Abstract
The ever-increasing worldwide energy demand and the limited resources of fossil have forced the urgent adoption of renewable energy sources. Additionally, concerns over CO2 emissions and potential increases in fuel prices have boosted technical efforts to make hybrid and electric vehicles more accessible to the public. Rechargeable batteries are undoubtedly a key player in this regard, especially lithium ion batteries (LIBs), which have high power capacity, a fast charge/discharge rate, and good cycle stability, while their further energy density improvement has been severely limited, because of the relatively low theoretical capacity of the graphite anode material which is mostly used. Among various high-capacity anode candidates, tin (II) sulfide (SnS2) has been attracted remarkable attention for high-energy LIBs due to its enormous resource and simplicity of synthesis, in addition to its high theoretical capacity. However, SnS2 has poor intrinsic conductivity, a big volume transition, and a low initial Coulombic efficiency, resulting in a short lifespan. SnS2/carbon composites have been considered to be a most promising approach to addressing the abovementioned issues. Therefore, this review summarizes the current progress in the synthesis of SnS2/carbon anode materials and their Li-ion storage properties, with special attention to the developments in Li-based technology, attributed to its immense current importance and promising prospects. Finally, the existing challenges within this field are presented, and potential opportunities are discussed.
1. Introduction
Lithium ion batteries (LIBs), with high energy density, extended cycle life, and environmental friendliness, have been considered to be one of the most appealing energy storage systems, and have played an increasingly significant role in modern civilization [1,2,3,4,5,6]. The progressing advancement of LIBs has brought exceptional enhancements in different parts of their activity [7,8,9,10,11,12], being widely involved on the market of compact electronic devices (e.g., cell phones, workstations, advanced cameras, etc.). Additionally, they have been distinguished as the favored force hotspot for electric vehicles (EVs) and fixed-vitality energy storage. However, state-of-the-art LIBs cannot fulfill the developing need for EVs and huge scope vitality energy storage [13,14,15,16,17], which is mainly caused by the limited capacity (372 mAh/g) of the mostly used graphite anode [13,14,17,18,19,20,21]. Thus, tremendous efforts have been made to fabricate high-capacity anode materials, including elementary substances (i.e., Ge, P, Sb, Si), transition metal oxides (i.e., MnO, V2O5), metal sulfides (i.e., ZnS, Cu2S), etc. [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Among them, SnS2 has been attracted remarkable attention because of its low cost, environmental friendliness, and high theoretical specific capacity [43,44,45,46,47]. SnS2 has the catenation ability of sulfur and contributes to the enrichment of the chemistry of tin sulfide. It is also possible to include other elements (metal and non-metal) to form trivalent and quadratic tin sulfide structures, as well as ternary and quaternary materials [48,49,50,51,52].
For SnS2, tin particles can exist in various oxidation states and different coordination structures, and sulfur ions have an enormous electronegativity and solid polarizability [16,53,54,55,56,57]. Li-ions diffuse from the octahedral gap position, where the energy is most supported, to the adjacent octahedral gap position, through the tetrahedral gap position. The expansion of the layer spacing is beneficial for reducing the diffusion barrier [58,59]. In addition, the energy density of SnS2-based LIBs can reach as high as 286 Wh/kg, which is much better than those of commercial graphite [60]. Despite these advantages, SnS2 has certain drawbacks that hinder its broad application, such as the non-negligible volume change issue and the comparatively poor initial Coulombic efficiency (CE), which is related to the irreversible synthesis of Li2S and LixSnS2 [61,62,63].
Overall, the commercial applications of SnS2-based anode materials are currently limited in large part by the following issues: (1) significant initial irreversible capacity loss as a result of the creation of thick solid electrolyte interphase (SEI) throughout the cycling process; (2) the large volume change that occurs during the charge/discharge process, which results in electrode pulverization and the loss of electrical contact with the current collector, leading to fast capacity fading along with poor cycling performance [64,65,66,67]. Many efforts have recently been concentrated on addressing the aforementioned difficulties, as well as advancing the use of SnS2-based anode materials in LIBs for practical applications. It has been found that hybridizing SnS2 with other materials—including nanocarbon [68,69], graphene [70,71], and MXene [72]—or doping SnS2 with other additives—such as Co [39], Ce [73], Mo [74], etc.—can significantly improve the overall conductivity and structural resilience of SnS2-based electrodes, thus resolving the issues mentioned above. As most commonly modified materials, carbon materials (i.e., amorphous carbon, carbon nanotubes, graphene, etc.) have been extensively explored because they can improve the electrical conductivity of the electrode, and can reduce the particle agglomeration of active materials, enhancing the utilization of active materials and extending their lifespan [75,76,77,78].
This paper has reviewed the most recent developments in SnS2/carbon anodes for LIBs. The structural properties of different composites using SnS2 clearly demonstrate the importance of preparation process. To fully use all the potential advantages of SnS2 in LIBs, endeavors have been made to handle the previously mentioned issues and push SnS2-based anode materials to handy applications. The morphological design and fabrication of electrode materials tremendously affect the electrochemical performance of LIBs; thus, the large-scale study of those material-based anodes is essential. This review highlights the most recent developments with thorough discussion in the microstructure, morphology, rational synthesis, and electrochemical performance of SnS2-based anode materials in LIBs with a goal to provide more insights in this area. The future challenges and research directions for practical, advanced SnS2-based anodes are also proposed at the end.
2. Working Mechanisms of SnS2-Based Anodes in LIBs
During the past decades, Sn-based materials, particularly SnS2, have played a major role in LIBs due to their layered structure, which allows them to provide optimum space for Li-ion intercalation [79,80,81,82,83]. It has also been demonstrated that the advantage of having layers in the structure of the crystal can be used to accommodate Li-ions [84]. The neighboring sulfur layers in SnS2 are held together by weak van der Waals contacts [85]. The lithiation procedure of SnS2 can be separated into two phases. When the Li content (x in LixSnS2) is under 1, the volume extension is not remarkable, and only the S particles trap electrons from Li-ions. When the Li content is more than 1, the Sn4+ cations are fundamentally decreased, the 3 S-Sn-S layers deteriorate bit by bit, and an LixS2 (1 ≤ x ≤ 3) layer is framed between the 2 Sn monolayers, and the volume expansion of SnS2 subtly reduces the intensity of Li 2p states. The anode’s stability may be jeopardized due to the lithiation-induced volume expansion and crystal structural change. During lithiation/delithiation, Sn-based anode materials always experience 200–300% volume expansion [61,62,63].
It has been proposed that the electrochemical reaction mechanisms of SnS2 with Li-ions are presented in Equations (1) and (2) [86]:
SnS2 + 4Li+ + 4e− → Sn + 2Li2S,
Sn + xLi+ + xe− ↔ LixSn (0 ≤ x ≤ 4.4),
The high theoretical capacity of 645 mAh/g is derived from the reversible reaction (1) of Sn in SnS2 with 4.4 mols of lithium. Despite this, during the conversion reaction of lithiated SnS2, 4 mols of lithium are consumed in the irreversible formation of Li2S. As a result, if the irreversible reaction is made reversible, then the theoretical capacity of SnS2 could be as high as 1231 mAh/g (8.4 mol Li+ per mol SnS2) [87]. Lithiation causes SnS2 to decompose into metallic tin and Li2S during the first discharge. Tin alloys/dealloys up to the theoretical limit of Li4.4Sn, and Li2S act as an inert matrix that surrounds the active Sn grains during substantial charge and discharge processes [86]. Li-ions can intercalate to some extent into the SnS2 layers without generating phase dissolution, according to earlier publications [45,50]; hence, the reaction can be separated into three phases, as follows in Equations (3)–(5):
SnS2 + xLi+ + xe− → LixSnS2,
LixSnS2 + (y − x)Li+ + (y − x)e− → LiySnS2,
LiySnS2 + (4 − y)Li+ + (4 − y)e− → Sn + 2Li2S (0 < x < y ≤ 2),
To enhance the energy density and capacity of battery materials, a detailed understanding of electrode thermodynamics and chemistry is required [83,84]. First principles have been utilized to study the Li-ion intercalation and diffusion in pristine and modified SnS2 interlayers. The data suggest that Li intercalation prefers the octahedral interstitial location. The minimum energy path of Li-ion diffusion in the SnS2 interlayer is explored. Researchers have discovered that Li atoms spread from one energetically favorable octahedral interstitial location to the next [63]. The results of this study suggest that regulating the inactive morphologies of SnS2-based anode materials may be a viable strategy for improving their electrochemical performances.
3. Pure SnS2
The SnS2 anode material for LIBs was first reported in 1998 by T. Brousse and his team [86]. SnS2 is an n-type semiconductor “layered compound”, with a hexagonal cadmium iodide (CdI2) structure that has the potential to own a high capacity [88,89,90,91,92]. SnS2 can host molecular guest species in vacancies between its neighboring sulfur layers because of its layered structure, similarly to how Li-ion is embedded in graphite [50,66,83,84,93]. For pure SnS2 anodes, different structures have been designed and investigated, such as nanoparticles [67,83], nanosheets [50,94,95,96,97], nanowalls [98,99], and nanoflowers [100,101,102,103], that show different capacities according to their morphologies.
For example, Momma et al. [92] observed that the amorphous SnS2 powder could be a viable candidate material for LIBs. An aqueous solution of SnCl4 and thioacetamide was sonicated in air at ambient temperature for 30 min to improve crystallinity before being annealed at 400 °C. The cell with the unannealed SnS2 electrode had an initial capacity of 300 mAh/g at a current density of 50 mA/g. After annealing, the capacity of SnS2 increased to above 600 mAh/g. The results showed that the crystalline morphology of annealed SnS2 has been revealed as a possible anode candidate for LIBs because it could accelerate the lithiation process.
Reducing the particle size of Sn-based materials to the nanoscale range is an efficient technique to improve cycling stability. Various methods have been developed for the synthesis of SnS2 nanostructures with various diameters (from 10 to 100 nm) and morphologies (i.e., nanoparticles, nanorods, nanobelts, nanotubes, and nanosheets) [104,105,106,107,108,109,110,111,112,113,114,115,116]. Kim et al. synthesized novel crystalline SnS2 nanosheets/nanoplates and applied them as anode materials for LIBs [50]. SnS2 nanosheets from ~1.6 to ~26 nm were successfully prepared via a simple, catalyst-free solvothermal route, without surfactants/functional groups. Ethylene glycol was used as a reducing agent by capping the Sn-ion source, resulting in creating a polymer network and the prevention of nanosheet aggregation. Li-ions could be embedded in the SnS2 layer to some extent without causing phase decomposition for nanosheet structures.
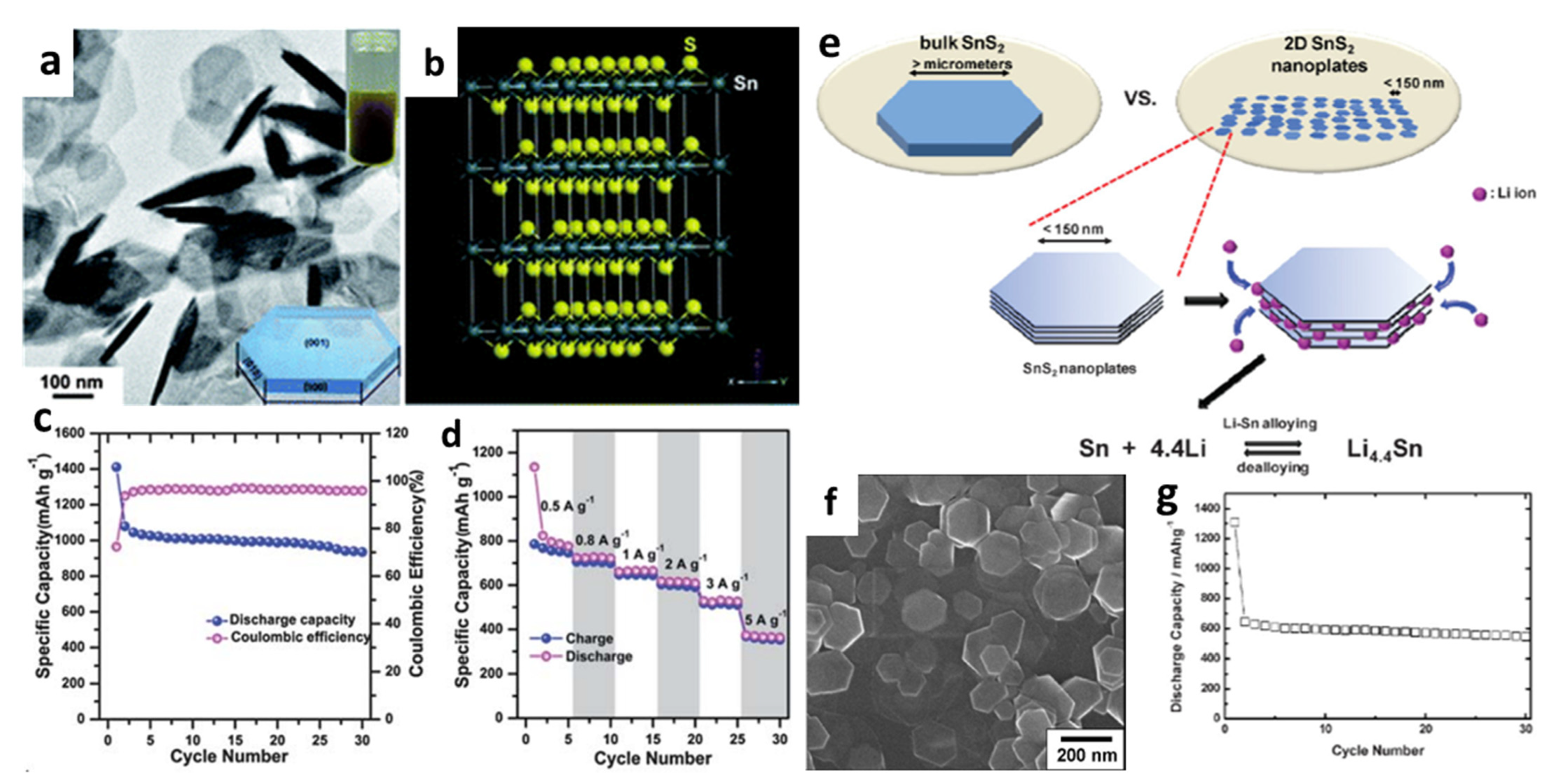
During the lithiation/delithiation process, many efforts have been undertaken to minimize the volume change and improve cycle performance. Du et al. [97] introduced an eco-accommodating and conservative manufacturing method for two-dimensional (2D) layered SnS2 nanoplates by one-pot synthesis using SnCl2·2H2O powder (Figure 1a). Figure 2b shows the crystal structure of SnS2 nanoplates with alternating S-Sn-S layers and S-S layers along the z-axis (c-axis). The final fabricated cell exhibited highly reversible capacity and good capacity retention after 30 cycles (Figure 1c,d). Seo et al. [94] discovered 2D layered nanostructures by thermal decomposition and provided better cyclability due to their unique nanoscale phenomena below 150 nm (Figure 1e,f). The determined average discharge capacity could be up to 583 mAh/g, which was 90% of the maximum theoretical reversible value and 1.6 times the commercial carbon electrode (372 mAh/g), as shown in Figure 1g. This exhibited greatly improved host capabilities as an active LIB electrode because of its unique shape, which consists of a finite, lateral sized, and well-defined layered structure.

Figure 1.
(a) A TEM image of hexagonal SnS2 nanoplates. Inset: (top) photograph of SnS2 nanoplate solution; (bottom) schematic diagram of a SnS2 nanoplate. (b) The supercell structure of a SnS2 crystal with A = 3a, B = 3b, C = 3c. (c) Cycling performance of the SnS2 nanoplate electrode at a current density of 0.2 A/g and (d) charge-discharge capacities at various current densities from 0.5 to 5 A/g. Reprinted with permission from Ref. [97]. Copyright 2013 RSC. (e) A schematic of lithiation processes for bulk versus nanoplates. (f) An SEM image of SnS2 nanoplates; (g) life cycle performance of the SnS2 electrode. Reprinted with permission from Ref. [94]. Copyright 2008 John Wiley and Sons, Inc.

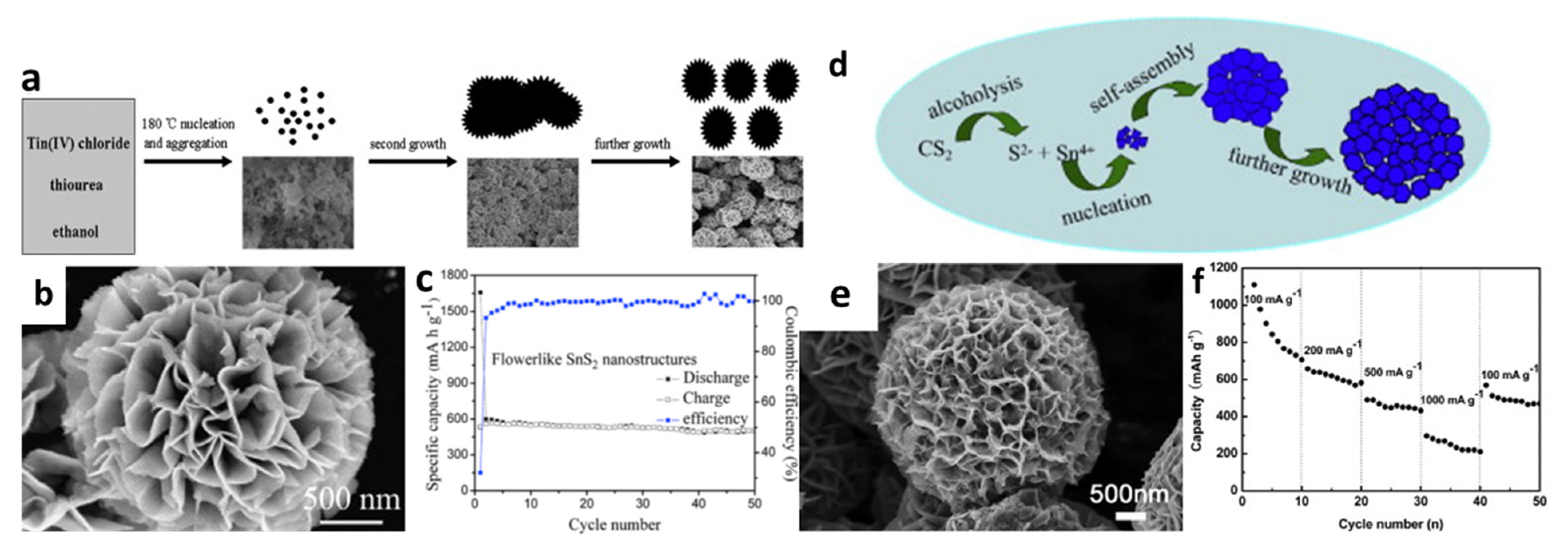
Figure 2.
(a) Schematic illustration of the morphological evolution process, (b) an SEM image, and (c) cycling performance of the 3D flower-like SnS2. Reprinted with permission from Ref. [66]. Copyright 2010 Elsevier. (d) Schematic illustration of the morphological formation process, (e) an SEM image, and (f) rate capabilities of SnS2 hierarchitectures. Reprinted with permission from Ref. [102]. Copyright 2013 Elsevier.
The vertically aligned 2D SnS2 nanowalls can also serve as an ideal anode material for LIBs. Liu et al. [98] performed a simple chemical bath deposition method to prepare SnS2 nanowall arrays grown directly on copper foils. The shape of these arrays offers numerous benefits for enhancing electrocatalytic activity. Because there are more space between neighboring nanowalls, the electrolyte may readily diffuse into the inner area of the electrode, and the volume change associated with Li+ insertion and extraction can be maintained. In the meantime, a simple, biomolecule-assisted technique was used to produce vertically aligned SnS2 ultrathin nanosheet arrays on Sn substrate by Zhong et al. [99]. A facile, L-cysteine-assisted hydrothermal strategy was devised to manufacture a graphene-like SnS2 film comprising 2–5 atomic layers on Sn foils. The electrochemical discharge capability of the cell with ultrathin SnS2 nanosheets was 690 mAh/g at 3C that was near to the theoretical limit.
The formation of 3D flower-like structures was initially proposed early in 2010 by Liu et al. [66]. The electrochemical characteristics of flower-like SnS2 systems were remarkable according to the achieved results. These flower-like SnS2 structures were prepared by a solvothermal ethanol method and produced nanoplates with thicknesses of about 5–10 nm, which revealed a reversible capacity of about 502 mAh/g after 50 cycles at a current density of 200 mA/g (Figure 2a–c). Wu et al. [102] used CS2 for the dissolve in ethanol to form a homogeneous solution, in which CS2 acted as a sulfur donor throughout the solvothermal process and S2- was released as a source of sulfides (Figure 2d,e). The cell with such prepared electrodes could have capacities of 706.7, 582.4, 432.8, and 210.8 mAh/g, at current densities of 100, 200, 500, and 1000 mA/g, respectively, and it was reversible back to 471 mAh/g when lowering the current density to 100 mA/g (Figure 2f). The overall capacity retained was 73% of the theoretical reversible capacity. The enhanced performance might be because the diffusion distance for ionic and electronic transport was greatly reduced, caused by the specific porous structures of the thin nanosheets, which were accessible for electrolytes and sufficiently dissipated the mechanical stress resulting from the severe volume change during Li-ion uptake/removal. Compared with SnS2 nanoparticle anodes, the layered porous structure and flower-like building blocks of these SnS2 nanoflowers make the redox reaction and charge transfer kinetics at the electrode faster, and show a much higher discharge capacity than SnS2 nanoparticles [116].
Among those morphologies, nanowall- and nanoflower-based SnS2 anodes provide more stable cyclic ability. However, within similar morphology, the particle or plate size has a significant impact on the lithiation and delithiation process, depending on the method, reaction time, reaction temperature, annealing, and crystallinity of the material. The electrochemical performance of pure SnS2-based anode is summarized in Table 1. For tin-based electrode materials, an increase in current density usually results in significant capacity fading. As can be seen, bare SnS2-based anode materials continue to be obviously harmed by large initial irreversible capacity losses, severe internal stress, and loss of electrical contact with the current collector. Some primary strategies have been adopted to address these current issues. The initial objective is to create a variety of novel, nanostructured SnS2 materials, including nanoparticles, nanosheets, and nanoflowers. Not only may nanoscale materials reduce the diffusion length of electrons and lithium ions, but they can also mitigate the large volume impact. However, the nanostructured or porous-structured material alone does not appear to be capable of completely resolving the abovementioned issues, particularly at long cycles and high rates. Thus, using cost-effective carbonaceous materials along with their easy processes is a unique technique which has been considered for increasing the capacity and cycling stability of SnS2.

Table 1.
Electrochemical performance of pure SnS2-based anodes with various morphologies.
4. SnS2/Carbon Composites
4.1. Amorphous Carbon/SnS2 Composites
Amorphous carbon has garnered considerable interest in energy-storage applications due to its high electrochemical activity and inexpensive cost [117,118,119,120,121,122,123,124,125]. It has been widely shown to improve the capacity and cycling stability of electrodes.
For instance, Kim et al. [121] first discovered carbon-coated SnS2 nanoparticles, in a study in which SnS2 powder was extracted from SnCl4·5H2O and thioacetamide by the solvothermal method and the carbon coating was derived from glucose. After 50 cycles, the C-SnS2 nanocomposite had a high reversible capacity of 668 mAh/g at a current density of 50 mA/g, superior to bare SnS2 nanoparticles in terms of cycle performance and rate capability, which owed to the conductive carbon shells and their close association with inert nanoscale SnS2 materials. Furthermore, a simple, high-energy ball-milling method was developed to synthesize SnS2/carbon (SnS2/C-x, x = 40, 50, 60 wt.%) nanocomposites in order to study the effect of carbon contents on the overall performance by Zhao et al. [122]. The results indicated that the SnS2/C-50 nanocomposite exhibited a remarkably high capacity of 700 mAh/g and stable cycle capacity of 540 mAh/g after 100 cycles at the same current rate of 100 mA/g. SnS2 NPs were uniformly implanted inside the graphite nanoparticles network after the ball-milling method, which could provide a large number of Li-ion storage sites, excellent electronic conductivity, and rapid ion diffusion, as well as a reduction in SnS2 volume expansion during cycling. Li et al. [123] prepared a 3D mesoporous carbon anchored with SnS2 nanosheets (MC-SnS2 NSs) by sonochemical reflux method with the structural features of both the 2D nanosheet and the 3D porous carbon matrix, which were expected to show improved Li storage efficiency. The composite showed better cyclic performance and improved structural stability compared with the bare-nanoplate-based SnS2-C anode. A stable discharge of 428.8 mAh/g at 100 mA/g after 50 cycles with a retention of 64.4% could be achieved by the MC-SnS2 NSs.
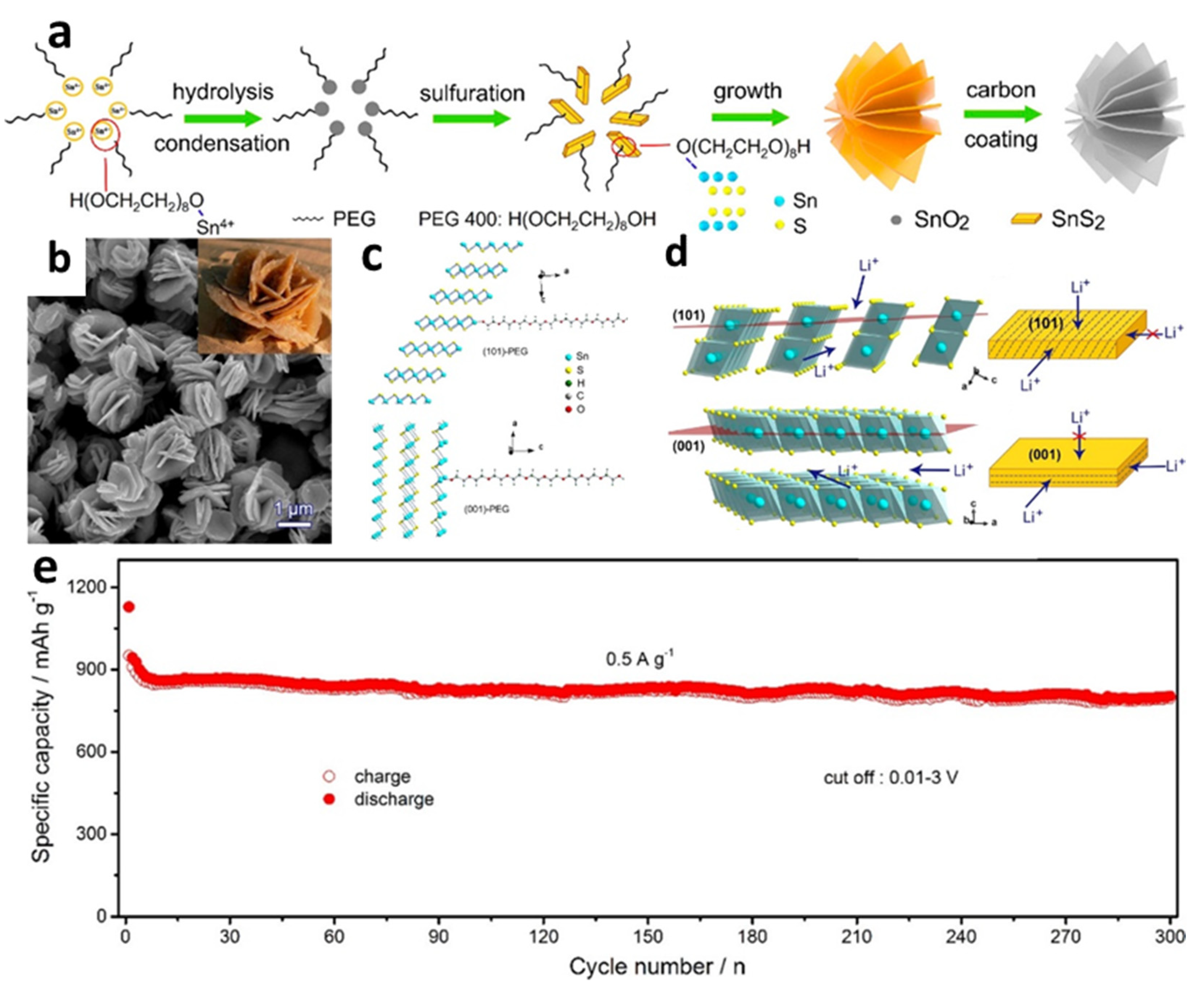
Compared with other regularly utilized carbon sources, different biomass-derived carbons have also been employed with the benefits of low cost and environmental friendliness [124]. An innovative SnS2/biochars (SnS2/B) composite with a hierarchical structure composed of SnS2 nanosheet arrays and biochars carbonized from chewed sugarcane was effectively generated using a simple one-step hydrothermal method [125]. The constructed cell with SnS2/B could deliver a high initial discharge specific capacity of 1107.4 mAh/g at 100 mA/g with a CE of 54.8%. Zhang et al. [56] fabricated carbon-encapsulated flower-like SnS2 nanoplates with (101) plane orientation by a hydrothermal method, with polyethylene glycol (PEG 400) as a surfactant (Figure 3a–c). The SnS2 nanoplates synthesized without PEG mainly grew along the (001) plane. The cell with the prepared material showed an excellent capability of 796 mAh/g at a current density of up to 2 A/g along with exceptional cycle stability. The cycle attenuation rate of the cell tested at 0.5 A/g for 300 cycles was only 0.05% (Figure 3e). The outstanding results might be ascribed to the use of highly (101) faceted preferred orientation in the design of the microstructures, creating a quick and long-lasting highway for Li-ion diffusion, resulting in rapid reaction kinetics (Figure 3d). Using a hydrothermal synthesis process coupled with membrane technology, flower-like SnS2 nanosheets, evenly fixed in the pores of the carbon membrane (SnS2-CM), were produced by Liu et al. [69]. The unique design proved that membrane technology supplied an abundant membrane pore space for uniform SnS2 nanosheet development via a C-S covalent connection. For LIBs at 50 mA/g, the highest reversible capacitance could be up to 808.9 mAh/g, which was because thin SnS2 nanosheets emerged in the membrane hole and surface, enabling the SnS2 cm a 3D interpenetrating network of porous morphology. The novel 3D porous structure not only assisted fast ion transit channels and lowered diffusion length, but also provided ample void space for SnS2 nanosheet volume growth during long-term cycles. The C-S covalent bond also maintained a close contact between SnS2 and the carbon membrane, contributing to structural stability.

Figure 3.
(a) Schematic illustration of the formation process; (b) SEM images of carbon-encapsulated SnS2 nanoplates; (c) structural models of terminated (101) and (001) surfaces of SnS2 with adsorbed PEG for first-principles calculation; (d) schematic illustration of Li-ion insertion; (e) long-term cycling performance of carbon-encapsulated SnS2 nanoplates with the (101)-oriented plane. Reprinted with permission from Ref. [56]. Copyright 2017 ACS.
However, SnS2 with amorphous carbon typically has a low reversible capacity. Besides, a better rate capabilty is desirable for next-generation LIBs. Thus, alternative carbon-based materials, such as carbon nanotubes (CNTs) and graphene, are being investigated in combination with SnS2 in order to increase their specific capacity and rate capability, and also resolve the issues related to pure SnS2-based anodes.
4.2. CNTs/SnS2 Composites
SnS2 combined with CNTs is another way to overcome the shortcomings associated with bare SnS2 anodes. CNT-based materials may be beneficial for charge transfer and electrode stability, improving the electrochemical performance [126,127,128,129,130,131,132,133,134,135].
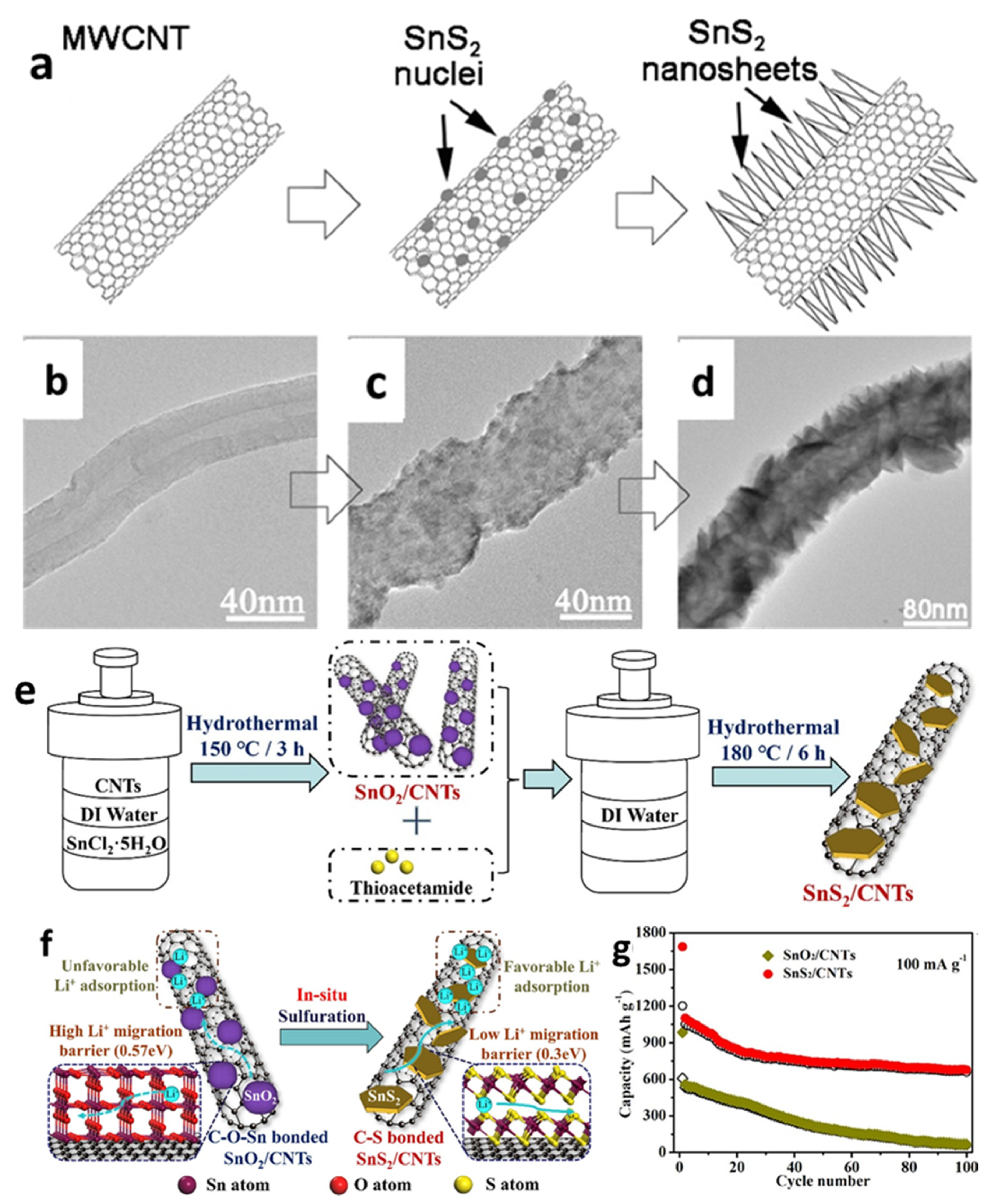
Zhai et al. [126] first reported SnS2 nanosheets on multiwall CNTs (MWCNTs) by chemical vapor deposition with a tube diameter around 80–90 nm. SnS2 nanosheets and nanoflakes were uniformly anchored on CNTs to form SnS2/CNT composite anodes with SnS2 sheaths of different thicknesses, which exhibited higher Li storage capacity and better cycle performance compared with pure SnS2 (Figure 4a–d). Sun et al. [133] also synthesized SnS2 nanoflakes decorated on a MWCNT structure through a simple solution–phase method. The cell with the SnS2/MWCNTs composite demonstrated initial discharge and charge capacities of 1416 and 518 mAh/g, respectively, and could maintain a reversible capacity of 510 mAh/g after 50 cycles at a current density of 100 mA/g. The improved performance might be attributed to the morphological properties of SnS2 flakes and the inclusion of MWCNT, that could reduce volume change throughout the cycle, offer more active sites to accept Li+, and accelerate the conductivity of the active material. Differently from the above fabricating processes, a SnS2/CNTs composite was also produced via a hydrothermal process by in situ vulcanization of SnO2/CNTs by Cheng et al. [48]. In the prefabricated SnO2/CNTs composite, SnO2 nanoparticles with diameters less than 5 nm were completely coated on the CNTs via Sn-O-C bonding. SnO2 nanoparticles were converted into SnS2 hexagonal nanosheets during the in situ sulphuration reaction, and the Sn-O-C bonding was replaced by C-S bonding. The cell with the obtained SnS2/CNTs exhibited superior electrochemical performance, which could deliver an initial reversible capacity of 1202 mAh/g and a capacity of around 660 mAh/g after 100 cycles at 100 mA/g (Figure 4e–g).

Figure 4.
(a) Schematic illustration for the growth process of the SnS2 NS@MWCNTs and (b–d) their TEM images. Reprinted with permission from Ref. [126]. Copyright 2011 ACS. (e) The synthesis procedures diagram; (f) illustration of the Li storage advantage; (g) cycling performance of the SnS2/CNTs composite. Reprinted with permission from Ref. [48]. Copyright 2021 Elsevier.
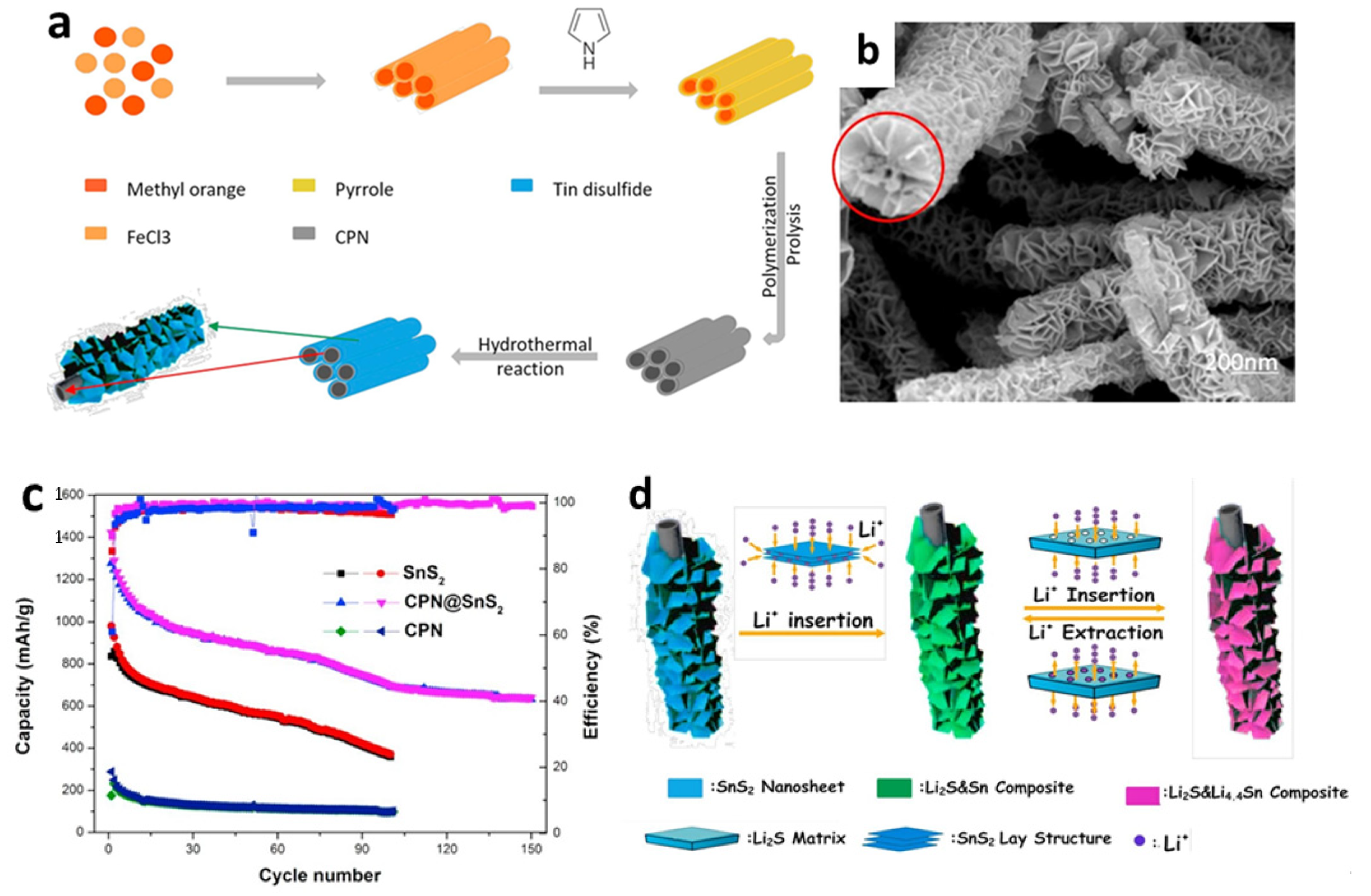
In addition, polypyrrole, which is a one kind of carbonaceous substance, is a prospective additive for improving the electrochemical performances of LIBs due to its ease of synthesis, low cost, strong electron conductivity, and environmental stability. Polypyrrole works as a matrix to support the internal stress of electrodes that experience extreme volume changes, as well as providing a conducting backbone for the active materials [135]. Chen et al. [127] prepared composites with a higher initial CE by combining polymerization and hydrothermal process (Figure 5a,b). Two-dimensional SnS2 nanosheets were used to adorn carbonaceous polypyrrole nanotubes with the interweaving twisted SnS2 nanosheets, reducing volume change during electrochemical cycling and providing more active sites to react with Li-ions. Figure 5c shows that the initial discharge capacity of carbon polypyrrole nanotubes (CPN)-coated SnS2 nanosheets was 1422 mAh/g at a current density of 60 mA/g, with a reversible capacity of 699.2 mAh/g after 100 cycles. The excellent electrochemical performance of CPN@SnS2 composite anode material derived from a unique structure was due to the insertion of conductive CPN, that substantially enhanced the electronic conductivity of the whole anode, allowing for fast electron transmission, as depicted in Figure 5d.
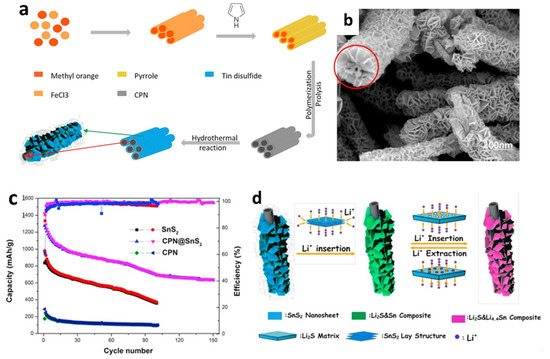
Figure 5.
(a) Formation mechanism, (b) an SEM image, (c) cycling performance, and (d) a schematic illustration of the Li insertion/extraction mechanism of CPN@SnS2 composites. Reprinted with permission from Ref. [127]. Copyright 2017 Elsevier.
The electrochemical performance of SnS2-CNT-based anodes has been widely explored, and their corresponding results are summarized in Table 2. In order to improve the conductivity of SnS2-based materials, it is believed that the combination of electronically conductive agents, such as CNTs, is an effective strategy. An additional effective route is through the morphology-controlled SnS2-CNT synthesis of nanostructured, active materials, such as nanowire, nanotubes, nanoflakes, and nanosheets. These nanostructures can shorten the pathway lengths of Li+ and compensate for volume change due to their large surface-to-volume ratio, which makes them ideal for use in LIBs. Additionally, SnS2-graphene-based composites are currently used for further improving electrochemical performance because they allow enormous concentrations of Li+ to adsorb and desorb during charging and discharging cycles.

Table 2.
Electrochemical performance of carbon-coated SnS2 and SnS2-CNT-based anodes.
4.3. Graphene/SnS2 Composites
Graphene is a novel, 2D, “aromatic” single molecule with high electron mobility, a unique electrical structure, high thermal conductivity, mechanical strength, and a large surface area, which has attracted unprecedented attention [136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167]. Many studies have been conducted to design novel SnS2/graphene anode materials for LIBs with different nanostructures to improve the electrochemical properties, including nanoparticles/nanocrystals [53,54,137,142,156,158,161,163], nanosheets/nanoplates [141,143,144,150,151,152,153], and nanoflowers [49].
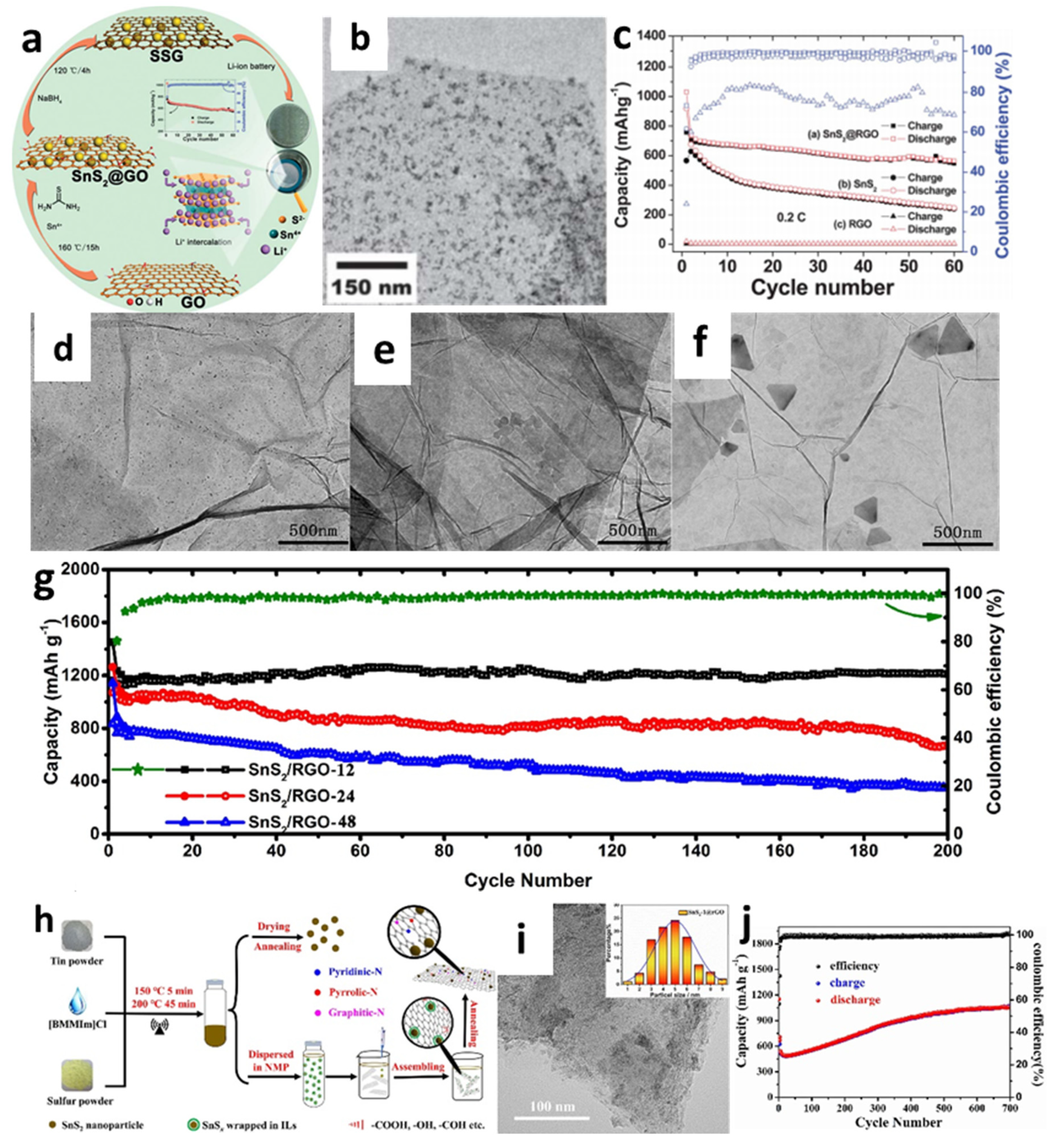
For instance, Yin et al. [138] decorated SnS2 nanocrystals on a reduced graphene oxide (RGO) sheet through the combination of hydrothermal and reduction methods (Figure 6a,b). The cell with the obtained composites showed better cyclic performance with a reversiable capacity of 820 mAh/g at a current rate of 0.2 C after 30 cycles compared with a pure SnS2 anode (Figure 6a–c). Controlling the particle size of electrode materials has been acknowledged as an effective approach for improving the cycle stability and rate characteristics of LIBs [67]. Thus, a simple, one-step hydrothermal process for fabricating composites containing size-tunable tin disulfide on SnS2-RGO (Figure 6d–f) was investigated by Zhao et al. to thoroughly explore the effect of particle size on the electrochemical properties of the material [161]. To demonstrate the morphological, size-dependent properties, the particle sizes of SnS2 nanoparticles were changed by varying the length of the hydrothermal process with three different heat-treatment times (12, 24, or 48 h). The collected samples were marked as SnS2/RGO-12, SnS2/RGO-24, and SnS2/RGO-48, respectively. After 12 h of hydrothermal treatment, the ultrafine SnS2 particles (12 nm) were evenly spread over the graphene nanosheets. It is seen from Figure 6g that, after 200 cycles at 0.1 A/g, a high reversible capacity of 1211 mAh/g remained, which was because the prepared samples had more active sites and increased transport kinetics, thus yielding significant enhancement in electrochemical performance. Mei et al. [156] reported ultrasmall SnS2 nanocrystals decorated on flexible RGO through a refluxing method. The supplied composite with a high surface-to-volume ratio could enhance Li atom absorption on both sides of the sheet and porous architectures, enabling the RGO nanosheet to offer enough room for Li+ storage. The cell with such materials exhibited good capacity retention even at high rates of 1 C and 5 C with the capacities of 773 mA h/g and 415 mAh/g, respectively, after 450 cycles, which were significantly better than the previous hydrothermal-based studies. SnS2@RGO nanocomposites were also created using a novel ionic-liquid-assisted method, which employed SnSx precursors by reacting elemental tin and sulfur in the ionic liquid, 1-butyl-2, 3-dimethylimidazolium chloride (Figure 6h,i) [163]. Exceptionally high reversible capacity and cycle stability could be achived by using the obtained composite. A discharge-specific capacity reached 1045.8 mAh/g, even after 700 cycles at a current density of 500 mA/g, as shown in Figure 6j. The improved reversible capacity of the SnS2@RGO electrode was explained by electrolyte breakdown at the low potential to create an organic polymeric/gel-like layer due to the “pseudo-capacitance-type behavior” that activated the active material under deep cycling.
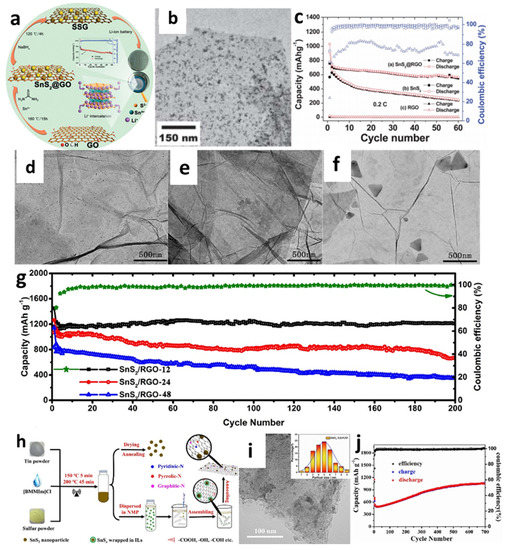
Figure 6.
(a) Synthetic process, (b) a TEM image, (c) cycling performance of SnS2@RGO composite. Reprinted with permission from Ref. [138]. Copyright 2012 RSC. TEM images of (d) SnS2/RGO-12, (e) SnS2/RGO-24, and (f) SnS2/RGO-48; (g) cycling performance of SnS2/RGO. Reprinted with permission from Ref. [161]. Copyright 2020 Elsevier. (h) Schematic illustration of the synthesis, (i) a TEM image, and (j) cycling performance at a current density of 500 mA/g of SnS2@RGO. Reprinted with permission from Ref. [163]. Copyright 2021 Elsevier.
Furthermore, a homogeneous layer of SnS2 nanoparticles was grown on graphene nanosheets (SnS2@GNS) and linked by covalent bonds using the solvothermal method (Figure 7a–c) [162]. The ID/IG values of SnS2@GNS and GNS were calculated to be 1.44 and 1.22, respectively, showing that SnS2@GNS had more flaws. High-level flaws in graphene can accelerate ion and electron migration, improve electrochemical reaction kinetics, and offer more active sites for Li-ion adsorption and intercalation [146]. As displayed in Figure 7d, the cell with SnS2@GNS delivered a capacity of 1250.8 mAh/g after 150 cycles at 0.1 A/g. In addition, Li et al. [166] prepared SnS2 nanocrystals (NCs) through the one-pot solvothermal method using carbon shells attached to RGO by C-S covalent bonding (Figure 7e). The well-controlled carbon shells offered long-term protection for SnS2 NCs against electrolyte corrosion and structural pulverization. Carbon shells could act as mediums, enhancing C-structural SnS2@RGO’s stability and conductivity. It is demonstrated that LIBs had superior rate capabilities and cycling stability (capacity retention of 74.7% after 1000 cycles at 2.0 A/g, as shown in Figure 7f).

Figure 7.
(a) Synthesis procedures diagram, (b) TEM and (c) HRTEM images, and (d) cycling performance of SnS2@GNS. Reprinted with permission from Ref. [162]. Copyright 2020 Elsevier. (e) An SEM image and EDX mapping of C, S, and Sn elements; (f) long-term cycle stability of C-SnS2@RGO. Reprinted with permission from Ref. [166]. Copyright 2020 RSC.
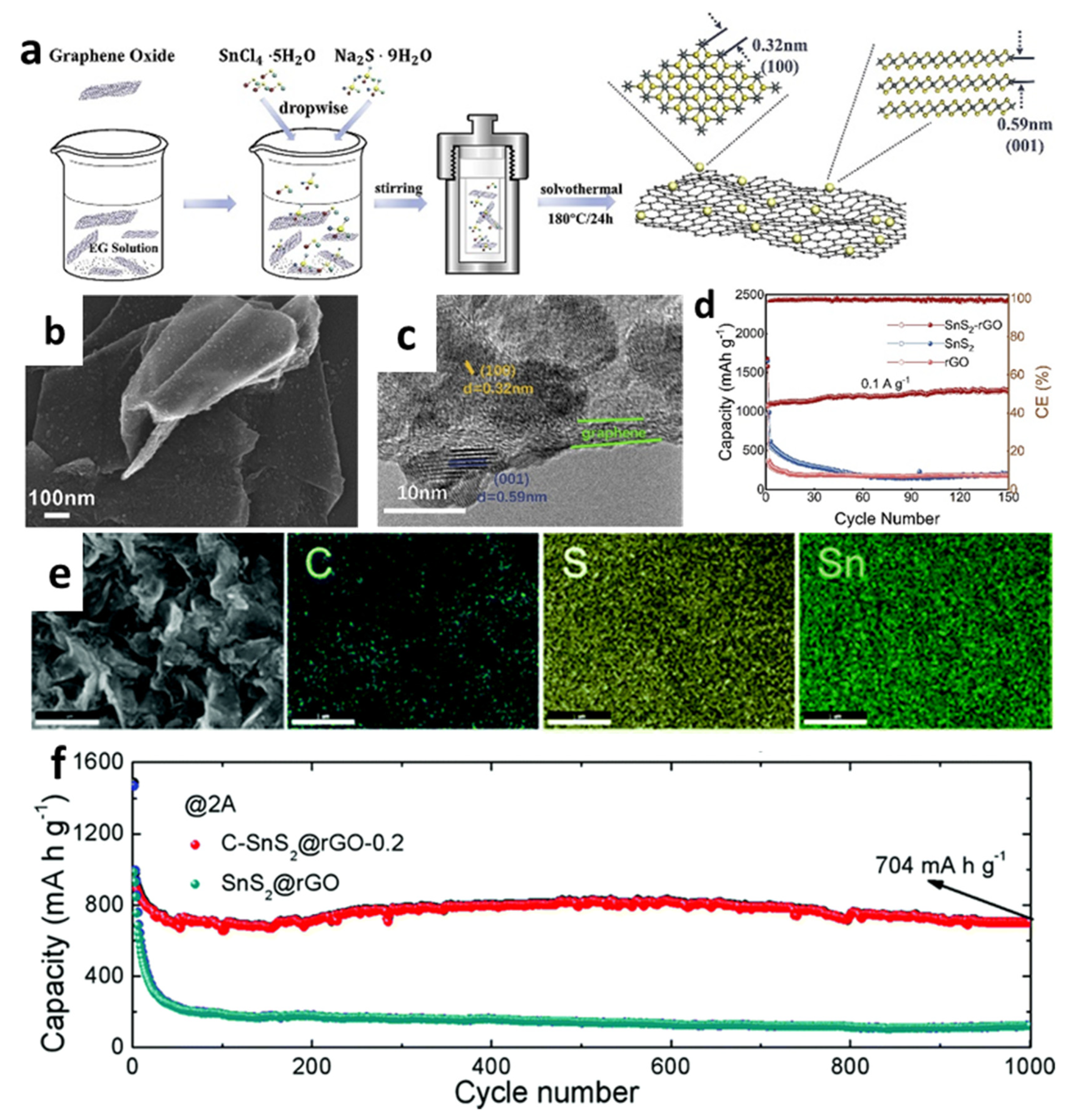
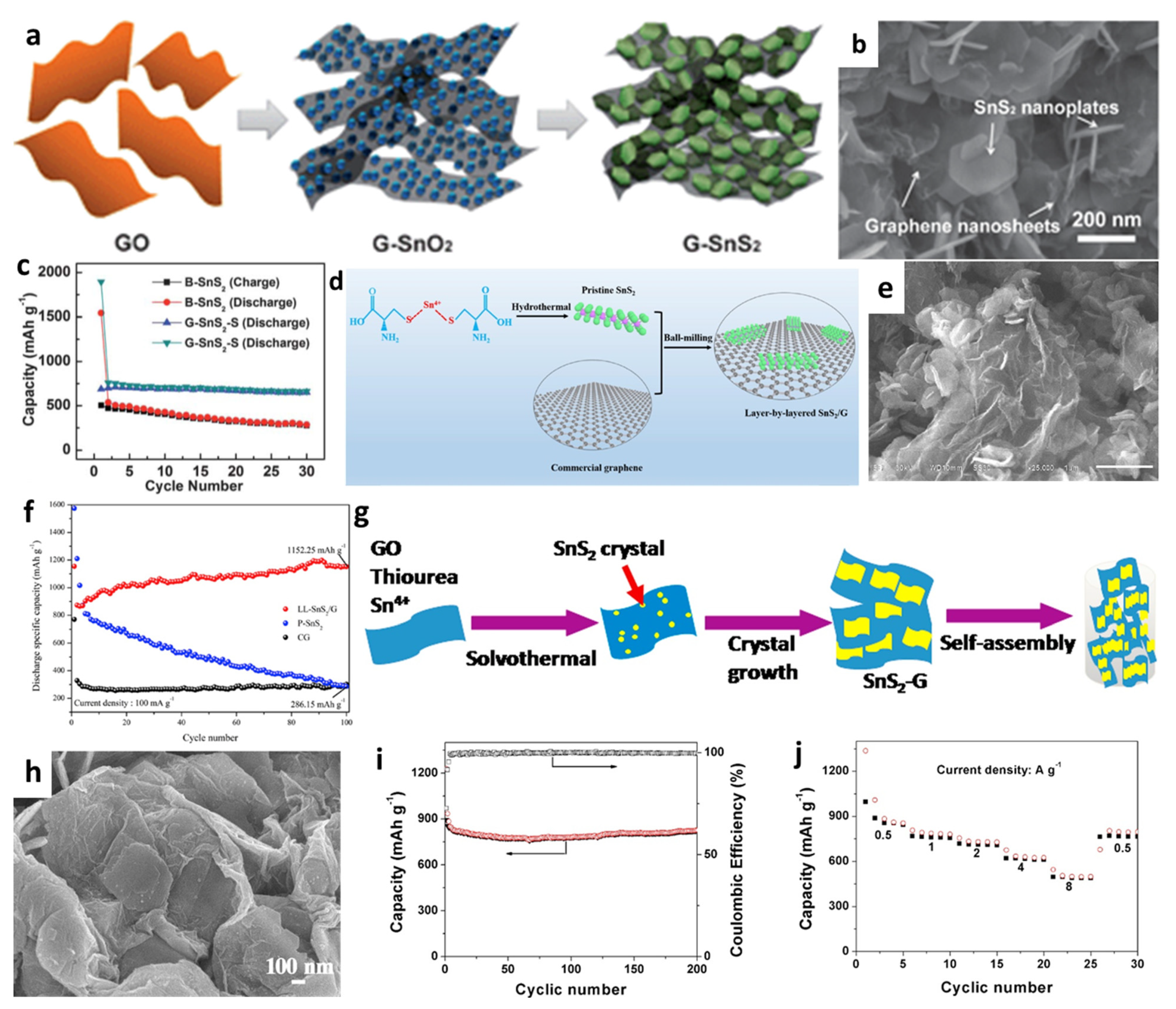
Moreover, Luo et al. [142] developed a new porous nanostructure composed of 2D graphene–SnS2 (G-SnS2) by transforming SnO2 nanoparticles into 2D SnS2 nanoplates directly on/between graphene nanosheets via a solution approach followed with a chemical vapor deposition (CVD) process (Figure 8a,b). The cycling performace in Figure 8c showed that the cell with the prepared G-SnS2 had a stable capacity of 650 mAh/g after 30 cycles at 50 mA/g, while the reversible capacity of bare SnS2 gradually decreased to 277 mAh/g. Xia et al. [146] synthesized pristine SnS2 nanosheets with a thickness of 5 nm by a hydrothermal process, and then uniformly layered SnS2 on graphene sheets to produce layer-by-layer nanosheets (LL-SnS2/G) through the ball-milling method (Figure 8d,e). When used as anodes for LIBs, the capacity reached 1152.25 mAh/g after 100 cycles at a current rate of 100 mA/g, as shown in Figure 8f. The excellent electrochemical performance was attributed to the synergistic effect between SnS2 nanoplates with high specific capacity and conductivity of graphene, which buffered the volume change and provided an effective physical barrier between the active materials and the electrolyte to suppress the shuttle effect of polysulfides formed during delithiation processes. Chen et al. [157] used reflux condensation and hydrothermal methods to grow SnS2 nanoplates on the surface of RGO nanosheets. When the GO concentration was 15%, the SnS2/RGO electrode exhibited the excellent electrochemical performance, which showed capacities of 776, 715, 635.6, 595.2, 517.5, and 447.1 mAh/g at current densities of 0.2, 0.5, 1, 2, 5, and 8 C, respectively. In addition, 3D nanoplate-based SnS2/graphene was synthesized through a facial solvothermal method by Zhang et al. [151], in which SnS2 nanoplates with an average thickness of 3.6 nm were well dispersed and tightly contacted onto graphene substrates (Figure 8g,h). The cell with SnS2-G achieved a very stable capacity of 826 mAh/g over 200 cycles at 500 mA/g. The specific capacities of 854, 780, 728, 625, and 498 mAh/g were obtained under the conditions of 0.5, 1, 2, 4, and 8 A/g, respectively (Figure 8i,j). The enhanced electrochemical performance of the cell was because of the enormous surface area of 2D hybrid materials, the highly conductive and flexible graphene matrix, the 3D design, the facilitated electrolyte filtration, and the smooth ion transport.

Figure 8.
(a) Illustration of the formation, (b) an SEM image, and (c) cycling performance of the G–SnS2. Reprinted with permission from Ref. [142]. Copyright 2012 RSC. (d) Schematic illustration of the formation, (e) an SEM image, and (f) cycling performance of LL-SnS2/G. Reprinted with permission from Ref. [146]. Copyright 2018 Elsevier. (g) Schematic formation, (h) an SEM image, (i) cycling performance, and (j) rate capability of SnS2-G. Reprinted with permission from Ref. [151]. Copyright 2016 Elsevier.
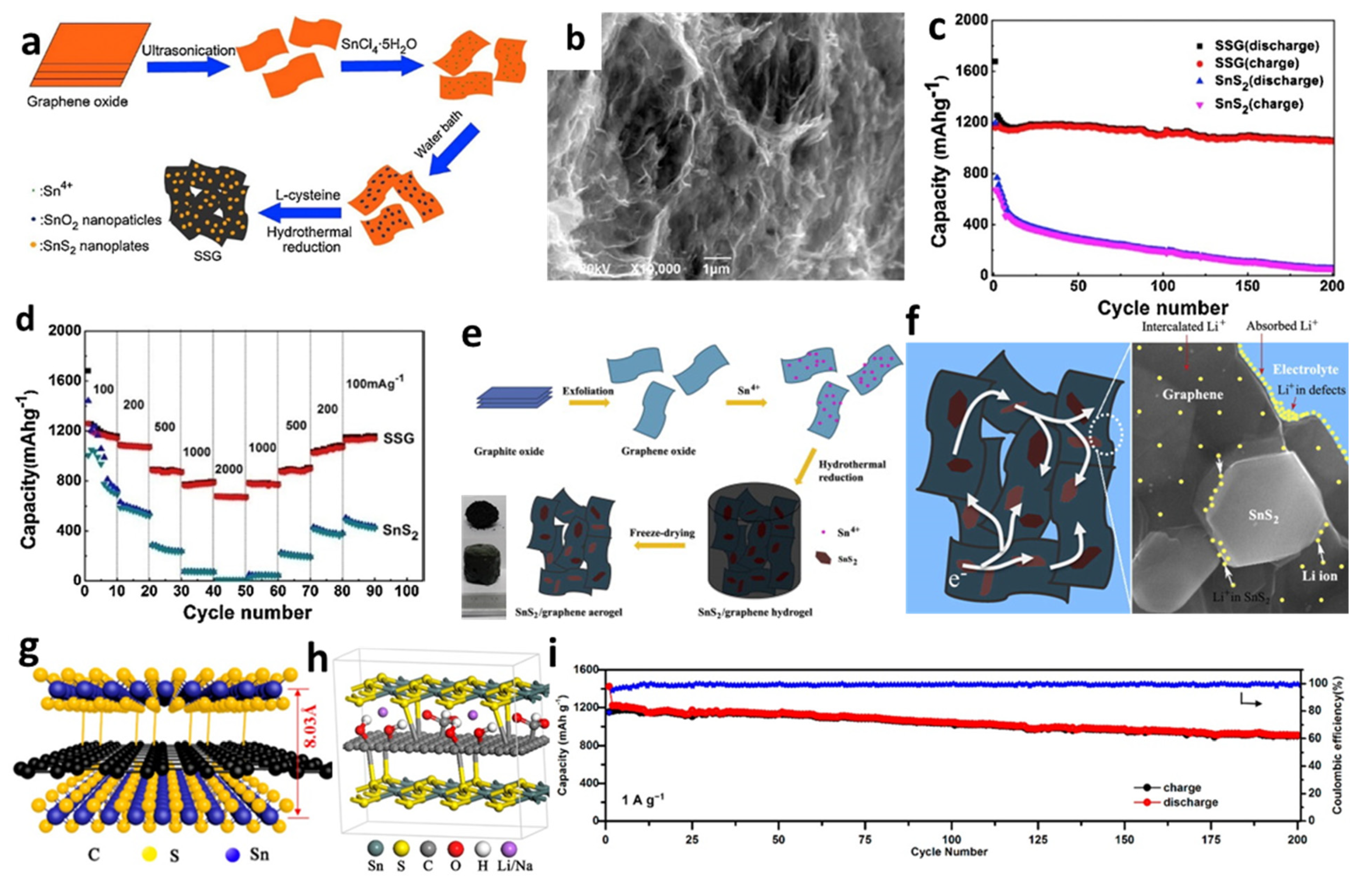
Meanwhile, due to the high porosity, low density, and large pore volume, several self-assembled graphene aerogels (GAs) and composites of 3D graphene-embedded metal or metal oxide nanopaticles have been successfully manufactured using various approaches [150,151,152,168,169,170]. For instance, Tang et al. [148] prepared a unique 3D SnS2/graphene (SSG) composite through transforming SnO2 nanoparticles anchored on GO sheets directly into SnS2 nanoplates, homogeneously embedded in the graphene frameworks (Figure 9a,b). The diameter of the obtained nanoplates on graphene was about 300 nm. The initial discharge and charge capacities of the cell were 1677 and 1159 mAh/g, respectively. A reversible capacity of 1060 mAh/g was retained after 200 cycles at a current density of 100 mA/g. When the current density declined from 2000 to 100 mA/g, it was found that the reversible capacity could be up to 1100 mAh/g (Figure 9c,d). Jiang et al. [150] successfully fabricated 3D SnS2/graphene aerogels (SnS2/GAs) via an in situ hydrothermal method for self-assembly of graphene sheets followed by freeze-drying to maintain a stable 3D structure (Figure 9e). Figure 9f illustrates that the cell with SnS2/GAs exhibited high-rate capability and cycling stability, which could be ascribed to the unique 3D interconnected architectures of the aerogels and the synergistic effects of the layered SnS2 and the graphene, providing enough sites for absorbing Li-ions and shortening transport distance between electrode and electrolyte. Additionally, 3D sandwich-like SnS2/graphene/SnS2 with expanded interlayer distance was introduced by Jiang et al. [149]. The covalently SnS2 nanosheets were decorated on both sides of RGO sheets to form an SnS2/RGO/SnS2 anode composite. The presence of GO could provide a nucleation site for SnS2 and promote SnS2 nanoplates aggregate and grow to form fewer layers. SnS2 nanosheets were chemically linked to graphene through the C-S bonds to produce a sandwich structure with specific capacities of 844 mAh/g after 200 cycles at a current density of 1 A/g (Figure 9g–i).
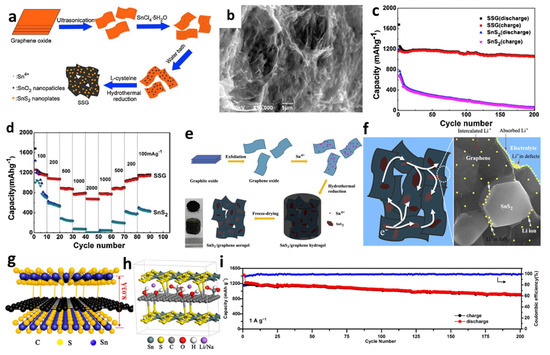
Figure 9.
(a) Schematic formation process, (b) an SEM image, (c) cycling performance, and (d) rate capability of SSG. Reprinted with permission from Ref. [148]. Copyright 2015 Elsevier. (e) Fabrication process, and (f) schematic representation of electron transmission and lithium ions storage of SnS2/GAs. Reprinted with permission from Ref. [150]. Copyright 2013 Elsevier. (g) Schematic illustration, (h) molecular model, and (i) high rate cycling performance of SnS2/RGO/SnS2. Reprinted with permission from Ref. [149]. Copyright 2019 ACS.
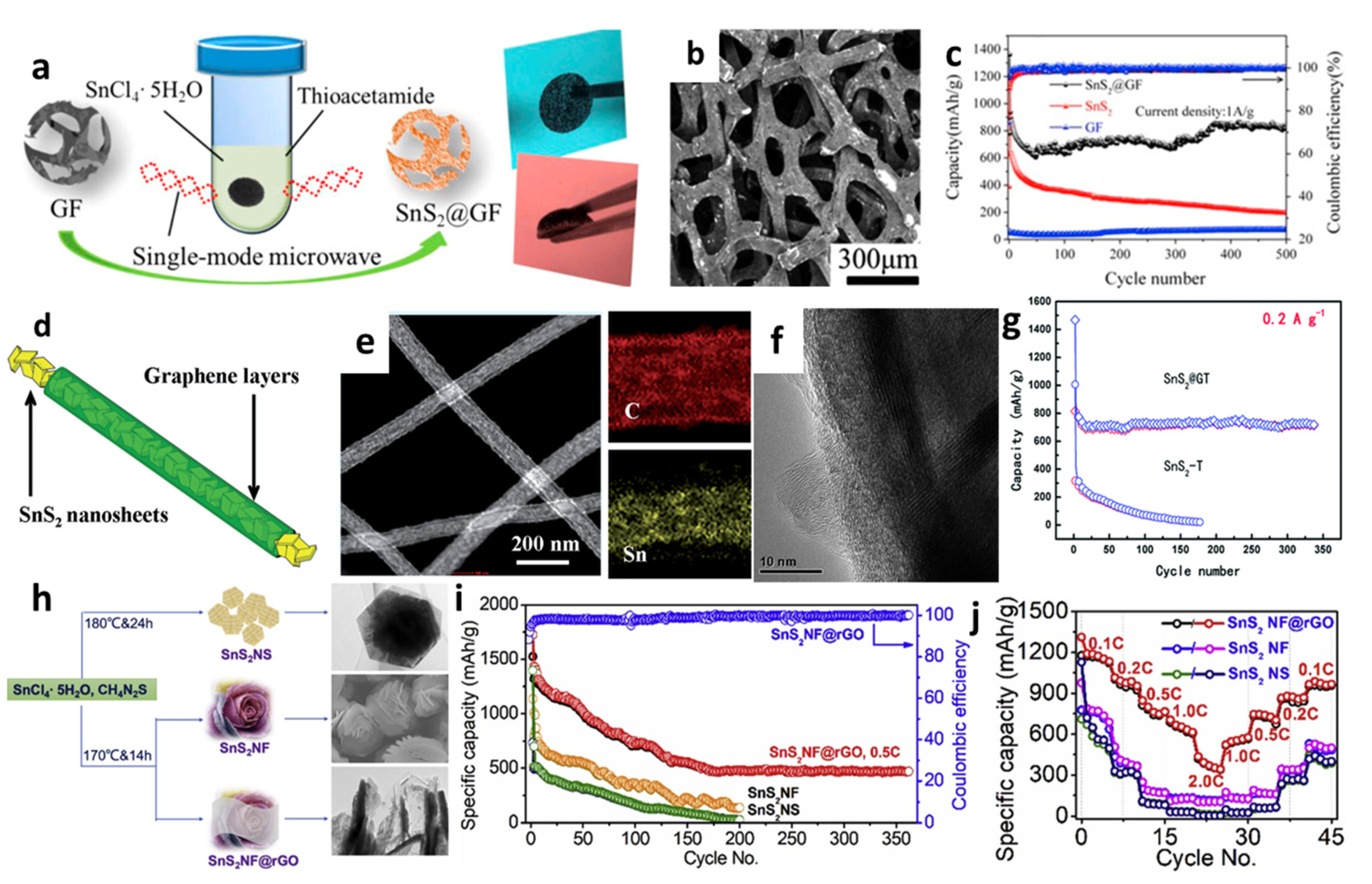
Apart from nanosheets, Ren et al. [144] introduced SnS2 nanoflakes on the 3D graphene foams (GFs) using a single-mode microwave hydrothermal technique (Figure 10a,b). The composite SnS2@GF electrode provided a high capacity of 818.4 mAh/g at a high current density of 1.0 A/g after 500 cycles, as seen from Figure 10c. The GF served as a 3D framework for SnS2 nanoflakes loading and this conductive porous matrix was convenient for rapid electron transport, reduced the strain during the intercalation/extraction process, and provided a large electrode/electrolyte contact area. A new nanocable-like structured SnS2–graphene network was fabricated by Kong et al. [158], in which graphene layers were rolled up to embody SnS2 nanosheets with a thickness of around 10 nm. SnS2@G nanocable showed the initial discharge and charge capacities of 1334 and 764 mAh/g, respectively (Figure 10d–f). Figure 10g presents that the composite maintained the reversible capacity of 720 mAh/g at 200 mA/g up to 350 cycles with over 93.5% capacity retention. This might be attributed to the unique structure design which released the volume change of SnS2 during discharge–charge cycles and promoted easy access of electrolytes to dynamic anode materials. Liu et al. [49] synthesized nanoflower-based SnS2@RGO (SnS2-NF@RGO) composite anodes for LIBs (Figure 10h). The initial specific capacities of SnS2-NS and SnS2-NF were 1300 and 1100 mAh/g, respectively, and gradually decreased to below 200 mAh/g after 200 cycles under 615.5 mA/g, while SnS2-NF@RGO maintained reversible capacity of 525 mAh/g after 360 cycles and capacities of 1211.8, 1021.7, 809.1, 708.1, 412.5, 509.6, 751.5, 820.3, and 923.5 mAh/g under 123.1, 246.2, 615.5, 1231, 2462, 1231, 615.5, 246.2, and 123.1 mA/g, respectively (Figure 10i,j). It revealed good capacity retention through the layer structure of RGO additives, which gave better conductivity between SnS2-NF/electrolyte interfaces and minimized the self-aggregation during the Li+ insertion/deinsertion processes.

Figure 10.
(a) Schematic illustration of the formation, (b) an SEM image, and (c) cycling performance of SnS2@GF. Reprinted with permission from Ref. [144]. Copyright 2016 Elsevier. (d) Schematic illustration, (e) an SEM image and mapping, (f) a TEM image, and (g) cycling performance of SnS2@G. Reprinted with permission from Ref. [158]. Copyright 2014 RSC. (h) Schematic diagram and SEM images, (i) cycling performance, and (j) rate capability of SnS2-NF@RGO. Reprinted with permission from Ref. [49]. Copyright 2019 Elsevier.
The electrochemical performance of SnS2/graphene anodes is summarized in Table 3. It can be seen that SnS2/graphene-based anodes have attracted great attention thanks to the synergistic interaction of SnS2 and graphene. On one hand, the graphene sheets could not only prevent the aggregation of microscopic SnS2, but also significantly improve the electrode’s electronic conductivity and buffer volume changes during charge/discharge processes. The inclusion of SnS2 between graphene sheets, on the other hand, could successfully prevent graphene restacking. In recent years, a variety of methods have been utilized to make SnS2-G nanocomposites, each with its own set of benefits. For example, by simply altering the reaction conditions and additives, hydrothermal/solvothermal methods and other solution-based methods are most typically employed to create SnS2/graphene nanocomposites with various nanostructures. SnS2 nanostructures, such as nanoparticles, nanorods/nanowire, 2D nanosheets/films, and 3D nanoflowers, can reduce volume change during charge/discharge and can shorten the diffusion length of Li-ions, which is critical for boosting cells’ rate capability and cycle stability.

Table 3.
Electrochemical performance of SnS2/graphene-based anodes.
5. Summary and Outlook
SnS2/carbon composites have been considered as an appealing family of high-capacity anode materials for next-generation LIBs. This review provides a comprehensive overview of the most significant advances in their microstructure, Li storage instrument, combination, and electrochemical characteristics. Specific accentuation has been put on handling the rest of the issues of SnS2-based anode materials through a reasonable basic structure (i.e., building remarkable nanostructures, different morphology, and creating SnS2/carbon-based composites). Besides, different procedures can be taken for electrochemical execution upgrade, such as securing controlled pre-lithiation and polymer fastener enhancement.
The creators suggested that the micropores could adequately mitigate the volume changes of SnS2 nanoparticles and forestall the breakdown of the permeable structure. The high explicit surface zone encourages the effective contact of dynamic materials with electrolytes. Among different carbon materials, compositing SnS2 with graphene sheets is one of the hotly debated ongoing exploration issues in recent years since the synergistic impact between SnS2 and graphene can strikingly improve the anode’s electrochemical performance. On the one hand, the graphene sheets could not just forestall the agglomeration of small SnS2 particles, cradle the volume change during charge–release forms, and essentially upgrade the terminal’s electronic conductivity. The diverse nanostructures of SnS2, including nanoparticles, nanorods/nanowires, nanosheets, nanoflowers, and 3D nanospheres, can also ease the volume change during the charge–release process.
In this paper, we focused on the morphological structure of the SnS2 material. In the case of pure SnS2-based anodes, particle size has a significant impact on discharging capacity. SnS2, with smaller particle size, showed better capacity retention and discharging capacity, and also provided more a specific area for volume change expansion. Nanoflower based structures with more active sites for Li-ion insertion significantly improve capacity retention and discharging capability at high current rates. In case of hybrid materials, the specific area and morphology of the other component also play a vital role in capacity performance. As we discussed in the graphene section, sandwich-like nanosheet structures could reduce the Li-ion diffusion distance and have shown excellent CE and rate capability. However, the dispersion length of Li particles is an extremely important factor in improving the reversible limit and cycling dependability. Graphene-based materials are growing rapidly as an incredibly adaptable 2D material for electrochemical energy storage systems, which have aided batteries in achieving excellent high capacities and rate capability due to their optimized interlayer spacing and proprietary chemistry. These accomplishments are a result of graphene’s inherent features, which include strong electrical conductivity, a defined structure, and the capacity to sustain adaptations, allowing for the electrodes to be tailored to a specific application.
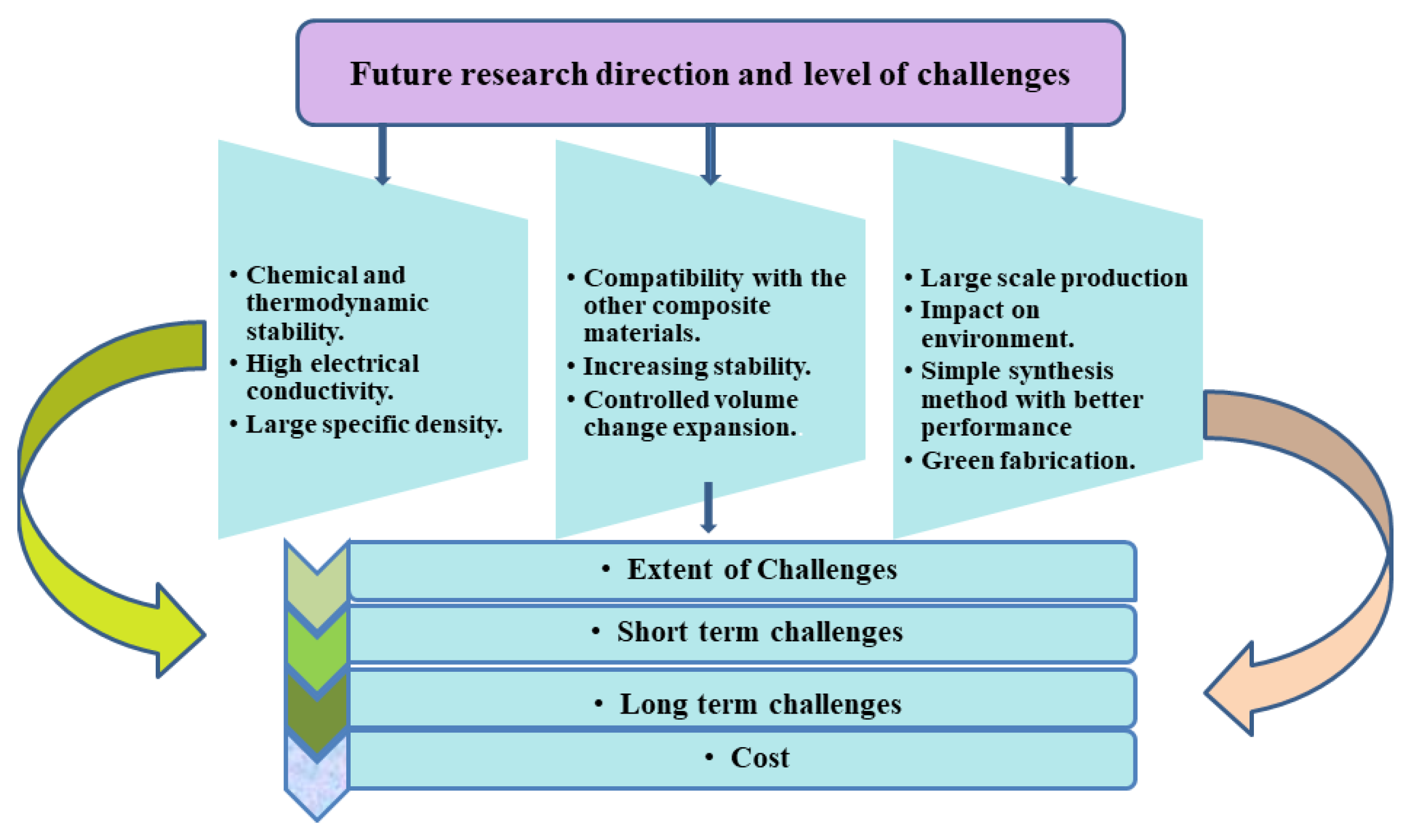
There is still a long way to go before the use of SnS2/graphene composites. Although some of the procedures listed are fairly easy after obtaining graphene or GO, one of the major difficulties is the question of how to further simplify the process of manufacturing graphene. Furthermore, further work is required to alter the mechanical characteristics of the SEI layer, such as SnS2/graphene-active materials, binders, electrolytes, and electrolyte additives, in order to achieve improved cycle performance and CE (Figure 11). Meanwhile, high energy consumption in material and battery production, depletion of critical raw material resources, and low degradation rates are incompatible with the current sustainability of LIBs, and could result in a severe environmental impact and uncertain production conditions in the future, which also need to be taken under consideration for future work. However, it is certain that SnS2-based anode materials will make tremendous advances in the near future due to the ongoing and unwavering efforts throughout the world, which will play an increasingly essential and active role in next-generation high-energy-density LIBs.

Figure 11.
Future research direction and level of challenges.
Author Contributions
Conceptualization, S.T.M. and L.L.; materials organization, S.T.M., R.M. and S.M.; writing—original draft preparation, S.T.M., R.M. and S.M.; writing—review and editing, M.Y., L.L. and J.Z.; supervision, S.S. and L.L.; funding acquisition, L.L., R.Z. and Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 61802285), the Natural Science Foundation of Hubei Province (No. 2021CFB478), the Scientific Research Project of Hubei Provincial Department of Education (No. D20201704), the Key Research and Development Project of Hubei Province (No. 2020BAB076), the Hubei Province Technical Innovation Special Project (No. 2019AAA005), the Chinese Government CSC Scholarship Program (CSC Number: 2019SLJ019819 and 2019SLJ019821), and the Chutian Scholar Program of Hubei Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, D.; Luo, L.; Zhu, J.; Qin, H.; Liu, P.; Sun, Z.; Lei, Y.; Jiang, M. A hybrid lithium sulfonated polyoxadiazole derived single-ion conducting gel polymer electrolyte enabled effective suppression of dendritic lithium growth. Chin. Chem. Lett. 2022, 33, 1025–1031. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Luo, L.; Zhu, J.; Li, J.; Liu, P.; Yu, Y.; Jiang, M. Electrospun Separator Based on Sulfonated Polyoxadiazole with Outstanding Thermal Stability and Electrochemical Properties for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 879–887. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, J.; Zeng, K.; Jiang, M. Mechanically robust and superior conductive n-type polymer binders for high-performance micro-silicon anodes in lithium-ion batteries. J. Mater. Chem. A 2021, 9, 3472–3481. [Google Scholar] [CrossRef]
- Han, L.; Lehmann, M.L.; Zhu, J.; Liu, T.; Zhou, Z.; Tang, X.; Heish, C.-T.; Sokolov, A.P.; Cao, P.; Chen, X.C. Recent Developments and Challenges in Hybrid Solid Electrolytes for Lithium-Ion Batteries. Front. Energy Res. 2020, 8, 202. [Google Scholar] [CrossRef]
- Gao, H.; Mao, J.; Li, D.; Yu, Y.; Yang, C.; Qi, S.; Liu, Q.; Zhu, J.; Jiang, M. Communication—Lithium sulfonated polyoxadiazole as a novel single-ion polymer electrolyte in lithium-ion batteries. J. Electrochem. Soc. 2020, 167, 070518. [Google Scholar] [CrossRef]
- Yang, C.; Li, D.; Gao, H.; Liu, Q.; Zhu, J.; Wang, F.; Jiang, M. Constructing High-Energy-Density Aqueous Supercapacitors with Potassium Iodide-Doped Electrolytes by a Precharging Method. ACS Appl. Energy Mater. 2020, 3, 2674–2681. [Google Scholar] [CrossRef]
- Huang, Z.X.; Wang, Y.; Liu, B.; Kong, D.; Zhang, J.; Chen, T.; Yang, H.Y. Unlocking the potential of SnS2: Transition metal catalyzed utilization of reversible conversion and alloying reactions. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Wang, Z.; Li, H.; Chen, L. Studies of stannic oxide as an anode material for lithium-ion batteries. J. Electrochem. Soc. 1998, 145, 59. [Google Scholar] [CrossRef]
- Palacin, M.R. Recent advances in rechargeable battery materials: A chemist’s perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef]
- Carny, O.; Shalev, D.E.; Gazit, E. Fabrication of coaxial metal nanocables using a self-assembled peptide nanotube scaffold. Nano Lett. 2006, 6, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yildirim, E.; Aly, K.; Shen, J.; Chen, C.; Lu, Y.; Jiang, M.; Kim, D.; Tonelli, A.E.; Pasquinelli, M.A. Hierarchical multi-component nanofiber separators for lithium polysulfide capture in lithium–sulfur batteries: An experimental and molecular modeling study. J. Mater. Chem. A 2016, 4, 13572–13581. [Google Scholar] [CrossRef]
- Yin, L.; Chai, S.; Ma, J.; Huang, J.; Kong, X.; Bai, P.; Liu, Y. Effects of binders on electrochemical properties of the SnS2 nanostructured anode of the lithium-ion batteries. J. Alloys Compd. 2017, 698, 828–834. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Hou, Z.; Zhang, W.; Zhu, Y.; Qian, Y.; Liang, J.; Qian, Y. SnS2-compared to SnO2-stabilized S/C composites toward high-performance lithium sulfur batteries. ACS Appl. Mater. Interfaces 2016, 8, 19550–19557. [Google Scholar] [CrossRef]
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.-G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene balls for lithium rechargeable batteries with fast charging and high volumetric energy densities. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- O’Heir, J. Building better batteries. Mech. Eng. 2017, 139, 10. [Google Scholar]
- Choi, S.; Kwon, T.-W.; Coskun, A.; Choi, J.W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef]
- Yazami, R.; Touzain, P. A reversible graphite-lithium negative electrode for electrochemical generators. J. Power Sources 1983, 9, 365–371. [Google Scholar] [CrossRef]
- Zaghib, K.; Simoneau, M.; Armand, M.; Gauthier, M. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries. J. Power Sources 1999, 81, 300–305. [Google Scholar] [CrossRef]
- Choi, S.; Cho, Y.G.; Kim, J.; Choi, N.S.; Song, H.K.; Wang, G.; Park, S. Mesoporous Germanium Anode Materials for Lithium-Ion Battery with Exceptional Cycling Stability in Wide Temperature Range. Small 2017, 13, 1603045. [Google Scholar] [CrossRef] [PubMed]
- Meister, P.; Jia, H.; Li, J.; Kloepsch, R.; Winter, M.; Placke, T. Best practice: Performance and cost evaluation of lithium ion battery active materials with special emphasis on energy efficiency. Chem. Mater. 2016, 28, 7203–7217. [Google Scholar] [CrossRef]
- Guo, J.; Dong, D.; Wang, J.; Liu, D.; Li, D.; Yu, X.; Zheng, Y.; Wen, Z.; Lei, W.; Deng, Y.; et al. Silicon-Based Lithium Ion Battery Systems: State-of-the-Art from Half and Full Cell Viewpoint. Adv. Funct. Mater. 2021, 31, 2102546. [Google Scholar] [CrossRef]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, X.; Li, J.; Sun, W.; Gao, J.; Guo, J.; Jiang, C. Nano-structured phosphorus composite as high-capacity anode materials for lithium batteries. Angew. Chem. 2012, 124, 9168–9171. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, T.; Zhu, J.; Huang, X.; Yin, Z.; Lu, G.; Fan, Z.; Yan, Q.; Hng, H.H.; Zhang, H. An effective method for the fabrication of few-layer-thick inorganic nanosheets. Angew. Chem. Int. Ed. 2012, 51, 9052–9056. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, P.; Wang, X.; Li, Q.; Dong, Y.; Hua, J.; Zhou, L.; Mai, L. Antimony nanoparticles anchored in three-dimensional carbon network as promising sodium-ion battery anode. J. Power Sources 2016, 304, 340–345. [Google Scholar] [CrossRef]
- Prikhodchenko, P.V.; Gun, J.; Sladkevich, S.; Mikhaylov, A.A.; Lev, O.; Tay, Y.Y.; Batabyal, S.K.; Yu, D.Y. Conversion of hydroperoxoantimonate coated graphenes to Sb2S3@ graphene for a superior lithium battery anode. Chem. Mater. 2012, 24, 4750–4757. [Google Scholar] [CrossRef]
- Li, J.; Tang, S.; Lu, L.; Zeng, H.C. Preparation of nanocomposites of metals, metal oxides, and carbon nanotubes via self-assembly. J. Am. Chem. Soc. 2007, 129, 9401–9409. [Google Scholar] [CrossRef]
- Hayner, C.M.; Zhao, X.; Kung, H.H. Materials for rechargeable lithium-ion batteries. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Tirado, J.L. Inorganic materials for the negative electrode of lithium-ion batteries: State-of-the-art and future prospects. Mater. Sci. Eng. R Rep. 2003, 40, 103–136. [Google Scholar] [CrossRef]
- Lee, W.-G.; Jang, H.S.; Raj, C.J.; Rajesh, M.; Kim, B.C.; Cho, W.-J.; Yu, K.H. Effect of proton irradiation on the structural and electrochemical properties of MnO2 nanosheets. J. Electroanal. Chem. 2018, 811, 16–25. [Google Scholar] [CrossRef]
- Gund, G.S.; Dubal, D.P.; Patil, B.H.; Shinde, S.S.; Lokhande, C.D. Enhanced activity of chemically synthesized hybrid graphene oxide/Mn3O4 composite for high performance supercapacitors. Electrochim. Acta 2013, 92, 205–215. [Google Scholar] [CrossRef]
- Li, W.; Shao, J.; Liu, Q.; Liu, X.; Zhou, X.; Hu, J. Facile synthesis of porous Mn2O3 nanocubics for high-rate supercapacitors. Electrochim. Acta 2015, 157, 108–114. [Google Scholar] [CrossRef]
- Deng, Y.; Wan, L.; Xie, Y.; Qin, X.; Chen, G. Recent advances in Mn-based oxides as anode materials for lithium ion batteries. RSC Adv. 2014, 4, 23914–23935. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; Dong, A.; Yang, D. Novel Fe3O4-CNTs nanocomposite for Li-ion batteries with enhanced electrochemical performance. Electrochim. Acta 2014, 144, 235–242. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Wu, Z.; Luo, Y.; Qiu, W.; Fan, X.; Long, B.; Huang, M.; Liu, P.; Tong, Y. High power density nitridated hematite (α-Fe2O3) nanorods as anode for high-performance flexible lithium ion batteries. J. Power Sources 2016, 308, 7–17. [Google Scholar] [CrossRef]
- Xiong, S.; Chen, J.S.; Lou, X.W.; Zeng, H.C. Mesoporous Co3O4 and CoO@C topotactically transformed from chrysanthemum-like Co(CO3)0.5(OH)·0.11 H2O and their lithium-storage properties. Adv. Funct. Mater. 2012, 22, 861–871. [Google Scholar] [CrossRef]
- Khalil, A.; Lalia, B.S.; Hashaikeh, R. Nickel oxide nanocrystals as a lithium-ion battery anode: Structure-performance relationship. J. Mater. Sci. 2016, 51, 6624–6638. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, L.; Zhang, Q.; Wang, C.; Xu, X. SnO2-based nanomaterials: Synthesis and application in lithium-ion batteries and supercapacitors. J. Nanomater. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Li, R.; Miao, C.; Yu, L.; Zhang, M.; Xiao, W. Novel self-assembled SnO2@ SnS2 hybrid microspheres as potential anode materials for lithium-ion batteries. Mater. Lett. 2020, 272, 127851. [Google Scholar] [CrossRef]
- Khan, Z.; Parveen, N.; Ansari, S.A.; Senthilkumar, S.T.; Park, S.; Kim, Y.; Cho, M.H.; Ko, H. Three-dimensional SnS2 nanopetals for hybrid sodium-air batteries. Electrochim. Acta 2017, 257, 328–334. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Zeng, L.; He, X.; Liu, J.; Huang, B.; Xiao, L.; Qian, Q.; Wei, M.; Chen, Q. SnS2 nanosheets anchored on porous carbon fibers for high performance of sodium-ion batteries. J. Electroanal. Chem. 2020, 862, 114021. [Google Scholar] [CrossRef]
- Lefebvre-Devos, I.; Olivier-Fourcade, J.; Jumas, J.; Lavela, P. Lithium insertion mechanism in SnS2. Phys. Rev. B 2000, 61, 3110. [Google Scholar] [CrossRef]
- Jiang, T.; Ozin, G.A. New directions in tin sulfide materials chemistry. J. Mater. Chem. 1998, 8, 1099–1108. [Google Scholar] [CrossRef]
- Morales, J.; Vicente, C.P.; Santos, J.; Tirado, J. Electrochemical characteristics of crystalline and amorphous SnS2 in lithium cells. J. Electrochem. Soc. 1996, 143, 2847. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, H.; Zhou, L.; Shi, B.; Guo, L.; Huang, J. In-situ liquid-phase transformation of SnS2/CNTs composite from SnO2/CNTs for high performance lithium-ion battery anode. Appl. Surf. Sci. 2021, 566, 150645. [Google Scholar] [CrossRef]
- Liu, J.; Qi, Y.; Fu, B.; Dai, J.; Wang, Q.; Zhu, X.; Shi, X. Li+ diffusion kinetics of SnS2 nanoflowers enhanced by reduced graphene oxides with excellent electrochemical performance as anode material for lithium-ion batteries. J. Alloys Compd. 2019, 794, 285–293. [Google Scholar] [CrossRef]
- Kim, T.-J.; Kim, C.; Son, D.; Choi, M.; Park, B. Novel SnS2-nanosheet anodes for lithium-ion batteries. J. Power Sources 2007, 167, 529–535. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Wu, J.; Chen, F.; Li, P.; Han, N.; Huang, W.; Liu, Y.; Ye, H.; Zhao, F.; et al. Engineering SnS2 nanosheet assemblies for enhanced electrochemical lithium and sodium ion storage. J. Mater. Chem. A 2017, 5, 25618–25624. [Google Scholar] [CrossRef]
- Cui, Z.; He, S.; Liu, Q.; Guan, G.; Zhang, W.; Xu, C.; Zhu, J.; Feng, P.; Hu, J.; Zou, R.; et al. Graphene-Like Carbon Film Wrapped Tin (II) Sulfide Nanosheet Arrays on Porous Carbon Fibers with Enhanced Electrochemical Kinetics as High-Performance Li and Na Ion Battery Anodes. Adv. Sci. 2020, 7, 1903045. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Xin, H.L.; Kuykendall, T.R.; Wu, S.-L.; Zheng, H.; Rao, M.; Cairns, E.J.; Battaglia, V.; Zhang, Y. SnS2 nanoparticle loaded graphene nanocomposites for superior energy storage. Phys. Chem. Chem. Phys. 2012, 14, 6981–6986. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ma, L.; Zheng, M.; Zhao, B.; Qiu, D.; Pan, L.; Cao, J.; Shi, Y. Synthesis and electrochemical properties of graphene-SnS2 nanocomposites for lithium-ion batteries. J. Solid State Electrochem. 2012, 16, 1999–2004. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.-Y.; Fang, Y.; Zhu, X.; Bao, J.; Zhou, X.; Lou, X.W.D. Confining SnS2 ultrathin nanosheets in hollow carbon nanostructures for efficient capacitive sodium storage. Joule 2018, 2, 725–735. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, H.; Du, Z.; Chang, X.; Zhao, L.; Du, X.; Li, Z.; Teng, Y.; Fang, J.; Świerczek, K. (101) Plane-oriented SnS2 nanoplates with carbon coating: A high-rate and cycle-stable anode material for lithium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 35880–35887. [Google Scholar] [CrossRef]
- Cui, J.; Yao, S.; Lu, Z.; Huang, J.; Chong, W.G.; Ciucci, F.; Kim, J. Revealing Pseudocapacitive Mechanisms of Meta Dichalcogenide SnS2/Graphene-CNT Aerogels for High-Energy Na Hybrid Capacitors. Adv. Energy Mater. 2018, 8, 1702488. [Google Scholar] [CrossRef]
- Hwang, S.; Yao, Z.; Zhang, L.; Fu, M.; He, K.; Mai, L.; Wolverton, C.; Su, D. Multistep lithiation of tin sulfide: An investigation using in situ electron microscopy. ACS Nano 2018, 12, 3638–3645. [Google Scholar] [CrossRef]
- Liang, C.; Gao, M.; Pan, H.; Liu, Y.; Yan, M. Lithium alloys and metal oxides as high-capacity anode materials for lithium-ion batteries. J. Alloys Compd. 2013, 575, 246–256. [Google Scholar] [CrossRef]
- Gao, C.; Li, L.; Raji, A.R.; Kovalchuk, A.; Peng, Z.; Fei, H.; He, Y.; Kim, N.D.; Zhong, Q.; Xie, E.; et al. Tin Disulfide Nanoplates on Graphene Nanoribbons for Full Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 26549–26556. [Google Scholar] [CrossRef]
- Bala, M.; Masarrat, A.; Bhogra, A.; Meena, R.; Lu, Y.-R.; Huang, Y.-C.; Chen, C.-L.; Dong, C.-L.; Ojha, S.; Avasthi, D. Structure and Transport Properties of Nickel-Implanted CoSb3 Skutterudite Thin Films Synthesized via Pulsed Laser Deposition. ACS Appl. Energy Mater. 2018, 1, 5879–5886. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, H.; Mukherjee, P.P. Evaluating pristine and modified SnS2 as a lithium-ion battery anode: A first-principles study. ACS Appl. Mater. Interfaces 2015, 7, 4000–4009. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Oleynik, I.I. Layer-dependent properties of SnS2 and SnSe2 two-dimensional materials. Phys. Rev. B 2016, 94, 125443. [Google Scholar] [CrossRef]
- Deng, Y.; Fang, C.; Chen, G. The developments of SnO2/graphene nanocomposites as anode materials for high performance lithium ion batteries: A review. J. Power Sources 2016, 304, 81–101. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.M.; Yakobson, B.I.; Wood, B.C. Assessing carbon-based anodes for lithium-ion batteries: A universal description of charge-transfer binding. Phys. Rev. Lett. 2014, 113, 028304. [Google Scholar] [CrossRef]
- Liu, S.; Yin, X.; Chen, L.; Li, Q.; Wang, T. Synthesis of self-assembled 3D flowerlike SnS2 nanostructures with enhanced lithium ion storage property. Solid State Sci. 2010, 12, 712–718. [Google Scholar] [CrossRef]
- Mukaibo, H.; Yoshizawa, A.; Momma, T.; Osaka, T. Particle size and performance of SnS2 anodes for rechargeable lithium batteries. J. Power Sources 2003, 119, 60–63. [Google Scholar] [CrossRef]
- Zuo, Y.; Xu, X.; Zhang, C.; Li, J.; Du, R.; Wang, X.; Han, X.; Arbiol, J.; Llorca, J.; Liu, J. SnS2/g-C3N4/graphite nanocomposites as durable lithium-ion battery anode with high pseudocapacitance contribution. Electrochim. Acta 2020, 349, 136369. [Google Scholar] [CrossRef]
- Liu, H.; Wei, C.; Ai, Z.; Li, M.; Xu, M.; Ma, C.; Shi, J. The positive effect of 3D interpenetrating network porous structure by carbon membranes on alleviating the volume expansion of SnS2 nanosheets for enhancing lithium and sodium storage. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125937. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Li, Y.; Zhang, Y.; Dong, Y.; Li, D.; Zhang, J. Construction of uniform SnS2/ZnS heterostructure nanosheets embedded in graphene for advanced lithium-ion batteries. J. Alloys Compd. 2020, 820, 153147. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, S.; Shao, Y.; Wu, Y.; Miao, S. High-Energy Density Li-Ion Capacitor with Layered SnS2/Reduced Graphene Oxide Anode and BCN Nanosheet Cathode. Adv. Energy Mater. 2020, 10, 1902836. [Google Scholar] [CrossRef]
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.-Z.; Zhou, A. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem. Commun. 2014, 47, 80–83. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Miao, J.; Zhao, Y.; Wang, Y. Synthesis and electrochemical characterizations of Ce doped SnS2 anode materials for rechargeable lithium ion batteries. Electrochim. Acta 2013, 93, 120–130. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, F.; Xia, Y.; Wang, H.; Kuang, X. Interlayer expansion of few-layered Mo-doped SnS2 nanosheets grown on carbon cloth with excellent lithium storage performance for lithium ion batteries. J. Mater. Chem. A 2017, 5, 4075–4083. [Google Scholar] [CrossRef]
- McNair, O.D.; Brent, D.P.; Sparks, B.J.; Patton, D.L.; Savin, D.A. Sequential thiol click reactions: Formation of ternary thiourethane/thiol–ene networks with enhanced thermal and mechanical properties. ACS Appl. Mater. Interfaces 2014, 6, 6088–6097. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, L.; Zhang, D.; Chen, S.; Coxon, P.R.; He, X.; Coto, M.; Kim, H.-K.; Xi, K.; Ding, S. A universal synthetic route to carbon nanotube/transition metal oxide nano-composites for lithium ion batteries and electrochemical capacitors. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Fang, H.; Zhao, L.; Yue, W.; Wang, Y.; Jiang, Y.; Zhang, Y. Facile and large-scale preparation of sandwich-structured graphene-metal oxide composites as anode materials for Li-ion batteries. Electrochim. Acta 2015, 186, 397–403. [Google Scholar] [CrossRef]
- Greenaway, D.L.; Nitsche, R. Preparation and optical properties of group IV–VI2 chalcogenides having the CdI2 structure. J. Phys. Chem. Solids 1965, 26, 1445–1458. [Google Scholar] [CrossRef]
- Deshpande, N.; Sagade, A.; Gudage, Y.; Lokhande, C.; Sharma, R. Growth and characterization of tin disulfide (SnS2) thin film deposited by successive ionic layer adsorption and reaction (SILAR) technique. J. Alloys Compd. 2007, 436, 421–426. [Google Scholar] [CrossRef]
- Huang, Y.; Ling, C.; Chen, X.; Zhou, D.; Wang, S. SnS2 nanotubes: A promising candidate for the anode material for lithium ion batteries. RSC Adv. 2015, 5, 32505–32510. [Google Scholar] [CrossRef]
- Ray, S.C.; Karanjai, M.K.; DasGupta, D. Structure and photoconductive properties of dip-deposited SnS and SnS2 thin films and their conversion to tin dioxide by annealing in air. Thin Solid Film 1999, 350, 72–78. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moyer, K.; Ramachandran, B.R.; Wick, C.D. Comparison of storage mechanisms in RuO2, SnO2, and SnS2 for lithium-ion battery anode materials. J. Phys. Chem. C 2016, 120, 2036–2046. [Google Scholar] [CrossRef]
- Francis, L. Crystallography and Crystal Chemistry of Materials with Layered Structures; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1976. [Google Scholar]
- Brousse, T.; Lee, S.; Pasquereau, L.; Defives, D.; Schleich, D. Composite negative electrodes for lithium ion cells. Solid State Ion. 1998, 113, 51–56. [Google Scholar] [CrossRef]
- Marestoni, L.D.; Barud, H.D.S.; Gomes, R.J.; Catarino, R.P.F.; Hata, N.N.Y.; Ressutte, J.B.; Spinosa, W.A. Commercial and potential applications of bacterial cellulose in Brazil: Ten years review. Polímeros 2021, 30, 1–19. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Meng, W.; Xie, Y.; Zhang, J.; He, C.; Zhao, D. Nanoplates-assembled SnS2 nanoflowers with carbon coating anchored on reduced graphene oxide for high performance Li-ion batteries. Appl. Surf. Sci. 2021, 539, 148283. [Google Scholar] [CrossRef]
- Soto, F.A.; Ma, Y.; Martinez De La Hoz, J.M.; Seminario, J.M.; Balbuena, P.B. Formation and growth mechanisms of solid-electrolyte interphase layers in rechargeable batteries. Chem. Mater. 2015, 27, 7990–8000. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- He, Y.-B.; Liu, M.; Huang, Z.-D.; Zhang, B.; Yu, Y.; Li, B.; Kang, F.; Kim, J.-K. Effect of solid electrolyte interface (SEI) film on cyclic performance of Li4Ti5O12 anodes for Li ion batteries. J. Power Sources 2013, 239, 269–276. [Google Scholar] [CrossRef]
- Aurbach, D. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 2000, 89, 206–218. [Google Scholar] [CrossRef]
- Momma, T.; Shiraishi, N.; Yoshizawa, A.; Osaka, T.; Gedanken, A.; Zhu, J.; Sominski, L. SnS2 anode for rechargeable lithium battery. J. Power Sources 2001, 97, 198–200. [Google Scholar] [CrossRef]
- Thangavel, R.; Samuthira Pandian, A.; Ramasamy, H.V.; Lee, Y.-S. Rapidly synthesized, few-layered pseudocapacitive SnS2 anode for high-power sodium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 40187–40196. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.w.; Jang, J.t.; Park, S.w.; Kim, C.; Park, B.; Cheon, J. Two-dimensional SnS2 nanoplates with extraordinary high discharge capacity for lithium ion batteries. Adv. Mater. 2008, 20, 4269–4273. [Google Scholar] [CrossRef]
- Zai, J.; Wang, K.; Su, Y.; Qian, X.; Chen, J. High stability and superior rate capability of three-dimensional hierarchical SnS2 microspheres as anode material in lithium ion batteries. J. Power Sources 2011, 196, 3650–3654. [Google Scholar] [CrossRef]
- Wang, L.; Zhuo, L.; Yu, Y.; Zhao, F. High-rate performance of SnS2 nanoplates without carbon-coating as anode material for lithium ion batteries. Electrochim. Acta 2013, 112, 439–447. [Google Scholar] [CrossRef]
- Du, Y.; Yin, Z.; Rui, X.; Zeng, Z.; Wu, X.-J.; Liu, J.; Zhu, Y.; Zhu, J.; Huang, X.; Yan, Q. A facile, relative green, and inexpensive synthetic approach toward large-scale production of SnS2 nanoplates for high-performance lithium-ion batteries. Nanoscale 2013, 5, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yin, X.; Hao, Q.; Zhang, M.; Li, L.; Chen, L.; Li, Q.; Wang, Y.; Wang, T. Chemical bath deposition of SnS2 nanowall arrays with improved electrochemical performance for lithium ion battery. Mater. Lett. 2010, 64, 2350–2353. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, G.; Song, H.; Liao, Q.; Cui, H.; Shen, P.; Wang, C.-X. Vertically aligned graphene-like SnS2 ultrathin nanosheet arrays: Excellent energy storage, catalysis, photoconduction, and field-emitting performances. J. Phys. Chem. C 2012, 116, 9319–9326. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y. Microwave solvothermal synthesis of flower-like SnS2 and SnO2 nanostructures as high-rate anodes for lithium ion batteries. Chem. Eng. J. 2013, 229, 183–189. [Google Scholar] [CrossRef]
- Guan, D.; Li, J.; Gao, X.; Xie, Y.; Yuan, C. Growth characteristics and influencing factors of 3D hierarchical flower-like SnS2 nanostructures and their superior lithium-ion intercalation performance. J. Alloys Compd. 2016, 658, 190–197. [Google Scholar] [CrossRef]
- Wu, Q.; Jiao, L.; Du, J.; Yang, J.; Guo, L.; Liu, Y.; Wang, Y.; Yuan, H. One-pot synthesis of three-dimensional SnS2 hierarchitectures as anode material for lithium-ion batteries. J. Power Sources 2013, 239, 89–93. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, H.; Pei, Y.; Chen, Y.; Zeng, Y.; Guo, H. Binder-free ultrathin SnS2 with superior reversibility of conversion reaction for high-rate lithium ion batteries. J. Alloys Compd. 2021, 873, 159623. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, X.; Wang, C.; Wang, W.; Xie, Y.; Qian, Y. Solvent–thermal preparation of nanocrystalline tin chalcogenide. J. Phys. Chem. Solids 1999, 60, 415–417. [Google Scholar] [CrossRef]
- Su, H.; Xie, Y.; Xiong, Y.; Gao, P.; Qian, Y. Preparation and morphology control of rod-like nanocrystalline tin sulfides via a simple ethanol thermal route. J. Solid State Chem. 2001, 161, 190–196. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, H.; Ma, X.; Xu, J.; Yang, D. Single-crystalline SnS2 nano-belts fabricated by a novel hydrothermal method. J. Phys. Condens. Matter 2003, 15, L661. [Google Scholar] [CrossRef]
- Chen, D.; Shen, G.-Z.; Tang, K.-B.; Liu, Y.-K.; Qian, Y.-T. Aligned SnS2 nanotubes fabricated via a template-assisted solvent-relief process. Appl. Phys. A 2003, 77, 747–749. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Y.; Wu, H.; Liu, X.; Qian, Y. Fabrication of layered nanocrystallites SnS and β-SnS2 via a mild solution route. Mater. Res. Bull. 2002, 37, 925–932. [Google Scholar] [CrossRef]
- Shen, G.; Chen, D.; Tang, K.; Huang, L.; Qian, Y.; Zhou, G. Novel polyol route to nanoscale tin sulfides flaky crystallines. Inorg. Chem. Commun. 2003, 6, 178–180. [Google Scholar] [CrossRef]
- Chen, D.; Shen, G.; Tang, K.; Lei, S.; Zheng, H.; Qian, Y. Microwave-assisted polyol synthesis of nanoscale SnSx (x = 1, 2) flakes. J. Cryst. Growth 2004, 260, 469–474. [Google Scholar] [CrossRef]
- Chen, X.; Lin, J.; Chen, Y.; Zhang, J.; Jiang, H.; Qiu, F.; Chu, R.; Guo, H. Binder-free and self-supported reduced graphene oxide coated Cu2SnS3/Carbon nanofibers for superior lithium storage. J. Alloys Compd. 2020, 842, 155619. [Google Scholar] [CrossRef]
- Nath, M.; Rao, C.N.R. New metal disulfide nanotubes. J. Am. Chem. Soc. 2001, 123, 4841–4842. [Google Scholar] [CrossRef] [PubMed]
- Mdleleni, M.M.; Hyeon, T.; Suslick, K.S. Sonochemical synthesis of nanostructured molybdenum sulfide. J. Am. Chem. Soc. 1998, 120, 6189–6190. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, B.; Yang, M.; Schmuki, P. MoS2 Decorated on Different Metal Oxide Nanotubular Structures with a High Density of Reactive Sites for HER Reactions. In Proceedings of the ECS Meeting Abstracts, Seattle, WA, USA, 13–17 May 2018; p. 1709. [Google Scholar]
- Guo, Y.G.; Hu, J.S.; Wan, L.J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 2008, 20, 2878–2887. [Google Scholar] [CrossRef]
- Ma, J.; Lei, D.; Duan, X.; Li, Q.; Wang, T.; Cao, A.; Mao, Y.; Zheng, W. Designable fabrication of flower-like SnS2 aggregates with excellent performance in lithium-ion batteries. RSC Adv. 2012, 2, 3615–3617. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Chi, C.; Wang, J.; Guo, Q.; Yang, Y.; Li, Y.; Liu, X.; Zhao, J. Enhanced storage capability by biomass-derived porous carbon for lithium-ion and sodium-ion battery anodes. Sustain. Energy Fuels 2018, 2, 2358–2365. [Google Scholar] [CrossRef]
- Hong, K.-l.; Qie, L.; Zeng, R.; Yi, Z.-q.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.-j.; Zhang, W.-x. Biomass derived hard carbon used as a high performance anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, X.; Wang, Q.; Xu, X.; Zhou, X.; Bao, J. Kelp-derived hard carbons as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 5761–5769. [Google Scholar] [CrossRef]
- Lu, P.; Sun, Y.; Xiang, H.; Liang, X.; Yu, Y. 3D amorphous carbon with controlled porous and disordered structures as a high-rate anode material for sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1702434. [Google Scholar] [CrossRef]
- Kim, H.S.; Chung, Y.H.; Kang, S.H.; Sung, Y.-E. Electrochemical behavior of carbon-coated SnS2 for use as the anode in lithium-ion batteries. Electrochim. Acta 2009, 54, 3606–3610. [Google Scholar] [CrossRef]
- Zhao, H.; Zeng, H.; Wu, Y.; Qi, W.; Zhang, S.; Li, B.; Huang, Y. Facile ball-milled synthesis of SnS2-carbon nanocomposites with superior lithium storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 676–682. [Google Scholar] [CrossRef]
- Li, J.; Wu, P.; Lou, F.; Zhang, P.; Tang, Y.; Zhou, Y.; Lu, T. Mesoporous carbon anchored with SnS2 nanosheets as an advanced anode for lithium-ion batteries. Electrochim. Acta 2013, 111, 862–868. [Google Scholar] [CrossRef]
- He, Y.; Xu, Z. The status and development of treatment techniques of typical waste electrical and electronic equipment in China: A review. Waste Manag. Res. 2014, 32, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Miao, C.; Zhang, M.; Xiao, W. Novel hierarchical structural SnS2 composite supported by biochar carbonized from chewed sugarcane as enhanced anodes for lithium ion batteries. Ionics 2020, 26, 1239–1247. [Google Scholar] [CrossRef]
- Zhai, C.; Du, N.; Zhang, H.; Yu, J.; Yang, D. Multiwalled carbon nanotubes anchored with SnS2 nanosheets as high-performance anode materials of lithium-ion batteries. ACS Appl. Mater. Interfaces 2011, 3, 4067–4074. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Zhang, K.; Feng, X.; Wang, M. Synthesis and high-performance of carbonaceous polypyrrole nanotubes coated with SnS2 nanosheets anode materials for lithium ion batteries. Chem. Eng. J. 2017, 330, 470–479. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, H.C.; Lee, J.Y. Highly reversible lithium storage in porous SnO2 nanotubes with coaxially grown carbon nanotube overlayers. Adv. Mater. 2006, 18, 645–649. [Google Scholar] [CrossRef]
- Shin, H.C.; Liu, M. Three-dimensional porous copper–tin alloy electrodes for rechargeable lithium batteries. Adv. Funct. Mater. 2005, 15, 582–586. [Google Scholar] [CrossRef]
- Lou, X.W.; Wang, Y.; Yuan, C.; Lee, J.Y.; Archer, L.A. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv. Mater. 2006, 18, 2325–2329. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Li, J.; Sun, Y.; Zhang, X.; Shen, L.; Lu, X.; Xiong, S.; Lou, X.W. Flexible hybrid paper made of monolayer Co3O4 microsphere arrays on rGO/CNTs and their application in electrochemical capacitors. Adv. Funct. Mater. 2012, 22, 2560–2566. [Google Scholar] [CrossRef]
- Han, K.; Liu, Z.; Ye, H.; Dai, F. Flexible self-standing graphene–Se@ CNT composite film as a binder-free cathode for rechargeable Li–Se batteries. J. Power Sources 2014, 263, 85–89. [Google Scholar] [CrossRef]
- Sun, H.; Ahmad, M.; Luo, J.; Shi, Y.; Shen, W.; Zhu, J. SnS2 nanoflakes decorated multiwalled carbon nanotubes as high performance anode materials for lithium-ion batteries. Mater. Res. Bull. 2014, 49, 319–324. [Google Scholar] [CrossRef]
- Mia, R.; Sultana, S. Fabrication and properties of silver nanowires (AgNWs) functionalized fabric. SN Appl. Sci. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Novák, P.; Müller, K.; Santhanam, K.; Haas, O. Electrochemically active polymers for rechargeable batteries. Chem. Rev. 1997, 97, 207–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Qian, Y.; Wu, H.; Kan, E. First-principles study on S and N doping graphene/SnS2 heterostructure for lithium-ion battery. Chem. Phys. Lett. 2021, 769, 138391. [Google Scholar] [CrossRef]
- Zhang, M.; Lei, D.; Yu, X.; Chen, L.; Li, Q.; Wang, Y.; Wang, T.; Cao, G. Graphene oxide oxidizes stannous ions to synthesize tin sulfide–graphene nanocomposites with small crystal size for high performance lithium ion batteries. J. Mater. Chem. 2012, 22, 23091–23097. [Google Scholar] [CrossRef]
- Yin, J.; Cao, H.; Zhou, Z.; Zhang, J.; Qu, M. SnS2@reduced graphene oxide nanocomposites as anode materials with high capacity for rechargeable lithium ion batteries. J. Mater. Chem. 2012, 22, 23963–23970. [Google Scholar] [CrossRef]
- Chang, K.; Wang, Z.; Huang, G.; Li, H.; Chen, W.; Lee, J.Y. Few-layer SnS2/graphene hybrid with exceptional electrochemical performance as lithium-ion battery anode. J. Power Sources 2012, 201, 259–266. [Google Scholar] [CrossRef]
- Wei, W.; Jia, F.-F.; Wang, K.-F.; Qu, P. SnS2/graphene nanocomposite: A high rate anode material for lithium ion battery. Chin. Chem. Lett. 2017, 28, 324–328. [Google Scholar] [CrossRef]
- Wang, H.-E.; Zhao, X.; Li, X.; Wang, Z.; Liu, C.; Lu, Z.; Zhang, W.; Cao, G. rGO/SnS 2/TiO2 heterostructured composite with dual-confinement for enhanced lithium-ion storage. J. Mater. Chem. A 2017, 5, 25056–25063. [Google Scholar] [CrossRef]
- Luo, B.; Fang, Y.; Wang, B.; Zhou, J.; Song, H.; Zhi, L. Two dimensional graphene–SnS2 hybrids with superior rate capability for lithium ion storage. Energy Environ. Sci. 2012, 5, 5226–5230. [Google Scholar] [CrossRef]
- Du, N.; Wu, X.; Zhai, C.; Zhang, H.; Yang, D. Large-scale synthesis and application of SnS2–graphene nanocomposites as anode materials for lithium-ion batteries with enhanced cyclic performance and reversible capacity. J. Alloys Compd. 2013, 580, 457–464. [Google Scholar] [CrossRef]
- Ren, Y.; Lv, W.; Wen, F.; Xiang, J.; Liu, Z. Microwave synthesis of SnS2 nanoflakes anchored graphene foam for flexible lithium-ion battery anodes with long cycling life. Mater. Lett. 2016, 174, 24–27. [Google Scholar] [CrossRef]
- Zhuo, L.; Wu, Y.; Wang, L.; Yu, Y.; Zhang, X.; Zhao, F. One-step hydrothermal synthesis of SnS2/graphene composites as anode material for highly efficient rechargeable lithium ion batteries. RSC Adv. 2012, 2, 5084–5087. [Google Scholar] [CrossRef]
- Xia, J.; Liu, L.; Xie, J.; Yan, H.; Yuan, Y.; Chen, M.; Huang, C.; Zhang, Y.; Nie, S.; Wang, X. Layer-by-layered SnS2/graphene hybrid nanosheets via ball-milling as promising anode materials for lithium ion batteries. Electrochim. Acta 2018, 269, 452–461. [Google Scholar] [CrossRef]
- Sathish, M.; Mitani, S.; Tomai, T.; Honma, I. Ultrathin SnS2 nanoparticles on graphene nanosheets: Synthesis, characterization, and Li-ion storage applications. J. Phys. Chem. C 2012, 116, 12475–12481. [Google Scholar] [CrossRef]
- Tang, H.; Qi, X.; Han, W.; Ren, L.; Liu, Y.; Wang, X.; Zhong, J. SnS2 nanoplates embedded in 3D interconnected graphene network as anode material with superior lithium storage performance. Appl. Surf. Sci. 2015, 355, 7–13. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, D.; Wu, J.; Wang, Z.; Huang, S.; Xu, Y.; Chen, Z.; Zhao, B.; Zhang, J. Sandwich-like SnS2/graphene/SnS2 with expanded interlayer distance as high-rate lithium/sodium-ion battery anode materials. ACS Nano 2019, 13, 9100–9111. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, X.; Zhu, Y.; Shen, J.; Fan, K.; Li, C. In situ assembly of graphene sheets-supported SnS2 nanoplates into 3D macroporous aerogels for high-performance lithium ion batteries. J. Power Sources 2013, 237, 178–186. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Zhang, X.; Guo, J. 3D architecture constructed by 2D SnS2-graphene hybrids towards large and fast lithium storage. Mater. Lett. 2016, 185, 311–314. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, J.; Mu, C.; Wen, F.; Yuan, S.; Zhao, J.; Xu, D.; Su, C.; Liu, Z. SnS2 nanoflakes anchored graphene obtained by liquid phase exfoliation and MoS2 nanosheet composites as lithium and sodium battery anodes. Electrochim. Acta 2017, 227, 203–209. [Google Scholar] [CrossRef]
- Wang, Q.; Nie, Y.-X.; He, B.; Xing, L.-L.; Xue, X.-Y. SnS2–graphene nanocomposites as anodes of lithium-ion batteries. Solid State Sci. 2014, 31, 81–84. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, Y.; Mahmood, N.; Liu, F.; Hou, Y. SnS2/graphene composites: Excellent anode materials for lithium ion battery and photolysis catalysts. Sci. Adv. Mater. 2013, 5, 1667–1675. [Google Scholar] [CrossRef]
- Liu, S.; Lu, X.; Xie, J.; Cao, G.; Zhu, T.; Zhao, X. Preferential c-axis orientation of ultrathin SnS2 nanoplates on graphene as high-performance anode for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 1588–1595. [Google Scholar] [CrossRef]
- Mei, L.; Xu, C.; Yang, T.; Ma, J.; Chen, L.; Li, Q.; Wang, T. Superior electrochemical performance of ultrasmall SnS2 nanocrystals decorated on flexible RGO in lithium-ion batteries. J. Mater. Chem. A 2013, 1, 8658–8664. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, B.; Zhang, J.; Yu, W.; Zheng, J.; Ding, Z.; Li, H.; Ming, L.; Bengono, D.; Chen, S. In-situ grown SnS2 nanosheets on rGO as an advanced anode material for lithium and sodium ion batteries. Front. Chem. 2018, 6, 629. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; He, H.; Song, Q.; Wang, B.; Yang, Q.-H.; Zhi, L. A novel SnS₂@ graphene nanocable network for high-performance lithium storage. RSC Adv. 2014, 4, 23372–23376. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Gao, Z.; Hu, X.; Ling, R.; Cai, S.; Zheng, C.; Hu, W. A Simple One-Pot Strategy for Synthesizing Ultrafine SnS2 Nanoparticle/Graphene Composites as Anodes for Lithium/Sodium-Ion Batteries. ChemSusChem 2018, 11, 1549–1557. [Google Scholar] [CrossRef]
- Yao, J.; Shen, X.; Wang, B.; Liu, H.; Wang, G. In situ chemical synthesis of SnO2–graphene nanocomposite as anode materials for lithium-ion batteries. Electrochem. Commun. 2009, 11, 1849–1852. [Google Scholar] [CrossRef]
- Zhao, B.; Song, D.; Ding, Y.; Li, W.; Wang, Z.; Jiang, Y.; Zhang, J. Size-tunable SnS2 nanoparticles assembled on graphene as anodes for high performance lithium/sodium-ion batteries. Electrochim. Acta 2020, 354, 136730. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Yang, Y.; Pu, H.; Gao, R.-Z.; Meng, W.-J.; Yang, H.-X.; Zhao, D.-L. SnS2 nanoparticle-integrated graphene nanosheets as high-performance and cycle-stable anodes for lithium and sodium storage. J. Alloys Compd. 2020, 822, 153686. [Google Scholar] [CrossRef]
- Cheng, M.; Hu, Q.; Du, C.; Li, J.; Liao, W.; Li, J.; Huang, X. An ionic liquid-assisted route towards SnS2 nanoparticles anchored on reduced graphene oxide for lithium-ion battery anode. J. Solid State Chem. 2021, 296, 122022. [Google Scholar] [CrossRef]
- Zhong, Y.; Mahmud, S.; He, Z.; Yang, Y.; Zhang, Z.; Guo, F.; Chen, Z.; Xiong, Z.; Zhao, Y. Graphene oxide modified membrane for highly efficient wastewater treatment by dynamic combination of nanofiltration and catalysis. J. Hazard. Mater. 2020, 397, 122774. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Al Mamun, M.A.; Rony, M.; Anchang, X.; Rashid, M.M. Effect of Different Solvent Systems on Fiber Morphology and Property of Electrospun PCL Nano Fibers. Tekst. Mühendis 2021, 28, 61–76. [Google Scholar] [CrossRef]
- Li, Y.-F.; Wang, S.-G.; Shi, Y.-H.; Fan, C.-Y.; Lin, J.; Wu, X.-L.; Sun, H.-Z.; Zhang, J.-P.; Xie, H.-M. In situ chemically encapsulated and controlled SnS2 nanocrystal composites for durable lithium/sodium-ion batteries. Dalton Trans. 2020, 49, 15874–15882. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, Y.; Huang, L.; Li, C.; Shi, G. Three-dimensional porous graphene/polyaniline composites for high-rate electrochemical capacitors. J. Mater. Chem. A 2014, 2, 17489–17494. [Google Scholar] [CrossRef]
- Tang, Z.; Shen, S.; Zhuang, J.; Wang, X. Noble-metal-promoted three-dimensional macroassembly of single-layered graphene oxide. Angew. Chem. 2010, 122, 4707–4711. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Zhang, Y.; Fan, W.; Liu, T. Three-dimensional nanoporous graphene-carbon nanotube hybrid frameworks for confinement of SnS2 nanosheets: Flexible and binder-free papers with highly reversible lithium storage. ACS Appl. Mater. Interfaces 2015, 7, 27823–27830. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).