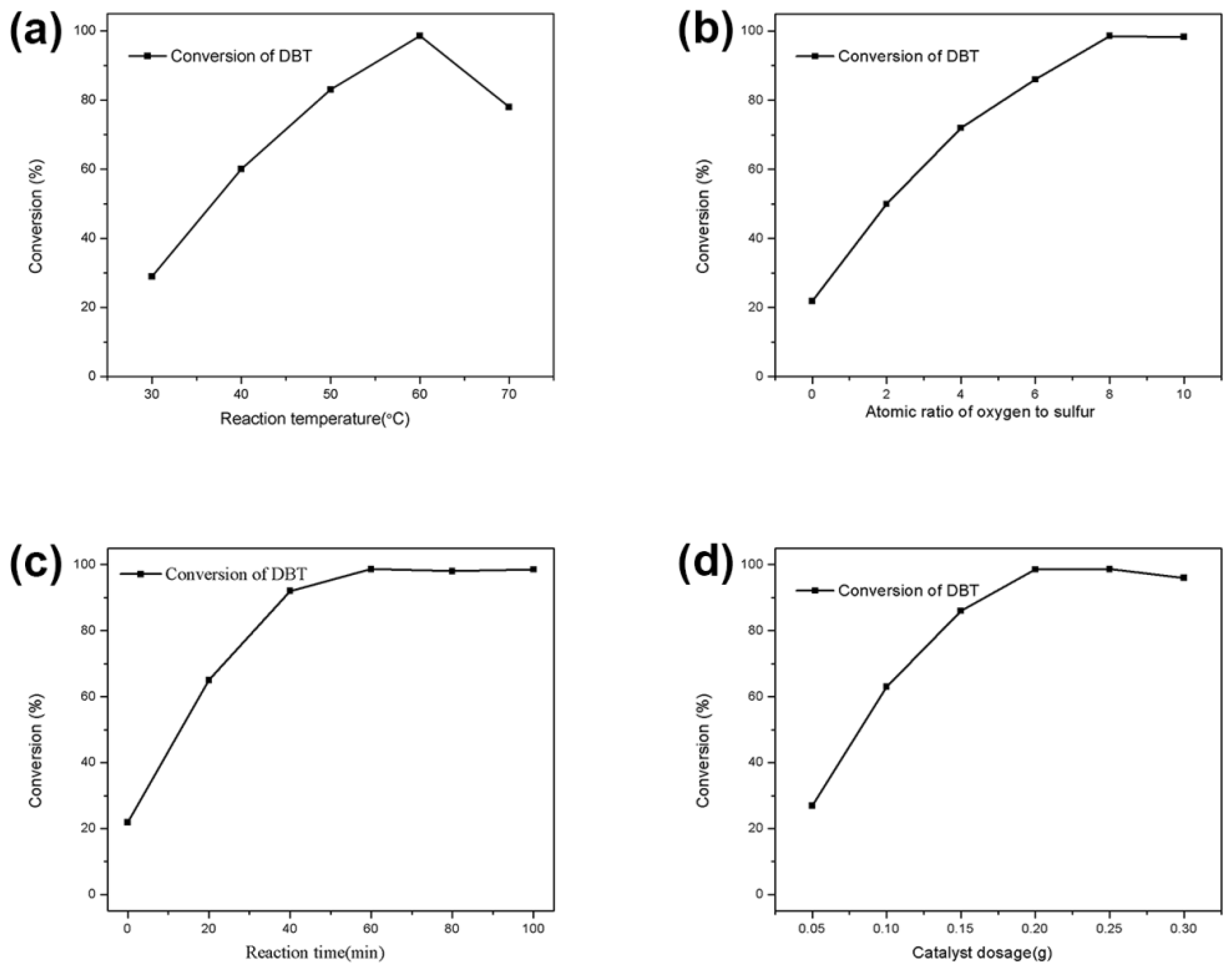
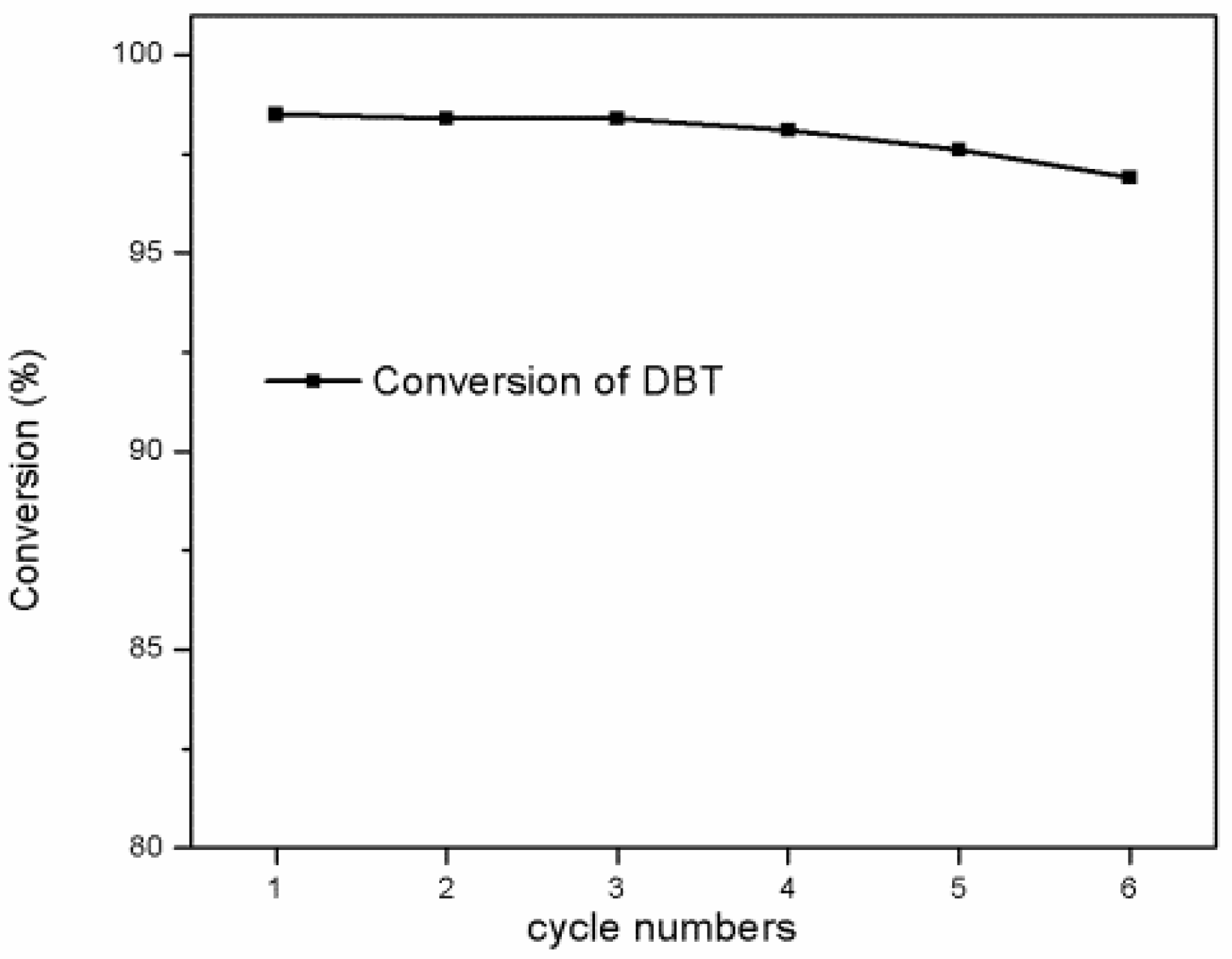
3.2. Oxidative Desulfurization Performance of the Catalyst
4. Conclusions
- (1)
- Three-dimensional mesoporous carbon materials were prepared by using histidine as the carbon source and silica microspheres as the hard template by adjusting the relative amounts of silica and histidine. Analysis using the nitrogen/adsorption measurement method showed that the three-dimensional mesoporous carbon materials have a typical porous structure with a high specific surface area of 893 m2·g−1.
- (2)
- Hollow niobium oxide nanospheres were successfully synthesized by using 3D mesoporous carbon as the hard template and niobium pentachloride as the precursor with a vacuum-assisted method. The prepared product has a hollow spherical structure with an outer diameter of about 45 nm, which is consistent with the spherical pore size of the carbon template. The calculated specific surface area is as high as 134.3 m2·g−1.
- (3)
- The oxidative desulfurization performance of the hollow niobium oxide nanosphere catalyst was investigated via the removal of DBT in simulated oil with hydrogen peroxide as the oxidant. Under suitable reaction conditions, the DBT conversion rate of the simulated oil was as high as 98.5%. The higher catalytic activity of the hollow niobium oxide nanosphere catalyst benefits from their larger specific surface area, unique hollow structure and hierarchical porous structure.
- (4)
- The reusability and stability of the catalyst were evaluated by cycling tests, and a possible reaction mechanism is proposed.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bertleff, B.; Claußnitzer, J.; Korth, W.; Wasserscheid, P.; Jess, A.; Albert, J. Extraction coupled oxidative desulfurization of fuels to sulfate and water-soluble sulfur compounds using polyoxometalate catalysts and molecular oxygen. ACS Sustain. Chem. Eng. 2017, 5, 4110–4118. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Synthesis of silica@C-dots/phosphotungstates core-shell microsphere for effective oxidative-adsorptive desulfurization of dibenzothiophene with less oxidant. Appl. Catal. B Environ. 2018, 234, 247–259. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, X.; Chen, J.; Wang, H. Electron-hole interactions in choline-phosphotungstic acid boosting molecular oxygen activation for fuel desulfurization. Appl. Catal. B Environ. 2019, 248, 573–586. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Helveg, S.; Lægsgaard, E.; Stensgaard, I.; Clausen, B.S.; Topsøe, H.; Besenbacher, F. Atomic-scale structure of Co–Mo–S nanoclusters in hydrotreating catalysts. J. Catal. 2001, 197, 1–5. [Google Scholar] [CrossRef]
- Chandra Srivastava, V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Gu, Q.; Wen, G.; Ding, Y.; Wu, K.-H.; Chen, C.; Su, D. Reduced graphene oxide: A metal-free catalyst for aerobic oxidative desulfurization. Green Chem. 2017, 19, 1175–1181. [Google Scholar] [CrossRef]
- Bösmann, A.; Datsevich, L.; Jess, A.; Lauter, A.; Schmitz, C.; Wasserscheid, P. Deep desulfurization of diesel fuel by extraction with ionic liquids. Chem. Commun. 2001, 23, 2494–2495. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Template-derived mesoporous carbons with highly dispersed transition metals as media for the reactive adsorption of dibenzothiophene. Langmuir 2007, 23, 6033–6041. [Google Scholar] [CrossRef]
- Jia, Y.; Li, G.; Ning, G. Efficient oxidative desulfurization (ODS) of model fuel with H2O2 catalyzed by MoO3/γ-Al2O3 under mild and solvent free conditions. Fuel Process. Technol. 2011, 92, 106–111. [Google Scholar] [CrossRef]
- Mohebali, G.; Ball, A.S. Biodesulfurization of diesel fuels–past, present and future perspectives. Int. Biodeterior. Biodegrad. 2016, 110, 163–180. [Google Scholar] [CrossRef]
- Li, L.; Deng, J.; Yu, R.; Chen, J.; Wang, Z.; Xing, X. Niobium pentoxide hollow nanospheres with enhanced visible light photocatalytic activity. J. Mater. Chem. A 2013, 1, 11894. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Block copolymer templating syntheses of mesoporous metal oxides with large ordering lengths and semicrystalline framework. Chem. Mater. 1999, 11, 2813–2826. [Google Scholar] [CrossRef]
- Tian, B.; Liu, X.; Solovyov, L.A.; Liu, Z.; Yang, H.; Zhang, Z.; Xie, S.; Zhang, F.; Tu, B.; Yu, C.; et al. Facile synthesis and characterization of novel mesoporous and mesorelief oxides with gyroidal structures. J. Am. Chem. Soc. 2003, 126, 865–875. [Google Scholar] [CrossRef]
- Roggenbuck, J.; Tiemann, M. Ordered mesoporous magnesium oxide with high thermal stability synthesized by exotemplating using CMK-3 carbon. J. Am. Chem. Soc. 2005, 127, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Shi, L.; Yoshida, H.; Li, M.; Zou, X. Synthesis of hollow spherical tantalum oxide nanoparticles and their photocatalytic activity for hydrogen production. J. Solid State Chem. 2013, 199, 15–20. [Google Scholar] [CrossRef]
- Jiao, F.; Bruce, P.G. Two- and three-dimensional mesoporous iron oxides with microporous walls. Angew. Chem. Int. Ed. 2004, 43, 5958–5961. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lee, E.J.; Choi, W.H.; Yoo, W.C.; Bang, J.H. Three-dimensionally ordered mesoporous carbons activated by hot ammonia treatment as high-performance anode materials in lithium-ion batteries. New J. Chem. 2015, 39, 6178–6185. [Google Scholar] [CrossRef]
- Vu, A.; Li, X.; Phillips, J.; Han, A.; Smyrl, W.H.; Bühlmann, P.; Stein, A. Three-dimensionally ordered mesoporous (3DOm) carbon materials as electrodes for electrochemical double-layer capacitors with ionic liquid electrolytes. Chem. Mater. 2013, 25, 4137–4148. [Google Scholar] [CrossRef]
- Sun, Y.; Piao, J.; Hu, L.; Bin, D.; Lin, X.; Duan, S.; Cao, A.; Wan, L. Controlling the reaction of nanoparticles for hollow metal oxide nanostructures. J. Am. Chem. Soc. 2018, 140, 9070–9073. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Xie, S.; Yue, B.; Qian, L.; Feng, S.; Tsang, S.C.; Li, Y.; He, H. Crystalline three-dimensional cubic mesoporous niobium oxide. CrystEngComm 2010, 12, 344–347. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, F.; Yin, X.; Song, Y.; Xiang, T.; Wang, J. Arginine-assisted hydrothermal synthesis of urchin-like Nb2O5 nanostructures composed of nanowires and their application in cyclohexanone ammoximation. J. Phys. Chem. C 2018, 122, 2155–2164. [Google Scholar] [CrossRef]
- Ye, J.; Wen, J.; Zhao, D.; Zhang, P.; Li, A.; Zhang, L.; Zhang, H.; Wu, M. Macroporous 3D carbon-nitrogen (CN) confined MoOx catalyst for enhanced oxidative desulfurization of dibenzothiophene. Chin. Chem. Lett. 2020, 31, 2819–2824. [Google Scholar] [CrossRef]
- Xiao, X.; Zhong, H.; Zheng, C.; Lu, M.; Zuo, X.; Nan, J. Deep oxidative desulfurization of dibenzothiophene using a flower-like WO3· H2O catalyst in an organic biphasic system. Chem. Eng. J. 2016, 304, 908–916. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, W.; Li, H.; Zhang, J.; Zou, F.; Shi, H.; Yan, Y. Oxidative desulfurization of fuels catalyzed by V2O5 in ionic liquids at room temperature. Energy Fuels 2009, 23, 5929–5933. [Google Scholar] [CrossRef]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]




| No. | Catalyst | DBT Conversion (%) |
|---|---|---|
| 1 | Commercial niobium oxide | 50.3 |
| 2 | Hollow niobium oxide | 98.5 |
| Catalyst | T (°C) | Time (min) | DBT Conversion (%) | Ref. |
|---|---|---|---|---|
| MoOx@CN-1 (C/A = 1) | 50 | 80 | 97.9 | [22] |
| MoO3/γ-Al2O3 | 60 | 15 | 100 | [9] |
| WO3 H2O | 70 | 60 | 95.1 | [23] |
| V2O5 | 30 | 150 | 98.7 | [24] |
| Hollow Nb2O5 nanospheres | 60 | 60 | 98.5 | This work |
| No. | Sulfur Compounds | Conversion (%) |
|---|---|---|
| 1 | BT | 84.3 |
| 2 | 4, 6-DMDBT | 32.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ren, L.; Li, Z.; Xin, F. Preparation of Hollow Niobium Oxide Nanospheres with Enhanced Catalytic Activity for Oxidative Desulfurization. Nanomaterials 2022, 12, 1106. https://doi.org/10.3390/nano12071106
Wang Y, Ren L, Li Z, Xin F. Preparation of Hollow Niobium Oxide Nanospheres with Enhanced Catalytic Activity for Oxidative Desulfurization. Nanomaterials. 2022; 12(7):1106. https://doi.org/10.3390/nano12071106
Chicago/Turabian StyleWang, Yong, Lei Ren, Zifeng Li, and Feng Xin. 2022. "Preparation of Hollow Niobium Oxide Nanospheres with Enhanced Catalytic Activity for Oxidative Desulfurization" Nanomaterials 12, no. 7: 1106. https://doi.org/10.3390/nano12071106
APA StyleWang, Y., Ren, L., Li, Z., & Xin, F. (2022). Preparation of Hollow Niobium Oxide Nanospheres with Enhanced Catalytic Activity for Oxidative Desulfurization. Nanomaterials, 12(7), 1106. https://doi.org/10.3390/nano12071106







