Detection of Nitroaromatic Explosives in Air by Amino-Functionalized Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
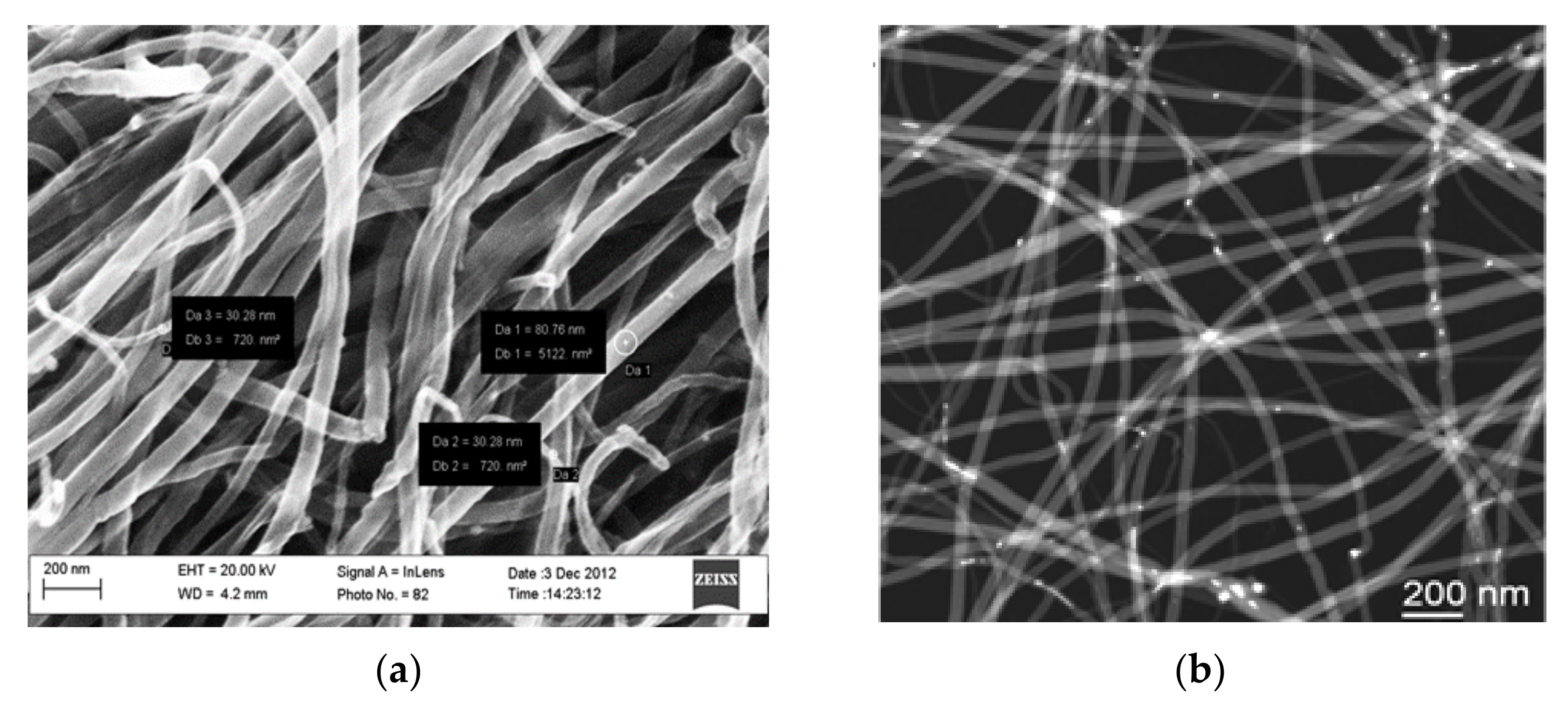
2.1. MWCNT Preparation and Characterization
2.2. Functionalization of MWCNTs
3. Results and Discussion
3.1. Infrared (IR) Analysis
3.2. X-ray Photoelectron Spectroscopy
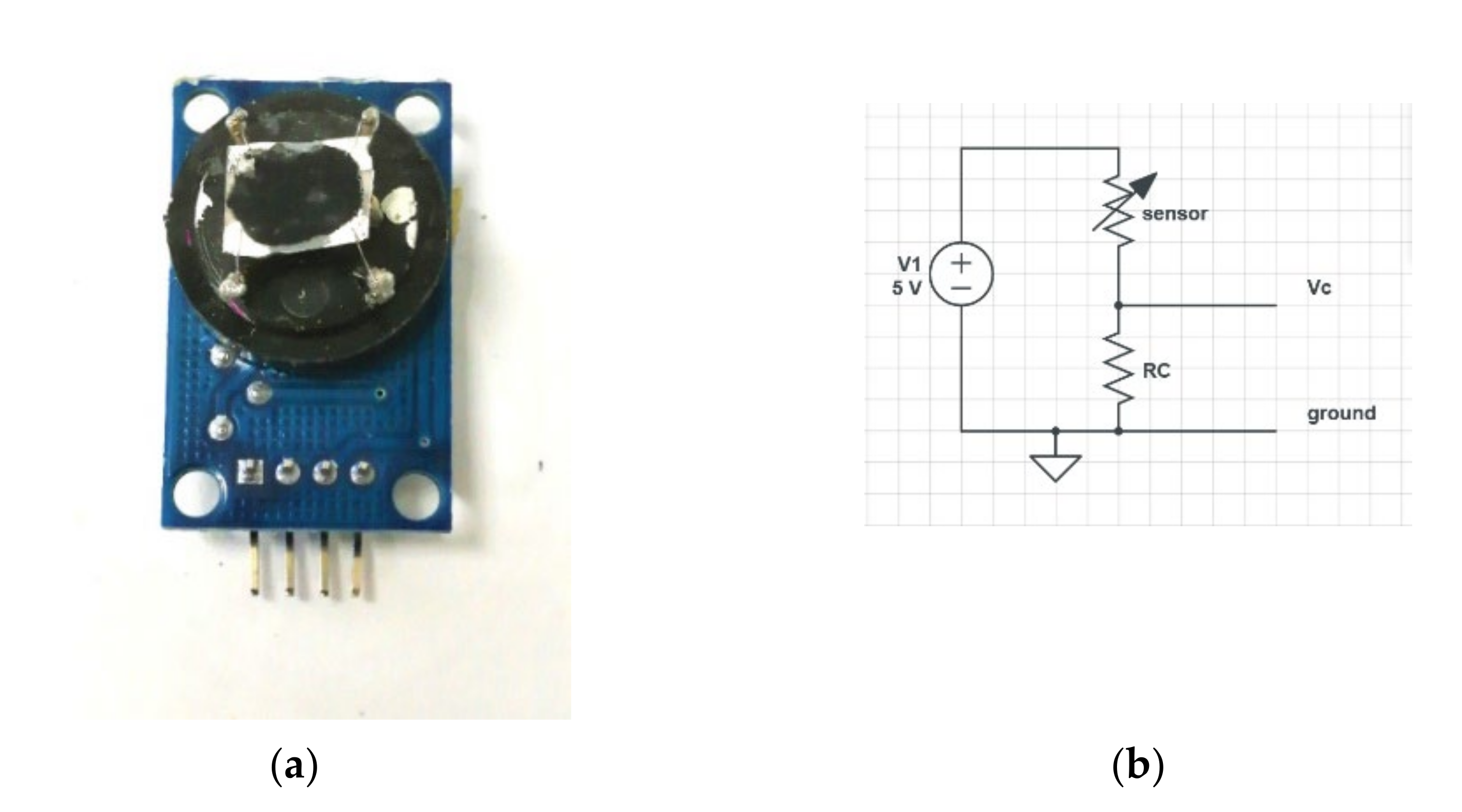
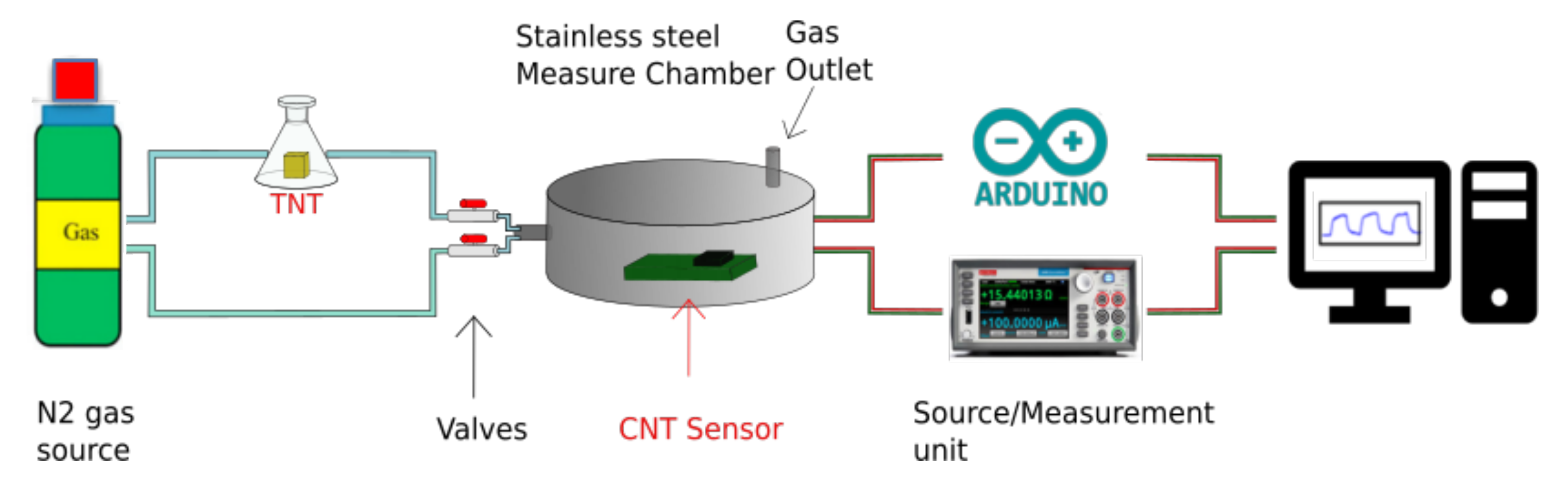
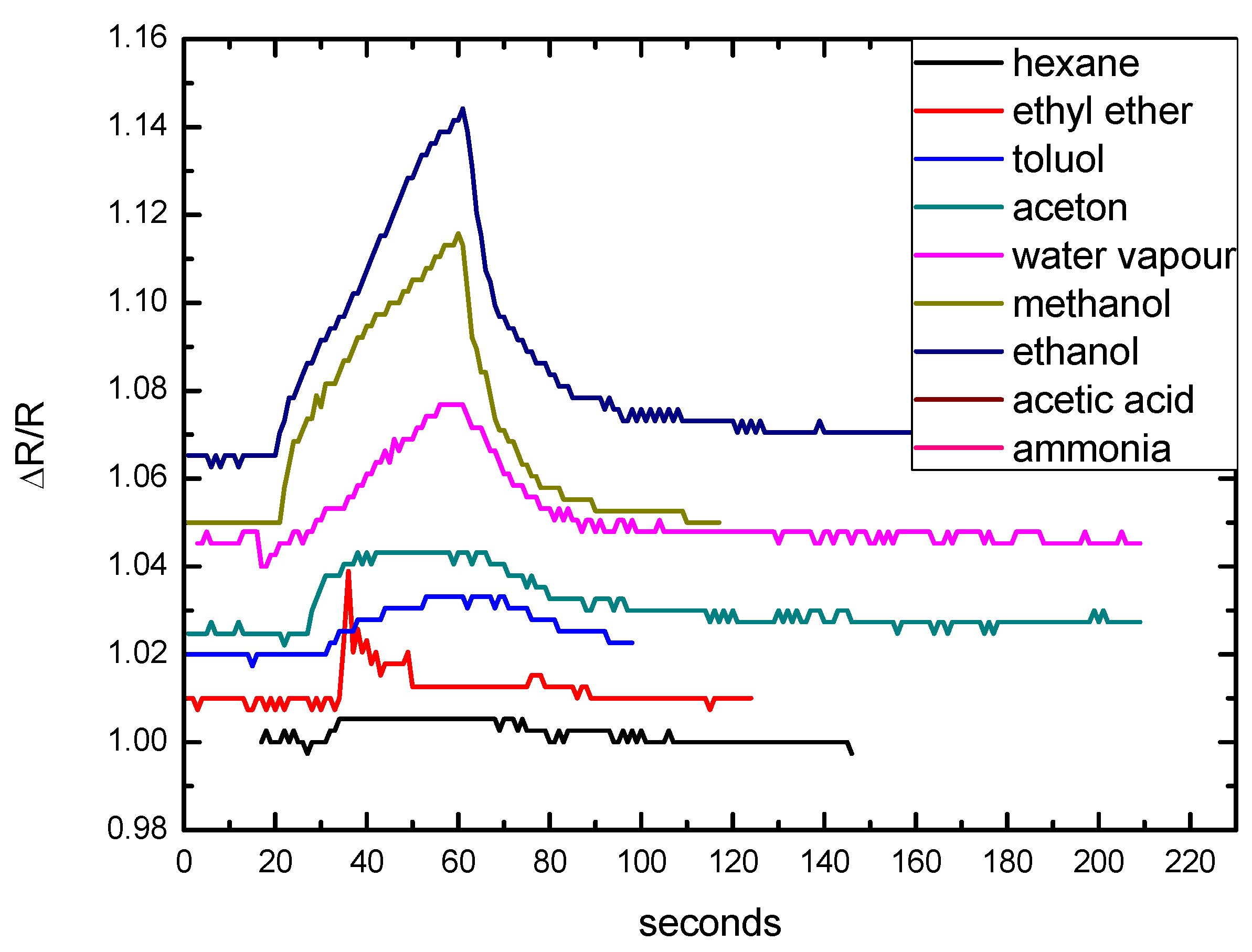
3.3. Sensor Structure Design and Test
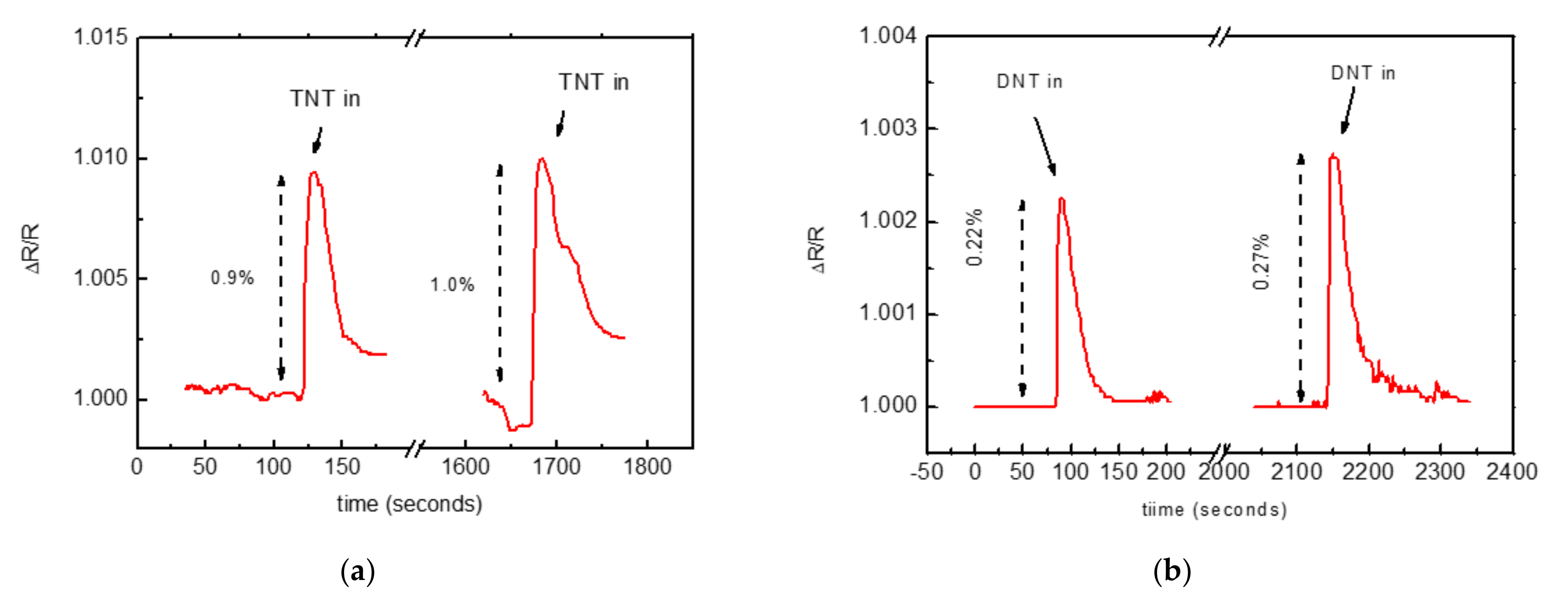
- The measuring chamber was kept in the dark and was shielded against electric noise. With or without N2 flux, the sensor signal was stable with noise of the order of 1 × 10−4.
- A sensor made with pristine MWCNTs that were tested with TNT and acetic acid did not show any change in resistivity.
- A test with a functionalized CNT network, performed by switching the gas flow through an empty ampoule, did not show any change in sensor resistivity.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ewing, R.J.; Waltman, M.J.; David, A.; Atkinson, D.A.; Grate, J.W. The vapor pressures of explosives. Trends Anal. Chem. 2013, 42, 35–48. [Google Scholar] [CrossRef]
- Rodacy, P.J.; Bender, S.; Bromenshenk, J.; Henderson, A.; Bender, G. Training and deployment of honeybees to detect explosives and other agents of harm. In Proceedings of the SPIE—The International Society for Optical Engineering, Orlando, FL, USA, 13 August 2002. [Google Scholar]
- Moore, D.S. Recent Advances in Trace Explosives Detection Instrumentation. Sens. Imaging 2007, 8, 9–38. [Google Scholar] [CrossRef]
- Kolla, P. The Application of Analytical Methods to the Detection of Hidden Explosives and Explosive Devices. Angew. Chem. Int. Ed. 1997, 36, 800–811. [Google Scholar] [CrossRef]
- Ouyang, Z.; Noll, R.J.; Cooks, R.G. Handheld Miniature Ion Trap Mass Spectrometry. Anal. Chem. 2009, 81, 2421–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrinskaya, A.; Kunz, R.R.; Clark, M.; Kingsborough, R.P.; Ong, T.-H.; Deneault, S. Rapid Quantitative Analysis of Multiple Explosive Compound Classes on a Single Instrument via Flow-Injection Analysis Tandem Mass Spectrometry. J. Forensic Sci. 2018, 64, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Yinon, Y. Detection of Explosives by Mass Spectrometry. In Counterterrorist Detection Techniques of Explosives; Yinon, J., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 41–59. [Google Scholar]
- Contreras, J.A.; Murray, J.A.; Tolley, S.E.; Oliphant, J.L.; Tolley, H.D.; Lammert, S.A.; Lee, E.D.; Later, D.W.; Lee, M.L. Hand-Portable Gas Chromatograph-Toroidal Ion Trap Mass Spectrometer (GC-TMS) For Detection of Hazardous Compounds. J. Am. Soc. Mass Spectrom. 2008, 19, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- Oxley, J.; Smith, J.; Brady, J.; Dubnikova, F.; Kosloff, R.; Zeiri, L.; Zeiri, Y. Raman and Infrared Fingerprint Spectroscopy of Peroxide- Based Explosives. Appl. Spectrosc. 2008, 62, 906–915. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Gong, W.; Wu, W.; Fan, M.; Wang, D.; Brolo, A.G. Detection of Buried Explosives Using a Surface-Enhanced Raman Scattering (SERS) Substrate Tailored for Miniaturized Spectrometers. ACS Sens. 2020, 5, 2933–2939. [Google Scholar] [CrossRef]
- Senesac, L.; Thundat, G.T. Nanosensors for trace explosive detection. Mater. Today 2008, 11, 28–36. [Google Scholar] [CrossRef]
- UnExplode (Unmanned Explosive Detection). Available online: www.unexplode.it (accessed on 1 January 2020).
- Zhang, D.; Jiang, J.; Chen, J.; Zhang, Q.; Lu, Y.; Yao, Y.; Li, S.; Logan, G.; Liu, Q. Smartphone-based portable bio sensing system using impedance measurement with printed electrodes for 2,4,6-trinitrotuluene (TNT) detection. Biosens. Bioelectron. 2015, 70, 81–88. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, D.; Zhang, D.; Li, C.; Sun, D.; Wen, S.; Chen, S.; Ruan, S. Gas Sensors Based on Metal Sulfide Zn1−xCdxS Nanowires with Excellent Performance. ACS Appl. Mater. Interfaces 2015, 7, 20793. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, C.; Zu, B.; Li, Y.; Dou, X. Contactless and Rapid Discrimination of Improvised Explosives Realized by Mn2+ Doping Tailored ZnS Nanocrystals. Adv. Funct. Mater. 2016, 26, 4578–4586. [Google Scholar] [CrossRef]
- Cognet, L.; Tsyboulski, D.A.; Rocha, J.D.R.; Doyle, C.D.; Tour, J.M.; Weisman, R.B. Stepwise quenching of exciton fluorescence in carbon nanotubes by single-molecule reactions. Science 2007, 316, 1465–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnorr, J.M.; Daan, V.D.Z.; Joseph, J.W.; Weizman, Y.; Swager, T.M. Sensory Arrays of Covalently Functionalized Single-Walled Carbon Nanotubes for Explosive Detection. Adv. Funct. Mater. 2013, 23, 5285–5291. [Google Scholar] [CrossRef] [Green Version]
- Chuen, T.K.; Ben-Jaber, S.; Parkin, I.P. Recent Developments in the Field of Explosive Trace Detection. ACS Nano 2020, 14, 10804–10833. [Google Scholar] [CrossRef]
- Musayeva, N.; Orujov, T.; Hasanov, R.; Sultanov, C.; Ferrari, C.; Frigeri, C.; Trevisi, G.; Beretta, S.; Bosi, M.; Verucchi, R.; et al. Growth and functionalization of carbon nanotubes for nitroaromatic explosive detection. Mater. Today Proc. 2020, 20, 46–49. [Google Scholar] [CrossRef]
- Solzi, M.; Cugini, F.; Sarzi Amadè, N.; Frigeri, C.; Attolini, G.; Musayeva, N.N.; Huseynov, A.B.; Abdullayeva, S.H.; Sultanov, C.A. High-temperature magnetic coercivity of CNTs filled with multi-phase Fe-based nanoparticles. J. Magn. Magn. Mater. 2020, 496, 165917. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Sience 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, A.B.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon N. Y. 2011, 46, 24–36. [Google Scholar] [CrossRef]
- Battigelli, A.; Ménard-Moyon, C.; Da Ros, T.; Prato, M.; Bianco, A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv. Drug Deliv. Rev. 2013, 65, 1899–1920. [Google Scholar] [CrossRef]
- Cavaliere, E.; Tatti, R.; Aversa, L.; Verucchi, R.; Garberoglio, G.; Pugno, N.M.; Speranza, G.; Taioli, S. Synthesis of single layer graphene on Cu(111) by C60 supersonic molecular beam epitaxy. RSC Adv. 2016, 6, 37982–37993. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.A.E.S. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Gershanik, A.P.; Yehuda, Z. Sublimation Rate of TNT Microcrystals in Air. J. Phys. Chem. 2010, 114, 12403–12410. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.; Havivi, E.; Shacham, R.; Hahamy, E.; Leibovich, R.; Pevzner, A.; Krivitsky, V.; Davivi, G.; Presman, I.; Elnathan, R.; et al. Supersensitive fingerprinting of explosives by chemically modified nanosensors arrays. Nat. Commun. 2014, 5, 4195. [Google Scholar] [CrossRef] [Green Version]
- Sablok, K.; Bhalla, V.; Sharma, P.; Kaushal, R.; Chaudhary, S.; Suri, C.R. Amine functionalized graphene oxide/CNT nanocomposite for ultrasensitive electrochemical detection of trinitrotoluene. J. Hazard. Mater. 2013, 248–249, 322–328. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Tran, T.H.; Lee, J.W.; Lee, K.; Lee, Y.D.; Ju, B.K. The gas sensing properties of single-walled carbon nanotubes deposited on an aminosilane monolayer. Sens. Actuators B 2008, 129, 67–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Bunes, B.R.; Wu, N.; Gross, D.E.; Moore, J.S.; Zang, L. Oligomer-Coated Carbon Nanotube Chemiresistive Sensors for Selective Detection of Nitroaromatic Explosives. ACS Appl. Mater. Interfaces 2015, 7, 7471–7475. [Google Scholar] [CrossRef]
- Cerruti, M.; Jaworski, J.; Raorane, D.; Zueger, C.; Varadarajan, J.; Carraro, C.; Lee, S.; Maboudian, R.; Majumdar, A. Polymer-Oligopeptide Composite Coating for Selective Detection of Explosives in Water. Anal. Chem. 2009, 81, 4192–4199. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Tutov, M.V.; Sergeev, A.A.; Mitsai, E.V.; Ustinov, A.Y.; Zhizhchenko, A.Y.; Linklater, D.P.; Bratskaya, S.Y.; Juodkazis, S.; Kuchmizhak, A.A. Ultratrace Nitroaromatic Vapor Detection via Surface-Enhanced Fluorescence on Carbazole-Terminated Black Silicon. ACS Sens. 2019, 4, 2879–2884. [Google Scholar] [CrossRef]
- Dhara, S.; Jawa, H.; Ghosh, S.; Varghese, A.; Karmakar, D.; Lodha, S. All-Electrical High-Sensitivity, Low-Power Dual-Mode Gas Sensing and Recovery with a WSe2/MoS2 pn Heterodiode. ACS Appl. Mater. Interfaces 2021, 13, 30785–30796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Swager, T.M. Sensory Responses in Solution vs Solid State: A Fluorescence Quenching Study of Poly(iptycenebutadiynylene)s. Macromolecules 2005, 38, 9377–9384. [Google Scholar] [CrossRef]
- Marchisio, A.; Tulliani, J. Semiconducting Metal Oxides Nanocomposites for Enhanced Detection of Explosive Vapors. Ceramics 2018, 1, 98–119. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Yang, Z.; Li, Y.; Zu, B.; Dou, X. Sensitive, real-time and anti-interfering detection of nitro-explosive vapors realized by ZnO/rGO core/shell micro-Schottky junction. Sens. Actuators B Chem. 2017, 239, 286–294. [Google Scholar] [CrossRef]
- Barata, P.D.; Prata, J.V. Fluorescent Calix[4]arene-Carbazole-Containing Polymers as Sensors for Nitroaromatic Explosives. Chemosensors 2020, 8, 128. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Zu, B.; Dou, X. Qualitative Detection Toward Military and Improvised Explosive Vapors by a Facile TiO2 Nanosheet-Based Chemiresistive Sensor Array. Front. Chem. 2020, 8, 29. [Google Scholar] [CrossRef]
- Kumar, V.; Maiti, B.; Chini, M.K.; De, P.; Satapathi, S. Multimodal Fluorescent Polymer Sensor for Highly Sensitive Detection of Nitroaromatics. Sci. Rep. 2019, 9, 7269. [Google Scholar] [CrossRef] [Green Version]
- Gradišek, A.; van Midden, M.; Koterle, M.; Prezelj, V.; Strle, D.; Štefane, B.; Brodnik, H.; Trifkovič, M.; Kvasić, I.; Zupanič, E.; et al. Improving the Chemical Selectivity of an Electronic Nose to TNT, DNT and RDX Using Machine Learning. Sensors 2019, 19, 5207. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, S.; Wang, H.; Gao, A.; Wang, Y.; Li, T.; Zhang, H.; Huang, Z.; Cheng, Z. Amino Monolayer Modified Nanowire Array for Trinitrotoluene Detection. Sens. Mater. 2018, 30, 2669–2677. [Google Scholar] [CrossRef]
- Eslami, M.; Alizadeh, N. Ultrasensitive and selective QCM sensor for detection of trace amounts of nitroexplosive vapors in ambient air based on polypyrrole—Bromophenol blue nanostructure. Sens. Actuators B Chem. 2018, 278, 55–63. [Google Scholar] [CrossRef]
- Ghoorchian, A.; Alizadeh, N. Chemiresistor gas sensor based on sulfonated dye-doped modified conducting polypyrrole film for high sensitive detection of 2,4,6-trinitrotoluene in air. Sens. Actuators B Chem. 2018, 255, 826–835. [Google Scholar] [CrossRef]
- Ge, Y.; Wei, Z.; Li, Y.; Qu, J.; Zu, B.; Dou, X. Highly sensitive and rapid chemiresistive sensor towards trace nitro-explosive vapors based on oxygen vacancy-rich and defective crystallized In-doped ZnO. Sens. Actuators B Chem. 2017, 244, 983–991. [Google Scholar] [CrossRef]



| % C | % O | % N | |
|---|---|---|---|
| Pristine MWCNTs | 94.0 | 6.0 | - |
| COOH−MWCNTs | 82.9 | 17.1 | - |
| NH2−C2−MWCNTs | 93.4 | 3.6 | 3.0 |
| NH2−C6−MWCNTs | 86.0 | 6.3 | 7.7 |
| Ligand | Sensing Device | Detection Limit | Reference |
|---|---|---|---|
| Amino-functionalized MWCNTs | Chemiresistive | Few ppb (T = 28 °C) | This work |
| Virus-phage litmus | Colorimetric | 300 ppb | Cerruti et al. [32] |
| Carbazole-terminated black silicon | Surface-enhanced fluorescence | 20 ppt (DNT) | Mironenko et al. [33] |
| WSe2/MoS2 | Two-dimensional MoS2 | 80 ppb | Dhara et al. [34] |
| Poly(iptycenebutadiynylene) | Florescence quenching | 5–7 ppb | Zhao [35] |
| Fe−ZnO | Chemoresistive | 9 ppb | Marchisio et al. [36] |
| Core-shell ZnO/reduced graphene oxide (rGO) | Chemoresistive | 1 ppb | Guo et al. [37] |
| Calix[4]arene-carbazole-containing polymers | Fluorescence-based | 9 ppb | Barata. et al. [38] |
| TiO2 nanosheet | Chemiresistive gas sensor | 9 ppb | Li et al. [39] |
| Alanine-based dansyl tagged copolymer | Fluorescence quenching | 9 ppb | Kumar et al. [40] |
| Organic silane | Microcapacitive detection of adsorbed molecules | 9 ppb | Gradišek et al. [41] |
| Silicon nanowires | Amino monolayer modified nanowire array | 9 ppb | Liu et al. [42] |
| Polypyrrole-bromophenol blue | Quartz crystal microbalance | 500 ppt | Eslami et al. [43] |
| Sulfonated dye-doped conducting polypyrrole | Chemiresistor | 200 ppt | Ghoorchian et al. [44] |
| ZnO nanoparticles (NPs) | Chemiresistor | 9 ppb | Ge et al. [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, C.; Attolini, G.; Bosi, M.; Frigeri, C.; Frigeri, P.; Gombia, E.; Lazzarini, L.; Rossi, F.; Seravalli, L.; Trevisi, G.; et al. Detection of Nitroaromatic Explosives in Air by Amino-Functionalized Carbon Nanotubes. Nanomaterials 2022, 12, 1278. https://doi.org/10.3390/nano12081278
Ferrari C, Attolini G, Bosi M, Frigeri C, Frigeri P, Gombia E, Lazzarini L, Rossi F, Seravalli L, Trevisi G, et al. Detection of Nitroaromatic Explosives in Air by Amino-Functionalized Carbon Nanotubes. Nanomaterials. 2022; 12(8):1278. https://doi.org/10.3390/nano12081278
Chicago/Turabian StyleFerrari, Claudio, Giovanni Attolini, Matteo Bosi, Cesare Frigeri, Paola Frigeri, Enos Gombia, Laura Lazzarini, Francesca Rossi, Luca Seravalli, Giovanna Trevisi, and et al. 2022. "Detection of Nitroaromatic Explosives in Air by Amino-Functionalized Carbon Nanotubes" Nanomaterials 12, no. 8: 1278. https://doi.org/10.3390/nano12081278
APA StyleFerrari, C., Attolini, G., Bosi, M., Frigeri, C., Frigeri, P., Gombia, E., Lazzarini, L., Rossi, F., Seravalli, L., Trevisi, G., Lolli, R., Aversa, L., Verucchi, R., Musayeva, N., Alizade, M., Quluzade, S., Orujov, T., Sansone, F., Baldini, L., & Rispoli, F. (2022). Detection of Nitroaromatic Explosives in Air by Amino-Functionalized Carbon Nanotubes. Nanomaterials, 12(8), 1278. https://doi.org/10.3390/nano12081278











