Nanomaterial Exposure, Extracellular Vesicle Biogenesis and Adverse Cellular Outcomes: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical and Methodological Framework
2.2. Focused Question
2.3. Search Strategy
2.4. Eligibility Criteria
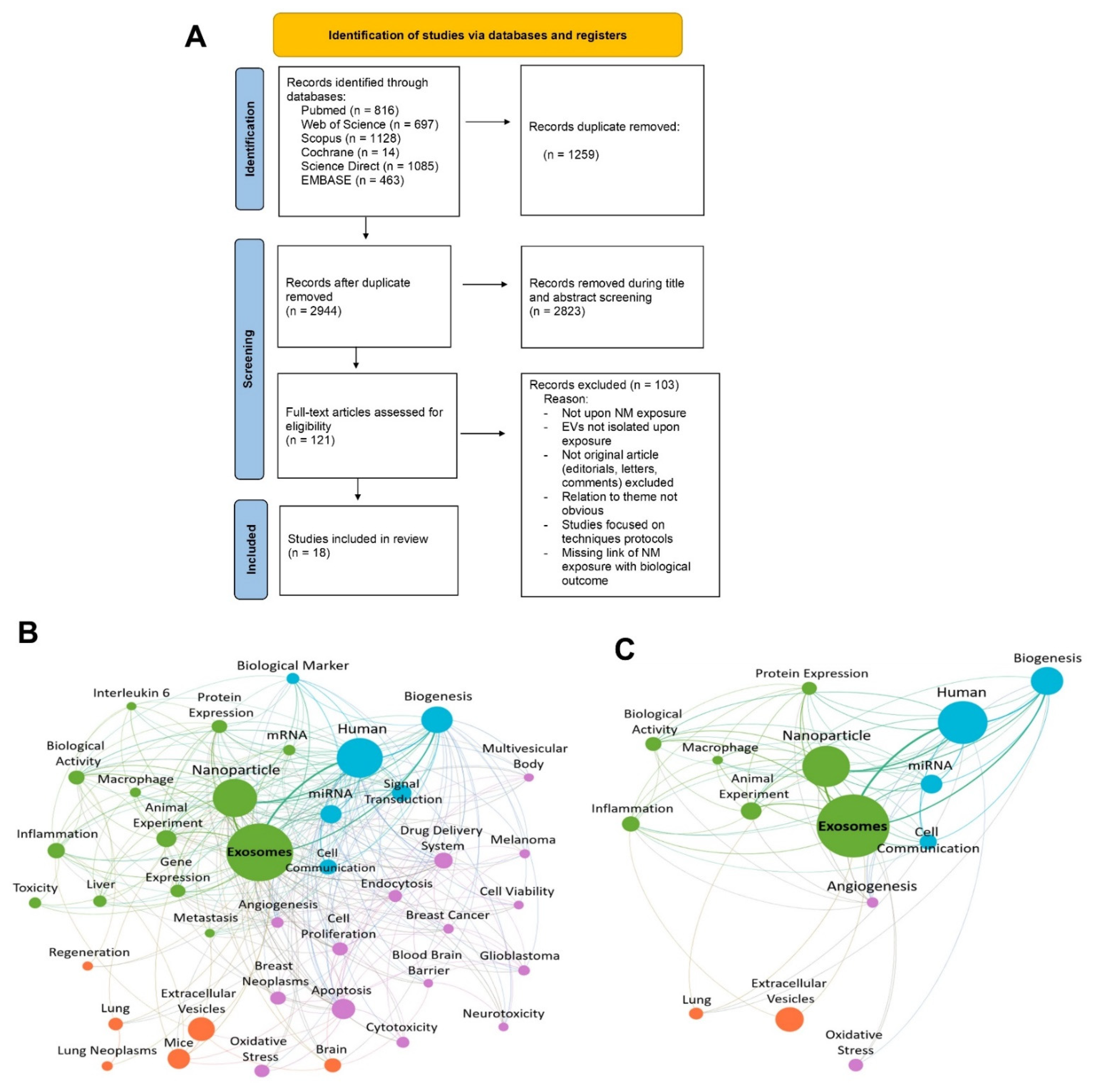
2.5. Article Selection, Screening Process, and Data Extraction
2.6. Assessment of Reliability
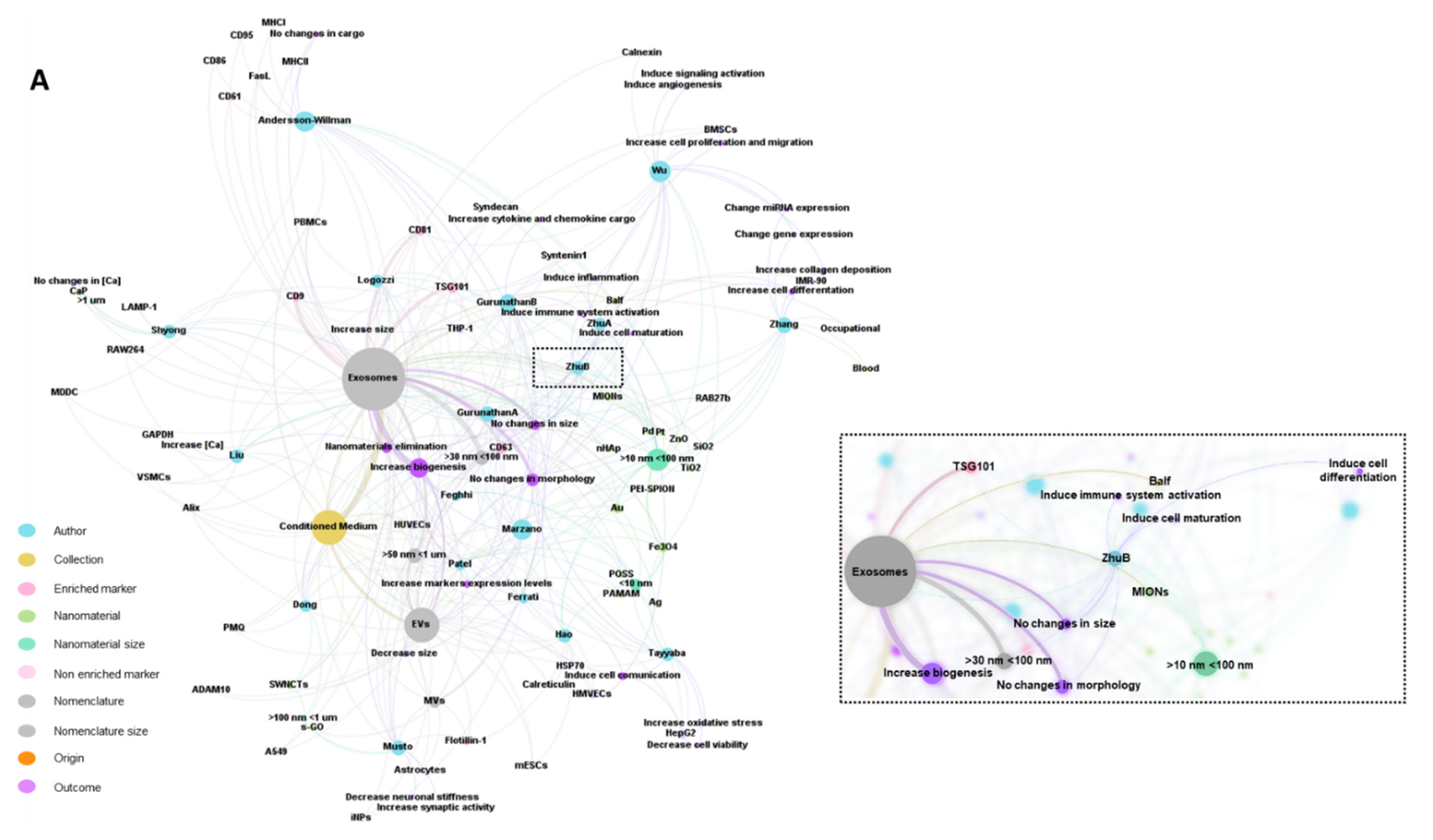
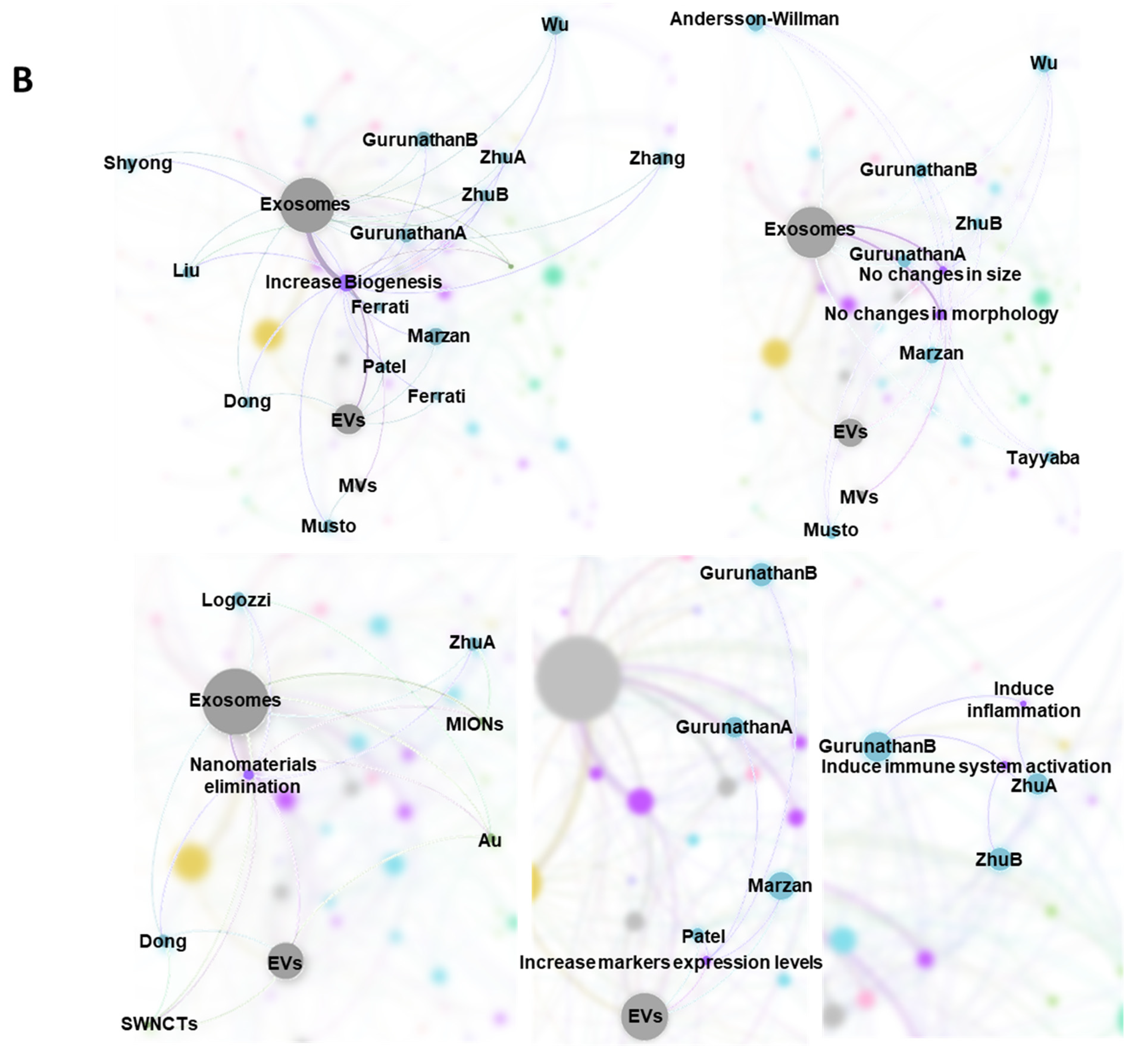
2.7. Bibliometric Data Analisys
2.8. Tabular Data Analysis
3. Results
3.1. Characteristics of the Studies
3.2. Biological Models, Exposure to NMs and Correlation with Physicochemical Characteristics
| Reference | Nanomaterial | Exposure Conditions | Biological Effects 4 | ||||
|---|---|---|---|---|---|---|---|
| Type 1 | Size 2 | Morphology/Crystalline Structure | Purity 3 | Z Potential | |||
| [20] | PEI-SPION NPs | 15 nm | - | - | Low density: 4.5 mV High density: 7.7 mV | Immersion CM 2–7 μg/mL 24 h | In vitro Human HMVECs: PEI-SPION NPs uptake did not impact cell viability |
| [22] | MIONs | - | - | LPS < 0.25 EU ml−1 | - | Respiratory exposure (Intratracheal instillation) 20 μg in 50 μL PBS Three times at daily intervals (days 0, 2, and 4). | In vivo Mouse BALB/c: NPs transferred across the pulmonary cell membrane and located in lysosomes |
| [24] | SiO2 NPs Occupational NPs | 10–20 nm | - | ≈ 99.5% | - | Immersion CM 100 µg/mL 24 h Exposed to occupational inhaling | In vitro: Human THP-1 Clinical: pneumoconiosis patients |
| [25] | Au NPs | P: 20 nm Water: 20.5 nm (T0 h), 20.2 nm (T24 h), CM: 19.5 nm (T0 h), 19.9 nm (T24 h) | - | - | - | Immersion CM 0.1, 1, 10 e 50 μM 24 h | In vitro Human PBMCs: Internalization of Au NPs in the early endosomes and/or in structures resembling MVB |
| [26] | SWNCTs | 200–1000 nm | Fiber-like | >95% | −44.1 mV (pH 12); −23.2 mv (pH 2) | Immersion CM 10 μg/mL 0–48 h | In vitro Mouse PMQ: ↑ SWCNTs uptake with prolonged exposure time No significant cell death. Alteration in primary macrophage morphology |
| [39] | TiO2 NPs ZnO NPs | P: 21 nm; CM: 28.6 nm P: 10 nm; CM: 16.9 nm | - | LPS: NPs < 50 pg/mL; Culture media < 5 pg/mL) | −12.2 ± 0.25 mV 11.4 ± 0.17 mV | Immersion CM 0.5–100 μg/mL 24 h | In vitro Human PBMC: No cell death MDDC: NPs active uptake. No alteration of surface markers In vitro Human MDDC: ↑ Cell death, ↑ Cas dose-dependent, ↑ DNA fragmentation. No NP uptake and no change in surface-marker expression PBMC: No differences in inactivation or expression of CD69 in T-cell. ↓ CD16 on NK-cells |
| [40] | Fe3O4 NPs | 100 nm | - | - | - | Immersion CM 400, 200, 100, 50, 25 μg/mL 1, 3, and 5 days | In vitro: Human BMSCs: ↑ Cell viability in the optimal working concentration (50 μg/mL) |
| [41] | MIONs | P: 43 nm Solution: 43 nm | Cubic | LPS < 0.25 EU ml−1) | - | Respiratory exposure (Intratracheal instillation) 20 μg in 50 μL PBS days 15, 17, and 19 | In vivo Mouse BALB/c: NPs transferred across the pulmonary cell membrane and located in lysosomes ↑ Th1 polarization ↑ Tc1 OVA-sensitized mouse BALB/c Mouse BALB/c: ↑ Activated Th + Tc ↑ Th1, ↑ Tc1 ↑ IFN-γ, ↑ IL-4 ↑ Inflammation |
| [42] | CaP | 1.84 ± 0.48 μm | Spherical or oval | - | −2.49 mV | Immersion CM 250, 500, 1000, 2000 μg/mL 1–3 days | In vitro Mouse RAW264.7: No alteration in proliferation In vitro Human THP-1: No alteration in proliferation |
| [43] | Au NPs | 5, 20, 80 nm | Spherical | - | AuNPs-5: −22.01 ± 1.81 mV, AuNPs-20: −32.17 ± 2.19, AuNPs-80: −55.21 ± 7.34 mV | Immersion CM 1 μg/mL 24–48 h | In vitro Mouse mESCs: LOEC: 5 μg/mL Non-cytotoxic and does not induce ROS. No interference in self-renewal or pluripotency |
| [44] | Pt NPs | 40–50 nm | Spherical, triangular, oval, and rod-shaped | - | Immersion CM 0, 2.5, 5, 10, 20, and 40 μmol/L 24 h | In vitro Human A549 monolayer culture: ↑Viability and proliferation Morphological signals of autophagy ↑ ROS, ↑ Cas3, ↑ LDH, ↑AchE | |
| [45] | Pd NPs | ~20 nm | Spherical | - | - | Immersion CM 5–25 μM 24 h | In vitro Human THP-1 Monolayer culture: |
| [46] | Fe3O4 NPs | 8, 15, 30 nm | Spherical | 8 nm (99.9%), 15–20 nm (99.5%) and 20–30 nm (99.0%) | - | Immersion CM 0, 1, 10 e 100 μM 48 h | In vitro Human iNPCs Cortical spheroids culture: Changes in morphology No effects on cell viability, metabolic activity, neurodegeneration, or oxidative stress |
| [47] | POSS NPs | 3–5 nm | Spherical | - | - | Immersion culture media 0- 600 ppm24 and 48 h | In vitro: Human HUVECs: ↑ Viability, ↑ Migration, ↑ Wound healing, ↑ VEGFR-2, HSP-70, Ang-1, and Ang-2, ↑ miRNA-21 and miRNA-155, ↑ VEGF-A and TGF-β, ↓ miRNA-182 |
| [48] | nHAp | <100 nm | Rod-like | 97% | - | Immersion CM 100 ug/mL 24 h, 7, and 14 days | In vitro Mice C57BL/6 VSMCs: ↑ ALP, Runx2, and OPN, ↑ Autophagic organelles, ↓ Lysosomal acidification, No effect on the viability, ↑ calcium deposition |
| [49] | s-GO | 50–500 nm | - | LPS free | −55.9 ± 1.4 mV | Immersion CM 10 μg/mL Six days | In vitro Rats Wistar Astrocytes: No impairment of astrocyte morphology or cell density No effect on viability Did not cytotoxic effect |
| [50] | PAMAM | G2:3 nm G7: 9 nm | - | - | G2-NH2: 19.8 mV; G2-COOH: −21.7 mV; G2-OH: 4.8 mV G7 NH2: 30.1 mV; G7-COOH: −19.5 mV; 2.8 mV; G7-OH: 2.8 mV | Immersion CM 1–100 μg/mL 24 h | In vitro Human HUVECs: Low cytotoxicity Moderate g1 arrest of cell cycle G2-NH2: ↑ICAM-1(CD54), ↑ Apoptosis G7-NH2: ↑ICAM-1(CD54), ↑PS ↑ Apoptosis ↑ Necrosis ↑ Plasma-membrane blebbing, disintegration, and permeability Moderate g1 arrest of cell cycle |
| [51] | NCs | Ag NCs: 1.3 nm; Fe3O4 NCs: 3.5 nm | - | - | - | Immersion CM 10–100 μmol/L 24 h | In vitro Human L02: Induce dose-dependent cytotoxicity ↓ Viability In vitro Human HepG2: Not difference in cytotoxicity |
3.3. EVs and Outcomes of NM Exposure
| Reference | Nanomaterials 1 | Biological Origin and Fluid Collection 2 | Isolation and Characterization 3 | EV Nomenclature and Size 4 | Ev Enriched and not Enriched Markers 5 | Biological Outcomes 6 |
|---|---|---|---|---|---|---|
| [20] | PEI-SPION NPs 15 nm | HMVECs; Cell-culture-conditioned media | HSC, MS; TEM, LSCM | EVs; 100 ± 1000 nm | - | ↑ EVs associated with apoptotic cell; Intercellular transfer of NMs through EVs PS+ in MCF7, 4T1 or HMVEC co-cultivated with EVs |
| [22] | MIONs | Mouse BALF | Centrifugation and ultracentrifugation; TEM, Protein dosage, FC | Exosomes 30–90 nm | - | ↑ Exosome biogenesis Exosomes MHCI H-2Kd+, MHCII I-Ad+, and CD80+ secreted are of APC origin. In vitro: Exosomes are internalized by AM φ, Raw264.7, and iDCs cells co-cultivated with exosomes. ↑ iDC maturation and secretion of cytokines DC1 and IL-12 exosomes mediated. ↑ Polarizing on T-cell differentiation in Th1 and Tc1 exosomes mediated. ↑ Phagocytic index of AM φ and secretion IL-12, and TNF- α exosomes mediated. In vivo: Exosomes were distributed in the liver and spleen of BALB/c mice |
| [24] | SiO2 NPs 10–20 nm Occupational NPs | IMR-90 and THP-1; Cell-culture-conditioned media Venous blood from patients (pneumoconiosis and control) | Ultracentrifugation; TEM, NTA, miRNA Isolation and High-Throughput Sequencing, Immunoblotting RT-qPCR, Immunohistochemistry, FC | Exosomes 30–150 nm | - | ↑ Exosome biogenesis; ↓ hsa-let-7a-5p, ↓ hsa-let-7i-5p ↑ WASL expression; ↑ Phagocytosis of NPs ↑ Fibroblast transdifferentiation in IMR-90 fibroblasts co-cultivated with exosomes. ↑ Collagen deposition in IMR-90 fibroblasts co-cultivated with exosomes |
| [25] | Au NPs 19.9 ± 3.3 nm | PBMCs Cell-culture-conditioned media | Centrifugation and ultracentrifugation. TEM, WB, Bradford protein assay, FC, SP-ICP-MS, NTA. | Exosomes 127.0 ± 3.8 nm | TSG101, CD9, and CD81 | ↑ Exosome size and refractive index; Au NPs internalized in exosomes. PBMC-derived exosomes eliminate Au NPs |
| [26] | SWNCTs 200–1000 nm | PMQ; Cell-culture-conditioned media | TEM, SEM, RAMAN | Exosomes; 50–100 nm EVs; 100–400 nm | - | ↑ Exosome biogenesis on the surface of macrophages; Exocytosis of SWCNTs through exosomes and EVs; Internalization sustained of SWCNTs in PMQ following exocytosis by exosomes. |
| [39] | TiO2 NPs 28.6 ± 3.2 nm ZnO NPs 16.9 ± 0.3 nm | PBMC and MDDC; Cell-culture-conditioned media | Ultracentrifugation, TEM, Protein concentration, NTA, FC | Exosomes; 30–100 nm | CD81, CD63, CD61, CD86, CD95/FasL MHCI and MHCII | No alterations in exosome secretion, morphology, size, number, or protein cargo |
| [40] | Fe3O4, NPs 100 nm | BMSCs Cell-culture-conditioned media | Centrifugation and ultracentrifugation TEM, NTA, WB | Exosomes 116.2 nm | CD9, CD63, CD81, TSG101 Calnexin | ↑ Exosome biogenesis. No morphological, size, shape, or electron-density alterations In vitro: ↑ Proliferation, migration, and angiogenesis in HUVECs and HSFs co-cultivated with exosomes ↑ miR-21-5p, ↓SPRY2, ↑PI3K/AKT and ERK1/2↑ Exosome biogenesis. No morphological, size, shape, or electron-density alterations In vitro: ↑ Proliferation, migration, and angiogenesis in HUVECs and HSFs co-cultivated with exosomes ↑ miR-21-5p, ↓SPRY2, ↑PI3K/AKT and ERK1/2↑ Migration, proliferation, and tube formation in HUVECs co-cultivated with exosomes ↑ Migration in HSFs co-cultivated with exosomes ↑ Mature miR-21-5p, VEGF, HIF-1α, PDGFRα, and bFGF in HUVECs and HSFs co-cultivated with exosomes In vivo: ↑ Wound closure, ↑ Density of blood vessels, ↑ Collagen deposition, ↓ Scar widths, ↑ Angiogenesis, ↑ Formation of sebaceous glands and hair follicles exosomes induce |
| [41] | MIONs 43 ± 5 nm | Mouse BALF | Centrifugation and ultracentrifugation; TEM, Protein dosage, WB, EDS, ICP-MS, FACS | Exosomes 30–90 nm | TSG101 | No morphological or size changes of exosomes In vitro: ↑ Exosome biogenesis. Exosomes induce iDC maturation. Exosomes induce sensitized T-cell activation and differentiation In vivo: ↑ Exosome biogenesis in the alveolar region of BALB/c mice. Exosomes MHCI H-2Kd+, MHCII I-Ad+, CD80+, and CD86- secreted are of APC origin. Exosomes induce a systemic immune response by being eliminated from alveolar spaces |
| [42] | CaP 1.84 ± 0.48 μm | RAW264.7 and THP-1 Cell-culture-conditioned media | Total Exosomes Isolation Kit EXOCET kit, WB, ICP-OES, DLS | Exosomes: 30.2 ± 8.6 nm Exosome aggregation: 196.3 ± 73.2 | CD9, LAMP-1 | ↑ Exosome biogenesis. No alterations in Ca content |
| [43] | Au NPs 5, 20, and 80 nm | mESCs; Cell-culture-conditioned media | Ultracentrifugation and filtration TEM, WB, NTA, QCM-D, LC-MS/MS | EVs 60–70 nm | CD63, HSP70, and Flotilina-1 Calreticulin | EVs-5: ↑ The rigidity of EVs, differentially expressed protein profile, and cellular uptake. ↓ Proliferation and migration of 4T1 cells co-cultivated with exosomes. ↓ Cofilin expression and Erk phosphorylation sEV-20 and sEV-80: negligible effects |
| [44] | Pt NPs 40–50 nm | A549 Cell-culture-conditioned media | Differential centrifugation and ExoQuick; DLS, NTA, TEM, SEM, EXOCETTM, FP, qRT-PCR, ELISA, BCA | Exosomes 90–100 nm | TSG101, CD81, CD63, CD9 | ↑ Exosome biogenesis ↑ Exosome total protein concentration ↑ Concentrations of TSG101, CD9, CD63, and CD81 proteins, Typical morphology and no significant difference in size were observed |
| [45] | Pd NPs ~20 nm | THP1; Cell-culture-conditioned media | Differential ultracentrifugation and ExoQuickTM; DLS, NTA, SEM, TEM, EXOCETTM, FP, BCA, qRT-PCR, Enzyme-linked immunosorbent assay, ELISA and WB | Exosomes 50–80 nm | TSG101, CD9, CD63 and CD81 | ↑ Exosome biogenesis. ↑ Exosome cytokine and chemokine levels (IL-6, MCP-1, IL-8, GM-CSF, TNF-α and IL-1β). ↑ TSG101, CD9, CD63, and CD81 Exosome markers expression levels; No morphological changes were observed |
| [46] | Fe3O4 NPs 8, 15, and 30 nm | iNPs; Cortical spheroids Culture-conditioned media | Differential ultracentrifugation and PEG-based method; RT-PCR, NTA, TEM | EVs 200–250 nm | CD63, CD81, Alix, TSG101, Syntenin1, ADAM10, RAB27b, and Syndecan | 8 and 15 nm: ↑ EV biogenesis. 30 nm: ↓ EV mean size No morphological changes were observed. Differential gene expression of EV biogenesis markers (CD63, CD81, Alix, TSG101, Syntenin1, ADAM10, RAB27b, and Syndecan) by different size NPs |
| [47] | POSS NPs 3–5 nm | HUVECs; Cell-culture-conditioned media | Exo-spin™ kit and centrifugation SEM, TEM, FC, AChE activity | Exosomes | CD63 | ↑ Exosome biogenesis |
| [48] | nHAp <100 nm | VSMCs; Cell-culture-conditioned media | Centrifugation and ultracentrifugation TEM, DLS, WB, granular analysis | Exosomes 100–133 nm | Alix, TSG101, and CD9 GAPDH | ↑ Exosome biogenesis. ↑ Ca content |
| [49] | s-GO 50–500 nm | Astrocytes; Cell-culture-conditioned media | Centrifugation WB, LSCM, AFM, NTA, FTIR-ATR spectroscopy, UVRR | MVs 50–500 nm | Flotillin-1 | ↑ MV biogenesis; Altered protein content in EVs; No alteration in EV morphology or size; ↑ PSCs in cortical neurons co-cultivated with MVs ↓ Neuronal stiffness. ↑ Synaptic activity |
| [50] | PAMAM G2:3 nm G7: 9 nm | HUVECs Cell-culture-conditioned media | Centrifugation NTA, TEM, FC | EVs 120 nm | - | ↑ EV biogenesis; ↑ EVs CD105+, PS+, TOM20+ |
| [51] | NCs Ag NCs: 1.3 nm Fe3O4 NCs: 3.5 nm | HepG2; Cell-culture-conditioned media | Centrifugation, filtration, and ultracentrifugation TEM, DLS, LSCM, EDS, SEM | Exosomes 50 nm | - | No changes in exosome morphology or size Change in exosome surface charge ↓ Viability in HepG2 and U87 co-cultivated with exosomes Cellular uptake of exosomes HepG2 and U87 ↑ ROS in HepG2 co-cultivated with exosomes |
4. Discussion
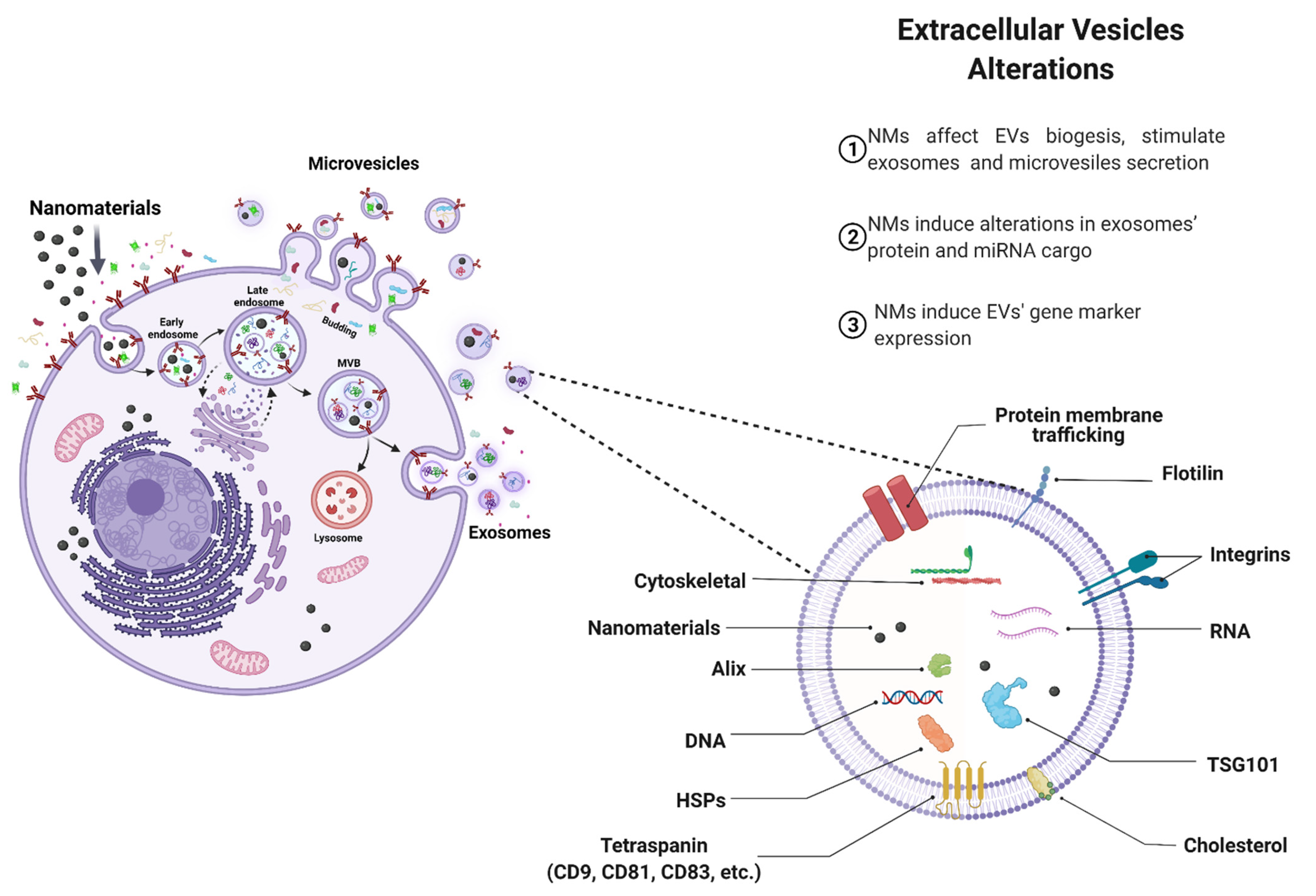
4.1. Effects of NMs on EVs Biogenesis
4.2. Effect of NMs on EV Cargo
4.3. Pathophysiological Implications
4.4. Challenges and Limitations on EV Biogenesis Studies upon NMs Exposure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Aker, W.; Huang, M.-J.; Watts, J.; Hwang, H.-M. Metal Oxide Nanomaterials in Nanomedicine: Applications in Photodynamic Therapy and Potential Toxicity. Curr. Top. Med. Chem. 2015, 15, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hwang, H.-M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Ma, Y.; Cao, Y.; Zhang, T. Mitochondrial toxicity of nanomaterials. Sci. Total Environ. 2020, 702, 134994. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fu, P.; Aker, W.G.; Hwang, H.-M. Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. J. Environ. Sci. Health Part C 2018, 36, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Garantziotis, S.; Rodrigues-Lima, F.; Dupret, J.-M.; Baeza-Squiban, A.; Boland, S. Intracellular Signal Modulation by Nanomaterials. Adv. Exp. Med. Biol. 2014, 801, 111–134. [Google Scholar] [CrossRef] [Green Version]
- Yah, C.S.; Simate, G.S.; Iyuke, S.E. Nanoparticles toxicity and their routes of exposures. Pak. J. Pharm. Sci. 2012, 25, 477–491. [Google Scholar] [PubMed]
- Missaoui, W.N.; Arnold, R.D.; Cummings, B.S. Toxicological status of nanoparticles: What we know and what we don’t know. Chem. Biol. Interact. 2018, 295, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Q.; Tang, R.-Z. Biological responses to nanomaterials: Understanding nano-bio effects on cell behaviors. Drug Deliv. 2017, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspec. Biol. 2016, 8, a006072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Huang, Y.; Zhang, F.; Chen, Q.; Wu, B.; Rui, W.; Zheng, J.C.; Ding, W. Macrophages treated with particulate matter PM 2.5 induce selective neurotoxicity through glutaminase-mediated glutamate generation. J. Neurochem. 2015, 134, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.A.; Westerhof, T.M.; Sabin, K.; Merajver, S.D.; Aguilar, C.A. Engineered Tools to Study Intercellular Communication. Adv. Sci. 2021, 8, 2002825. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, D.S.; Ghaisas, S.; Rokad, D.; Kanthasamy, A.G. Exosomes in Toxicology: Relevance to Chemical Exposure and Pathogenesis of Environmentally Linked Diseases. Toxicol. Sci. 2017, 158, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chidester, S.; Livinski, A.A.; Fish, A.F.; Joseph, P.V. The Role of Extracellular Vesicles in β-Cell Function and Viability: A Scoping Review. Front. Endocrinol. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Stępień, E.Ł.; Durak-Kozica, M.; Kamińska, A.; Targosz-Korecka, M.; Libera, M.; Tylko, G.; Opalinska, A.; Kapusta, M.; Solnica, B.; Georgescu, A.; et al. Circulating ectosomes: Determination of angiogenic microRNAs in type 2 diabetes. Theranostics 2018, 8, 3874–3890. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Long, L.; Huang, Q. Extracellular vesicles in toxicological studies: Key roles in communication between environmental stress and adverse outcomes. J. Appl. Toxicol. 2020, 40, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Stępień, E.Ł.; Rząca, C.; Moskal, P. Novel biomarker and drug delivery systems for theranostics—Extracellular vesicles. Bio-Algorithms Med-Systems 2022, 17, 301–309. [Google Scholar] [CrossRef]
- Wang, S.E. Extracellular Vesicles and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037275. [Google Scholar] [CrossRef] [PubMed]
- Ferrati, S.; McConnell, K.I.; Mack, A.C.; Sirisaengtaksin, N.; Diaz, R.; Bean, A.J.; Ferrari, M.; Serda, R.E. Cellular communication via nanoparticle-transporting biovesicles. Nanomedicine 2014, 9, 581–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, M.Z.; Ratajczak, J. Innate Immunity Communicates Using the Language of Extracellular Microvesicles. Stem Cell Rev. Rep. 2021, 17, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tian, X.; Song, X.; Li, Y.; Tian, Y.; Zhao, Y.; Nie, G. Nanoparticle-Induced Exosomes Target Antigen-Presenting Cells to Initiate Th1-Type Immune Activation. Small 2012, 8, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Malcor, J.-D.M.; Harper, M.T. Lipid rafts are essential for release of phosphatidylserine-exposing extracellular vesicles from platelets. Sci. Rep. 2018, 8, 9987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Hao, C.; Yao, S.; Tang, R.; Guo, W.; Cong, H.; Li, J.; Bao, L.; Wang, D.; Li, Y.; et al. Exosomal miRNA Profiling to Identify Nanoparticle Phagocytic Mechanisms. Small 2018, 14, 1704008. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Bocca, B.; di Raimo, R.; Petrucci, F.; Caimi, S.; Alimonti, A.; Falchi, M.; Cappello, F.; Campanella, C.; et al. Human primary macrophages scavenge AuNPs and eliminate it through exosomes. A natural shuttling for nanomaterials. Eur. J. Pharm. Biopharm. 2019, 137, 23–36. [Google Scholar] [CrossRef]
- Dong, P.-X.; Song, X.; Wu, J.; Cui, S.; Wang, G.; Zhang, L.; Sun, H. The Fate of SWCNTs in Mouse Peritoneal Macrophages: Exocytosis, Biodegradation, and Sustainable Retention. Front. Bioeng. Biotechnol. 2020, 8, 211. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Inter. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef] [PubMed]
- Hartling, L.; Milne, A.; Hamm, M.P.; Vandermeer, B.; Ansari, M.; Tsertsvadze, A.; Druden, D.M. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J. Clin. Epidemiol. 2013, 66, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eck, N.J.; Waltman, L. Visualizing Bibliometric Networks. Measuring Scholarly Impact; Springer International Publishing: Cham, Germany, 2014; pp. 285–320. [Google Scholar] [CrossRef]
- Clauset, A.; Newman, M.E.J.; Moore, C. Finding community structure in very large networks. Phys. Rev. E 2004, 70, 066111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks; Association for the Advancement of Artificial Intelligence: Menlo Park, CA, USA, 2009. [Google Scholar]
- Andersson-Willman, B.; Gehrmann, U.; Cansu, Z.; Buerki-Thurnherr, T.; Krug, H.F.; Gabrielsson, S.; Scheynius, A. Effects of subtoxic concentrations of TiO2 and ZnO nanoparticles on human lymphocytes, dendritic cells and exosome production. Toxicol. Appl. Pharmacol. 2012, 264, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kang, L.; Tian, J.; Wu, Y.; Liu, J.; Li, Z.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated miR-21-5p. Int. J. Nanomed. 2020, 15, 7979–7993. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Shi, J.; Feng, W.; Nie, G.; Zhao, Y. Exosomes as Extrapulmonary Signaling Conveyors for Nanoparticle-Induced Systemic Immune Activation. Small 2012, 8, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Shyong, Y.-J.; Chang, K.-C.; Lin, F.-H. Calcium phosphate particles stimulate exosome secretion from phagocytes for the enhancement of drug delivery. Colloids Surf. B Biointerfaces 2018, 171, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Ku, T.; Yang, X.; Liu, Q.S.; Zhao, X.; Faiola, F.; Zhou, Q.; Jiang, G. Gold nanoparticles change small extracellular vesicle attributes of mouse embryonic stem cells. Nanoscale 2020, 12, 15631–15637. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Kim, J.-H. Platinum Nanoparticles Enhance Exosome Release in Human Lung Epithelial Adenocarcinoma Cancer Cells (A549): Oxidative Stress and the Ceramide Pathway are Key Players. Int. J. Nanomed. 2021, 16, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Kim, J.-H. Palladium Nanoparticle-Induced Oxidative Stress, Endoplasmic Reticulum Stress, Apoptosis, and Immunomodulation Enhance the Biogenesis and Release of Exosome in Human Leukemia Monocytic Cells (THP-1). Int. J. Nanomed. 2021, 16, 2849–2877. [Google Scholar] [CrossRef] [PubMed]
- Marzano, M.; Bou-Dargham, M.J.; Cone, A.S.; York, S.; Helsper, S.; Grant, S.C.; Meckes, D.G., Jr.; Sang, Q.-X.A.; Li, Y. Biogenesis of Extracellular Vesicles Produced from Human-Stem-Cell-Derived Cortical Spheroids Exposed to Iron Oxides. ACS Biomater. Sci. Eng. 2021, 7, 1111–1122. [Google Scholar] [CrossRef]
- Feghhi, M.; Rezaie, J.; Akbari, A.; Jabbari, N.; Jafari, H.; Seidi, F.; Szafert, S. Effect of multi-functional polyhydroxylated polyhedral oligomeric silsesquioxane (POSS) nanoparticles on the angiogenesis and exosome biogenesis in human umbilical vein endothelial cells (HUVECs). Mater. Des. 2021, 197, 109227. [Google Scholar] [CrossRef]
- Liu, Q.; Xiang, P.; Chen, M.; Luo, Y.; Zhao, Y.; Zhu, J.; Jing, W.; Yu, H. Nano-Sized Hydroxyapatite Induces Apoptosis and Osteogenic Differentiation of Vascular Smooth Muscle Cells via JNK/c-JUN Pathway. Int. J. Nanomed. 2021, 16, 3633–3648. [Google Scholar] [CrossRef] [PubMed]
- Musto, M.; Parisse, P.; Pachetti, M.; Memo, C.; di Mauro, G.; Ballesteros, B.; Lazano, N.; Kostarelos, K.; Casalis, L.; Ballerini, L. Shedding plasma membrane vesicles induced by graphene oxide nanoflakes in brain cultured astrocytes. Carbon 2021, 176, 458–469. [Google Scholar] [CrossRef]
- Patel, M.; de Paoli, S.H.; Elhelu, O.K.; Farooq, S.; Simak, J. Cell membrane disintegration and extracellular vesicle release in a model of different size and charge PAMAM dendrimers effects on cultured endothelial cells. Nanotoxicology 2019, 13, 664–681. [Google Scholar] [CrossRef] [PubMed]
- Tayyaba; Rehman, F.U.; Shaikh, S.; Semcheddine, F.; Du, T.; Jiang, H.; Wang, X. In situ self-assembled Ag–Fe3O4 nanoclusters in exosomes for cancer diagnosis. J. Mater. Chem. B 2020, 8, 2845–2855. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef]
- Wu, X.; Tang, T.; Wei, Y.; Cummins, K.A.; Wood, D.K.; Pang, H. Extracellular Vesicles Mediate the Intercellular Exchange of Nanoparticles. Adv. Sci. 2022, 9, 2102441. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.D.T.; Alves, L.R. Extracellular Vesicles in Viral Infections: Two Sides of the Same Coin? Front. Cell. Infect. Microbiol. 2020, 10, 593170. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, J.; Hao, C.; Guo, W.; Wang, D.; Zhang, J.; Zhao, Y.; Duan, S.; Yao, W. Up-regulation of exosomal miR-125a in pneumoconiosis inhibits lung cancer development by suppressing expressions of EZH2 and hnRNPK. RSC Adv. 2018, 8, 26538–26548. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Chen, D.; Ma, H.; Li, Y. LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axis. OncoTargets Ther. 2017, 10, 5137–5149. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Zhu, K.; Wang, Y.; Yu, H.; Guo, J. Overexpression of miR-21-5p promotes proliferation and invasion of colon adenocarcinoma cells through targeting CHL1. Mol. Med. 2018, 24, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, D.; Li, M.; Gao, X.; Shi, G.; Zhao, H. The CADM2/Akt pathway is involved in the inhibitory effect of miR-21-5p downregulation on proliferation and apoptosis in esophageal squamous cell carcinoma cells. Chem. Interact. 2018, 288, 76–82. [Google Scholar] [CrossRef]
- Qiu, F.; Tong, H.; Wang, Y.; Tao, J.; Wang, H.; Chen, L. Inhibition of miR-21-5p suppresses high glucose-induced proliferation and angiogenesis of human retinal microvascular endothelial cells by the regulation of AKT and ERK pathways via maspin. Biosci. Biotechnol. Biochem. 2018, 82, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Báez-Vega, P.M.; Echevarría Vargas, I.M.; Valiyeva, F.; Encarnación-Rosado, J.; Roman, A.; Flores, J.; Marcos-Martínez, M.J.; Vivas-Mejía, P.E. Targeting miR-21-3p inhibits proliferation and invasion of ovarian cancer cells. Oncotarget 2016, 7, 36321–36337. [Google Scholar] [CrossRef]
- Muro, S.; Miyake, Y.; Kato, H.; Tsutsumi, K.; Yamamoto, K. Serum Anti-60S Ribosomal Protein L29 Antibody as a Novel Prognostic Marker for Unresectable Pancreatic Cancer. Digestion 2015, 91, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, A.W.; Gomes, F.A.; Rode, M.P.; da Silva, M.M.; Veleirinho, M.B.D.R.; Maraschin, M.; Hayashi, L.; Calloni, G.W.; Stimamiglio, M.A. The skin regeneration potential of a pro-angiogenic secretome from human skin-derived multipotent stromal cells. J. Tissue Eng. 2019, 10, 204173141983339. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, J.; Thomas, G.; Volarević, S. Ribosome biogenesis in cancer: New players and therapeutic avenues. Nat. Cancer 2017, 18, 51–63. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Ni, Z.; Qian, J.; Fang, W. Calcium Phosphate Crystals from Uremic Serum Promote Osteogenic Differentiation in Human Aortic Smooth Muscle Cells. Calcif. Tissue Res. 2016, 99, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.; Forde, H.; Davenport, C.; Rochfort, K.; Smith, D.; Cummins, P.M. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL. Vasc. Pharmacol. 2016, 82, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.M.; Asotra, K.; Fitzpatrick, L.A.; Qiao, J.-H.; Wilkin, D.J.; Detrano, R.C.; Dunstan, C.R.; Shah, P.K.; Rajavashisth, T.B. Calcification in atherosclerosis: Bone biology and chronic inflammation at the arterial crossroads. Proc. Natl. Acad. Sci. USA 2003, 100, 11201–11206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leopold, J.A. Vascular Calcification. Circulation 2013, 127, 2380–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadhil, R.S.; Wei, M.Q.; Nikolarakos, D.; Good, D.; Nair, R.G. Salivary microRNA miR-let-7a-5p and miR-3928 could be used as potential diagnostic bio-markers for head and neck squamous cell carcinoma. PLoS ONE 2020, 15, e0221779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agnelli, S.; Gerra, M.C.; Bignami, E.; Arendt-Nielsen, L. Exosomes as a new pain biomarker opportunity. Mol. Pain 2020, 16, 174480692095780. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Ochiya, T. Circulating microRNAs and extracellular vesicles as potential cancer biomarkers: A systematic review. Int. J. Clin. Oncol. 2017, 22, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin. Immunol. 2018, 35, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mango, D.; Saidi, A.; Cisale, G.Y.; Feligioni, M.; Corbo, M.; Nisticò, R. Targeting Synaptic Plasticity in Experimental Models of Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hao, C.; Zhang, L.; Zhang, J.; Liu, S.; Li, Y.; Qu, Y.; Zhao, Y.; Huang, R.; Wei, J.; et al. Exosomal miR-125a-5p derived from silica-exposed macrophages induces fibroblast transdifferentiation. Ecotoxicol. Environ. Saf. 2020, 192, 110253. [Google Scholar] [CrossRef]
- Ferrati, S.; Shamsudeen, S.; Summers, H.; Rees, P.; Abbey, J.V.A.; Schmulen, J.; Liu, X.; Wong, S.T.C.; Bean, A.J.; Ferrari, M.; et al. Inter-endothelial Transport of Microvectors using Cellular Shuttles and Tunneling Nanotubes. Small 2012, 8, 3151–3160. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and Biomarkers of Endothelium: When Something Is Rotten in the State. Oxid. Med. Cell. Longev. 2017, 2017, 9759735. [Google Scholar] [CrossRef] [PubMed]
- Zifkos, K.; Dubois, C.; Schäfer, K. Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 9317. [Google Scholar] [CrossRef]
- Nair, A.; Ingram, N.; Verghese, E.T.; Wijetunga, I.; Markham, A.F.; Wyatt, J.; Prasad, K.R.; Coletta, P.L. CD105 is a prognostic marker and valid endothelial target for microbubble platforms in cholangiocarcinoma. Cell. Oncol. 2020, 43, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; EL Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, 492. [Google Scholar] [CrossRef]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived from Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surman, M.; Drozdz, A.; Stępień, E.; Przybyło, M. Extracellular Vesicles as Drug Delivery Systems—Methods of Production and Potential Therapeutic Applications. Curr. Pharm. Des. 2019, 25, 132–154. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.P.; Cockburn, I.M.; Simcoe, T.S. The Economics of Reproducibility in Preclinical Research. PLoS Biol. 2015, 13, e1002165. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef] [PubMed]

| Acronym | Definition | Description |
|---|---|---|
| P | Population | Extracellular vesicles |
| E | Exposure | Nanomaterials |
| C | Comparison | Extracellular vesicles without nanomaterials exposure |
| O | Outcome | Cellular response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, T.S.M.; Souza, W.; Geaquinto, L.R.O.; Sanches, P.L.; Stepień, E.L.; Meneses, J.; Fernández-de Gortari, E.; Meisner-Kober, N.; Himly, M.; Granjeiro, J.M.; et al. Nanomaterial Exposure, Extracellular Vesicle Biogenesis and Adverse Cellular Outcomes: A Scoping Review. Nanomaterials 2022, 12, 1231. https://doi.org/10.3390/nano12071231
Lima TSM, Souza W, Geaquinto LRO, Sanches PL, Stepień EL, Meneses J, Fernández-de Gortari E, Meisner-Kober N, Himly M, Granjeiro JM, et al. Nanomaterial Exposure, Extracellular Vesicle Biogenesis and Adverse Cellular Outcomes: A Scoping Review. Nanomaterials. 2022; 12(7):1231. https://doi.org/10.3390/nano12071231
Chicago/Turabian StyleLima, Thais S. M., Wanderson Souza, Luths R. O. Geaquinto, Priscila L. Sanches, Ewa. L. Stepień, João Meneses, Eli Fernández-de Gortari, Nicole Meisner-Kober, Martin Himly, José M. Granjeiro, and et al. 2022. "Nanomaterial Exposure, Extracellular Vesicle Biogenesis and Adverse Cellular Outcomes: A Scoping Review" Nanomaterials 12, no. 7: 1231. https://doi.org/10.3390/nano12071231
APA StyleLima, T. S. M., Souza, W., Geaquinto, L. R. O., Sanches, P. L., Stepień, E. L., Meneses, J., Fernández-de Gortari, E., Meisner-Kober, N., Himly, M., Granjeiro, J. M., & Ribeiro, A. R. (2022). Nanomaterial Exposure, Extracellular Vesicle Biogenesis and Adverse Cellular Outcomes: A Scoping Review. Nanomaterials, 12(7), 1231. https://doi.org/10.3390/nano12071231












