Lipid-Based Nano-Sized Cargos as a Promising Strategy in Bone Complications: A Review
Abstract
:1. Introduction
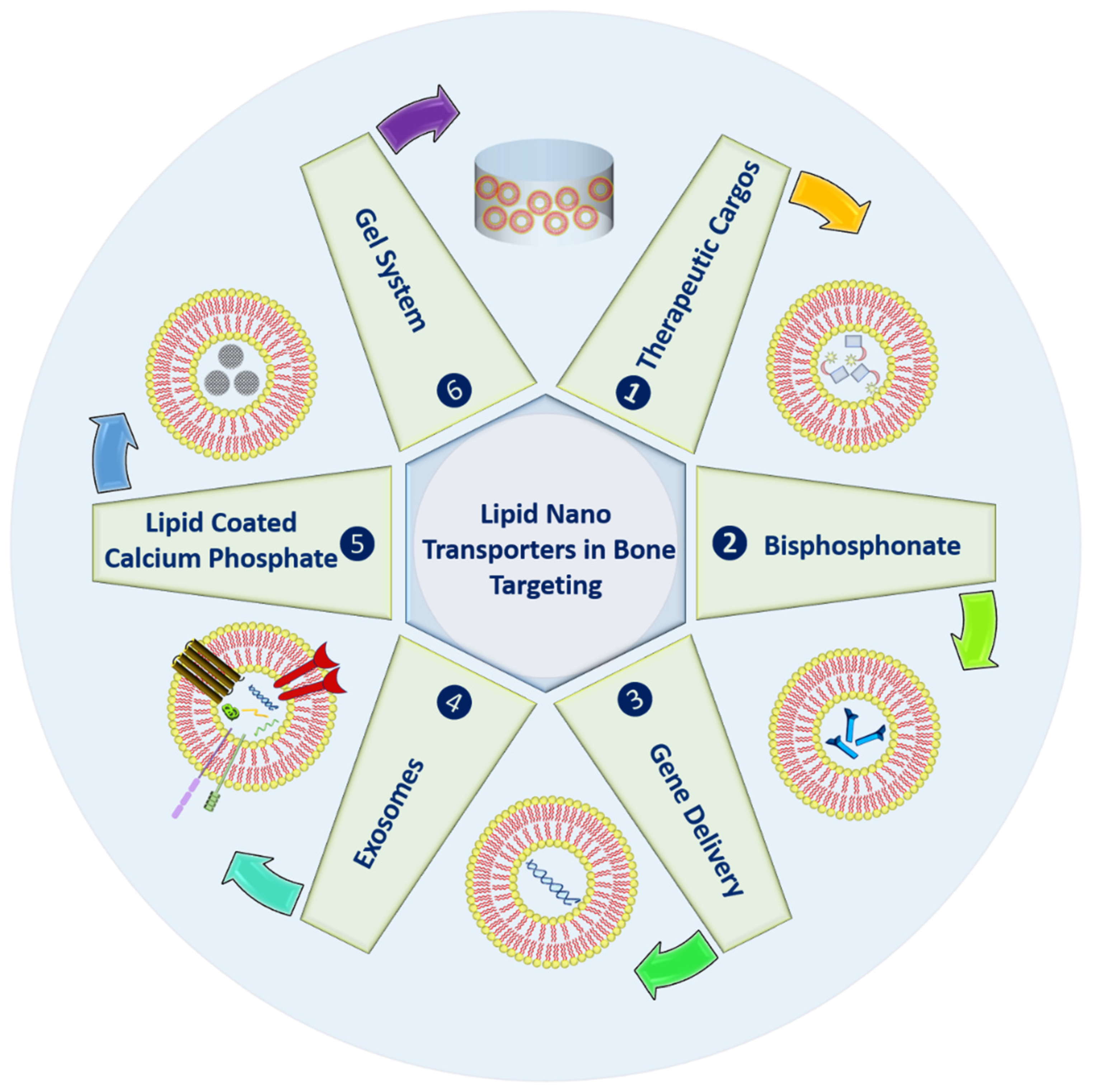
2. Strategies in Bone Targeting
2.1. Therapeutic Cargoes
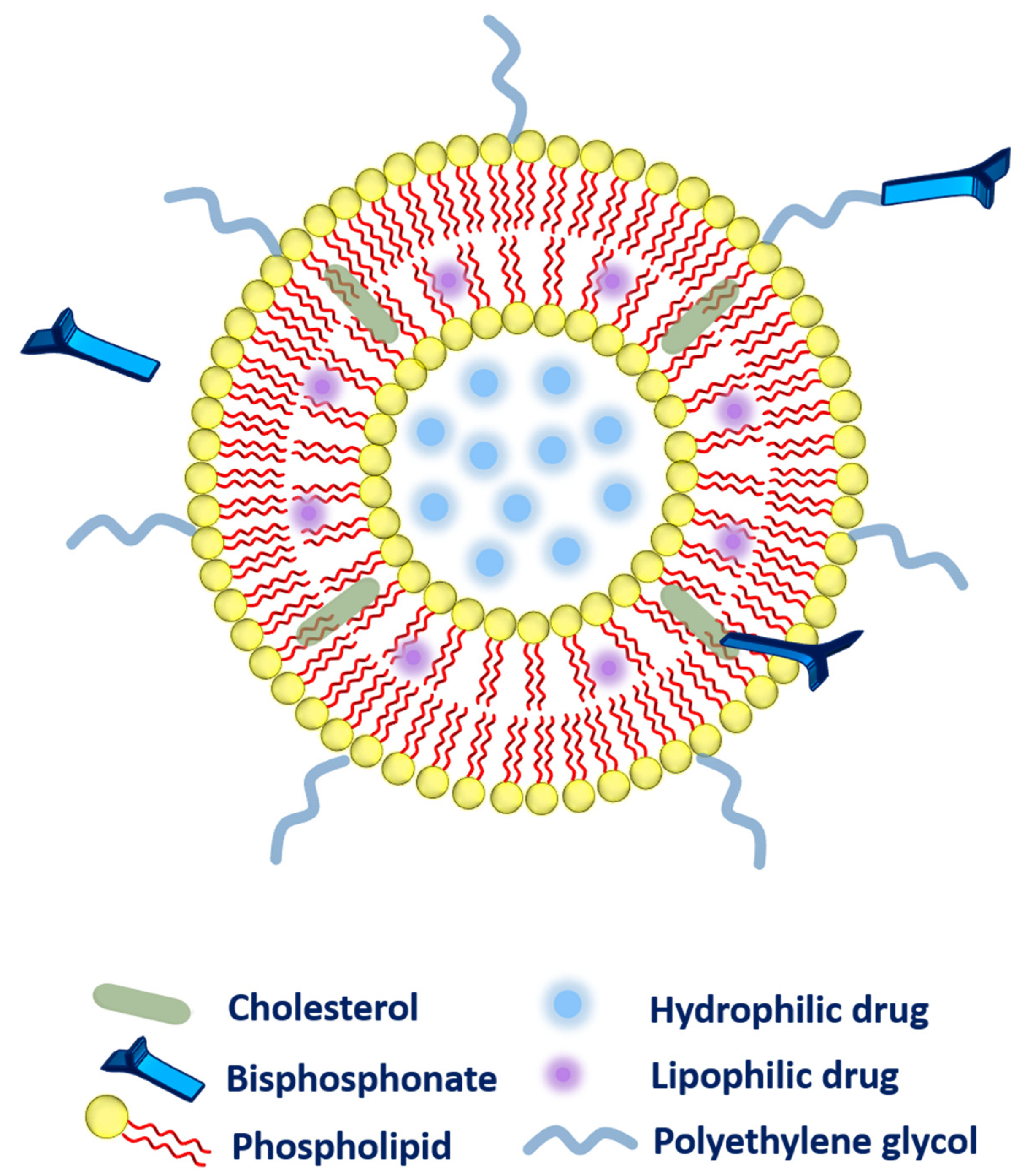
2.2. Bisphosphonate Delivery
- ➢
- ➢
- Secondly, the bisphosphonate can also be conjugated along with the cholesterol (as a main component of liposomal composition via a click reaction known as Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition). The aforementioned system has exhibited a very strong affinity toward bones [40].
- ➢
- Thirdly, bisphosphonate can be associated with the polyethylene glycol (PEG) chain to provide higher circulation time to the liposomes. More precisely, phospholipid-PEG-bisphosphonate conjugation can be employed in liposomes [41]. Polyethylene-glycol-conjugated phospholipid was used to embed zoledronic acid (as a potent inhibitor of farnesyl-pyrophosphate synthase). The fabricated liposomes were subjected to biodistribution studies that evidenced higher drug accumulation in the liver, spleen, bones, and tumor as compared to zoledronic acid in free form or entrapped in non-PEGylated liposomes. However, toxicity was the main concern as these liposomes were found very toxic to rodents [36].
2.3. Gene Delivery
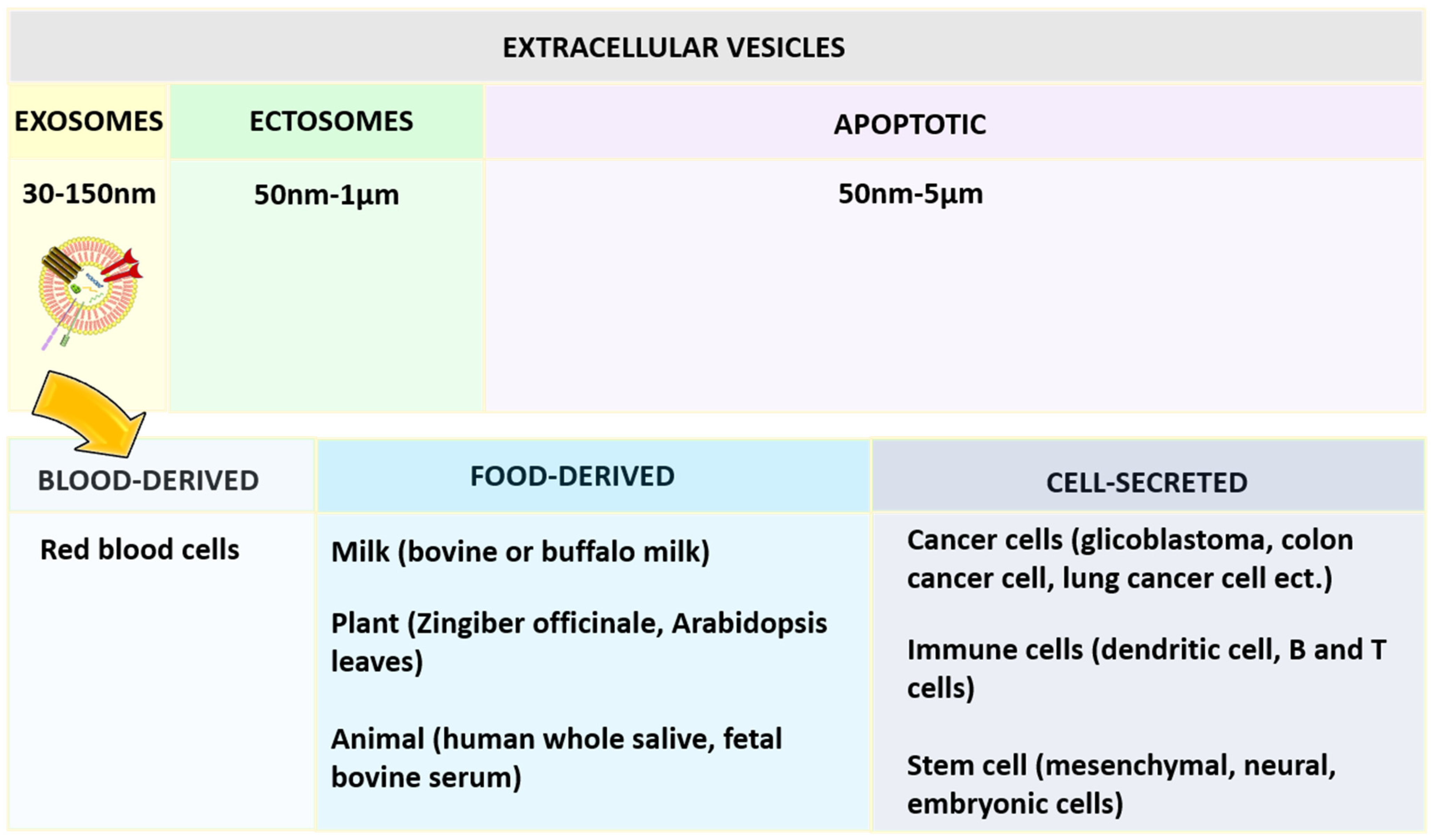
2.4. Exosomes
2.5. Lipid Coated Calcium Phosphate
2.6. Gel System
3. Composition of Lipid-Based Systems in Bone Metastasis and Bone Regeneration
| Carrier | Drug | Composition | Targeting Moiety | Outcome | Ref. |
|---|---|---|---|---|---|
| Liposomes | Paclitaxel | Soybean phospholipids | Glutamic oligopeptides-RGD peptide | High hydroxyapatite binding efficiency, improved cytotoxicity | [127] |
| Doxorubicin | Hydrogenated soy phosphatidylcholine, cholesterol and DSPE-mPEG2000 | Aspartate and folate | relieve pain and improve survival in a mice model | [128] | |
| Doxorubicin | Distearoylphosphotidylcholine, cholesterol | Thiol-bisphosphonate | A good candidate in bone regeneration with higher retention | [129] | |
| Lipid Nanoparticles | Glucocorticoid prednisolone | Glyceryl monostearate, dimethyldioctadecylammonium bromide, cholesterol | Hyaluronic acid | Reduced joint swelling, bone erosion, and levels of cytokines in serum | [130] |
| Simvastatin | monostearin, polyethylene glycol monostearate, oleic acid | Aspartic oligopeptide | Induced osteoblast differentiation, biocompatible with MC3T3-E1 cells | [27] | |
| Bone morphogenetic protein-9 gene | DOPE (1,2-dioleoyl-sn-glycero-3- phosphoethanolamine), mPEG2000-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolaminemethoxypolyethyleneglycol 2000), hydrogenated soy phosphatidylcholine, and cholesterol | Bone-homing peptide | Effective in vitro and in vivo gene delivery, no toxicity | [131] | |
| siRNA | Dilinoleylmethyl-4-dimethylaminobutyrate, distearoylphosphatidylcholine, cholesterol, and polyethylene glycol-dimyristol glycerol | N/A | Prolonged knockdown, accumulation of osteocytes | [132] |
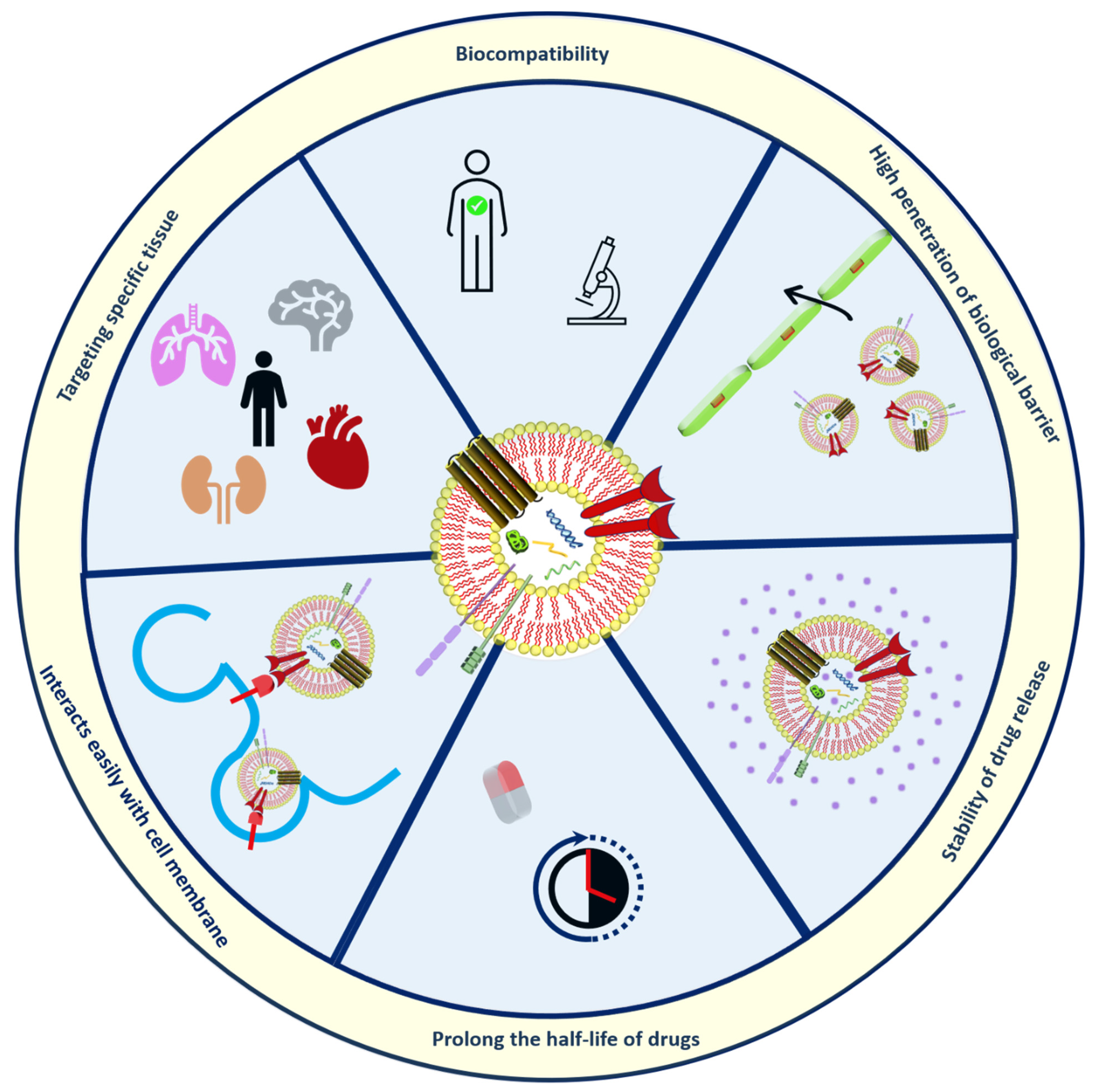
4. Characterization of Lipid-Based Nano-Sized Cargos
5. Challenges and Future Prospectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer Statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Metastatic Bone Disease: Clinical Features, Pathophysiology and Treatment Strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Gdowski, A.S.; Ranjan, A.; Vishwanatha, J.K. Current Concepts in Bone Metastasis, Contemporary Therapeutic Strategies and Ongoing Clinical Trials. J. Exp. Clin. Cancer Res. 2017, 36, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Probert, C.; Dottorini, T.; Speakman, A.; Hunt, S.; Nafee, T.; Fazeli, A.; Wood, S.; Brown, J.E.; James, V. Communication of Prostate Cancer Cells with Bone Cells via Extracellular Vesicle RNA; a Potential Mechanism of Metastasis. Oncogene 2019, 38, 1751–1763. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T. Multidisciplinary Approach for Bone Metastasis: A Review. Cancers 2018, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Lalle, M.; De Rosa, L.; Marzetti, L.; Montuoro, A. Detection of Breast Cancer Cells in the Bone Marrow or Peripheral Blood: Methods and Prognostic Significance. Tumori 2000, 86, 183–190. [Google Scholar] [CrossRef]
- Van Der Pluijm, G.; Sijmons, B.; Vloedgraven, H.; Deckers, M.; Papapoulos, S.; Löwik, C. Monitoring Metastatic Behavior of Human Tumor Cells in Mice with Species-Specific Polymerase Chain Reaction: Elevated Expression of Angiogenesis and Bone Resorption Stimulators by Breast Cancer in Bone Metastases. J. Bone Miner. Res. 2001, 16, 1077–1091. [Google Scholar] [CrossRef]
- Sottnik, J.L.; Keller, E.T. Understanding and Targeting Osteoclastic Activity in Prostate Cancer Bone Metastases. Curr. Mol. Med. 2013, 13, 626–639. [Google Scholar] [CrossRef] [Green Version]
- PKarr, J.P.; Yamanaka, H. (Eds.) Prostate Cancer and Bone Metastasis. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1992; Volume 324, ISBN 978-1-4613-6501-3. [Google Scholar]
- Giancotti, F.G. Mechanisms Governing Metastatic Dormancy and Reactivation. Cell 2013, 155, 750–764. [Google Scholar] [CrossRef] [Green Version]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human Prostate Cancer Metastases Target the Hematopoietic Stem Cell Niche to Establish Footholds in Mouse Bone Marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A Review of Diagnosis, Management, and Treatment Strategies. Clin. Adv. Hematol. Oncol. 2010, 8, 705–718. [Google Scholar] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallan, S.S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.S.; Marchetti, P.; Bortolotti, D.; Sguizzato, M.; Esposito, E.; Mariani, P.; Trapella, C.; Rizzo, R.; Cortesi, R. Design of Nanosystems for the Delivery of Quorum Sensing Inhibitors: A Preliminary Study. Molecules 2020, 25, 5655. [Google Scholar] [CrossRef]
- Wang, T.; Suita, Y.; Miriyala, S.; Dean, J.; Tapinos, N.; Shen, J. Advances in Lipid-Based Nanoparticles for Cancer Chemoimmunotherapy. Pharmaceutics 2021, 13, 520. [Google Scholar] [CrossRef]
- Rahdar, A.; Taboada, P.; Hajinezhad, M.R.; Barani, M.; Beyzaei, H. Effect of Tocopherol on the Properties of Pluronic F127 Microemulsions: Physico-Chemical Characterization and In Vivo Toxicity. J. Mol. Liq. 2019, 277, 624–630. [Google Scholar] [CrossRef]
- Razzaq, S.; Rauf, A.; Raza, A.; Akhtar, S.; Tabish, T.A.; Sandhu, M.A.; Zaman, M.; Ibrahim, I.M.; Shahnaz, G.; Rahdar, A.; et al. A Multifunctional Polymeric Micelle for Targeted Delivery of Paclitaxel by the Inhibition of the P-Glycoprotein Transporters. Nanomaterials 2021, 11, 2858. [Google Scholar] [CrossRef]
- Arshad, R.; Tabish, T.A.; Kiani, M.H.; Ibrahim, I.M.; Shahnaz, G.; Rahdar, A.; Kang, M.; Pandey, S. A Hyaluronic Acid Functionalized Self-Nano-Emulsifying Drug Delivery System (SNEDDS) for Enhancement in Ciprofloxacin Targeted Delivery against Intracellular Infection. Nanomaterials 2021, 11, 1086. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to Drug Delivery in Solid Tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug Delivery to Solid Tumors: The Predictive Value of the Multicellular Tumor Spheroid Model for Nanomedicine Screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.R.; Goyal, A.K. Surfactant-Based Drug Delivery Systems for Treating Drug-Resistant Lung Cancer. Drug Deliv. 2016, 23, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; Ruiz, M.C.; Scioli-Montoto, S.; Ruiz, M.E.; Fernández, M.A.; Torres-Sanchez, R.M.; Baran, E.J.; Castro, G.R.; León, I.E. Lipid Nanoparticles–Metvan: Revealing a Novel Way to Deliver a Vanadium Compound to Bone Cancer Cells. New J. Chem. 2019, 43, 17726–17734. [Google Scholar] [CrossRef]
- Sun, X.; Wei, J.; Lyu, J.; Bian, T.; Liu, Z.; Huang, J.; Pi, F.; Li, C.; Zhong, Z. Bone-Targeting Drug Delivery System of Biomineral-Binding Liposomes Loaded with Icariin Enhances the Treatment for Osteoporosis. J. Nanobiotechnol. 2019, 17, 10. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Chen, S.; Zhou, W.; Yu, F.; Bao, L.; Qiu, G.; Qiao, Q.; Hu, F.; Wang, J.; Yuan, H. A Novel Biocompatible, Simvastatin-Loaded, Bone-Targeting Lipid Nanocarrier for Treating Osteoporosis More Effectively. RSC Adv. 2020, 10, 20445–20459. [Google Scholar] [CrossRef]
- Emanuel, N.; Rosenfeld, Y.; Cohen, O.; Applbaum, Y.H.; Segal, D.; Barenholz, Y. A Lipid-and-Polymer-Based Novel Local Drug Delivery System—BonyPidTM: From Physicochemical Aspects to Therapy of Bacterially Infected Bones. J. Control. Release 2012, 160, 353–361. [Google Scholar] [CrossRef]
- González-Fernández, Y.; Brown, H.K.; Patiño-García, A.; Heymann, D.; Blanco-Prieto, M.J. Oral Administration of Edelfosine Encapsulated Lipid Nanoparticles Causes Regression of Lung Metastases in Pre-Clinical Models of Osteosarcoma. Cancer Lett. 2018, 430, 193–200. [Google Scholar] [CrossRef]
- Aldayel, A.M.; O’Mary, H.L.; Valdes, S.A.; Li, X.; Thakkar, S.G.; Mustafa, B.E.; Cui, Z. Lipid Nanoparticles with Minimum Burst Release of TNF-α SiRNA Show Strong Activity against Rheumatoid Arthritis Unresponsive to Methotrexate. J. Control. Release 2018, 283, 280–289. [Google Scholar] [CrossRef]
- Riaz, M.; Riaz, M.; Zhang, X.; Lin, C.; Wong, K.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [Green Version]
- Zylberberg, C.; Matosevic, S. Pharmaceutical Liposomal Drug Delivery: A Review of New Delivery Systems and a Look at the Regulatory Landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallan, S.S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simelière, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and Characterization of Ethosomes for Transdermal Delivery of Caffeic Acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Coxon, F.P.; Thompson, K.; Roelofs, A.J.; Ebetino, F.H.; Rogers, M.J. Visualizing Mineral Binding and Uptake of Bisphosphonate by Osteoclasts and Non-Resorbing Cells. Bone 2008, 42, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Kaur, V.; Kumar, M.; Kaur, P.; Murthy, R.S.R.; Rawal, R.K. The Critical Role of Bisphosphonates to Target Bone Cancer Metastasis: An Overview. J. Drug Target. 2015, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shmeeda, H.; Amitay, Y.; Tzemach, D.; Gorin, J.; Gabizon, A. Liposome Encapsulation of Zoledronic Acid Results in Major Changes in Tissue Distribution and Increase in Toxicity. J. Control. Release 2013, 167, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Epstein, H.; Gutman, D.; Cohen-Sela, E.; Haber, E.; Elmalak, O.; Koroukhov, N.; Danenberg, H.D.; Golomb, G. Preparation of Alendronate Liposomes for Enhanced Stability and Bioactivity: In Vitro and In Vivo Characterization. AAPS J. 2008, 10, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Hodgins, N.O.; Al-Jamal, W.T.; Wang, J.T.-W.; Parente-Pereira, A.C.; Liu, M.; Maher, J.; Al-Jamal, K.T. In Vitro Potency, In Vitro and In Vivo Efficacy of Liposomal Alendronate in Combination with Γδ T Cell Immunotherapy in Mice. J. Control. Release 2016, 241, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Gutman, D.; Golomb, G. Liposomal Alendronate for the Treatment of Restenosis. J. Control. Release 2012, 161, 619–627. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/Tricalcium Phosphate Combination Scaffolds Can Enhance Bone Regeneration by Activating the PI3K/Akt Signaling Pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zheng, C.; Li, Y.; Bian, S.; Pan, H.; Zhao, X.; Lu, W.W. Bone Targeted Delivery of SDF-1 via Alendronate Functionalized Nanoparticles in Guiding Stem Cell Migration. ACS Appl. Mater. Interfaces 2018, 10, 23700–23710. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Deng, T.; Yao, P.; Song, H.; Zhou, S.; Yan, W. Development of Drug Loaded Nanoparticles Binding to Hydroxyapatite Based on a Bisphosphonate Modified Nonionic Surfactant. J. Nanomater. 2015, 2015, 145. [Google Scholar] [CrossRef]
- Amirian, J.; Tripathi, G.; Kang, H.-J.; Lee, B.-T. Porous BMP-2 Immobilized PLGA/Glycol Chitosan Scaffold with Enhanced Hydrophilicity, Mineralization and Osteogenesis. Mater. Lett. 2022, 308, 131140. [Google Scholar] [CrossRef]
- Jung, A.; Makkar, P.; Amirian, J.; Lee, B.-T. A Novel Hybrid Multichannel Biphasic Calcium Phosphate Granule-Based Composite Scaffold for Cartilage Tissue Regeneration. J. Biomater. Appl. 2018, 32, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-J.; Amirian, J.; Linh, N.T.B.; Lee, B.-T. Bone Morphogenetic Protein-2 Immobilization on Porous PCL-BCP-Col Composite Scaffolds for Bone Tissue Engineering. J. Appl. Polym. Sci. 2017, 134, 45186. [Google Scholar] [CrossRef]
- Amirian, J.; Linh, N.T.B.; Min, Y.K.; Lee, B.-T. Bone Formation of a Porous Gelatin-Pectin-Biphasic Calcium Phosphate Composite in Presence of BMP-2 and VEGF. Int. J. Biol. Macromol. 2015, 76, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Dirzu, N.; Lucaciu, O.; Dirzu, D.S.; Soritau, O.; Cenariu, D.; Crisan, B.; Tefas, L.; Campian, R.S. BMP-2 Delivery through Liposomes in Bone Regeneration. Appl. Sci. 2022, 12, 1373. [Google Scholar] [CrossRef]
- Balmayor, E.R.; van Griensven, M. Gene Therapy for Bone Engineering. Front. Bioeng. Biotechnol. 2015, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.H. Gene Delivery to Bone. Adv. Drug Deliv. Rev. 2012, 64, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.-Q.; Liu, D.-Z.; Cui, H.; Cheng, Y.; Liu, M.; Zhang, B.-L.; Mei, Q.-B.; Zhou, S.-Y. Charge Reversible Calcium Phosphate Lipid Hybrid Nanoparticle for SiRNA Delivery. Oncotarget 2017, 8, 42772–42788. [Google Scholar] [CrossRef]
- Xu, P.; Van Kirk, E.A.; Zhan, Y.; Murdoch, W.J.; Radosz, M.; Shen, Y. Targeted Charge-Reversal Nanoparticles for Nuclear Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 4999–5002. [Google Scholar] [CrossRef]
- Miyata, K.; Oba, M.; Nakanishi, M.; Fukushima, S.; Yamasaki, Y.; Koyama, H.; Nishiyama, N.; Kataoka, K. Polyplexes from Poly(Aspartamide) Bearing 1,2-Diaminoethane Side Chains Induce PH-Selective, Endosomal Membrane Destabilization with Amplified Transfection and Negligible Cytotoxicity. J. Am. Chem. Soc. 2008, 130, 16287–16294. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal Stem Cells: Environmentally Responsive Therapeutics for Regenerative Medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.-Y. The Meaning, the Sense and the Significance: Translating the Science of Mesenchymal Stem Cells into Medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent Advancements in the Use of Exosomes as Drug Delivery Systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Boorn, J.G.; Daßler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as Nucleic Acid Nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Rufino-Ramos, D.; Albuquerque, P.R.; Carmona, V.; Perfeito, R.; Nobre, R.J.; Pereira de Almeida, L. Extracellular Vesicles: Novel Promising Delivery Systems for Therapy of Brain Diseases. J. Control. Release 2017, 262, 247–258. [Google Scholar] [CrossRef]
- Mandal, S. Curcumin, a Promising Anti-Cancer Therapeutic: It’s Bioactivity and Development of Drug Delivery Vehicles. Int. J. Drug Res.Technol. 2017, 6, 14. [Google Scholar]
- Osteikoetxea, X.; Sódar, B.; Németh, A.; Szabó-Taylor, K.; Pálóczi, K.; Vukman, K.V.; Tamási, V.; Balogh, A.; Kittel, Á.; Pállinger, É.; et al. Differential Detergent Sensitivity of Extracellular Vesicle Subpopulations. Org. Biomol. Chem. 2015, 13, 9775–9782. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Salem, K.Z.; Moschetta, M.; Sacco, A.; Imberti, L.; Rossi, G.; Ghobrial, I.M.; Manier, S.; Roccaro, A.M. Exosomes in Tumor Angiogenesis. In Tumor Angiogenesis Assays; Ribatti, D., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; Volume 1464, pp. 25–34. ISBN 978-1-4939-3997-8. [Google Scholar]
- Lässer, C.; O’Neil, S.E.; Shelke, G.V.; Sihlbom, C.; Hansson, S.F.; Gho, Y.S.; Lundbäck, B.; Lötvall, J. Exosomes in the Nose Induce Immune Cell Trafficking and Harbour an Altered Protein Cargo in Chronic Airway Inflammation. J. Transl. Med. 2016, 14, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular Vesicles: Structure, Function, and Potential Clinical Uses in Renal Diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möbius, W.; Ohno-Iwashita, Y.; van Donselaar, E.G.; Oorschot, V.M.J.; Shimada, Y.; Fujimoto, T.; Heijnen, H.F.G.; Geuze, H.J.; Slot, J.W. Immunoelectron Microscopic Localization of Cholesterol Using Biotinylated and Non-Cytolytic Perfringolysin O. J. Histochem. Cytochem. 2002, 50, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An Integrated Database of High-Throughput Data for Systemic Analyses of Extracellular Vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of Exosomal Proteins, RNA and Lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stremersch, S.; Vandenbroucke, R.E.; Van Wonterghem, E.; Hendrix, A.; De Smedt, S.C.; Raemdonck, K. Comparing Exosome-like Vesicles with Liposomes for the Functional Cellular Delivery of Small RNAs. J. Control. Release 2016, 232, 51–61. [Google Scholar] [CrossRef]
- Antimisiaris, S.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef]
- Liu, C.; Su, C. Design Strategies and Application Progress of Therapeutic Exosomes. Theranostics 2019, 9, 1015–1028. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering Hybrid Exosomes by Membrane Fusion with Liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [Green Version]
- Elkhoury, K.; Koçak, P.; Kang, A.; Arab-Tehrany, E.; Ellis Ward, J.; Shin, S.R. Engineering Smart Targeting Nanovesicles and Their Combination with Hydrogels for Controlled Drug Delivery. Pharmaceutics 2020, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, J.; Xu, F.; Lin, X.; Zhong, J.; Wu, F.; Yuan, L. Role of Tumor-derived Exosomes in Bone Metastasis. Oncol. Lett. 2019, 18, 3935–3945. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sui, B.; Fan, W.; Lei, L.; Zhou, L.; Yang, L.; Diao, Y.; Zhang, Y.; Li, Z.; Liu, J.; et al. Exosomes Derived from Osteogenic Tumor Activate Osteoclast Differentiation and Concurrently Inhibit Osteogenesis by Transferring COL1A1-targeting MiRNA-92a-1-5p. J. Extracell. Vesicles 2021, 10, e12056. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, L.; De Luca, A.; Gallo, A.; Costa, V.; Russelli, G.; Cuscino, N.; Manno, M.; Raccosta, S.; Carina, V.; Bellavia, D.; et al. Osteosarcoma Cell-Derived Exosomes Affect Tumor Microenvironment by Specific Packaging of MicroRNAs. Carcinogenesis 2020, 41, 666–677. [Google Scholar] [CrossRef]
- Roudier, M.P.; Morrissey, C.; True, L.D.; Higano, C.S.; Vessella, R.L.; Ott, S.M. Histopathological Assessment of Prostate Cancer Bone Osteoblastic Metastases. J. Urol. 2008, 180, 1154–1160. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A New Self-Healing Hydrogel Containing HucMSC-Derived Exosomes Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef]
- Huang, J.-L.; Chen, H.-Z.; Gao, X.-L. Lipid-Coated Calcium Phosphate Nanoparticle and beyond: A Versatile Platform for Drug Delivery. J. Drug Target. 2018, 26, 398–406. [Google Scholar] [CrossRef]
- Placente, D.; Benedini, L.A.; Baldini, M.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Multi-Drug Delivery System Based on Lipid Membrane Mimetic Coated Nano-Hydroxyapatite Formulations. Int. J. Pharm. 2018, 548, 559–570. [Google Scholar] [CrossRef]
- Brangule, A.; Gross, K.; Komarovska, L.; Viksna, A. Exploring Zinc Apatites through Different Synthesis Routes. Key Eng. Mater. 2013, 587, 171–176. [Google Scholar] [CrossRef]
- Brangule, A.; Gross, K.; Skadiņš, I.; Reinis, A.; Kroiča, J. Simultaneous Identification of Amorphous Calcium Phosphate and S. Epidermidis Bacteria by Photoacoustic Spectroscopy. Key Eng. Mater. 2016, 720, 125–129. [Google Scholar] [CrossRef]
- Brangule, A.; Gross, K. Effect on Drying Conditions on Amorphous Calcium Phosphate. Key Eng. Mater. 2014, 631, 99–103. [Google Scholar] [CrossRef]
- Brangule, A.; Gross, K.A. Importance of FTIR Spectra Deconvolution for the Analysis of Amorphous Calcium Phosphates. IOP Conf. Ser. Mater. Sci. Eng. 2015, 77, 012027. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium Phosphate Cements as Drug Delivery Materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, D.; Xie, F.; Qin, M.; Lin, Z.; Wang, Y. Fabrication and Characterization of Calcium-Phosphate Lipid System for Potential Dental Application. Front. Chem. 2020, 8, 161. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous Calcium (Ortho)Phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Dorozhkin, S. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Li, W.; Lenzo, J.C.; Holden, J.A.; McCullough, M.J.; O’Connor, A.J.; O’Brien-Simpson, N.M. The Potential of Calcium Phosphate Nanoparticles as Adjuvants and Vaccine Delivery Vehicles. Front. Mater. 2021, 8, 788373. [Google Scholar] [CrossRef]
- Niu, X.-Q.; Zhang, D.-P.; Bian, Q.; Feng, X.-F.; Li, H.; Rao, Y.-F.; Shen, Y.-M.; Geng, F.-N.; Yuan, A.-R.; Ying, X.-Y.; et al. Mechanism Investigation of Ethosomes Transdermal Permeation. Int. J. Pharm. X 2019, 1, 100027. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Niu, B.; Guo, X.; Rao, C.; Li, W. Hyaluronic Acid-Amorphous Calcium Phosphate Nanoparticles for Drug Delivery and Anticancer. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 561–567. [Google Scholar] [CrossRef]
- Olton, D.; Li, J.; Wilson, M.E.; Rogers, T.; Close, J.; Huang, L.; Kumta, P.N.; Sfeir, C. Nanostructured Calcium Phosphates (NanoCaPs) for Non-Viral Gene Delivery: Influence of the Synthesis Parameters on Transfection Efficiency. Biomaterials 2007, 28, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous Calcium Phosphates: Synthesis, Properties and Uses in Biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Li, W.; Wang, H.; Fan, Y.-Y.; Wang, H.; Gao, X.; Niu, B.; Gong, X. Preparation, Characterization, Release and Antioxidant Activity of Curcumin-Loaded Amorphous Calcium Phosphate Nanoparticles. J. Non-Cryst. Solids 2018, 500, 317–325. [Google Scholar] [CrossRef]
- Amirian, J.; Makkar, P.; Lee, G.H.; Paul, K.; Lee, B.T. Incorporation of Alginate-Hyaluronic Acid Microbeads in Injectable Calcium Phosphate Cement for Improved Bone Regeneration. Mater. Lett. 2020, 272, 127830. [Google Scholar] [CrossRef]
- Amirian, J.; Van, T.T.T.; Bae, S.-H.; Jung, H.-I.; Choi, H.-J.; Cho, H.-D.; Lee, B.-T. Examination of In Vitro and In Vivo Biocompatibility of Alginate-Hyaluronic Acid Microbeads as a Promising Method in Cell Delivery for Kidney Regeneration. Int. J. Biol. Macromol. 2017, 105, 143–153. [Google Scholar] [CrossRef]
- Sapino, S.; Chindamo, G.; Chirio, D.; Manzoli, M.; Peira, E.; Riganti, C.; Gallarate, M. Calcium Phosphate-Coated Lipid Nanoparticles as a Potential Tool in Bone Diseases Therapy. Nanomaterials 2021, 11, 2983. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, Y.; Rao, N.; Li, J.; Li, X.; Fang, T.; Zhao, Y.; Ge, L. Activation and Biological Properties of Human β Defensin 4 in Stem Cells Derived From Human Exfoliated Deciduous Teeth. Front. Physiol. 2019, 10, 1304. [Google Scholar] [CrossRef]
- Huang, J.-L.; Jiang, G.; Song, Q.-X.; Gu, X.; Hu, M.; Wang, X.-L.; Song, H.-H.; Chen, L.-P.; Lin, Y.-Y.; Jiang, D.; et al. Lipoprotein-Biomimetic Nanostructure Enables Efficient Targeting Delivery of siRNA to Ras-Activated Glioblastoma Cells via Macropinocytosis. Nat. Commun. 2017, 8, 15144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Wang, W.; Liu, X.; Xu, C.; Wang, Y.; Wang, Z.; Xu, J.; Guan, J.; Zhou, P.; Mao, Y. Gelatin Methacrylate Hydrogel Scaffold Carrying Resveratrol-Loaded Solid Lipid Nanoparticles for Enhancement of Osteogenic Differentiation of BMSCs and Effective Bone Regeneration. Regen. Biomater. 2021, 8, rbab044. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef]
- Das, T.; Venkatesh, M.P.; Pramod Kumar, T.M.; Koland, M. SLN Based Alendronate in Situ Gel as an Implantable Drug Delivery System—A Full Factorial Design Approach. J. Drug Deliv. Sci. Technol. 2020, 55, 101415. [Google Scholar] [CrossRef]
- Shan, H.; Li, K.; Zhao, D.; Chi, C.; Tan, Q.; Wang, X.; Yu, J.; Piao, M. Locally Controlled Release of Methotrexate and Alendronate by Thermo-Sensitive Hydrogels for Synergistic Inhibition of Osteosarcoma Progression. Front. Pharmacol. 2020, 11, 573. [Google Scholar] [CrossRef]
- Sun, Y.; Li, K.; Li, C.; Zhang, Y.; Zhao, D. Thermogel Delivers Oxaliplatin and Alendronate In Situ for Synergistic Osteosarcoma Therapy. Front. Bioeng. Biotechnol. 2020, 8, 573962. [Google Scholar] [CrossRef]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-Situ Crosslinked Hydrogel Based on Amidated Pectin/Oxidized Chitosan as Potential Wound Dressing for Skin Repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Pandey, A.; Yang, T.-S.; Cheng, S.-L.; Huang, C.-S.; Brangule, A.; Kareiva, A.; Yang, J.-C. A Novel One-Pot Synthesis and Characterization of Silk Fibroin/α-Calcium Sulfate Hemihydrate for Bone Regeneration. Polymers 2021, 13, 1996. [Google Scholar] [CrossRef]
- Cheng, R.; Yan, Y.; Liu, H.; Chen, H.; Pan, G.; Deng, L.; Cui, W. Mechanically Enhanced Lipo-Hydrogel with Controlled Release of Multi-Type Drugs for Bone Regeneration. Appl. Mater. Today 2018, 12, 294–308. [Google Scholar] [CrossRef]
- Patterson, J.; Siew, R.; Herring, S.W.; Lin, A.S.P.; Guldberg, R.; Stayton, P.S. Hyaluronic Acid Hydrogels with Controlled Degradation Properties for Oriented Bone Regeneration. Biomaterials 2010, 31, 6772–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirian, J.; Abdi, G.; Shekh, M.I.; Zendehdel, E.A.; Du, B.; Stadler, F.J. Gelatin Based Hydrogels for Tissue Engineering and Drug Delivery Applications. Mater. Res. Found. 2021, 81, 244–270. [Google Scholar]
- Liu, L.; Xiang, Y.; Wang, Z.; Yang, X.; Yu, X.; Lu, Y.; Deng, L.; Cui, W. Adhesive Liposomes Loaded onto an Injectable, Self-Healing and Antibacterial Hydrogel for Promoting Bone Reconstruction. NPG Asia Mater. 2019, 11, 81. [Google Scholar] [CrossRef]
- Gradauer, K.; Barthelmes, J.; Vonach, C.; Almer, G.; Mangge, H.; Teubl, B.; Roblegg, E.; Dünnhaupt, S.; Fröhlich, E.; Bernkop-Schnürch, A.; et al. Liposomes Coated with Thiolated Chitosan Enhance Oral Peptide Delivery to Rats. J. Control. Release 2013, 172, 872–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Dhand, C.; Ong, S.T.; Dwivedi, N.; Diaz, S.M.; Venugopal, J.R.; Navaneethan, B.; Fazil, M.H.U.T.; Liu, S.; Seitz, V.; Wintermantel, E.; et al. Bio-Inspired In Situ Crosslinking and Mineralization of Electrospun Collagen Scaffolds for Bone Tissue Engineering. Biomaterials 2016, 104, 323–338. [Google Scholar] [CrossRef]
- Hallan, S.S.; Kaur, P.; Kaur, V.; Mishra, N.; Vaidya, B. Lipid Polymer Hybrid as Emerging Tool in Nanocarriers for Oral Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 334–349. [Google Scholar] [CrossRef]
- Singh Hallan, S.; Sguizzato, M.; Pavoni, G.; Baldisserotto, A.; Drechsler, M.; Mariani, P.; Esposito, E.; Cortesi, R. Ellagic Acid Containing Nanostructured Lipid Carriers for Topical Application: A Preliminary Study. Molecules 2020, 25, 1449. [Google Scholar] [CrossRef] [Green Version]
- Ezzati Nazhad Dolatabadi, J.; Hamishehkar, H.; Eskandani, M.; Valizadeh, H. Formulation, Characterization and Cytotoxicity Studies of Alendronate Sodium-Loaded Solid Lipid Nanoparticles. Colloids Surf. B Biointerfaces 2014, 117, 21–28. [Google Scholar] [CrossRef]
- Ajiboye, A.L.; Nandi, U.; Galli, M.; Trivedi, V. Olanzapine Loaded Nanostructured Lipid Carriers via High Shear Homogenization and Ultrasonication. Sci. Pharm. 2021, 89, 25. [Google Scholar] [CrossRef]
- Hallan, S.S.; Nidhi; Kaur, V.; Jain, V.; Mishra, N. Development and Characterization of Polymer Lipid Hybrid Nanoparticles for Oral Delivery of LMWH. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1631–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, K.; Hallan, S.S.; Lal, B.; Bhardwaj, A.; Mishra, N. Development and Characterization of Floating Spheroids of Atorvastatin Calcium Loaded NLC for Enhancement of Oral Bioavailability. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1448–1456. [Google Scholar] [CrossRef]
- Singh, D.; Rashid, M.; Hallan, S.S.; Mehra, N.K.; Prakash, A.; Mishra, N. Pharmacological Evaluation of Nasal Delivery of Selegiline Hydrochloride-Loaded Thiolated Chitosan Nanoparticles for the Treatment of Depression. Artif. Cells Nanomed. Biotechnol. 2016, 44, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, Y.; Xie, C.; Chen, C.; Lin, D.; Wang, S.; Lin, D.; Cui, X.; Guo, Z.; Zhou, J. Dual-Active Targeting Liposomes Drug Delivery System for Bone Metastatic Breast Cancer: Synthesis and Biological Evaluation. Chem. Phys. Lipids 2019, 223, 104785. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Lin, W.; Li, X.; Wang, H.; Xiao, X.; Guo, Z. Synergistic Dual-Modified Liposome Improves Targeting and Therapeutic Efficacy of Bone Metastasis from Breast Cancer. Drug Deliv. 2017, 24, 1680–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Mostafa, N.Z.; Incani, V.; Kucharski, C.; Uludağ, H. Bisphosphonate-Decorated Lipid Nanoparticles Designed as Drug Carriers for Bone Diseases. J. Biomed. Mater. Res. Part A 2012, 100, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hou, J.; Zhong, Z.; Hao, N.; Lin, Y.; Li, C. Targeted Delivery of Hyaluronic Acid-Coated Solid Lipid Nanoparticles for Rheumatoid Arthritis Therapy. Drug Deliv. 2018, 25, 716–722. [Google Scholar] [CrossRef]
- Vhora, I.; Lalani, R.; Bhatt, P.; Patil, S.; Misra, A. Lipid-Nucleic Acid Nanoparticles of Novel Ionizable Lipids for Systemic BMP-9 Gene Delivery to Bone-Marrow Mesenchymal Stem Cells for Osteoinduction. Int. J. Pharm. 2019, 563, 324–336. [Google Scholar] [CrossRef]
- Basha, G.; Ordobadi, M.; Scott, W.R.; Cottle, A.; Liu, Y.; Wang, H.; Cullis, P.R. Lipid Nanoparticle Delivery of siRNA to Osteocytes Leads to Effective Silencing of SOST and Inhibition of Sclerostin In Vivo. Mol. Ther.-Nucleic Acids 2016, 5, e363. [Google Scholar] [CrossRef] [Green Version]
- Haber, E.; Afergan, E.; Epstein, H.; Gutman, D.; Koroukhov, N.; Ben-David, M.; Schachter, M.; Golomb, G. Route of Administration-Dependent Anti-Inflammatory Effect of Liposomal Alendronate. J. Control. Release 2010, 148, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Kharshoum, R.M.; Mahmoud, M.; Azim, S.A.; Ebeid, E.-Z.M. Development and Characterization of a Novel Nano-Liposomal Formulation of Alendronate Sodium Loaded with Biodegradable Polymer. Ars Pharm. Internet 2018, 59, 9–20. [Google Scholar] [CrossRef]
- da Rocha, M.C.O.; da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; de Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-Loaded Solid Lipid Nanoparticles Prevent Tumor Growth and Lung Metastasis of 4T1 Murine Mammary Carcinoma Cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Parhi, P.; Suklabaidya, S.; Kumar Sahoo, S. Enhanced Anti-Metastatic and Anti-Tumorigenic Efficacy of Berbamine Loaded Lipid Nanoparticles in Vivo. Sci. Rep. 2017, 7, 5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granja, A.; Lima-Sousa, R.; Alves, C.G.; de Melo-Diogo, D.; Pinheiro, M.; Sousa, C.T.; Correia, I.J.; Reis, S. Mitoxantrone-Loaded Lipid Nanoparticles for Breast Cancer Therapy—Quality-by-Design Approach and Efficacy Assessment in 2D and 3D In Vitro Cancer Models. Int. J. Pharm. 2021, 607, 121044. [Google Scholar] [CrossRef] [PubMed]
- Guney Eskiler, G.; Cecener, G.; Dikmen, G.; Egeli, U.; Tunca, B. Solid Lipid Nanoparticles: Reversal of Tamoxifen Resistance in Breast Cancer. Eur. J. Pharm. Sci. 2018, 120, 73–88. [Google Scholar] [CrossRef]
- Sguizzato, M.; Drechsler, M.; Baldisserotto, A.; Cortesi, R.; Esposito, E. Antioxidant-Containing Monoolein Aqueous Dispersions: A Preliminary Study. Drug Deliv. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A Brief Review on Solid Lipid Nanoparticles: Part and Parcel of Contemporary Drug Delivery Systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Huang, X.; Guo, H.; Wang, L.; Yang, W.; Wu, W.; Jing, D.; Shao, Z. Exosomes as Efficient Nanocarriers in Osteosarcoma: Biological Functions and Potential Clinical Applications. Front. Cell Dev. Biol. 2021, 9, 737314. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Lee, C.-S.; Lee, M. Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering 2021, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef]
- Tang, J.; Cai, L.; Xu, C.; Sun, S.; Liu, Y.; Rosenecker, J.; Guan, S. Nanotechnologies in Delivery of DNA and MRNA Vaccines to the Nasal and Pulmonary Mucosa. Nanomaterials 2022, 12, 226. [Google Scholar] [CrossRef]
- Yuan, Z.; Das, S.; Do, C.; Park, Y.C. Effect of Cholesterol on Nano-Structural Alteration of Light-Activatable Liposomes via Laser Irradiation: Small Angle Neutron Scattering Study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128548. [Google Scholar] [CrossRef]
- Chaves, M.A.; Baldino, L.; Pinho, S.C.; Reverchon, E. Supercritical CO2 Assisted Process for the Production of Mixed Phospholipid Nanoliposomes: Unloaded and Vitamin D3-Loaded Vesicles. J. Food Eng. 2022, 316, 110851. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Niosomes Formation Using a Continuous Supercritical CO2 Assisted Process. J. CO2 Util. 2021, 52, 101669. [Google Scholar] [CrossRef]
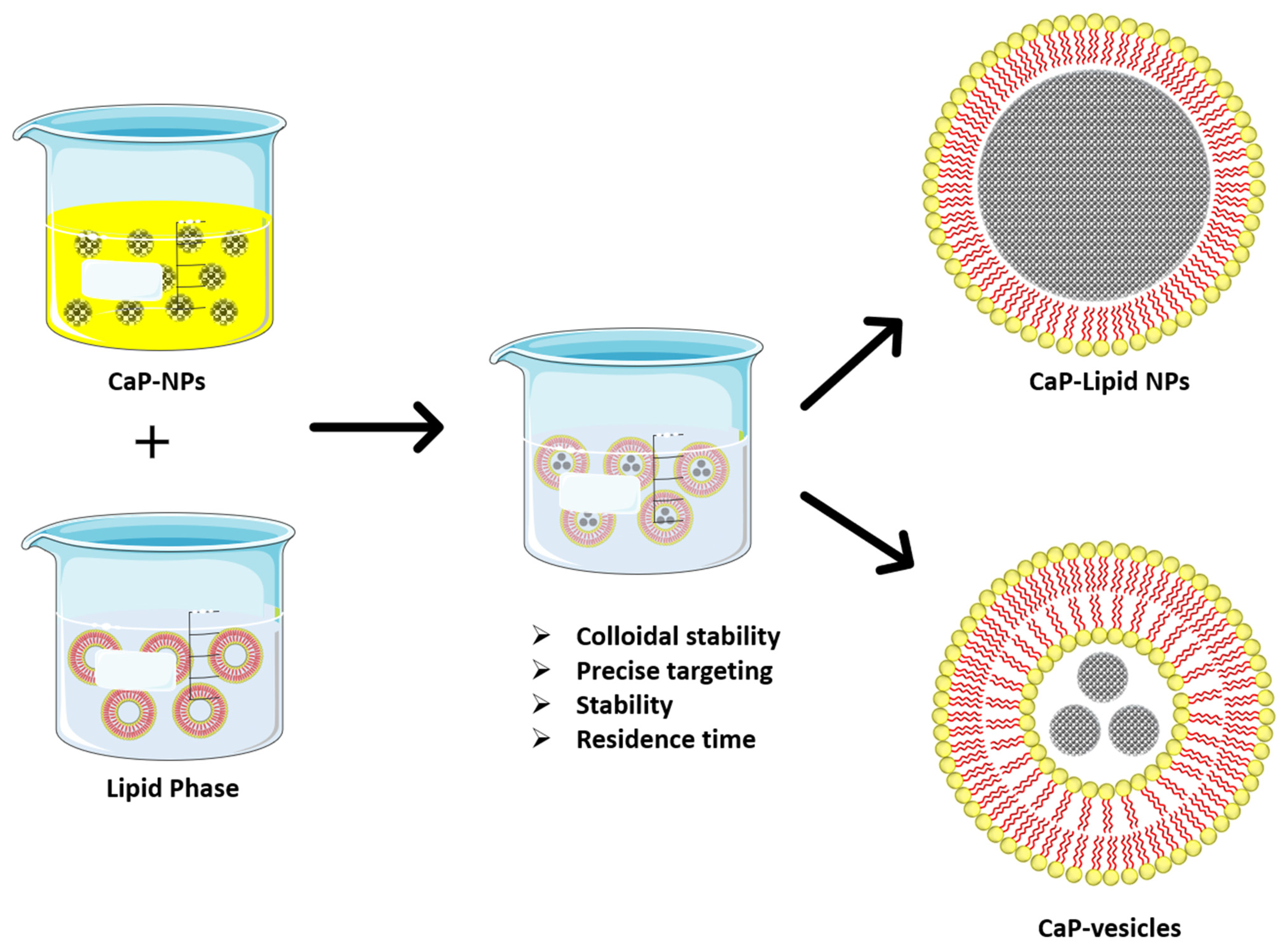
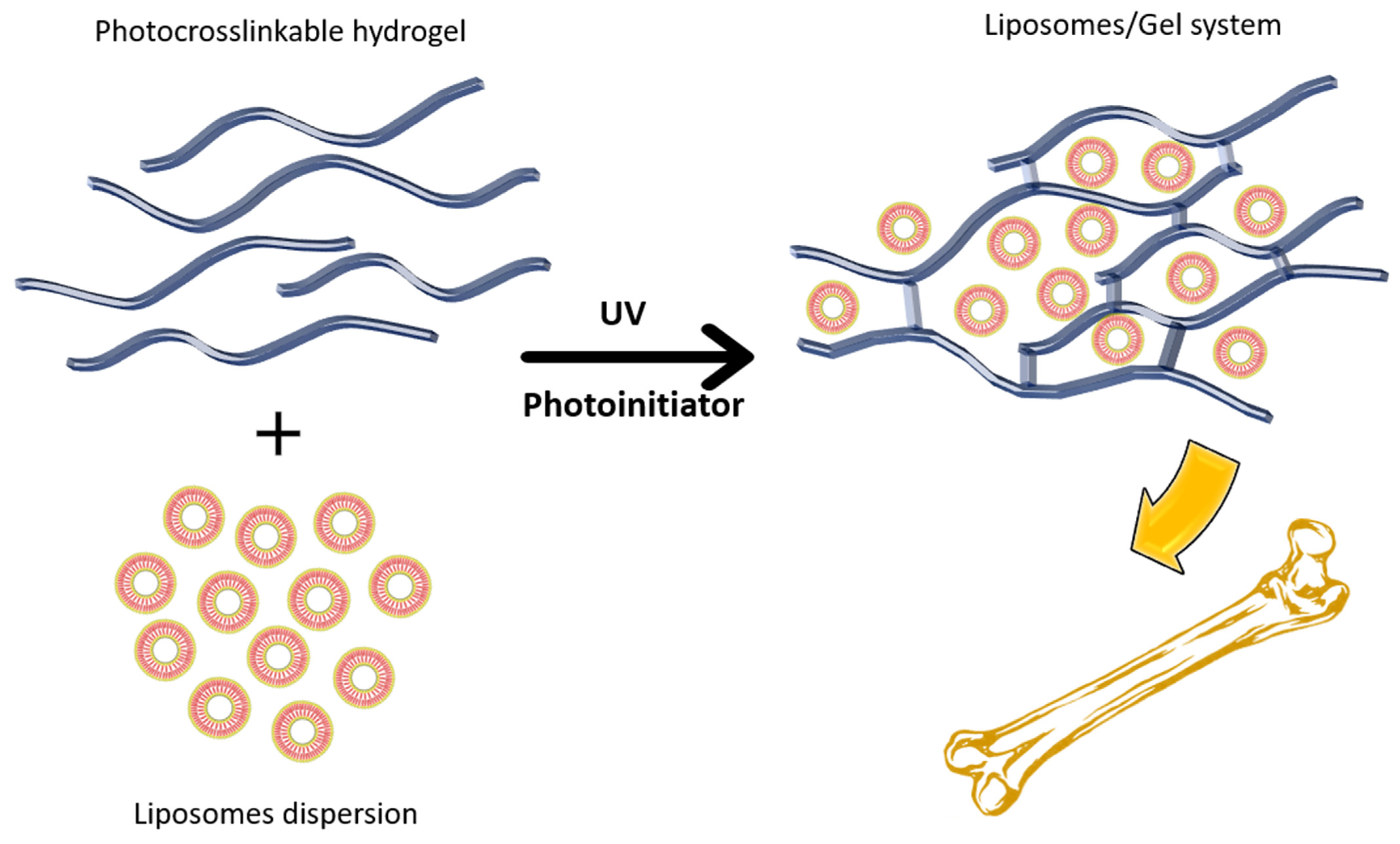
| Drug | Issue | Formulation | Outcome | Ref. |
|---|---|---|---|---|
| Metvan | Rapid oxidation, interference with blood components | Nanostructured Lipid Carriers | Quantitative encapsulation efficiency, sustained-release within 48 h, high cytotoxic effects | [25] |
| Icariin | Low water-solubility, susceptible to first-pass metabolism, and low bioavailability | Liposomes | Amplified the mechanical strength of femoral midshaft, triggered bone turnover/remodeling | [26] |
| Simvastatin | Deterioration at a physiological pH, low water solubility, low bioavailability, high toxicity | Lipid nanoparticles | Higher encapsulation efficiency with a sustained release of 70% within 50 h, reduction in cytotoxicity | [27] |
| Doxycycline | Degradation in the anhydrous environment, poor bone penetration | Lipid- Polymer hybrid system | Zero-order release rate up to one month, eradicate bacterial bone infections | [28] |
| Edelfosine | Poor oral bioavailability, dose-dependent hemolysis | Lipid nanoparticles | Shows immediate cytotoxicity to human osteosarcoma cells, negligible tumor growth with declining of tumor volume by five-fold | [29] |
| TNF-α small interfering RNA | Short half-life, deprived extravasation from blood vessels to target cells, low cellular uptake | PEGylated solid-lipid nanoparticles | Encapsulation efficiency more than 90%, precise targeting to inflamed sites in a mouse model, declined bone loss, | [30] |
| Formulation | Cargo | Avg. Diameter (nm) | PDI | Zeta Potential (mV) | %EE | Ref. |
|---|---|---|---|---|---|---|
| Liposomes | Sodium-alendronate | 185.2 ± 22 | <0.3 | −27.4 ± 1 | N/A | [133] |
| 160 ± 24 | <0.1 | –29.2 ± 1.9 | 30 ± 5 | [37] | ||
| 298 ± 3.5 | 0.07 ± 0.2 | −39 ± 2.19 | 78.5 | [134] | ||
| Lipid nanoparticles | Edelfosine | 124 ± 12 | 0.16 ± 0.01 | −14.5 | N/A | [29] |
| Docetaxel | 128 ± 2.2 | 0.153 ± 0.02. | − 15 ± 0.5 | 86 ± 2.4 | [135] | |
| Berbamine | 75 | <0.3 | −16 | 87 | [136] | |
| Mitoxantrone | 230 ± 17 | 0.16 ± 0.01 | −3 ± 1 | 93 ± 6 | [137] | |
| Tamoxifen | 277.4 ± 1.26 | 0.298 ± 0.05 | −40.5 ± 1.61 | N/A | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallan, S.S.; Amirian, J.; Brangule, A.; Bandere, D. Lipid-Based Nano-Sized Cargos as a Promising Strategy in Bone Complications: A Review. Nanomaterials 2022, 12, 1146. https://doi.org/10.3390/nano12071146
Hallan SS, Amirian J, Brangule A, Bandere D. Lipid-Based Nano-Sized Cargos as a Promising Strategy in Bone Complications: A Review. Nanomaterials. 2022; 12(7):1146. https://doi.org/10.3390/nano12071146
Chicago/Turabian StyleHallan, Supandeep Singh, Jhaleh Amirian, Agnese Brangule, and Dace Bandere. 2022. "Lipid-Based Nano-Sized Cargos as a Promising Strategy in Bone Complications: A Review" Nanomaterials 12, no. 7: 1146. https://doi.org/10.3390/nano12071146
APA StyleHallan, S. S., Amirian, J., Brangule, A., & Bandere, D. (2022). Lipid-Based Nano-Sized Cargos as a Promising Strategy in Bone Complications: A Review. Nanomaterials, 12(7), 1146. https://doi.org/10.3390/nano12071146








