Recent Trends and Advances of Co3O4 Nanoparticles in Environmental Remediation of Bacteria in Wastewater
Abstract
:1. Introduction
2. Synthesis
2.1. Physicochemical Methods
2.2. Biological Methods
2.2.1. Bio-Synthesis Using Plant Extracts
2.2.2. Bio-Synthesis Using Microbes
2.2.3. Characterization Methods
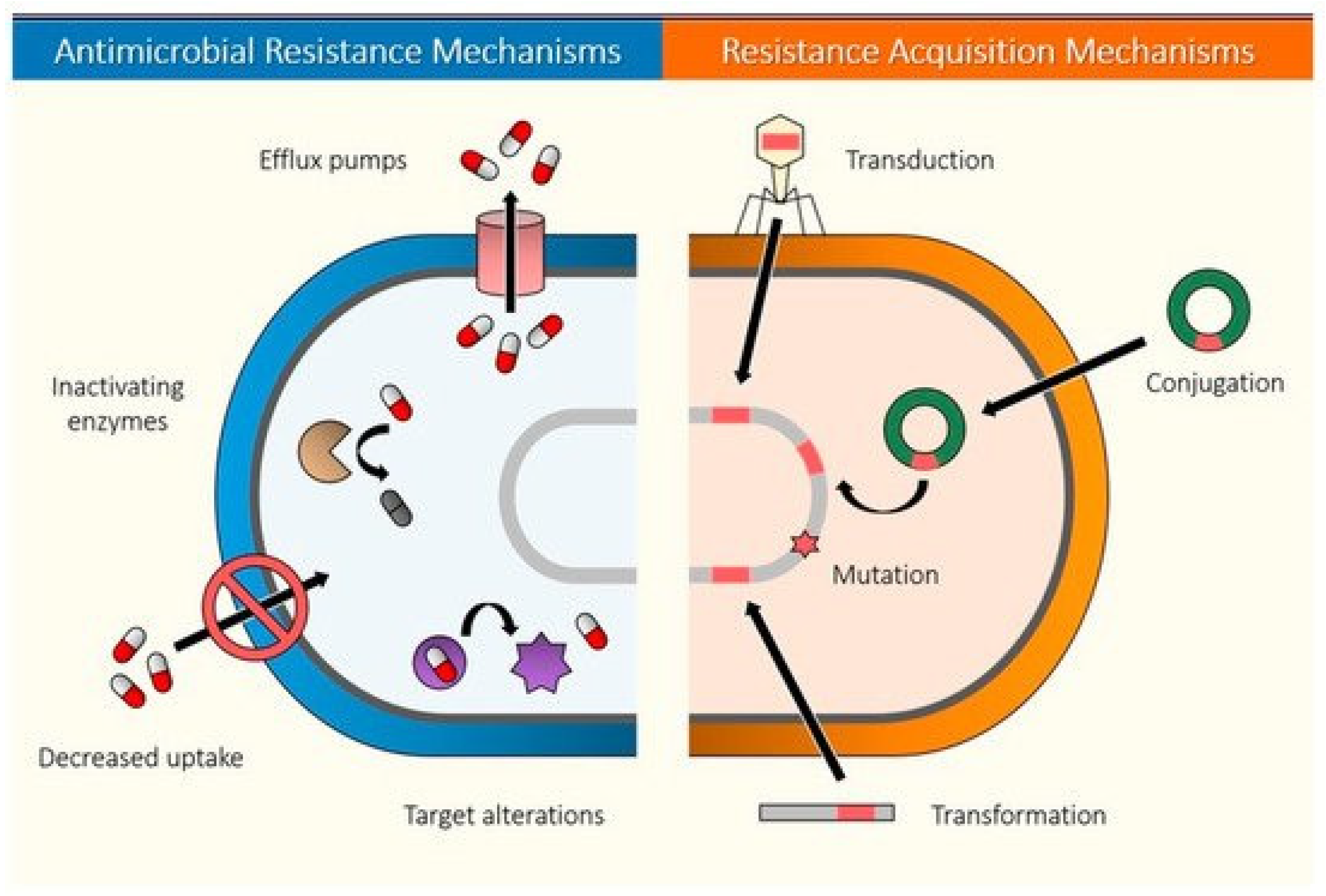
3. Antibacterial Resistance and Antibacterial Activity
3.1. Antibacterial Resistance
3.2. Nanoparticles and Wastewater Remediation
3.2.1. Nanoparticles and Remediation of Bacteria
3.2.2. Metal Oxide Nanoparticles and Remediation of Bacteria
3.3. Cobalt Oxide-Based Nanoparticles and Their Antibacterial Applications
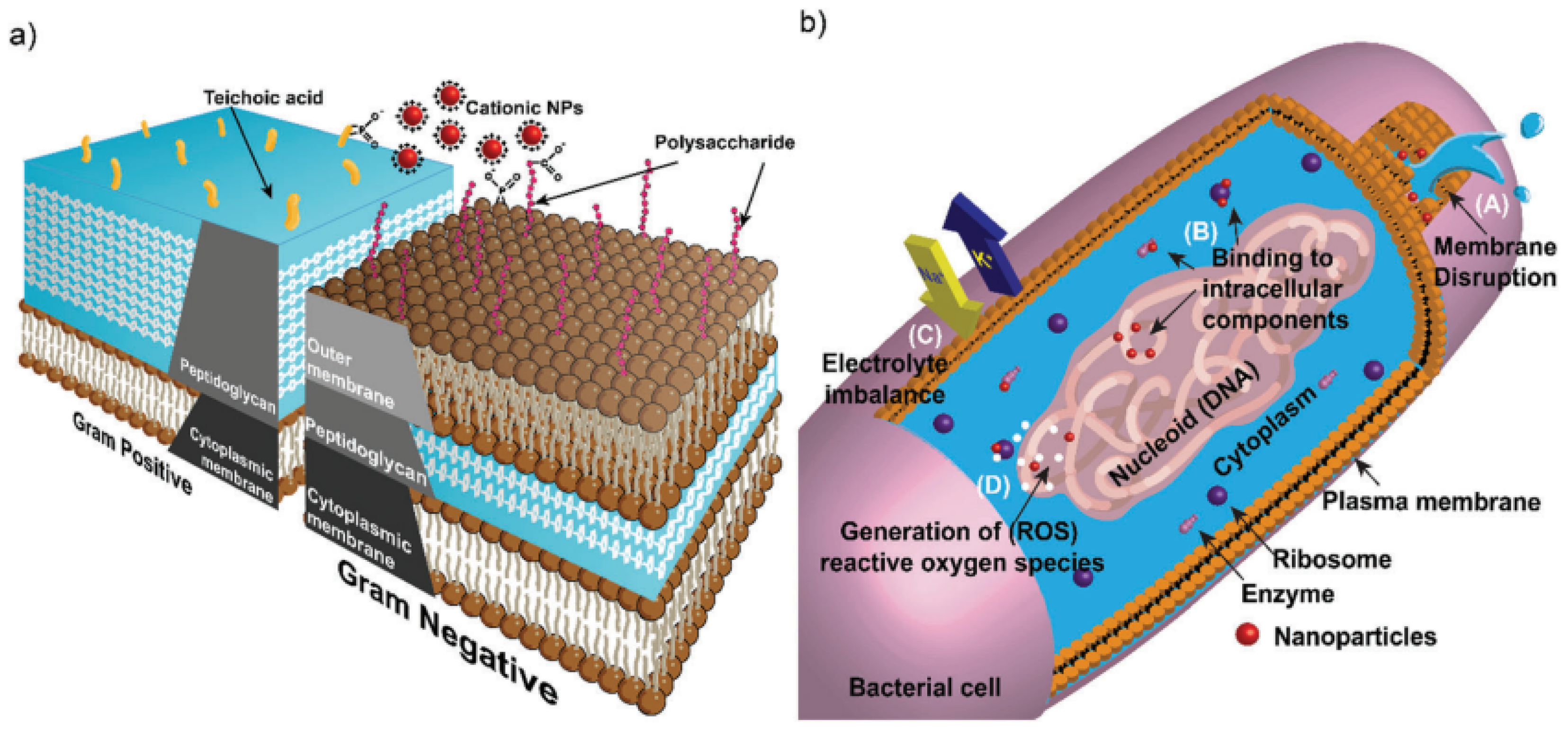
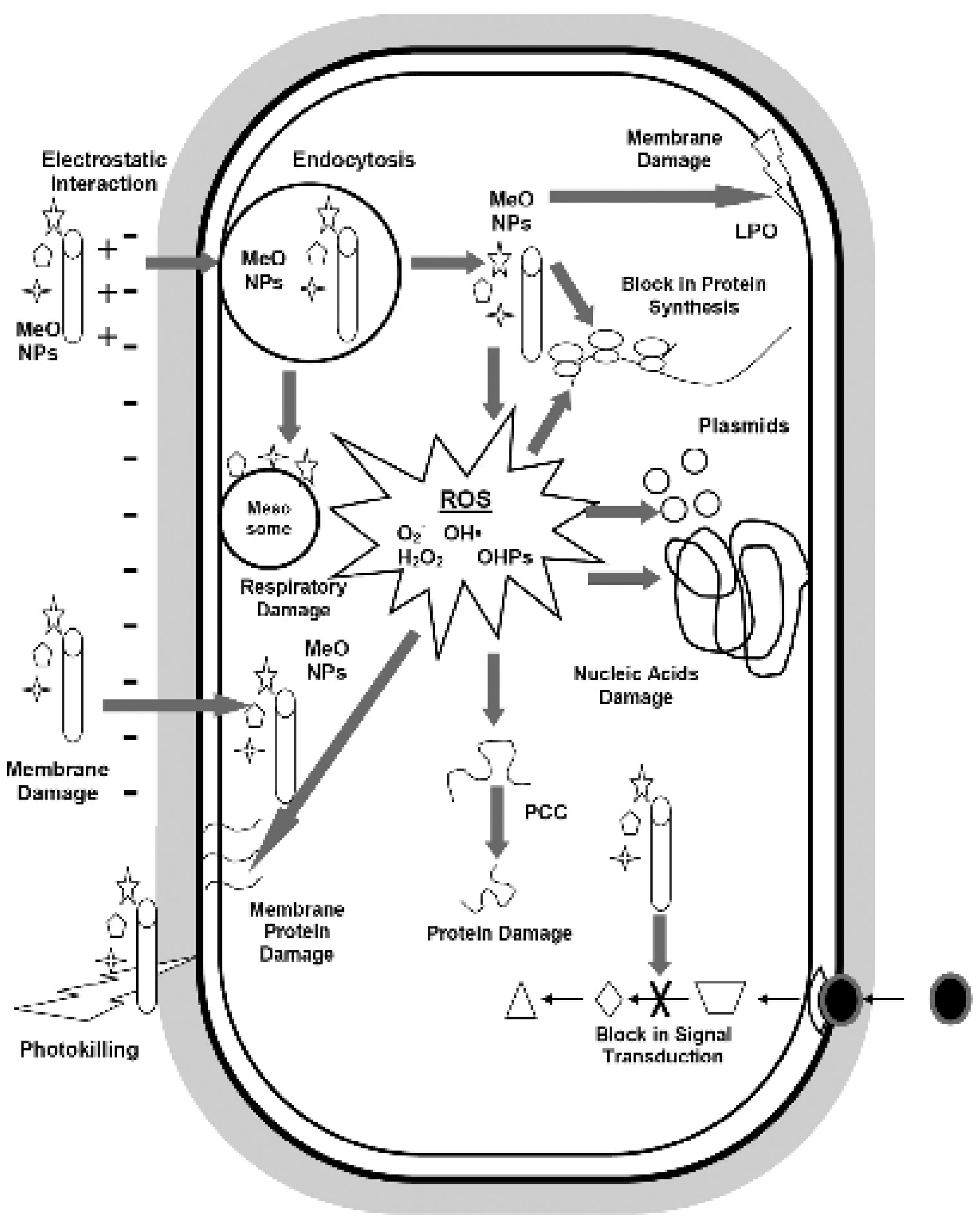
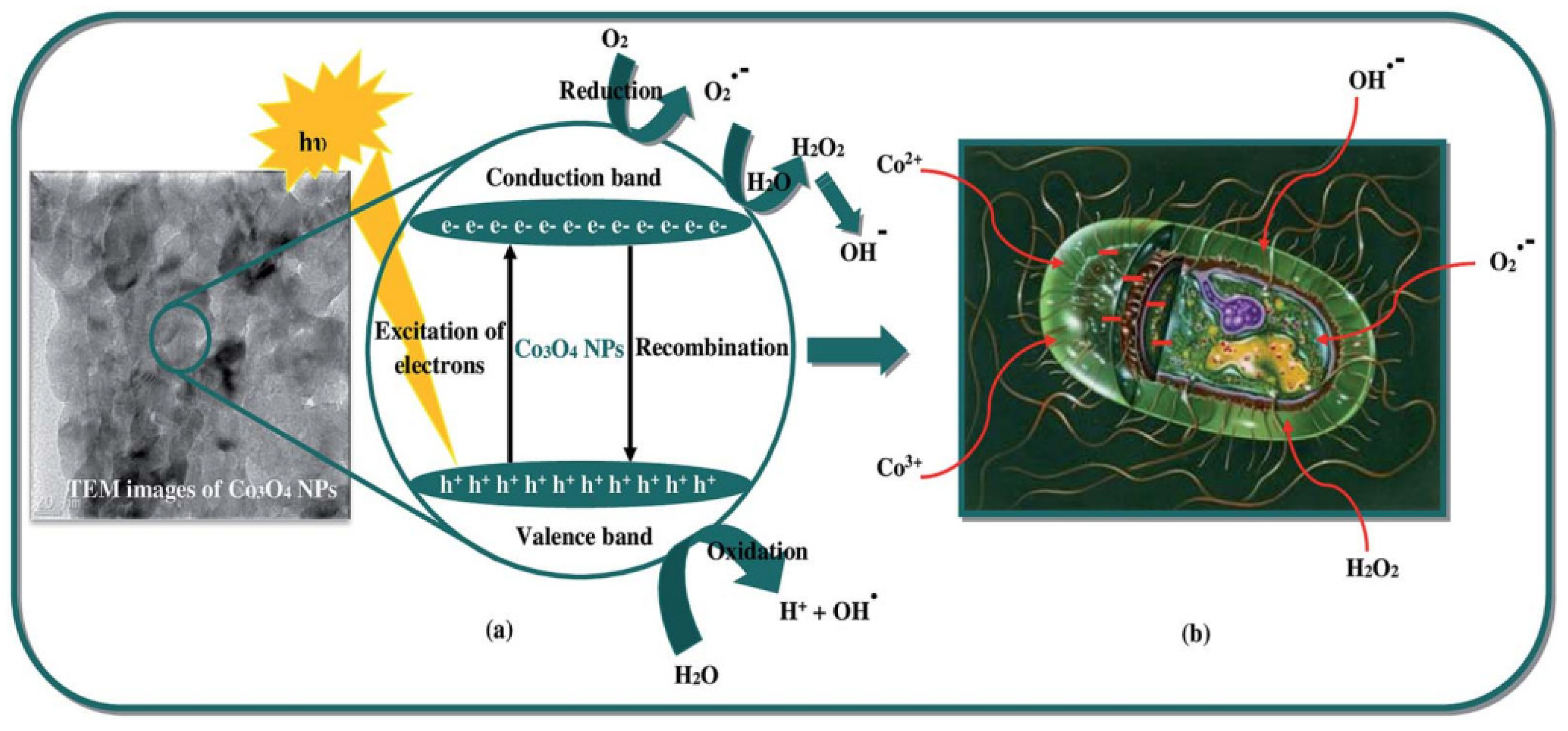
3.3.1. Antibacterial Activity of Cobalt Oxide Nanoparticles and Mechanisms
3.3.2. Antibacterial Activity of Cobalt Oxide-Based Nanocomposites and Mechanisms
4. Environmental Impact
5. Future Directions and Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| CLSI | Clinical and Laboratory Standards Institute |
| CV | Cyclic voltammetry |
| DLS | Dynamic light scattering |
| DNA | Deoxyribonucleic acid |
| DRS | Diffuse reflectance spectroscopy |
| DTA | Differential thermal analysis |
| EDS | Energy-dispersive x-ray spectroscopy |
| FESEM | Field emission scanning electron microscope |
| Fluor | Fluorescent spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| HRSEM | High-resolution scanning electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| IR | Infra-red spectroscopy |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MLC | Minimum lethal concentration |
| PL | Photoluminescence spectroscopy |
| PSA | Particle size analysis |
| Raman | Raman spectroscopy |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| UNEP | United Nations Environment Program |
| UV | Ultraviolet spectroscopy |
| UV-Vis | Ultraviolet-visible spectroscopy |
| UV-Vis-NIR | Ultraviolet-visible-near-infrared spectroscopy |
| VSM | Vibrating sample magnetometry |
| WHO | World Health Organization |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction spectroscopy |
References
- Jesudoss, S.K.; Judith Vijaya, J.; Iyyappa Rajan, P.; Kaviyarasu, K.; Sivachidambaram, M.; John Kennedy, L.; Al-Lohedan, H.A.; Jothiramalingam, R.; Munusamy, M.A. High Performance Multifunctional Green Co3O4 Spinel Nanoparticles: Photodegradation of Textile Dye Effluents, Catalytic Hydrogenation of Nitro-Aromatics and Antibacterial Potential. Photochem. Photobiol. Sci. 2017, 16, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Sustainable Synthesis of Cobalt and Cobalt Oxide Nanoparticles and Their Catalytic and Biomedical Applications. Green Chem. 2020, 22, 2643–2661. [Google Scholar] [CrossRef]
- Omran, B.A.; Nassar, H.N.; Younis, S.A.; El-Salamony, R.A.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Novel Mycosynthesis of Cobalt Oxide Nanoparticles Using Aspergillus Brasiliensis ATCC 16404—Optimization, Characterization and Antimicrobial Activity. J. Appl. Microbiol. 2020, 128, 438–457. [Google Scholar] [CrossRef] [PubMed]
- Raveau, B.; Seikh, M.M. Charge Ordering in Cobalt Oxides: Impact on Structure, Magnetic and Transport Properties. Z. Fur. Anorg. Und. Allg. Chem. 2015, 641, 1385–1394. [Google Scholar] [CrossRef]
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green Fabrication of Co and Co3O4 nanoparticles and Their Biomedical Applications: A Review. Open Life Sci. 2021, 16, 14–30. [Google Scholar] [CrossRef]
- Ma, Z. Cobalt Oxide Catalysts for Environmental Remediation. Curr. Catal. 2014, 3, 15–26. [Google Scholar] [CrossRef]
- Hafeez, M.; Shaheen, R.; Akram, B.; Zain-Ul-Abdin; Haq, S.; Mahsud, S.; Ali, S.; Khan, R.T. Green Synthesis of Cobalt Oxide Nanoparticles for Potential Biological Applications. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Varghese, B.; Teo, C.H.; Zhu, Y.; Reddy, M.V.; Chowdari, B.V.; Wee, A.T.S.; Tan, V.B.C.; Lim, C.T.; Sow, C.-H. Co3O4 Nanostructures with Different Morphologies and Their Field-Emission Properties. Adv. Funct. Mater. 2007, 17, 1932–1939. [Google Scholar] [CrossRef]
- Han, L.; Yang, D.-P.; Liu, A. Leaf-Templated Synthesis of 3D Hierarchical Porous Cobalt Oxide Nanostructure as Direct Electrochemical Biosensing Interface with Enhanced Electrocatalysis. Biosens. Bioelectron. 2015, 63, 145–152. [Google Scholar] [CrossRef]
- Dewi, N.O.M.; Yulizar, Y.; Bagus Apriandanu, D.O. Green Synthesis of Co3O4 Nanoparticles Using Euphorbia Heterophylla L. Leaves Extract: Characterization and Photocatalytic Activity. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012105. [Google Scholar] [CrossRef]
- Anuradha, C.T.; Raji, P. Effect of Annealing Temperature on Antibacterial, Antifungal and Structural Properties of Bio-Synthesized Co3O4 Nanoparticles Using Hibiscus Rosa-Sinensis. Mater. Res. Express 2019, 6, 095063. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kaviyarasu, K.; Arularasu, M.V.; Kanimozhi, K.; Ramalingam, G. Structural and Morphological Properties of Co3O4 Nanostructures: Investigation of Low Temperature Oxidation for Photocatalytic Application for Waste Water Treatment. Surf. Interfaces 2019, 17, 100369. [Google Scholar] [CrossRef]
- Shaheen, I.; Ahmad, K.S. Green Synthesis of Doped Co3O4 Nanocatalysts Using Organic Template for Fast Azo Dye Degradation from Aqueous Environment. J. Chem. Technol. Biotechnol. 2020, 95, 2898–2910. [Google Scholar] [CrossRef]
- Saeed, M.; Haq, A.U.; Muneer, M.; Usman, M.; Naqvi, S.A.R.; Adeel, M.; Nisar, A. Helianthus Annuus Assisted Green Synthesis of Co3O4 and Ag-Co3O4 and Evaluation of Their Catalytic Activities toward Photodegradation of Crystal Violet Dye. Environ. Prog. Sustain. Energy 2021, 40, e13591. [Google Scholar] [CrossRef]
- Khalil, A.; Ali, N.; Khan, A.; Asiri, A.M.; Kamal, T. Catalytic Potential of Cobalt Oxide and Agar Nanocomposite Hydrogel for the Chemical Reduction of Organic Pollutants. Int. J. Biol. Macromol. 2020, 164, 2922–2930. [Google Scholar] [CrossRef]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.E.; Behroozian, S. An Ancient Solution to a Modern Problem. Mol. Microbiol. 2020, 113, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Molina, D.; Mang, P.; Schmitt, H.; Chifiriuc, M.C.; Radon, K.; Wengenroth, L. Do Wastewater Treatment Plants Increase Antibiotic Resistant Bacteria or Genes in the Environment? Protocol for a Systematic Review. Syst. Rev. 2019, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Manaia, C.M.; Rocha, J.; Scaccia, N.; Marano, R.; Radu, E.; Biancullo, F.; Cerqueira, F.; Fortunato, G.; Iakovides, I.C.; Zammit, I.; et al. Antibiotic Resistance in Wastewater Treatment Plants: Tackling the Black Box. Environ. Int. 2018, 115, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Madhura, L.; Singh, S.; Kanchi, S.; Sabela, M.; Bisetty, K. Inamuddin Nanotechnology-Based Water Quality Management for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 65–121. [Google Scholar] [CrossRef]
- Naseem, T.; Waseem, M. A Comprehensive Review on the Role of Some Important Nanocomposites for Antimicrobial and Wastewater Applications. Int. J. Environ. Sci. Technol. 2022, 19, 2221–2246. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Adefisoye, M.A.; Okoh, A.I. Nanotechnology as a Viable Alternative for the Removal of Antimicrobial Resistance Determinants from Discharged Municipal Effluents and Associated Watersheds: A Review. J. Environ. Manag. 2020, 275, 111234. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, Characteristics, and Applications in Analytical and Other Sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, J.D.; Gray, H.B.; Winkler, J.R.; Müller, A.M. Co3O4 Nanoparticle Water-Oxidation Catalysts Made by Pulsed-Laser Ablation in Liquids. ACS Catalysis 2013, 3, 2497–2500. [Google Scholar] [CrossRef]
- Ghaem, E.N.; Dorranian, D.; Sari, A.H. External Magnetic Field Effects on the Characteristics of Cobalt Nanoparticles Prepared by Pulsed Laser Ablation. Opt. Quantum Electron. 2021, 53, 1–14. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, C.-K.; Han, L.-L.; Yang, J.; Du, X.-W. Top-Down Preparation of Active Cobalt Oxide Catalyst. ACS Catal. 2016, 6, 6699–6703. [Google Scholar] [CrossRef]
- Mahmoud, K.H. Synthesis and Spectroscopic Investigation of Cobalt Oxide Nanoparticles. Polym. Compos. 2016, 37, 1881–1885. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Durosinmi, L.M.; Oluwafemi, O.S.; Olayanju, D.S.; Akinola, A.S.; Obisesan, O.R.; Akinyele, O.F.; Ajayeoba, T.A. Potential of Cobalt and Cobalt Oxide Nanoparticles as Nanocatalyst towards Dyes Degradation in Wastewater. Nano-Struct. Nano-Objects 2020, 21, 100405. [Google Scholar] [CrossRef]
- Chinnathambi, A.; Nasif, O.; Alharbi, S.A.; Khan, S.S. Enhanced Optoelectronic Properties of Multifunctional MnFe2O4 Nanorods Decorated Co3O4 Nanoheterostructure: Photocatalytic Activity and Antibacterial Behavior. Mater. Sci. Semicond. Process. 2021, 134, 105992. [Google Scholar] [CrossRef]
- Dogra, V.; Kaur, G.; Jindal, S.; Kumar, R.; Kumar, S.; Singhal, N.K. Bactericidal Effects of Metallosurfactants Based Cobalt Oxide/Hydroxide Nanoparticles against Staphylococcus aureus. Sci. Total Environ. 2019, 681, 350–364. [Google Scholar] [CrossRef]
- Mayakannan, M.; Gopinath, S.; Vetrivel, S. Synthesis and Characterization of Antibacterial Activities Nickel Doped Cobalt Oxide Nano Particles. Mater. Chem. Phys. 2020, 242, 122282. [Google Scholar] [CrossRef]
- Ahmadov, T.O.; Durmus, Z.; Baykal, A.; Kavas, H. A Simple Approach for the Synthesis of Co3O4 Nanocrystals. Inorg. Mater. 2011, 47, 426–430. [Google Scholar] [CrossRef]
- Hosein, H.-A.; Strongin, D.R.; Allen, M.; Douglas, T. Iron and Cobalt Oxide and Metallic Nanoparticles Prepared from Ferritin. Langmuir 2004, 20, 10283–10287. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.A.; Awwad, N.S.; Ibrahium, H.A.; Ahmed, M.K. Casted Polymeric Blends of Carboxymethyl Cellulose/Polyvinyl Alcohol Doped with Gold Nanoparticles via Pulsed Laser Ablation Technique; Morphological Features, Optical and Electrical Investigation. Radiat. Phys. Chem. 2020, 177, 109155. [Google Scholar] [CrossRef]
- Gholami, A.; Moghadassi, A.R.; Hosseini, S.M.; Shabani, S.; Gholami, F. Preparation and Characterization of Polyvinyl Chloride Based Nanocomposite Nanofiltration-Membrane Modified by Iron Oxide Nanoparticles for Lead Removal from Water. J. Ind. Eng. Chem. 2014, 20, 1517–1522. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal Oxide Nanoparticles as Antimicrobial Agents: A Promise for the Future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Ahmed, J.; Ahmad, T.; Ramanujachary, K.V.; Lofland, S.E.; Ganguli, A.K. Development of a Microemulsion-Based Process for Synthesis of Cobalt (Co) and Cobalt Oxide (Co3O4) Nanoparticles from Submicrometer Rods of Cobalt Oxalate. J. Colloid Interface Sci. 2008, 321, 434–441. [Google Scholar] [CrossRef]
- Shah, R.M.; Malherbe, F.; Eldridge, D.; Palombo, E.A.; Harding, I.H. Physicochemical Characterization of Solid Lipid Nanoparticles (SLNs) Prepared by a Novel Microemulsion Technique. J. Colloid Interface Sci. 2014, 428, 286–294. [Google Scholar] [CrossRef]
- Morsy, S.M.I.; Shaban, S.A.; Ibrahim, A.M.; Selim, M.M. Characterization of Cobalt Oxide Nanocatalysts Prepared by Microemulsion with Different Surfactants, Reduction by Hydrazine and Mechanochemical Method. J. Alloys Compd. 2009, 486, 83–87. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Biologically Synthesized Metal Nanoparticles: Recent Advancement and Future Perspectives in Cancer Theranostics. Future Sci. OA 2017, 3, FSO203. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.; Kumar, J.; Kumar, A.; Sharma, Y.C. Facile and Green Synthesis of Highly Dispersed Cobalt Oxide (Co3O4) Nano Powder: Characterization and Screening of Its Eco-Toxicity. Adv. Powder Technol. 2018, 29, 2583–2590. [Google Scholar] [CrossRef]
- Bhushan, M.; Kumar, Y.; Periyasamy, L.; Viswanath, A.K. Antibacterial Applications of α-Fe2O3/Co3O4 Nanocomposites and Study of Their Structural, Optical, Magnetic and Cytotoxic Characteristics. Appl. Nanosci. Switz. 2018, 8, 137–153. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, S.R.; Alshamsi, H.A.; Amiri, O.; Salavati-Niasari, M. Synthesis, Characterization and Application of Co/Co3O4 Nanocomposites as an Effective Photocatalyst for Discoloration of Organic Dye Contaminants in Wastewater and Antibacterial Properties. J. Mol. Liq. 2021, 337, 116405. [Google Scholar] [CrossRef]
- Safaei, M.; Taran, M.; Jamshidy, L.; Imani, M.M.; Mozaffari, H.R.; Sharifi, R.; Golshah, A.; Moradpoor, H. Optimum Synthesis of Polyhydroxybutyrate-Co3O4 Bionanocomposite with the Highest Antibacterial Activity against Multidrug Resistant Bacteria. Int. J. Biol. Macromol. 2020, 158, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; LewisOscar, F.; Shanmugam, S.; Thajuddin, N.; Alharbi, S.A.; Alharbi, N.S.; Brindhadevi, K.; Pugazhendhi, A. Core/Shell Nanoparticles: Synthesis, Investigation of Antimicrobial Potential and Photocatalytic Degradation of Rhodamine B. J. Photochem. Photobiol. B Biol. 2020, 202, 111729. [Google Scholar] [CrossRef] [PubMed]
- Stekel, D. First Report of Antimicrobial Resistance Pre-Dates Penicillin. Nature 2018, 562, 192. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S. Zur Frage Der Salvarsanresistenten Lues. Arch. Für. Dermatol. Und. Syph. 1924, 147, 116–130. [Google Scholar] [CrossRef]
- Beckh, W.; Kulchar, G.V. Treatment-Resistant Syphilis: An Evaluation of the Causative Factors in Eighteen Cases. Arch. Dermatol. Syphilol. 1939, 40, 1–12. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How Antibiotics Kill Bacteria: From Targets to Networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme. UNEP Frontiers 2017 Emerging Issues of Environmental Concern; United Nations Environment Programme: Nairobi, Kenya, 2017. [Google Scholar]
- World Health Organization. World Health Organization Global Action plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Prochaska, C.; Zouboulis, A. A Mini-Review of Urban Wastewater Treatment in Greece: History, Development and Future Challenges. Sustain. Switz. 2020, 12, 6133. [Google Scholar] [CrossRef]
- Salgot, M.; Folch, M. Wastewater Treatment and Water Reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Barancheshme, F.; Munir, M. Strategies to Combat Antibiotic Resistance in the Wastewater Treatment Plants. Front. Microbiol. 2018, 8, 2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Liu, L.; Wang, D.-N.; Yang, D.; Liu, W.-L.; Yin, J.; Yang, Z.-W.; Wang, H.-R.; Qiu, Z.-G.; Shen, Z.-Q.; et al. Chlorine Disinfection Promotes the Exchange of Antibiotic Resistance Genes across Bacterial Genera by Natural Transformation. ISME J. 2020, 14, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Lu, J.; Yu, Z.; Song, H.; Bond, P.L.; Guo, J. Chlorine Disinfection Facilitates Natural Transformation through ROS-Mediated Oxidative Stress. ISME J. 2021, 15, 2969–2985. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanomaterials for Removal of Toxic Elements from Water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-Synthesized Nanocatalysts and Nanomaterials for Water Treatment: Current Challenges and Future Perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered Nanomaterials for Antimicrobial Applications: A Review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- El-sayed, M.E.A. Nanoadsorbents for Water and Wastewater Remediation. Sci. Total Environ. 2020, 739, 133013. [Google Scholar] [CrossRef]
- Gautam, P.K.; Singh, A.; Misra, K.; Sahoo, A.K.; Samanta, S.K. Synthesis and Applications of Biogenic Nanomaterials in Drinking and Wastewater Treatment. J. Environ. Manag. 2019, 231, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting Antibiotic-Resistant Bacteria Using Nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Vijayanandan, A.S.; Balakrishnan, R.M. Photostability and Electrical and Magnetic Properties of Cobalt Oxide Nanoparticles through Biological Mechanism of Endophytic Fungus Aspergillus Nidulans. Appl. Phys. A: Mater. Sci. Process. 2020, 126. [Google Scholar] [CrossRef]
- Sivachidambaram, M.; Vijaya, J.J.; Kaviyarasu, K.; Kennedy, L.J.; Al-Lohedan, H.A.; Jothi Ramalingam, R. A Novel Synthesis Protocol for Co3O4 Nanocatalysts and Their Catalytic Applications. RSC Adv. 2017, 7, 38861–38870. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.K.; Baig, U. Synthesis of Co3O4 Nanoparticles and Their Performance towards Methyl Orange Dye Removal: Characterisation, Adsorption and Response Surface Methodology. J. Clean. Prod. 2019, 211, 1141–1153. [Google Scholar] [CrossRef]
- Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Taher, M.M.; Khalaf, M. Benign Synthesis of Cobalt Oxide Nanoparticles Containing Red Algae Extract: Antioxidant, Antimicrobial, Anticancer, and Anticoagulant Activity. J. Clust. Sci. 2021, 32, 712–718. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Javed, R.; Mahfooz-Ur-Rehman; Shahid, M. Effect of Heating on the Structural and Optical Properties of TiO2 Nanoparticles: Antibacterial Activity. Appl. Nanosci. Switz. 2018, 8, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Uglov, V.V.; Doroshevich, I.L.; Kvasov, N.T.; Remnev, G.E.; Shymanski, V.I. On Physical Properties of Nanoparticles: Size Effect and Scale of Nanoobjects. Phys. Status Solidi C Curr. Top. Solid State Phys. 2016, 13, 903–907. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial Resistance to Silver Nanoparticles and How to Overcome It. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Jin, M.; Yuan, Z.; Bond, P.; Guo, J. Both Silver Ions and Silver Nanoparticles Facilitate the Horizontal Transfer of Plasmid-Mediated Antibiotic Resistance Genes. Water Res. 2020, 169, 115229. [Google Scholar] [CrossRef]
- Zhang, R.; Carlsson, F.; Edman, M.; Hummelgård, M.; Jonsson, B.-G.; Bylund, D.; Olin, H. Escherichia Coli Bacteria Develop Adaptive Resistance to Antibacterial ZnO Nanoparticles. Adv. Biosyst. 2018, 2, 1800019. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, F.; Zhao, J.; Xu, Y.; Mao, D.; Zhu, X.; Luo, Y.; Alvarez, P.J.J. Bacterial Exposure to ZnO Nanoparticles Facilitates Horizontal Transfer of Antibiotic Resistance Genes. NanoImpact 2018, 10, 61–67. [Google Scholar] [CrossRef]
- Liu, X.; Tang, J.; Song, B.; Zhen, M.; Wang, L.; Giesy, J.P. Exposure to Al2O3 Nanoparticles Facilitates Conjugative Transfer of Antibiotic Resistance Genes from Escherichia Coli to Streptomyces. Nanotoxicology 2019, 13, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Ge, Y.; Xia, T.; He, J.-Z.; Shen, C.; Wang, J.; Liu, Y.-J. Rare Earth Oxide Nanoparticles Promote Soil Microbial Antibiotic Resistance by Selectively Enriching Antibiotic Resistance Genes. Environ. Sci. Nano 2019, 6, 456–466. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Li, J.; Hao, Q.; Li, X.; Liu, F. Heterogeneous Activation of Peroxymonosulfate by a Biochar-Supported Co3O4 Composite for Efficient Degradation of Chloramphenicols. Environ. Pollut. 2020, 257, 113610. [Google Scholar] [CrossRef]
- Yu, W.; Zhan, S.; Shen, Z.; Zhou, Q. A Newly Synthesized Au/GO-Co3O4 Composite Effectively Inhibits the Replication of Tetracycline Resistance Gene in Water. Chem. Eng. J. 2018, 345, 462–470. [Google Scholar] [CrossRef]
- Mobeen Amanulla, A.; Jasmine Shahina, S.K.; Sundaram, R.; Maria Magdalane, C.; Kaviyarasu, K.; Letsholathebe, D.; Mohamed, S.B.; Kennedy, J.; Maaza, M. Antibacterial, Magnetic, Optical and Humidity Sensor Studies of β-CoMoO4-Co3O4 Nanocomposites and Its Synthesis and Characterization. J. Photochem. Photobiol. B: Biol. 2018, 183, 233–241. [Google Scholar] [CrossRef]
- Hlongwane, G.N.; Sekoai, P.T.; Meyyappan, M.; Moothi, K. Simultaneous Removal of Pollutants from Water Using Nanoparticles: A Shift from Single Pollutant Control to Multiple Pollutant Control. Sci. Total Environ. 2019, 656, 808–833. [Google Scholar] [CrossRef]
- Malakar, A.; Kanel, S.R.; Ray, C.; Snow, D.D.; Nadagouda, M.N. Nanomaterials in the Environment, Human Exposure Pathway, and Health Effects: A Review. Sci. Total Environ. 2021, 759, 143470. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, Incidental, and Engineered Nanomaterials and Their Impacts on the Earth System. Science 2019, 363, aau8299. [Google Scholar] [CrossRef] [Green Version]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the Environment: Where Do We Come from, Where Do We Go To? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, G.; He, Y.; Zhao, M.; Yu, T.; Qin, Y.; Lin, S. Differential Effects of Metal Oxide Nanoparticles on Zebrafish Embryos and Developing Larvae. Environ. Sci. Nano 2018, 5, 1200–1207. [Google Scholar] [CrossRef]
- Bouguerra, S.; Gavina, A.; Natal-da-Luz, T.; Sousa, J.P.; Ksibi, M.; Pereira, R. The Use of Soil Enzymes Activity, Microbial Biomass, and Basal Respiration to Assess the Effects of Cobalt Oxide Nanomaterial in Soil Microbiota. Appl. Soil Ecol. 2022, 169, 104246. [Google Scholar] [CrossRef]
- Sharan, A.; Nara, S. Exposure of Synthesized Co3O4 Nanoparticles to Chlorella Minutissima: An Ecotoxic Evaluation in Freshwater Microalgae. Aquat. Toxicol. 2020, 224, 105498. [Google Scholar] [CrossRef] [PubMed]
- Sharan, A.; Nara, S. Exposure-Based Ecotoxicity Assessment of Co3O4 Nanoparticles in Marine Microalgae. Environ. Sci. Pollut. Res. 2021, 28, 54802–54810. [Google Scholar] [CrossRef] [PubMed]

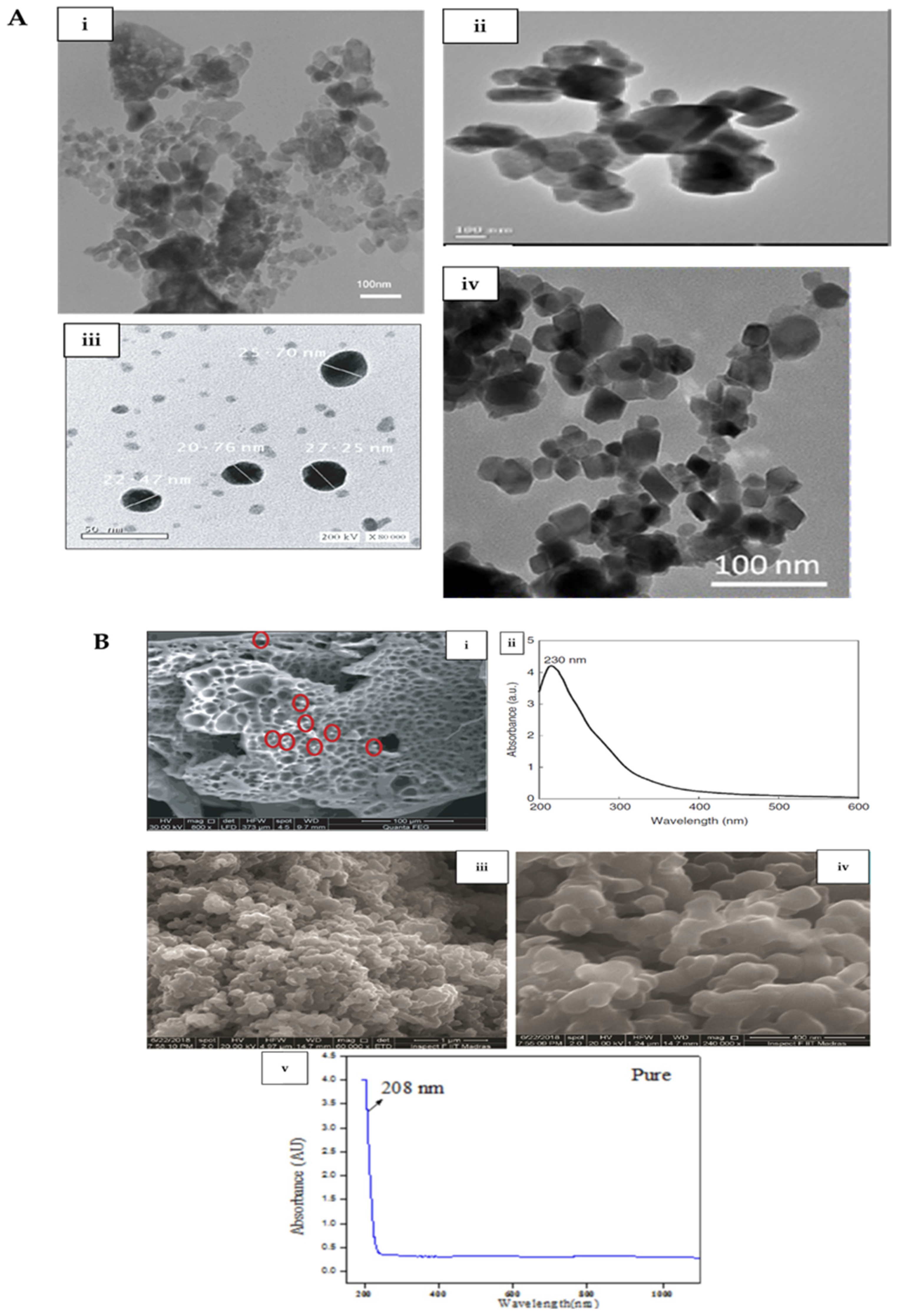
| Material | Synthesis Method | Characterization Method | Morphology | Size | Reference |
|---|---|---|---|---|---|
| Co3O4 | Biological (plant extract) synthesis and hot plate combustion method | XRD, FTIR, Raman, HRTEM, EDS, and UV-Vis | Quasi-spherical shape and high agglomeration | 1–7 nm | [1] |
| Co3O4 | Biological (myco-) synthesis | DLS, EDS, FTIR, VSM, FESEM, HRTEM | Quasi-spherical shape and monodispersed | 20–27 nm | [3] |
| Co3O4 | Biological (plant extract) synthesis | TEM, SEM, XRD, FTIR | Square-shaped, and aggregated | 15–35 nm | [7] |
| Co3O4 | Biological molecule-based synthesis | FTIR, XRD, SEM, TGA | Mixture of octahedron, tetrahedron, spheroidal, flakelike morphologies | 20 nm–2 µm | [40] |
| Co3O4 | Biological molecule-based synthesis | AFM, XPS | Spherical | 2.5–3 nm | [41] |
| Co3O4 | Microwave synthesis and calcination | XRD, UV, FTIR, HRSEM, PL, TEM | Spherical and agglomerated | 13 nm | [39] |
| Co3O4 | Precipitation and calcination | FTIR, SEM, TEM, XRD, UV-Vis | Spherical, interconnected, layered structure | 32.66 nm | [36] |
| Co3O4 | Microwave synthesis and calcination | FTIR, SEM, TEM, XRD, UV-Vis | Spherical, interconnected, layered structure | 72.43 nm | [36] |
| Co3O4 | Casting technique and calcination | XRD, TEM, IR, UV-Vis | Cubic, no agglomeration | 13 nm | [35] |
| Co3O4 | Microemulsion quenching technique | TEM, FESEM, EDS, XRD, UV-Vis | Spherical | 1–5 nm | [38] |
| Co3O4 | Laser ablation | UV-Vis-NIR, TEM, SEM, XRD, FTIR, PL, DLS, VSM | Spherical with some agglomeration | 10 nm | [33] |
| Co3O4 | Laser ablation | TEM, Raman, UV-Vis, XPS, CV | Spherical, agglomerated | ∼2.5 nm | [32] |
| Co3O4 | Laser fragmentation | XRD, TEM, EDS, XPS, Raman, FTIR | Uniform, spherical, well dispersed | ∼5.8 nm | [34] |
| Material | Synthesis Method | Characterization Method | Morphology | Size | Reference |
|---|---|---|---|---|---|
| α-Fe2O3-Co3O4 | Co-precipitation and calcination | XRD, TEM, EDS, VSM, Raman | Mixture of rod-shaped and hexagonal | 25.34 nm (crystallite size) | [50] |
| Ni doped-Co3O4 | Microwave synthesis and annealing | XRD, UV-Vis-NIR, FTIR, HRSEM, TEM, Fluor, EDS | Nanocubes | 15–41 nm | [39] |
| Co/Co3O4 | Sonochemical method | SEM, FTIR, XRD, VSM, EDS, CV | Snowballs | ∼20 nm | [51] |
| MnFe2O4-Co3O4 | Sonochemical co-precipitation method | HRTEM, EDS, XRD, PL, DRS, VSM, FTIR | MnFe2O4 nanorods attached to Co3O4 nanostructures | Not indicated | [37] |
| polyhydroxybutyrate-Co3O4 | Co-precipitation method | FTIR, UV-Vis, XRD, SEM, EDS, TEM, TGA, DTA | Uneven surfaced structure, agglomerated; well dispersed Co3O4 in biopolymer | Not indicated | [52] |
| Co3O4@ZrO2 | Sol-gel method | UV-Vis, FTIR, CV, FESEM, XRD | Spherical with irregular morphology; agglomerated | 378.8 nm and 681.4 nm | [53] |
| Target Bacteria in Study | Method of Assessing Activity on Bacteria | Concentration Used | Contact Time and Other Conditions | Antibacterial/Inhibitory Activity | Summary of Mechanism of Antibacterial/Inhibitory Activity | Reference |
|---|---|---|---|---|---|---|
| S. aureus | Disc diffusion method | 0.001 g/10 mL | Incubated at 37 °C for 24 h | 18.6 mm zone of inhibition | Probably cell membrane disruption and oxidative stress from ROS | [1] |
| B. subtilis | Disc diffusion method | 0.001 g/10 mL | Incubated at 37 °C for 24 h | 20.8 mm zone of inhibition | Probably cell membrane disruption and oxidative stress from ROS | |
| P. aeruginosa | Disc diffusion method | 0.001 g/10 mL | Incubated at 37 °C for 24 h | 18.5 mm zone of inhibition | Probably cell membrane disruption and oxidative stress from ROS | |
| E. coli | Disc diffusion method | 0.001 g/10 mL | Incubated at 37 °C for 24 h | 25.1 mm zone of inhibition | Probably cell membrane disruption and oxidative stress from ROS | |
| S. aureus | Disc diffusion method | 0.001 g/10 mL | Incubated at 37 °C for 24 h | 16.3 mm zone of inhibition | Probably cell membrane disruption and oxidative stress from ROS | [76] |
| B. subtilis | Disc diffusion method | 0.001 g/10 mL | Incubated at 37 °C for 24 h | 22.2 mm zone of inhibition | Probably cell membrane disruption and oxidative stress from ROS | |
| P. aeruginosa | Disc diffusion method | 0.001 g/10 mL | Incubated at 37 °C for 24 h | 34.5 mm zone of inhibition | Probably cell membrane disruption and oxidative stress from ROS | |
| E. coli | Disc diffusion method | 0.001 g/10 mL | Incubated at 37 °C for 24 h | 16.4 mm zone of inhibition | Probably cell membrane disruption and oxidative stress from ROS | |
| B. subtilis ATCC 6633 | Agar plate well diffusion method | 5 mg mL−1 | Not indicated | 15.6 mm zone of inhibition | Attributed to size effects | [3] |
| S. aureus ATCC 35556 | Agar plate well diffusion method | 5 mg mL−1 | Not indicated | 20 mm zone of inhibition | Attributed to size effects | |
| P. aeruginosa ATCC 10145 | Agar plate well diffusion method | 5 mg mL−1 | Not indicated | 11.3 mm zone of inhibition | Attributed to size effects | |
| E. coli ATCC 23282 | Agar plate well diffusion method | 5 mg mL−1 | Not indicated | 12 mm zone of inhibition | Attributed to size effects | |
| B. subtilis ATCC 6633 | MIC and MLC | 0.035–5 mg mL−1 | Optical density (OD600) taken after incubation at 24 h | 2.5 mg mL−1 | Attributed to size effects | [3] |
| S. aureus ATCC 35556 | MIC and MLC | 0.035–5 mg mL−1 | Optical density (OD600) taken after incubation at 24 h | 5 mg mL−1 | Attributed to size effects | |
| P. aeruginosa ATCC 10145 | MIC and MLC | 0.035–5 mg mL−1 | Optical density (OD600) taken after incubation at 24 h | 2.5 mg mL−1 | Attributed to size effects | |
| E. coli ATCC 23282 | MIC and MLC | 0.035–5 mg mL−1 | Optical density (OD600) taken after incubation at 24 h | 2.5 mg mL−1 | Attributed to size effects | |
| E. coli | Agar plate well diffusion method | 2, 4, and 8 mg mL−1 | Incubated at 37 °C for 24 h | 23.5 mm zone of inhibition at a dose of 8 mg mL−1 | Attributed to size effects and ROS damage to bacteria DNA, protein, and cell membrane | [7] |
| Klebsiella pneumoniae | Agar plate well diffusion method | 2, 4, and 8 mg mL−1 | Incubated at 37 °C for 24 h | 27.2 mm zone of inhibition at a dose of 8 mg mL−1 | Attributed to size effects and ROS damage to bacteria DNA, protein, and cell membrane | |
| B. subtilis | Agar plate well diffusion method | 2, 4, and 8 mg mL−1 | Incubated at 37 °C for 24 h | 25.3 mm zone of inhibition at a dose of 8 mg mL−1 | Attributed to size effects and ROS damage to bacteria DNA, protein, and cell membrane | |
| Bacillus licheniformis | Agar plate well diffusion method | 8 mg mL−1 | Incubated at 37 °C for 24 h | 24.2 mm zone of inhibition at a dose of 8 mg mL−1 | Attributed to size effects and ROS damage to bacteria DNA, protein, and cell membrane | |
| E. coli | Disc diffusion method | 31.25–500 µg/mL | Incubated at 37 °C for 24 h | 22.8 mm zone of inhibition at a dose of 500 µg/mL | Attributed to size effects and ROS effects on cellular contents | [78] |
| P. aeruginosa | Disc diffusion method | 31.25–500 µg/mL | Incubated at 37 °C for 24 h | 28.4 mm zone of inhibition at a dose of 500 µg/mL | Attributed to size effects and ROS effects on cellular contents | |
| S. aureus | Disc diffusion method | 31.25–500 µg/mL | Incubated at 37 °C for 24 h | 29.2 mm zone of inhibition at a dose of 500 µg/mL | Attributed to size effects and ROS effects on cellular contents |
| Material Used in Study | Target Bacteria in Study | Method of Assessing Activity on Bacteria | Concentration Used | Contact Time and Other Conditions | Antibacterial/Inhibitory Activity | Summary of Mechanism of Antibacterial/Inhibitory Activity | Reference |
|---|---|---|---|---|---|---|---|
| α-Fe2O3/Co3O4 | B. subtilis | Disc diffusion method | 400, 600 and 800 µg | Incubated at 37 °C for 24 h | 21 mm zone of inhibition at a dose of 800 µg | Attributed to ROS effects on cellular contents | [50] |
| S. aureus | 24 mm zone of inhibition at a dose of 800 µg | ||||||
| E. coli | 26 mm zone of inhibition at a dose of 800 µg | ||||||
| S. typhi | 19 mm zone of inhibition at a dose of 800 µg | ||||||
| α-Fe2O3/Co3O4 | B. subtilis | Growth curve analysis | 45, 60, 75, 90 and 120 mg/dL | Incubated at 37 °C for 24 h (reading taken at 6 h intervals) | OD600 = ∼0.3 at a concentration of 120 mg/dL after 24 h; MIC = 90 mg/dL | Attributed to ROS effects on cellular contents | [50] |
| S. aureus | OD600 = 0 at a concentration of 120 mg/dL after 24 h; MIC = 75 mg/dL | ||||||
| E. coli | OD600 = 0 at a concentration of 120 mg/dL after 24 h; MIC = 45 mg/dL | ||||||
| S. typhi | OD600 = ∼0.01 at a concentration of 120 mg/dL after 24 h; MIC = 60 mg/dL | ||||||
| β-CoMoO4-Co3O4 | E. coli | Agar plate well diffusion method | 1.56–50 mg/mL | Incubated at 37 °C for 24 h | 17 mm zone of inhibition at a dose of 50 mg/mL | Electrostatic interactions with bacteria and ROS effects | [89] |
| P. aeruginosa | 19 mm zone of inhibition at a dose of 50 mg/mL | ||||||
| S. aureus | 18 mm zone of inhibition at a dose of 50 mg/mL | ||||||
| Co/Co3O4 | B. subtilis | MIC and MBC | ∼0–2000 µg/mL | CLSI guidelines | MIC = ∼125 µg/mL MBC = 2000 µg/mL | Not indicated | [51] |
| S. aureus | MIC = ∼500 µg/mL MBC = 2000 µg/mL | ||||||
| P. aeruginosa | MIC = 31.25 µg/mL MBC = ∼500 µg/mL | ||||||
| K. pneumonia | MIC = ∼500 µg/mL MBC = 1000 µg/mL | ||||||
| E. coli | MIC = ∼500 µg/mL MBC = 1000 µg/mL | ||||||
| Ni doped-Co3O4 (20 wt% of Ni) | E. coli MTCC 443 | Agar plate well diffusion method | 100 µg/mL | Incubated at 37 °C for 24 h | 20 mm zone of inhibition | Attributed to interactions of nanoparticle with bacteria cell membrane | [39] |
| P. aeruginosa MTCC 2453 | 14 mm zone of inhibition | ||||||
| B. subtilis MTCC 441 | 18 mm zone of inhibition | ||||||
| S. aureus MTCC 96 | 13 mm zone of inhibition | ||||||
| Co3O4@ZrO2 | E. coli | Agar plate well diffusion method | 50, 100 and 200 µg/mL | Incubated at 37 °C for 24 h | ∼˂1 mm zone of inhibition at a dose of 200 µg/mL | Attributed to cell wall penetration and genotoxicity resulting in cell deformation | [53] |
| P. aeruginosa | ∼13 mm zone of inhibition at a dose of 200 µg/mL | ||||||
| B. subtilis | ∼1 mm zone of inhibition at a dose of 200 µg/mL | ||||||
| S. aureus | ∼12 mm zone of inhibition at a dose of 200 µg/m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anele, A.; Obare, S.; Wei, J. Recent Trends and Advances of Co3O4 Nanoparticles in Environmental Remediation of Bacteria in Wastewater. Nanomaterials 2022, 12, 1129. https://doi.org/10.3390/nano12071129
Anele A, Obare S, Wei J. Recent Trends and Advances of Co3O4 Nanoparticles in Environmental Remediation of Bacteria in Wastewater. Nanomaterials. 2022; 12(7):1129. https://doi.org/10.3390/nano12071129
Chicago/Turabian StyleAnele, Anuoluwapo, Sherine Obare, and Jianjun Wei. 2022. "Recent Trends and Advances of Co3O4 Nanoparticles in Environmental Remediation of Bacteria in Wastewater" Nanomaterials 12, no. 7: 1129. https://doi.org/10.3390/nano12071129
APA StyleAnele, A., Obare, S., & Wei, J. (2022). Recent Trends and Advances of Co3O4 Nanoparticles in Environmental Remediation of Bacteria in Wastewater. Nanomaterials, 12(7), 1129. https://doi.org/10.3390/nano12071129







