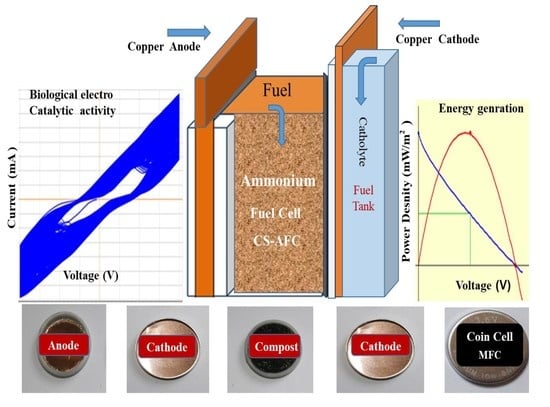
High Power Generation with Reducing Agents Using Compost Soil as a Novel Electrocatalyst for Ammonium Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation and Fabrication of Fuel Cell
2.2. Electrochemical and Electrical Characterization
3. Results and Discussion
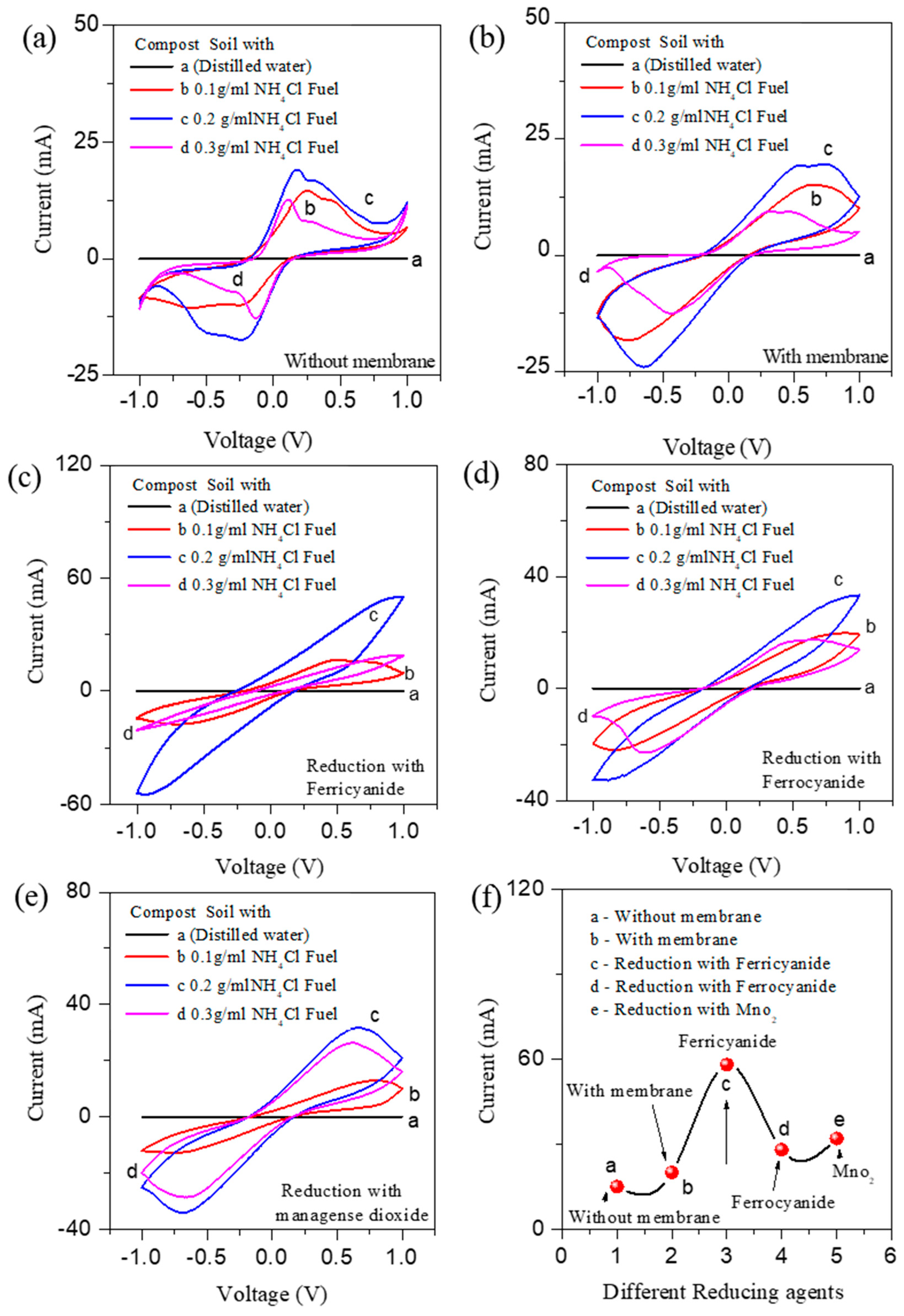
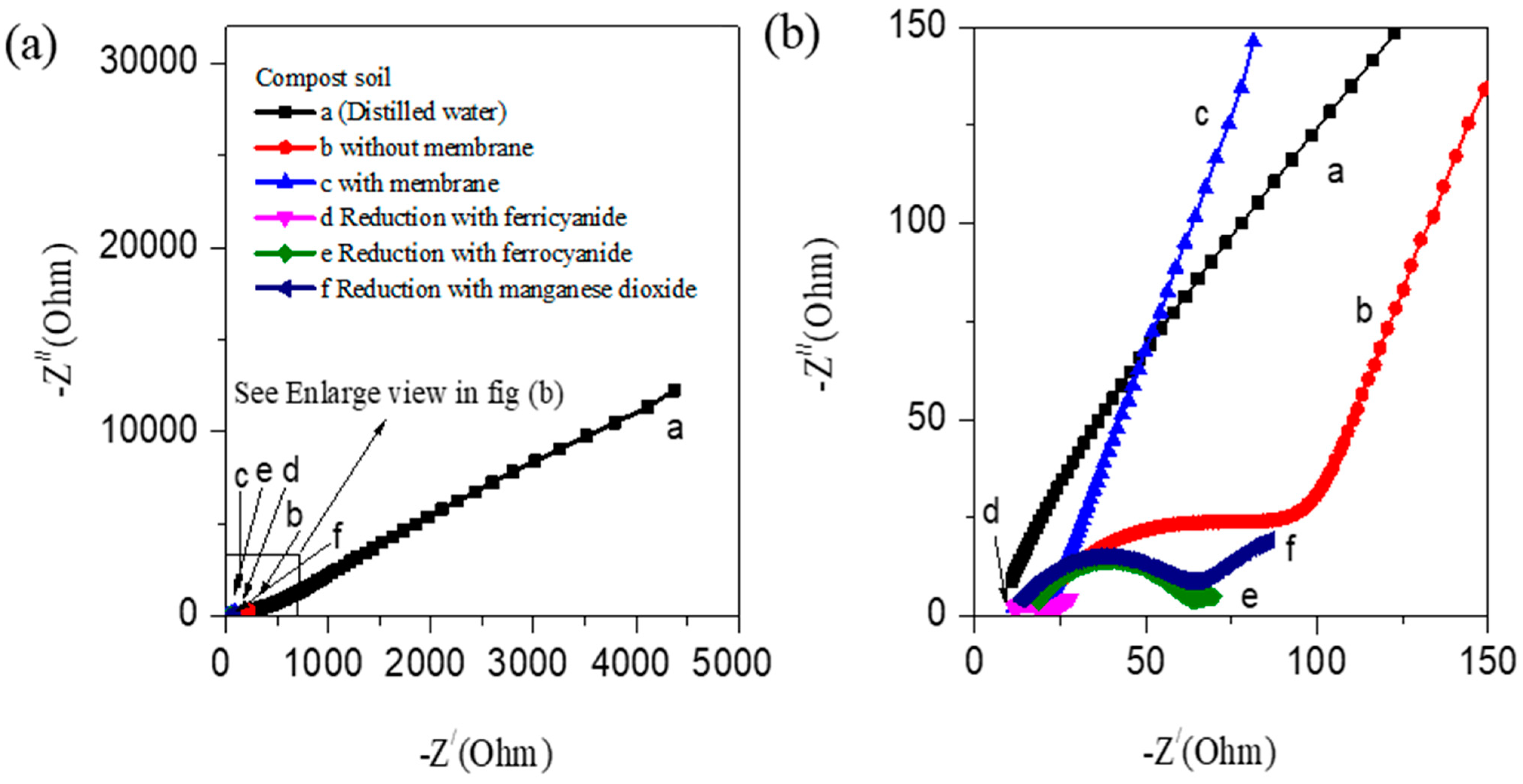
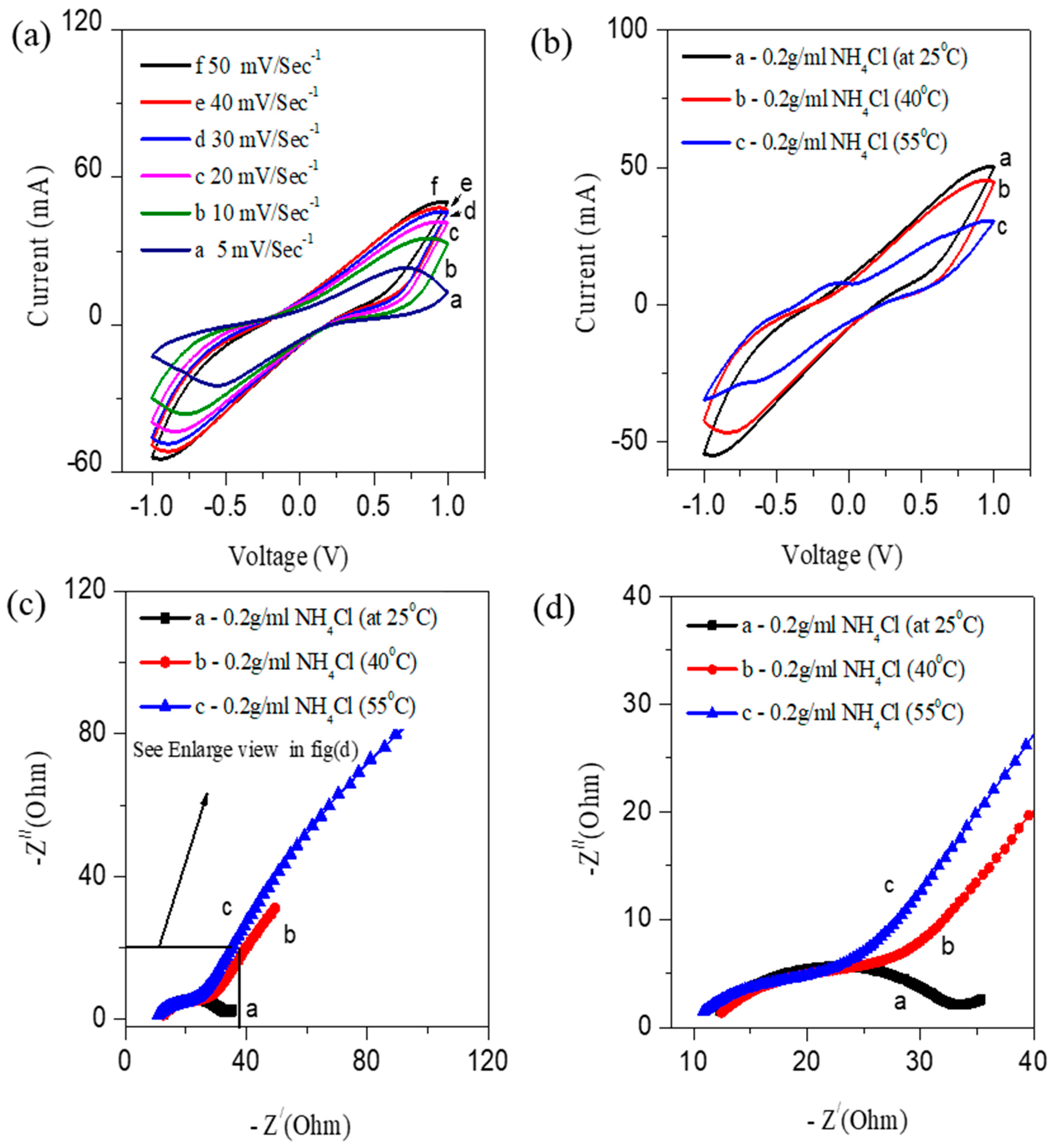
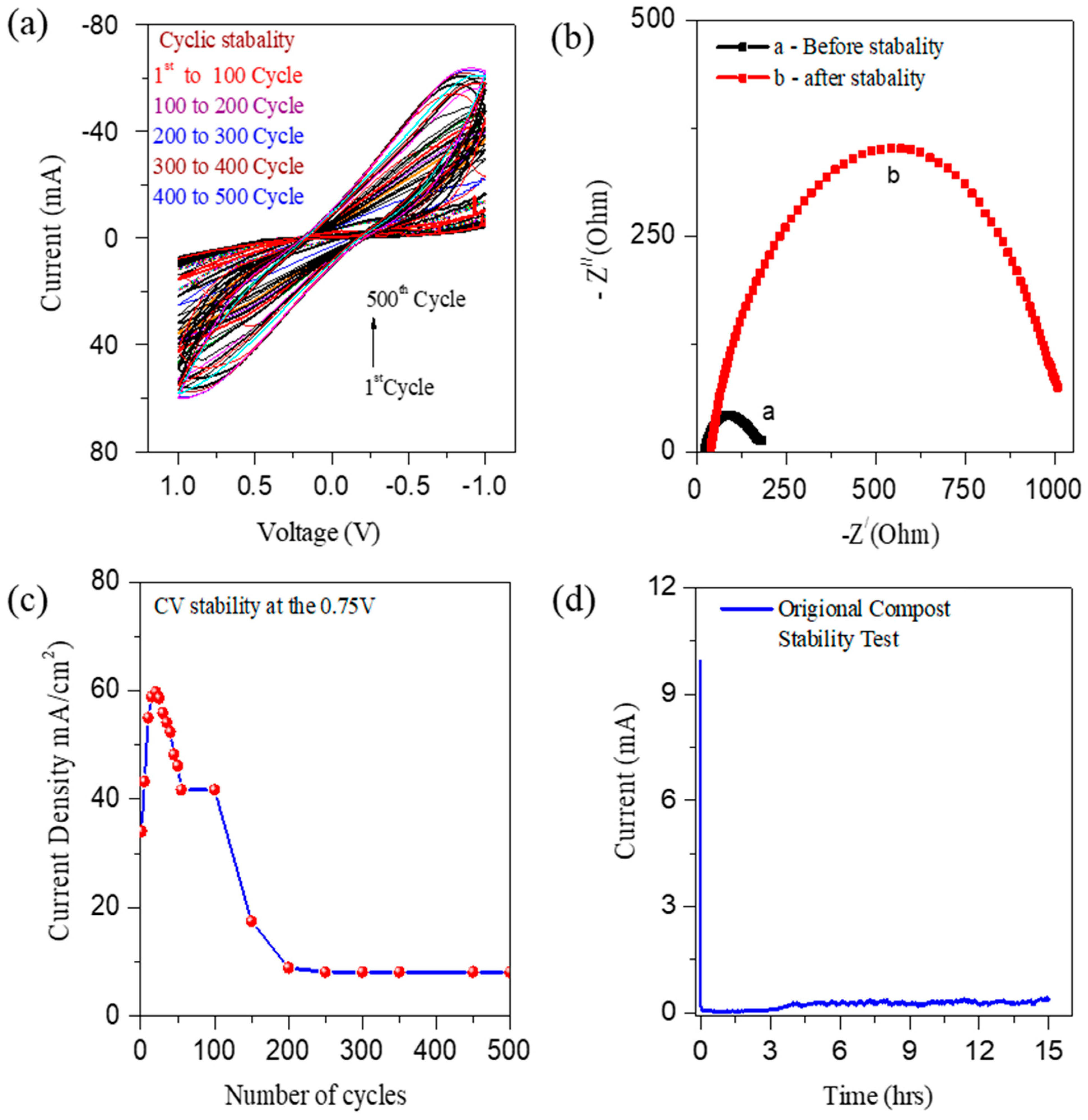
3.1. Electrochemical Studies
3.2. Polarization and Power Studies
3.3. Bacterial and Autoclaved Sterilisation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannan, A.M.; Renugopalakrishnan, V.; Filipek, S.; Li, P.; Audette, G.F.; Munukutla, L. Bio-batteries and bio-fuel cells: Leveraging on electronic charge transfer proteins. J. Nanosci. Nanotechnol. 2009, 9, 1665–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Kan, J.; Yanbing, W.; Huang, Y.; Mansfeld, F.; Nelson, K.H. Electricity production coupled to ammonium in a microbial fuel cell. Environ. Sci. Technol. 2009, 43, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Angar, Y.; Djelali, N.E. Influence of the anode nature in the ammonium electro-oxidation. Rev. Roum. Chim. 2015, 60, 1039–1046. [Google Scholar]
- Kumar, S.; Magotra, V.K.; Jeon, H.C.; Kang, T.W.; Inamdar, A.I.; Aqueel, A.T.; Im, H.; Ahuja, R. Multifunctional ammonium fuel cell using compost as an oval electrocatalyst. J. Power Sources 2018, 402, 221–228. [Google Scholar] [CrossRef]
- Anglada, A.; Ibanez, R.; Uriega, A.; Ortiz, I. Electrochemical oxidation of saline industrial wastewaters using boron-doped diamond anodes. Catal. Today 2010, 151, 178–184. [Google Scholar] [CrossRef]
- Perez, G.; Saiz, J.; IBanez, R.; Urtiaga, A.M.; Ortiz, I. Assessment of the formation of Inorganic oxidation by-products electrocatalytic treatment of ammonium from Landfill leachates. Water Res. 2012, 46, 2579–2590. [Google Scholar] [CrossRef]
- Kapałk, A.; Fierro, A.; Frontistis, Z.; Katsaounis, S.A.; Neodo, S.; Frey, O.; Rooij, N.D.; Udert, K.M.; Comninellis, C. Electrochemical oxidation of ammonia (NH4+/NH3)on thermally and electrochemically prepared IrO2 electrodes. Electrochim. Acta. 2011, 56, 1361–1365. [Google Scholar] [CrossRef]
- Bunce, N.J.; Bejan, D. Mechanism of electrochemical oxidation of ammonia. Electrochim. Acta. 2011, 56, 8085–8093. [Google Scholar] [CrossRef]
- Zöllig, H.; Morgenroth, E.; Udder, K.M. Inhibition of direct electrolytic ammonia oxidation due to a change in local pH. Electrochim. Acta. 2015, 165, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetton, M.M.S. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidising microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Jetten, M.S.; Wagner, M.; Fuerst, J.; Loosdrecht, M.V.; Kuenen, G.; Strous, M. Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Biotechnology 2001, 122, 83–288. [Google Scholar] [CrossRef]
- Thamdrup, B.; Dalsgaard, T. Production of N2 through anaerobic ammonium Oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 2002, 68, 1312–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fux, C.; Boehler, M.; Huber, P.; Brunner, I.; Siegrist, H. Biological treatment of ammonium-rich wastewater by partial nitration and subsequent anaerobic ammonium oxidation (anammox) in a pilot plant. J. Biotechnol. 2002, 99, 295–306. [Google Scholar] [CrossRef]
- Kuntke, P.; Smiech, K.M.; Bruning, H.; Zeeman, G.; Saakes, M.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 2012, 46, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Wolinska, A.; Stepniewska, Z.; Bielecka, A.; Ciesielski, J. Bioelectricity production from soil using microbial fuel cell. Appl. Biochem. Biotechnol. 2014, 173, 2287–2296. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Zhong, W.H.; Han, C.; Deng, H. Characterization of electricity generated by the soil in microbial fuel cells and the isolation of soil source exoelectrogenic Bacteria. Front. Microbiol. 2016, 7, 1776. [Google Scholar] [CrossRef]
- Huang, D.Y.; Zhou, S.G.; Chen, Q.; Zhao, B.; Yuan, Y.; Zhuang, L. Enhanced anaerobic degradation of organic pollutants soil microbial fuel cell. Chem. Eng. J. 2011, 172, 647–653. [Google Scholar] [CrossRef]
- Kim, J.R.; Jung, S.H.; Regan, J.M.; Logan, E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 2007, 98, 2568–2577. [Google Scholar] [CrossRef]
- Raj, S.; Solomon, J.; Prathipa Kumar, A. Production of electricity from agricultural soil and dye industrial effluent soil using microbial fuel cell. IJRET 2013, 10, 2321–7308. [Google Scholar]
- Wang, C.T.U.; Liao, F.Y.; Liu, K.S. Electrical analysis of compost solid-phase microbial fuel cell. Int. J. Hydrog. Energy 2013, 38, 11124–11130. [Google Scholar] [CrossRef]
- Li, J. An Experimental Study of Microbial Fuel Cells for Electricity Generating: Performance Characterisation and Capacity Improvement. J. Sustain. Bioenergy Syst. 2013, 3, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Roche, I.; Katuri, K.; Scott, K. A microbial fuel cell using manganese oxide reduction catalysts. J. Appl. Electrochem. 2010, 40, 13–21. [Google Scholar] [CrossRef]
- Gustafsson, A.M.K.; Björefors, F.; Steineri, B.M.; Ekbarg, C. Investigation of an electrochemical method for separating copper, indium, and gallium from pre-treated CIGS solar cell waste materials. Sci. World J. 2015, 49, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Han, H.; Shen, J. Effects of cathodic electron acceptors and potassium ferricyanide concentrations on the performance of microbial fuel cell. Int. J. Hydrogen Energy 2012, 37, 12980–12986. [Google Scholar] [CrossRef]
- Gladkova, M.M.; Terekhova, V.A. Engineered nanomaterials in soil: Sources of entry and migration pathways. Mosc. Univ. Soil Sci. Bull. 2013, 68, 129–134. [Google Scholar] [CrossRef]
- Zhang, G. Soil nanoparticles and their influence on engineering properties of soils. Adv. Meas. Modeling Soil Behav. 2017, 1–13. [Google Scholar] [CrossRef]
- Song, E.; Goyne, K.W.; Kremer, R.J.; Anderson, S.H.; Xiong, X. Certain Soil Surfactants Could Become a Source of Soil Water Repellency after Repeated Application. Nanomaterials 2021, 11, 2577. [Google Scholar] [CrossRef]
- Shrivastava, M.; Srivastav, A.; Gandhi, S.; Rao, S.; Roychoudhury, A.; Kumar, A.; Singh, S.D. Monitoring of engineered nanoparticles in soil-plant system: A review. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100218. [Google Scholar] [CrossRef]
- Oh, S.; Min, B.; Logan, B.E. Cathode performance as a factor in electricity generation in microbial fuel cells. Environ. Sci. Technol. 2004, 38, 4900–4904. [Google Scholar] [CrossRef]
- Cai, J.; Zheng, P.; Mahmood, Q. Effect of cathode electron acceptors on simultaneous anaerobic sulfide and nitrate removal in a microbial fuel cell. Water Sci. Technol. 2016, 73, 947–954. [Google Scholar] [CrossRef]
- Lawson, K.; Rossi, R.; Regan, J.M.; Logan, B.E. Impact of cathodic electron acceptor on microbial fuel cell internal resistance. Bioresour. Technol. 2020, 316, 123919–123927. [Google Scholar] [CrossRef] [PubMed]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schroder, U. Do it have to be carbon? Metal anodes in microbial fuel cells and related bio electrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Sam, A.; Hu, B.; DeBruler, C.; Wei, X.; Wang, W.; Liu, T.L. Unraveling pH Dependent Cycling Stability of Ferricyanide/Ferrocyanide in Redox Flow Batteries. Nano Energy 2017, 42, 215–221. [Google Scholar] [CrossRef]
- Mrlík, M.; Osička, J.; Cvek, M.; Ilčíková, M.; Srnec, P.; Gorgol, D.; Tofel, P. Comparative Study of PVDF Sheets and Their Sensitivity to Mechanical Vibrations: The Role of Dimensions, Molecular Weight, Stretching and Poling. Nanomaterials 2021, 11, 1637. [Google Scholar] [CrossRef]
- Magotra, V.K.; Kang, T.W.; Inamdar, I.A.; Ahmed, A.T.A.; Im, H.; Ghodake, G.; Choubey, R.K.; Kumar, V.; Kumar, S. Effect of gold nanoparticles laced anode on the bio-electro-catalytic activity and power generation ability of compost based microbial fuel cell as a coin cell sized device. Biomass Bioenergy 2021, 152, 106200. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Sushkova, S.; Singh, R.K.; Verma, K.K. Nanotechnology in the Restoration of Polluted Soil. Nanomaterials 2022, 12, 769. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Ahmed, A.T.A.; Cho, S.; Kim, J.; Jo, Y.; Pawar, S.M.; Spark, Y.K.; Im, H. Nano grain tungsten oxide with excess as a highly reversible anode material for high-performance Li-ion batteries. Mater. Lett. 2018, 215, 233–237. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Kalubarme, R.S.; Kim, J.; Jo, Y.; Woo, H.; Cho, S.; Pawar, S.M.; Park, C.J.; Lee, Y.W.; Sohn, J.I.; et al. Nickel titanate lithium-ion battery anodes with high reversible capacity and high rate long cycle life performance. J. Mater. Chem. A 2016, 4, 4691–4699. [Google Scholar] [CrossRef]
- Heijne, A.N.; Hamelers, H.V.M.; Wilde, V.; Rozendal, R.A.; Buisman, C.J.N. A bipolar membrane combined with ferric iron Reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Ahmed, A.T.A.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Kim, H.; Im, H. Microporous Cu(OH)2 nanorod network fabricated directly on Cu foil as binder-free Lithium-ion battery anode with the ultrahigh capacity. J. Alloys Compd. 2020, 829, 154593. [Google Scholar] [CrossRef]
- Fricke, K.; Harnisch, F.; Schroder, U. The use of cyclic voltammetry for the study of anodic electron transfer in microbial fuel cells. Energy Environ. 2008, 1, 144–147. [Google Scholar] [CrossRef]
- Magotra, V.K.; Kumar, S.; Kang, T.W.; Inamdar, I.A.; Ahmed, A.T.A.; Im, H.; Ghodake, G.; Shinde, S.K.; Waghmode, D.P.; Jeon, H.C. Compost soil microbial fuel cell using urea as fuel. Sci. Rep. 2020, 10, 4154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulu, F.; Kobya, M. Ammonia removal from wastewater by air stripping and recovery struvite and calcium sulphate precipitations from anesthetic gases manufacturing wastewater. J. Water Process. Eng. 2020, 38, 101641. [Google Scholar] [CrossRef]
- Geissler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Kim, J.R.; Zuo, Y.; Regan, M.; Logan, B.E. Analysis of ammonia loss mechanisms in microbial fuel cells treating animal waste water. Biotechnol. Bioeng. 2008, 99, 1120–1127. [Google Scholar] [CrossRef]
- Anger, Y.; Daoudi, S.; Gana, S.K.; Djelali, N.E. The electrocatalytic activity survey of some cathode materials to reduce nitrites and nitrates in aqueous solution. Rev. Roum. Chim. 2020, 65, 1019–1029. [Google Scholar] [CrossRef]
- Rysgaard, S.; Risgaard, N.; Sloth, N.P. Nitrification, denitrification and nitrate ammonification in sediments of two coastal lagoons in Southern France. Hydrobiologia 1996, 329, 133–141. [Google Scholar] [CrossRef]
- Szpyrkowicz, L. Application of electrochemical oxidation for treatment wastewater The influence of rector hydrodynamics on direct and mediated processes. J. Chem. Technol. Biotechnol. 2006, 81, 1375–1383. [Google Scholar] [CrossRef]
- Bavasso, I.; Montanaro, D.; Petrucci, E.; Di Palma, L. Shortcut Biological Nitrogen Removal (SBNR) in an MFC anode chamber under microaerobic conditions: The effect of C/N ratio and kinetic study. Sustainability 2018, 10, 1062. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.R.; Ali, M.; Katuri, K.P.; Gralnick, J.A.; Reimann, J.; Mesman, R.; Niftrik, L.V.; Jetten, M.S.M.; Saikaly, P.E. Extracellular electron transfer-dependent anaerobic oxidation of ammonium by anammox bacteria. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magotra, V.K.; Lee, S.J.; Kang, T.W.; Inamdar, A.I.; Kim, D.Y.; Im, H.; Jeon, H.C. High Power Generation with Reducing Agents Using Compost Soil as a Novel Electrocatalyst for Ammonium Fuel Cells. Nanomaterials 2022, 12, 1281. https://doi.org/10.3390/nano12081281
Magotra VK, Lee SJ, Kang TW, Inamdar AI, Kim DY, Im H, Jeon HC. High Power Generation with Reducing Agents Using Compost Soil as a Novel Electrocatalyst for Ammonium Fuel Cells. Nanomaterials. 2022; 12(8):1281. https://doi.org/10.3390/nano12081281
Chicago/Turabian StyleMagotra, Verjesh Kumar, Seung Joo Lee, Tae Won Kang, Akbar I. Inamdar, Deuk Young Kim, Hyunsik Im, and Hee Chang Jeon. 2022. "High Power Generation with Reducing Agents Using Compost Soil as a Novel Electrocatalyst for Ammonium Fuel Cells" Nanomaterials 12, no. 8: 1281. https://doi.org/10.3390/nano12081281