Creation and Magnetic Study of Ferrites with Magnetoplumbite Structure Multisubstituted by Al3+, Cr3+, Ga3+, and In3+ Cations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation Methods
→ BaFe6Al1.5Cr1.5Ga1.5In1.5O19 + CO2↑.
2.2. Structure and Elemental Composition Study Methods
2.3. Magnetic Study Methods
3. Results and Discussion
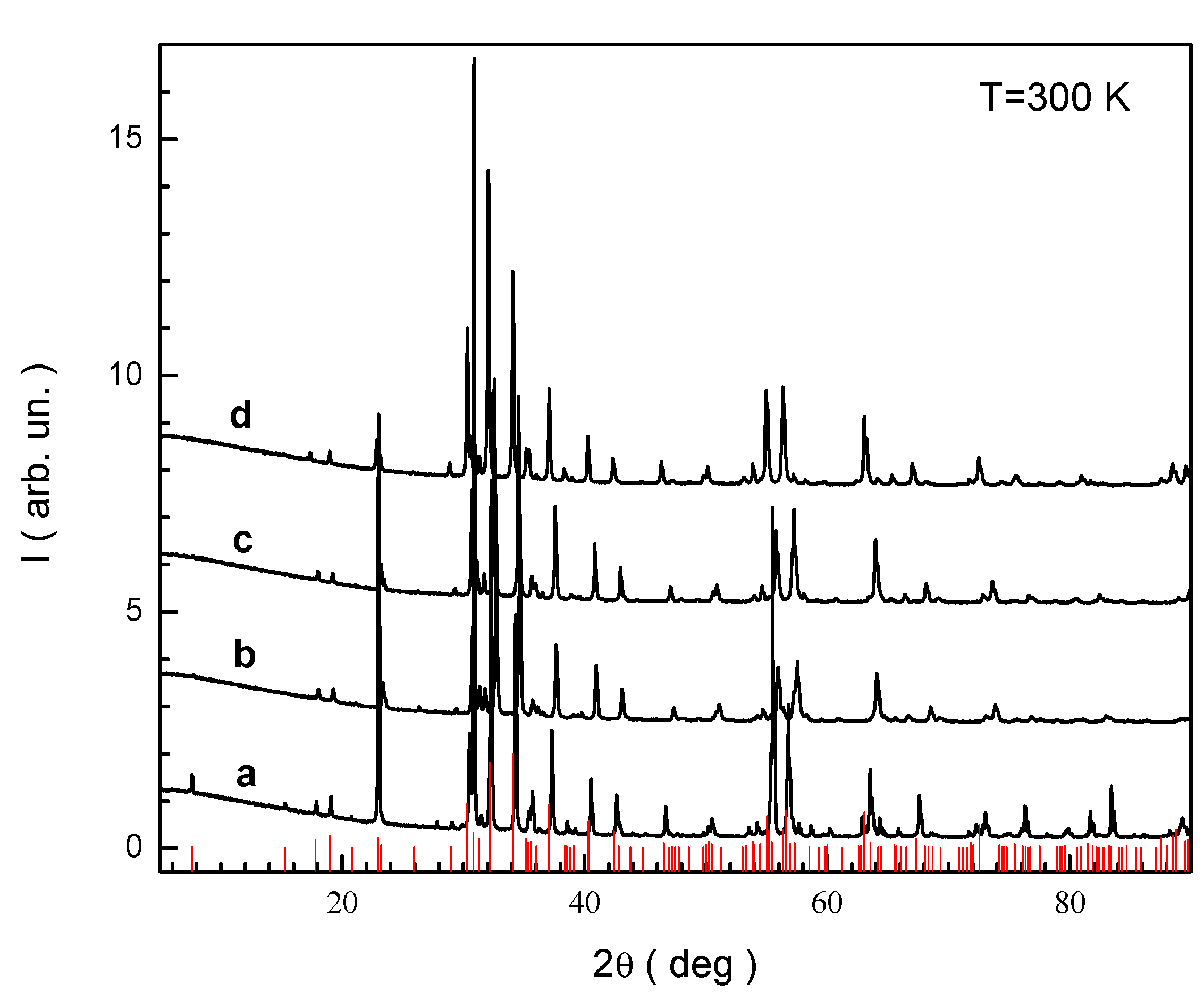
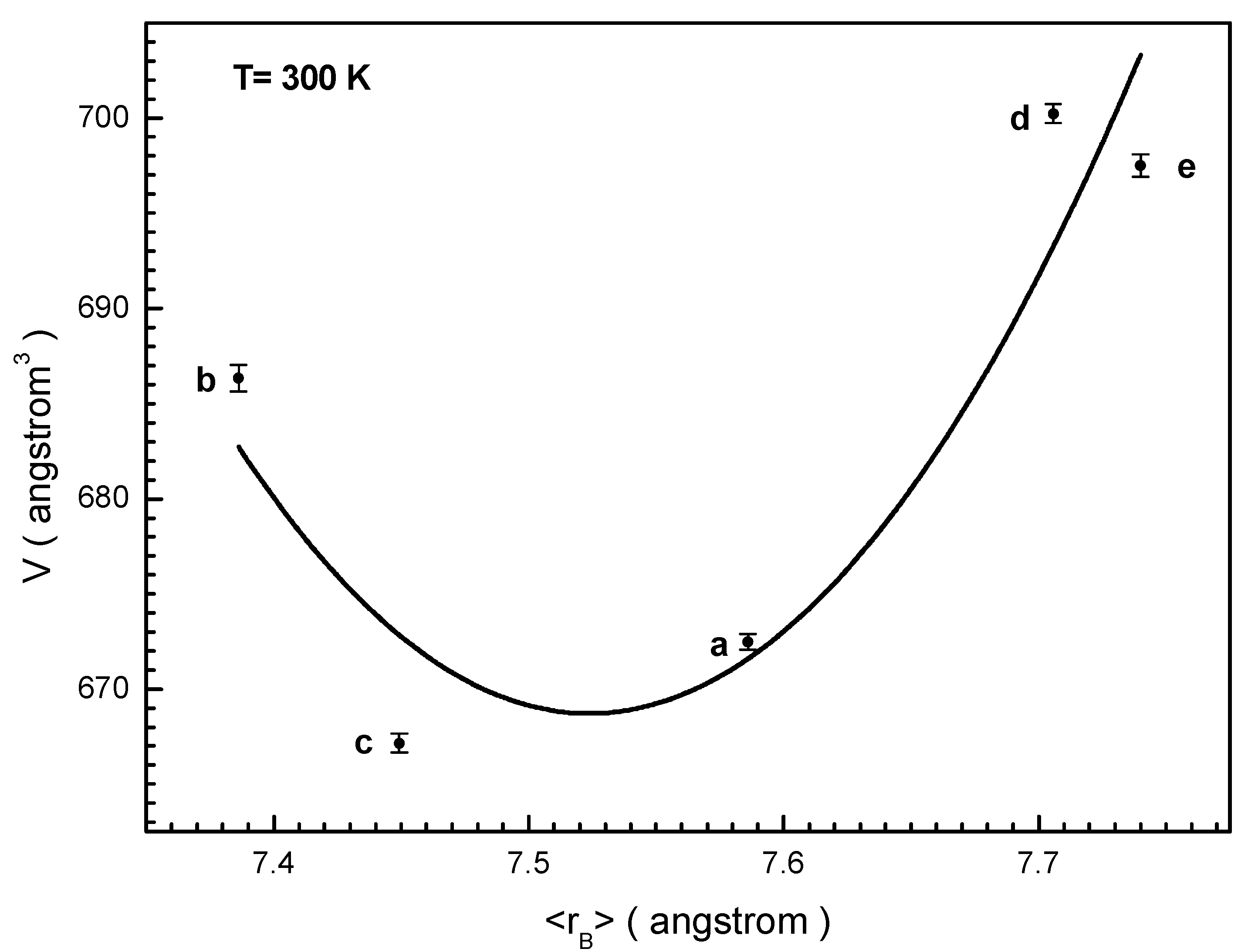
3.1. Study of the Crystal Structure
< r(In3+,V) = 0.800 Å.
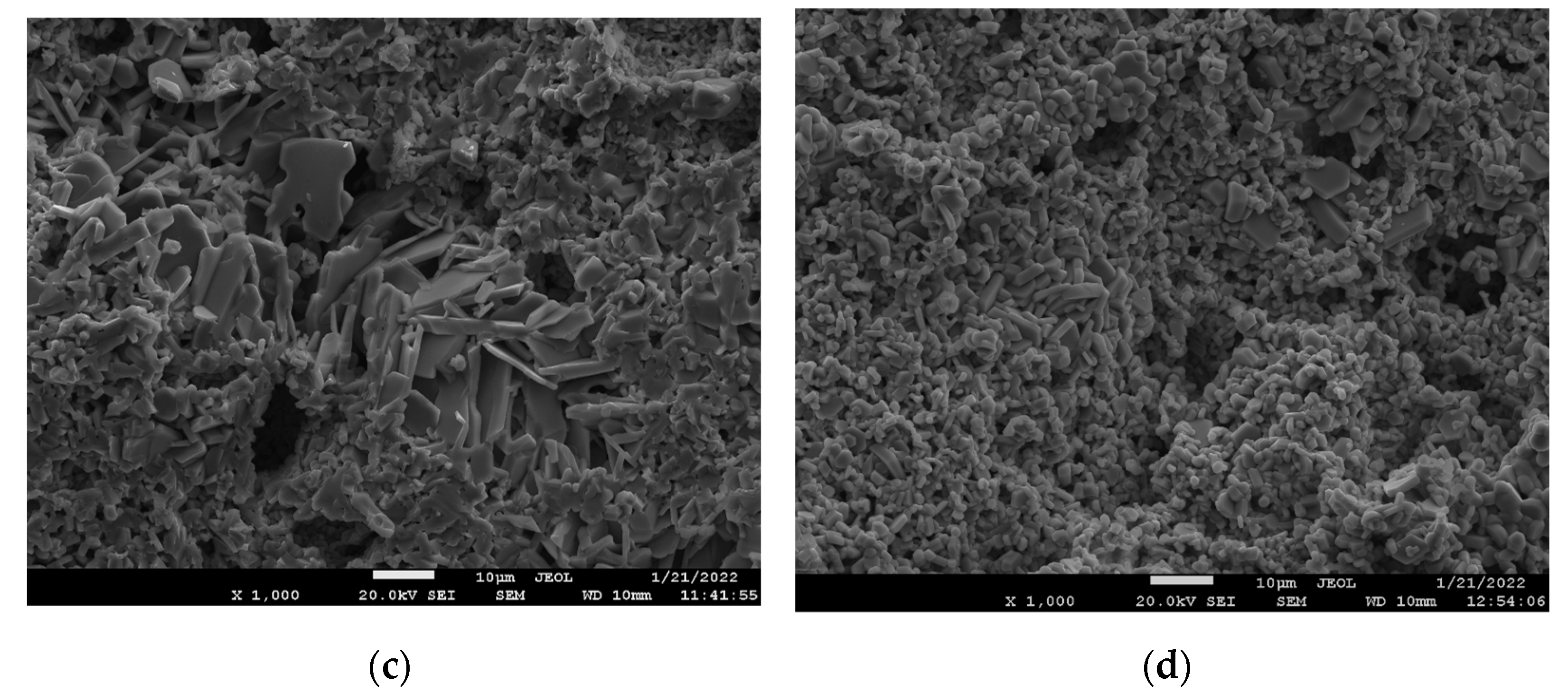
3.2. Study of the Surface Morphology and Elemental Composition
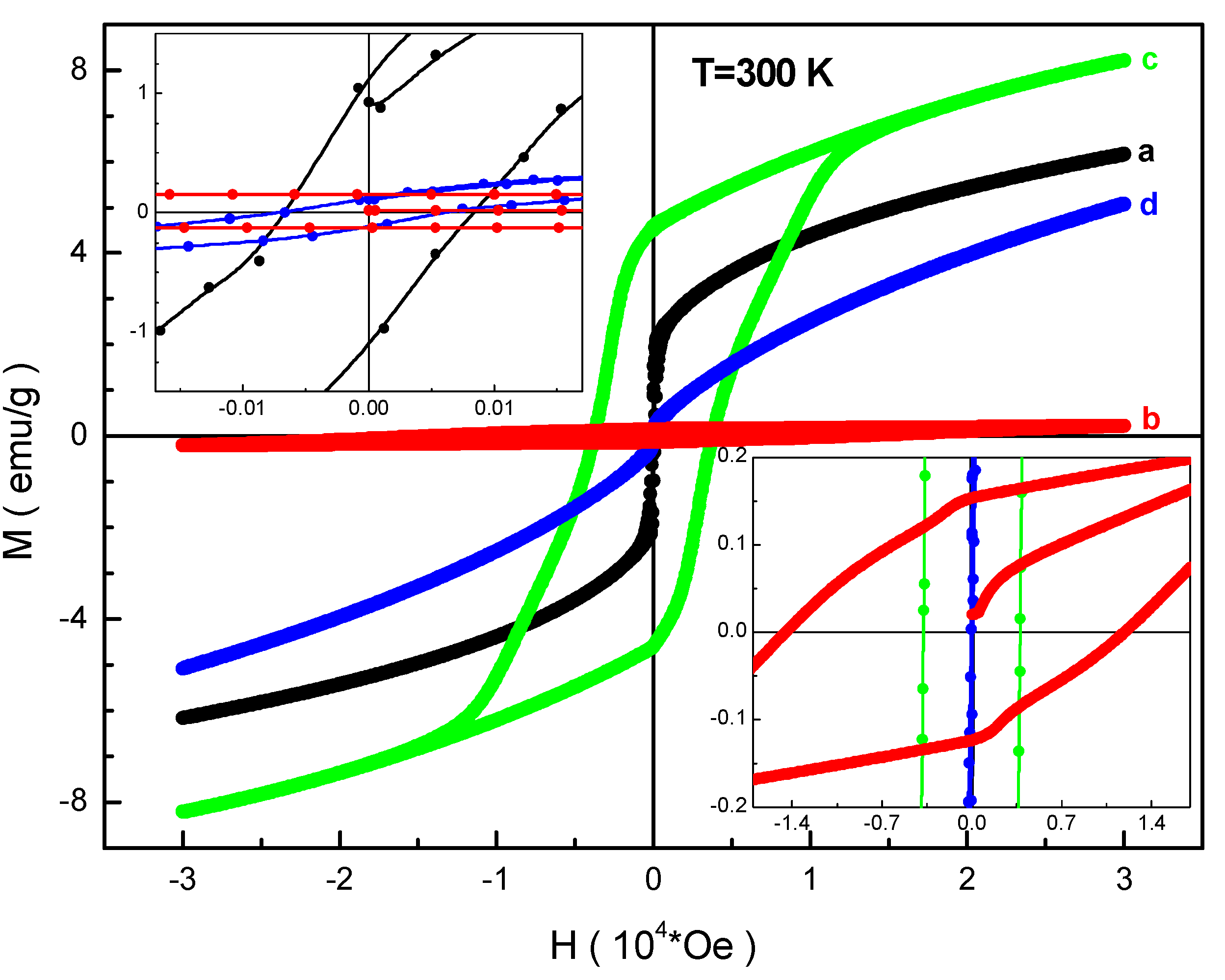
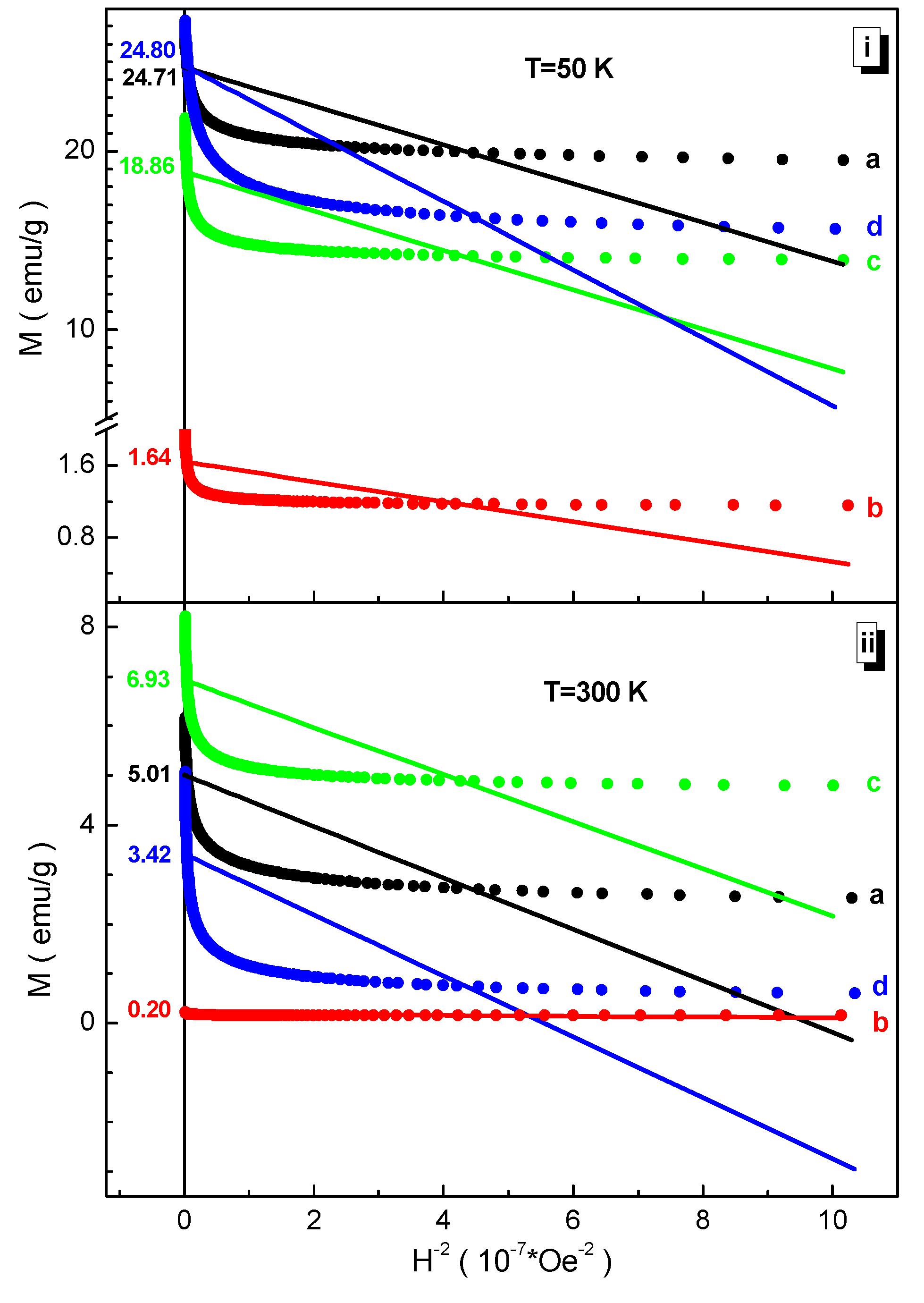
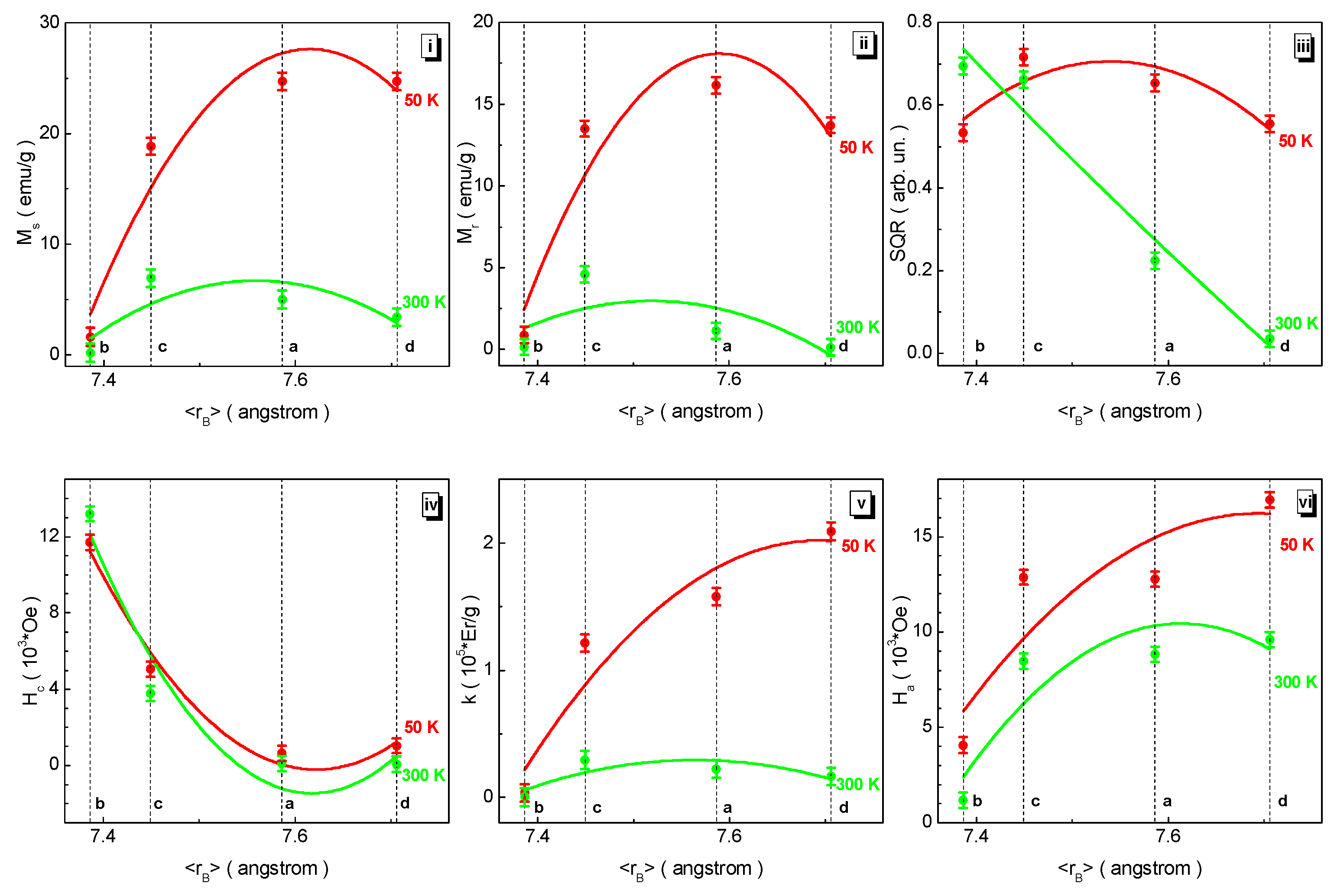
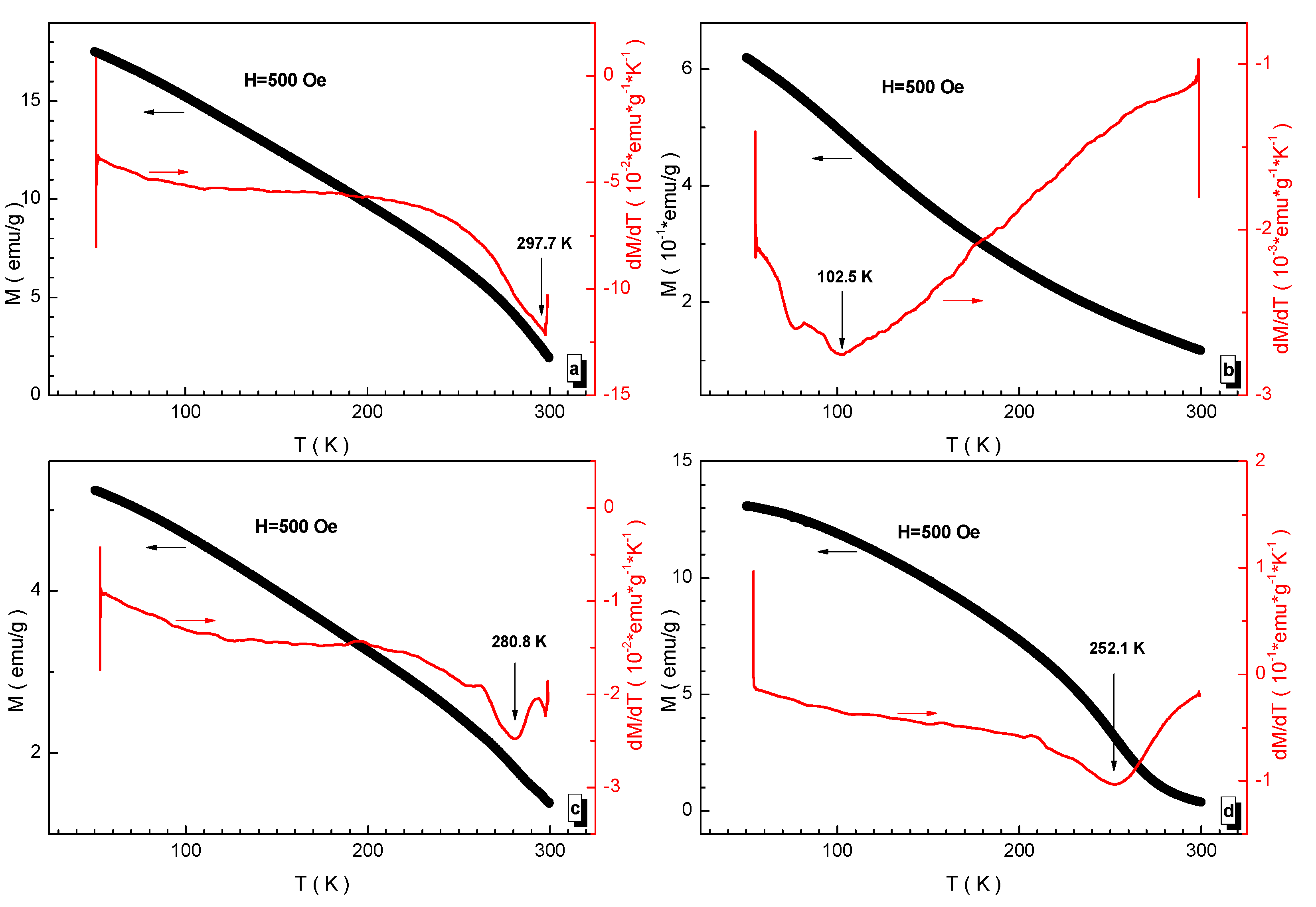
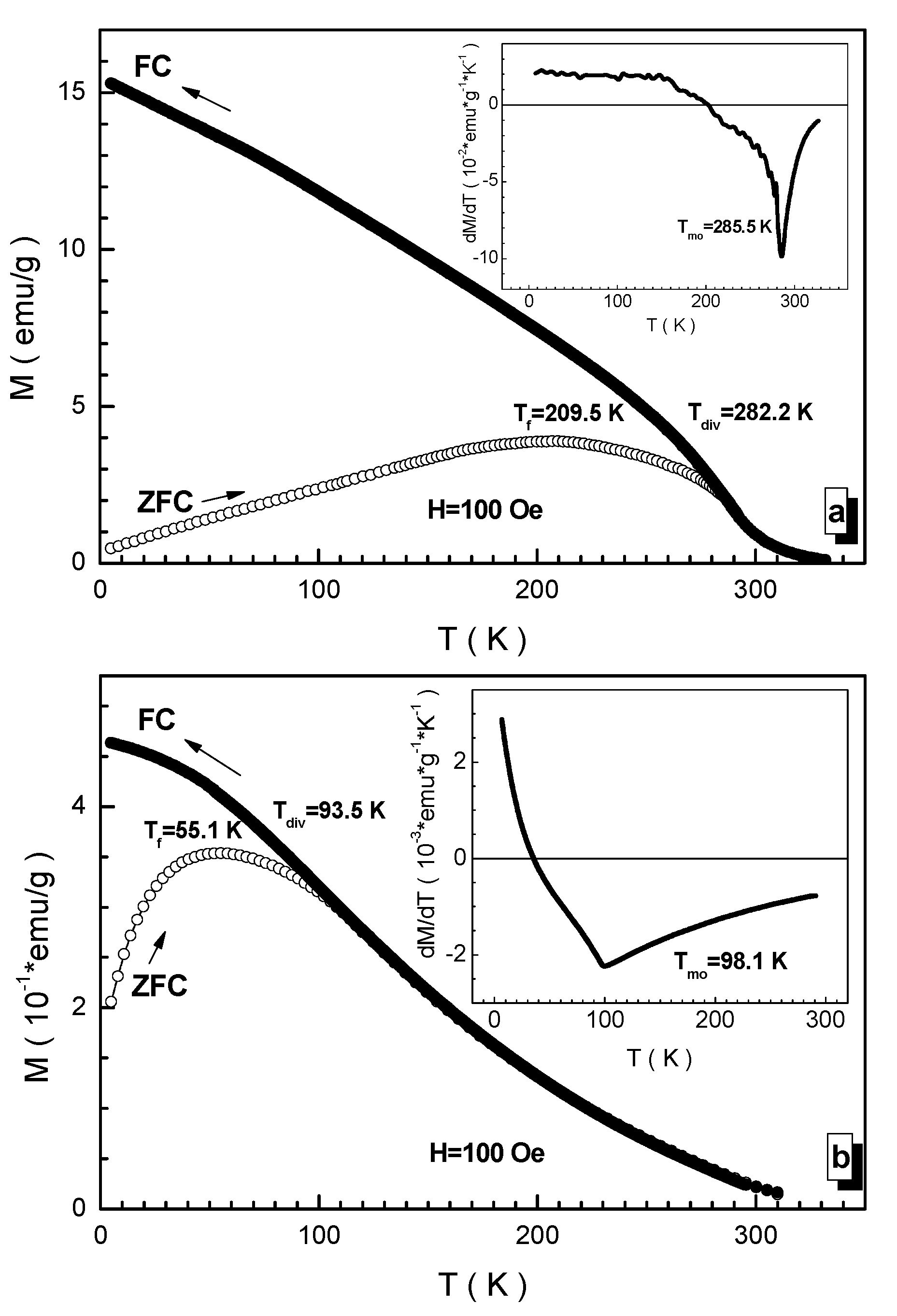
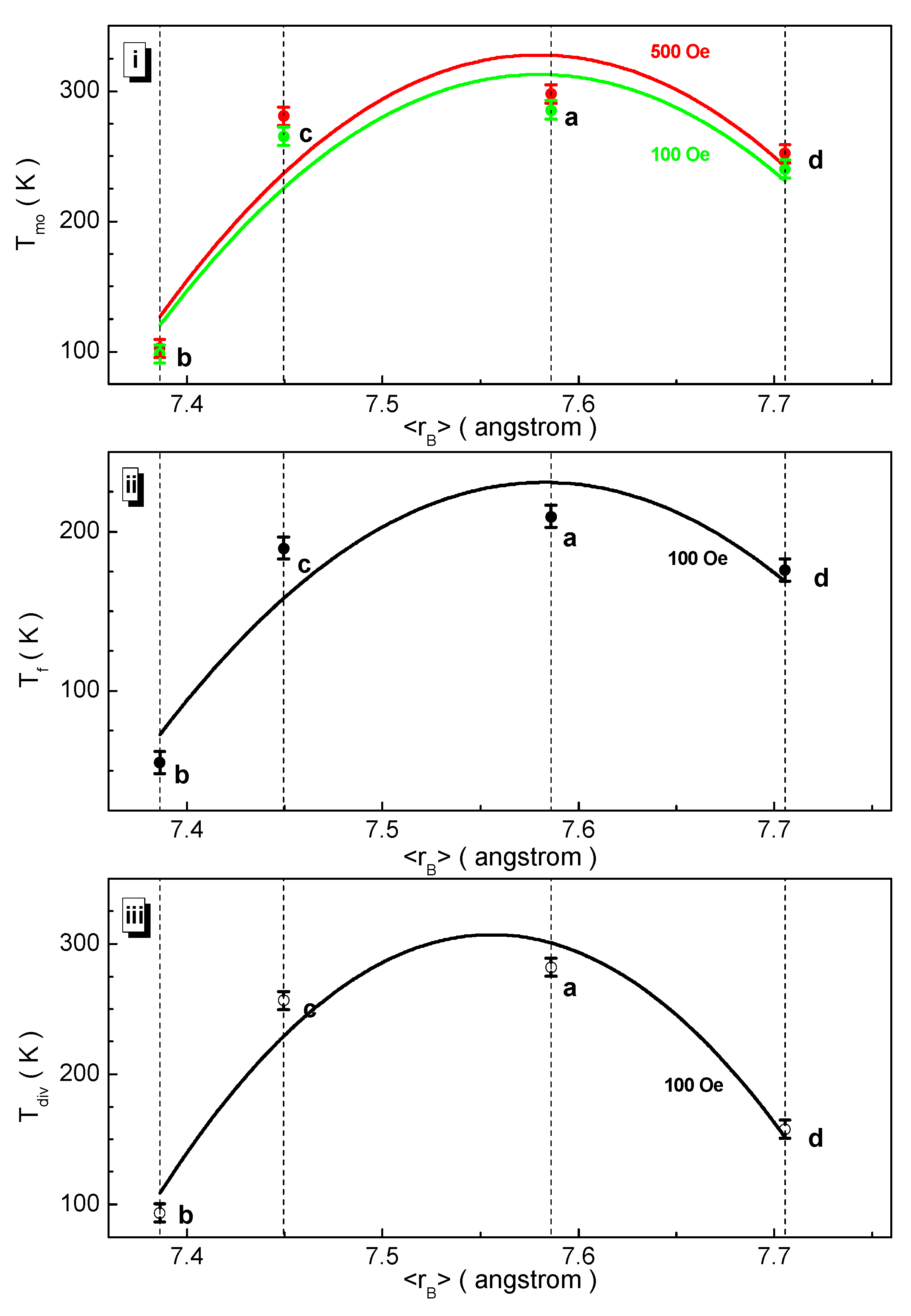
3.3. Magnetic Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillissen, J.; Van Rysselberghe, P.J. Studies on zinc and barium ferrites. J. Electrochem. Soc. 1931, 59, 95–106. [Google Scholar] [CrossRef]
- Watson, M.L.; Beard, R.A.; Kientz, S.M.; Feebeck, T.W. Investigation of thermal demagnetization effects in data recorded on advanced barium ferrite recording media. IEEE Trans. Magn. 2008, 44, 3568–3571. [Google Scholar] [CrossRef]
- Huang, K.; Yu, J.; Zhang, L.; Xu, J.; Yang, Z.; Liu, C.; Wang, W.; Kan, X. Structural and magnetic properties of Gd–Zn substituted M-type Ba–Sr hexaferrites by sol-gel auto-combustion method. J. Alloys Compd. 2019, 803, 971–980. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Yi, S.; Zu, P.; Wu, J.; Lin, J.; Li, M.; Su, W. Influence of La-Nb co-substituted Sr ferrite on microstructure, spectrum and magnetic properties of hexaferrites. J. Alloys Compd. 2021, 871, 159563. [Google Scholar] [CrossRef]
- Liu, J.; Peng, X.; Li, J.; Yang, Y.; Xu, J.; Hong, B.; Gong, J.; Han, Y.; Ge, H.; Wang, X. Highly improved middle to higher frequency magnetic performance of Fe-based soft magnetic composites with insulating Co2Z hexaferrites. J. Magn. Magn. Mater. 2021, 538, 168297. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Algarou, N.A.; Gondal, M.A.; Wudil, Y.S.; Younas, M.; Auwal, I.A.; Baykal, A.; Manikandan, A.; Zubar, T.I.; et al. Electronic, magnetic, and microwave properties of hard/soft nanocomposites based on hexaferrite SrNi0.02Zr0.02Fe11.96O19 with variable spinel phase MFe2O4 (M = Mn, Co, Cu, and Zn). Ceram. Int. 2021, 47, 35209–35223. [Google Scholar] [CrossRef]
- Darwish, M.A.; Turchenko, V.A.; Morchenko, A.T.; Kostishyn, V.G.; Timofeev, A.V.; Sayyed, M.I.; Sun, Z.; Podgornaya, S.V.; Trukhanova, E.L.; Kaniukov, E.Y.; et al. Heterovalent substituted BaFe12-xSnxO19 (0.1 ≤ x ≤ 1.2) M-type hexaferrite: Chemical composition, phase separation, magnetic properties and electrodynamics features. J. Alloys Compd. 2022, 896, 163117. [Google Scholar] [CrossRef]
- Ismail, I.; Muhammad Zulkimi, M.M.; Azis, R.S.; Mustaffa, M.S.; Hamidon, M.N.; Ertugrul, M.; Yesilbag, Y.O.; Tuzluca, F.N.; Hasan, I.H.; Ozturk, G.; et al. Effect of Mn and Zn doping on natural resonance frequency of strontium U-type hexaferrite and its performance as electromagnetic wave absorbers. J. Alloys Compd. 2022, 898, 163246. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Xu, C.; Chen, H.; Chen, Y.; Chen, F.; Kang, B.; Lu, W.; Zhang, J.; Cao, S. Magnetic field-induced polarization reversal in Y-type hexaferrites Ba0.7Sr1.3CoZnFe11AlO22 single crystals. Ceram. Int. 2021, 47, 19356–19361. [Google Scholar] [CrossRef]
- Bhaduri, A.; Singh, S.; Thapa, K.B.; Yadav, B.C. Visible light-induced, highly responsive, below lower explosive limit (LEL) LPG sensor based on hydrothermally synthesized barium hexaferrite nanorods. Sens. Actuators B Chem. 2021, 348, 130714. [Google Scholar] [CrossRef]
- Rajaji, U.; Chinnapaiyan, S.; Chen, T.-W.; Chen, S.-M.; Mani, G.; Mani, V.; Ali, M.A.; Al-Hemaid, F.M.A.; El-Shikh, M.S. Rational construction of novel strontium hexaferrite decorated graphitic carbon nitrides for highly sensitive detection of neurotoxic organophosphate pesticide in fruits. Electrochim. Acta 2021, 371, 137756. [Google Scholar] [CrossRef]
- Granja-Banguera, C.P.; Silgado-Cortázar, D.G.; Morales-Morales, J.A. Transition metal substituted barium hexaferrite-modified electrode: Application as electrochemical sensor of acetaminophen. Molecules 2022, 27, 1550. [Google Scholar] [CrossRef] [PubMed]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Kostishyn, V.G.; Panina, L.V.; Kazakevich, I.S.; Balagurov, A.M. Structure and magnetic properties of BaFe11.9In0.1O19 hexaferrite in a wide temperature range. J. Alloys Compd. 2016, 689, 383–393. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Y.; Han, X.; Wang, L.; Lu, X.; Qiao, L. Magnetic properties of Co–Ti substituted barium hexaferrite. J. Alloys Compd. 2013, 546, 234–238. [Google Scholar] [CrossRef]
- Han, G.; Sui, R.; Yu, Y.; Wang, L.; Li, M.; Li, J.; Liu, H.; Yang, W. Structure and magnetic properties of the porous Al-substituted barium hexaferrites. J. Magn. Magn. Mater. 2021, 528, 167824. [Google Scholar] [CrossRef]
- Lohmaah, A.; Chokprasombat, K.; Pinitsoontorn, S.; Sirisathitkul, C. Magnetic properties and morphology copper-substituted barium hexaferrites from sol-gel auto-combustion synthesis. Materials 2021, 14, 5873. [Google Scholar] [CrossRef] [PubMed]
- Trukhanov, S.V.; Trukhanov, A.V.; Kostishin, V.G.; Panina, L.V.; Kazakevich, I.S.; Turchenko, V.A.; Oleinik, V.V.; Yakovenko, E.S.; Matsui, L.Y. Magnetic and absorbing properties of M-type substituted hexaferrites BaFe12−xGaxO19 (0.1 < x < 1.2). J. Exp. Theor. Phys. 2016, 123, 461–469. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Kostishyn, V.G.; Zabeivorota, N.I.; Panina, L.V.; Trukhanov, A.V.; Turchenko, V.A.; Trukhanova, E.L.; Oleynik, V.V.; Yakovenko, O.S.; et al. High-frequency absorption properties of gallium weakly doped barium hexaferrites. Philos. Mag. 2019, 99, 585–605. [Google Scholar] [CrossRef]
- Gorbachev, E.A.; Trusov, L.A.; Wu, M.; Vasiliev, A.V.; Svetogorov, R.D.; Alyabyeva, L.N.; Lebedev, V.A.; Sleptsova, A.E.; Karpov, M.A.; Mozharov, Y.M.; et al. Submicron particles of Ga-substituted strontium hexaferrite obtained by a citrate auto-combustion method. J. Mater. Chem. C 2021, 9, 13832–13840. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.O.; Bobrikov, I.A.; Trukhanov, S.V.; Kazakevich, I.S.; Balagurov, A.M. Crystal structure and magnetic properties of the BaFe12−xAlxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Ahmed, A.G.; Prokhorov, A.S.; Anzin, V.B.; Vinnik, D.A.; Bush, A.; Gorshunov, B.P.; Alyabyeva, L.N. Origin of terahertz excitations in single-crystalline lead substituted M-type barium hexaferrite doped with Al. J. Phys. Conf. Ser. 2021, 1984, 012015. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Q.; Yin, Q.; Chen, J.; Zhang, H.; Liu, Y. Room-temperature multiferroic properties of Al-doped hexaferrites sintered at high oxygen atmospheric concentrations. Ceram. Int. 2021, 47, 21398–21403. [Google Scholar] [CrossRef]
- Kumar, S.; Supriya, S.; Kar, M. Correlation between temperature dependent dielectric and DC resistivity of Cr substituted barium hexaferrite. Mater. Res. Express 2017, 4, 126302. [Google Scholar] [CrossRef]
- Sözeri, H.; Genç, F.; Almessiere, M.A.; Ünver, İ.S.; Korkmaz, A.D.; Baykal, A. Cr3+-substituted Ba nanohexaferrites as high-quality microwave absorber in X band. J. Alloys Compd. 2019, 779, 420–426. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, S.; Kan, X.; Lv, Q.; Trukhanov, A.V.; Trukhanov, S.V. Synthesis, Magnetic and electrical characteristics of Ba-Sr hexaferrites substituted with samarium, chromium and aluminum. ChemistrySelect 2021, 6, 470–479. [Google Scholar] [CrossRef]
- Turchenko, V.; Kostishin, V.G.; Trukhanov, S.; Damay, F.; Balasoiu, M.; Bozzo, B.; Fina, I.; Burkhovetsky, V.V.; Polosan, S.; Zdorovets, M.V.; et al. Structural features, magnetic and ferroelectric properties of SrFe10.8In1.2O19 compound. Mater. Res. Bull. 2021, 138, 111236. [Google Scholar] [CrossRef]
- Turchenko, V.A.; Trukhanov, S.V.; Kostishin, V.G.; Damay, F.; Porcher, F.; Klygach, D.S.; Vakhitov, M.G.; Lyakhov, D.; Michels, D.; Bozzo, B.; et al. Features of structure, magnetic state and electrodynamic performance of SrFe12−xInxO19. Sci. Rep. 2021, 11, 18342. [Google Scholar] [CrossRef]
- Turchenko, V.A.; Trukhanov, S.V.; Kostishin, V.G.; Damay, F.; Porcher, F.; Klygach, D.S.; Vakhitov, M.G.; Matzui, L.Y.; Yakovenko, O.S.; Bozzo, B.; et al. Impact of In3+ cations on structure and electromagnetic state of M-type hexaferrites. J. Energy Chem. 2022, 69, 667–676. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zhivulin, V.E.; Trofimov, E.A.; Starikov, A.Y.; Zherebtsov, D.A.; Zaitseva, O.V.; Gudkova, S.A.; Taskaev, S.V.; Klygach, D.S.; Vakhitov, M.G.; et al. Extremely polysubstituted magnetic material based on magnetoplumbite with a hexagonal structure: Synthesis, structure, properties, prospects. Nanomaterials 2019, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- McCormack, S.J.; Navrotsky, A. Thermodynamics of high entropy oxides. Acta Mater. 2021, 202, 1–21. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Musicó, B.L.; Gilbert, D.; Ward, T.Z.; Page, K.; George, E.; Yan, J.; Mandrus, D.; Keppens, V. The emergent field of high entropy oxides: Design, prospects, challenges, and opportunities for tailoring material properties featured. APL Mater. 2020, 8, 040912. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yao, J.; Wang, J.; Chang, A. Spinel-type high-entropy (Co0.2Mn0.2Fe0.2Zn0.2Ti0.2)3O4 oxides constructed from disordered cations and oxygen vacancies. J. Alloys Compd. 2022, 897, 163188. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Punda, A.Y.; Valiulina, A.N.; Gavrilyak, M.; Zaitseva, O.V.; Taskaev, S.V.; Khandaker, M.U.; et al. A-site cation size effect on structure and magnetic properties of Sm(Eu,Gd)Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3 high-entropy solid solutions. Nanomaterials 2022, 12, 36. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, J.; Zajusz, M.; Moździerz, M.; Berent, K.; Mikuła, A.; Stępień, A.; Świerczek, K. Structure and transport properties of the novel (Dy,Er,Gd,Ho,Y)3Fe5O12 and (Dy,Gd,Ho,Sm,Y)3Fe5O12 high entropy garnets. J. Eur. Ceram. Soc. 2021, 41, 3844–3849. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Gudkova, S.A.; Starikov, A.Y.; Zherebtsov, D.A.; Kirsanova, A.A.; Häßner, M.; Niewa, R. High-entropy oxide phases with magnetoplumbite structure. Ceram. Int. 2019, 45, 12942–12948. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Zherebtsov, D.A.; Starikov, A.Y.; Sherstyuk, D.P.; Gudkova, S.A.; Taskaev, S.V. The new extremely substituted high entropy (Ba,Sr,Ca,La)Fe6−x(Al,Ti,Cr,Ga,In,Cu,W)xO19 microcrystals with magnetoplumbite structure. Ceram. Int. 2020, 46, 9656–9660. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Trukhanov, A.V.; Podgornov, F.V.; Trofimov, E.A.; Zhivulin, V.E.; Starikov, A.Y.; Zaitseva, O.V.; Gudkova, S.A.; Kirsanova, A.A.; Taskaev, S.V.; et al. Correlation between entropy state, crystal structure, magnetic and electrical properties in M-type Ba-hexaferrites. J. Eur. Ceram. Soc. 2020, 40, 4022–4028. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Taskaev, S.V.; Zhou, D.; Astapovich, K.A.; Trukhanov, S.V.; Yang, Y. Correlation of the Fe content and entropy state in multiple substituted hexagonal ferrites with magnetoplumbite structure. Ceram. Int. 2021, 47, 17684–17692. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R 2021, 146, 100644. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Salem, M.M.; Trukhanova, E.L.; Panina, L.V.; Kostishyn, V.G.; Darwish, M.A.; Trukhanov, A.V.; Zubar, T.I.; Tishkevich, D.I.; et al. Preparation and investigation of structure, magnetic and dielectric properties of (BaFe11.9Al0.1O19)1-x-(BaTiO3)x bicomponent ceramics. Ceram. Int. 2018, 44, 21295–21302. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Algarou, N.A.; Vakhitov, M.G.; Klygach, D.S.; Baykal, A.; Zubar, T.I.; Trukhanov, S.V.; Trukhanov, A.V.; Attia, H.; et al. Tuning the structure, magnetic and high frequency properties of Sc-doped Sr0.5Ba0.5ScxFe12-xO19/NiFe2O4 hard/soft nanocomposites. Adv. Electr. Mater. 2022, 8, 2101124. [Google Scholar] [CrossRef]
- Trukhanov, S.V. Peculiarities of the magnetic state in the system La0.70Sr0.30MnO3−γ (0 ≤ γ ≤ 0.25). J. Exp. Theor. Phys. 2005, 100, 95–105. [Google Scholar] [CrossRef]
- Grossinger, R. Correlation between the inhomogeneity and the magnetic anisotropy in polycrystalline ferromagnetic materials. J. Magn. Magn. Mater. 1982, 28, 137–142. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Troyanchuk, I.O.; Trukhanov, A.V.; Szymczak, H.; Szymczak, R.; Baran, M. Magnetic and electrotransport properties of the anion-deficient manganites with perovskite structure. J. Low Temp. Phys. 2005, 139, 2005. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. B 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Kostishin, V.G.; Panina, L.V.; Kazakevich, I.S.; Balagurov, A.M. Crystal structure and magnetic properties of the BaFe12-xInxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 2016, 417, 130–136. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Kostishyn, V.G.; Panina, L.V.; Turchenko, V.A.; Kazakevich, I.S.; Trukhanov, A.V.; Trukhanova, E.L.; Natarov, V.O.; Balagurov, A.M. Thermal evolution of exchange interactions in lightly doped barium hexaferrites. J. Magn. Magn. Mater. 2017, 426, 554–562. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Trukhanov, S.V.; Kostishyn, V.G.; Panina, L.V.; Korovushkin, V.V.; Turchenko, V.A.; Vinnik, D.A.; Yakovenko, E.S.; Zagorodnii, V.V.; Launetz, V.L.; et al. Correlation of the atomic structure, magnetic properties and microwave characteristics in substituted hexagonal ferrites. J. Magn. Magn. Mater. 2018, 462, 127–135. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Panina, L.V.; Kostishyn, V.G.; Turchenko, V.A.; Trukhanova, E.L.; Trukhanov, A.V.; Zubar, T.I.; Ivanov, V.M.; Tishkevich, D.I.; et al. Temperature evolution of the structure parameters and exchange interactions in BaFe12-xInxO19. J. Magn. Magn. Mater. 2018, 466, 393–405. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.A.; Kostishin, V.G.; Damay, F.; Porcher, F.; Lupu, N.; Bozzo, B.; Fina, I.; Polosan, S.; Silibin, M.V.; et al. The origin of the dual ferroic properties in quasi-centrosymmetrical SrFe12-xInxO19 hexaferrites. J. Alloys Compd. 2021, 886, 161249. [Google Scholar] [CrossRef]
- Topkaya, R. Effect of composition and temperature on the magnetic properties of BaBixLaxFe(12−2x)O19 (0.0 ≤ x ≤ 0.2) hexaferrites. Appl. Phys. A 2017, 123, 488. [Google Scholar] [CrossRef]
- Gorter, E.W. Saturation magnetization of some ferrimagnetic oxides with hexagonal crystal structures. Proc. IEE Part B 1957, 104, 255–260. [Google Scholar] [CrossRef]
- Herbst, J.F.; Pinkerton, F.E. Law of approach to saturation for polycrystalline ferromagnets: Remanent initial state. Phys. Rev. B 1998, 57, 10733–10739. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güngüneş, H.; Korkmaz, A.D.; Zubar, T.; Trukhanov, S.; Trukhanov, A.; Manikandan, A.; Alahmari, F.; Baykal, A. Influence of Dy3+ ions on microstructure, magnetic, electrical and microwave properties of [Ni0.4Cu0.2Zn0.4](Fe2−xDyx)O4 (0.00 < x < 0.04) spinel ferrites. ACS Omega 2021, 6, 10266–10280. [Google Scholar] [CrossRef]
- Bean, C.P.; Livingstone, J.D. Superparamagnetism. J. Appl. Phys. 1959, 30, S120–S129. [Google Scholar] [CrossRef]
- Cornia, A.; Barra, A.-L.; Bulicanu, V.; Clérac, R.; Cortijo, M.; Hillard, E.A.; Galavotti, R.; Lunghi, A.; Nicolini, A.; Rouzières, M.; et al. The origin of magnetic anisotropy and single-molecule magnet behavior in chromium(II)-based extended metal atom chains. Inorg. Chem. 2020, 59, 1763–1777. [Google Scholar] [CrossRef]


| No | Chemical Formula | Mass Percentage of the Original Component, % | ||||||
|---|---|---|---|---|---|---|---|---|
| 1. | BaFe6Al6O19 | BaCO3 | Fe2O3 | Al2O3 | 0.693147 | |||
| 20.09 | 48.77 | 31.14 | ||||||
| 2. | BaFe6Cr6O19 | BaCO3 | Fe2O3 | Cr2O3 | 0.693147 | |||
| 17.43 | 42.31 | 40.27 | ||||||
| 3. | BaFe6Ga6O19 | BaCO3 | Fe2O3 | Ga2O3 | 0.693147 | |||
| 15.93 | 38.67 | 45.39 | ||||||
| 4. | BaFe6Al3Cr3O19 | BaCO3 | Fe2O3 | Al2O3 | Cr2O3 | 1.039721 | ||
| 18.66 | 45.31 | 14.46 | 21.56 | |||||
| 5. | BaFe6Al2Gr2Ga2O19 | BaCO3 | Fe2O3 | Al2O3 | Cr2O3 | Ga2O3 | 1.242453 | |
| 17.66 | 42.86 | 9.12 | 13.60 | 1.04 | ||||
| 6. | BaFe6Al1.5Cr1.5Ga1.5In1.5O19 | BaCO3 | Fe2O3 | Al2O3 | Cr2O3 | In2O3 | Ga2O3 | 1.386294 |
| 16.40 | 39.80 | 6.35 | 9.47 | 16.30 | 11.68 | |||
| No | Chemical Formula and Label | Unit Cell Parameters | ||
|---|---|---|---|---|
| a, Å | c, Å | V, Å3 | ||
| 1. | BaFe5.84Ga6.19O19 (a) | 5.8515(2) | 23.1460(19) | 686.33(7) |
| 2. | BaFe6.26Al2.27Cr3.47O19 (b) | 5.8077(14) | 22.8396(7) | 667.15(2) |
| 3. | BaFe6.11Al1.56Cr2.17Ga2.16O19 (c) | 5.8148(13) | 22.9660(5) | 672.47(19) |
| 4. | BaFe6.19Al1.25Cr1.57Ga1.74In1.26O19 (d) | 5.8884(2) | 23.3191(12) | 700.23(5) |
| 5. | BaFe12O19 [3] | 5.8930(3) | 23.1940(2) | 697.50(13) |
| Elemental Composition of the Obtained Samples, Atom % | Gross Formula and Label | |||||
|---|---|---|---|---|---|---|
| BaFe6Ga6O19 | BaFe5.84Ga6.16O19 (a) | |||||
| Ba | Fe | Ga | ||||
| 3.3 | 20.1 | 21.3 | ||||
| BaFe6Al3Cr3O19 | BaFe6.26Al2.27Cr3.47O19 (b) | |||||
| Ba | Fe | Al | Cr | |||
| 4.0 | 21.4 | 7.8 | 11.9 | |||
| BaFe6Al2Cr2Ga2O19 | BaFe6.11Al1.56Cr2.17Ga2.16O19 (c) | |||||
| Ba | Fe | Al | Cr | Ga | ||
| 3.7 | 21.0 | 5.4 | 7.5 | 7.4 | ||
| BaFe6Al1.5Cr1.5Ga1.5In1.5O19 | BaFe6.19Al1.25Cr1.57Ga1.74In1.26O19 (d) | |||||
| Ba | Fe | Al | Cr | Ga | In | |
| 3.5 | 21.4 | 4.3 | 5.4 | 6.0 | 4.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhivulin, V.E.; Sherstyuk, D.P.; Zaitseva, O.V.; Cherkasova, N.A.; Vinnik, D.A.; Taskaev, S.V.; Trofimov, E.A.; Trukhanov, S.V.; Latushka, S.I.; Tishkevich, D.I.; et al. Creation and Magnetic Study of Ferrites with Magnetoplumbite Structure Multisubstituted by Al3+, Cr3+, Ga3+, and In3+ Cations. Nanomaterials 2022, 12, 1306. https://doi.org/10.3390/nano12081306
Zhivulin VE, Sherstyuk DP, Zaitseva OV, Cherkasova NA, Vinnik DA, Taskaev SV, Trofimov EA, Trukhanov SV, Latushka SI, Tishkevich DI, et al. Creation and Magnetic Study of Ferrites with Magnetoplumbite Structure Multisubstituted by Al3+, Cr3+, Ga3+, and In3+ Cations. Nanomaterials. 2022; 12(8):1306. https://doi.org/10.3390/nano12081306
Chicago/Turabian StyleZhivulin, Vladimir E., Daria P. Sherstyuk, Olga V. Zaitseva, Natalia A. Cherkasova, Denis A. Vinnik, Sergey V. Taskaev, Evgeny A. Trofimov, Sergei V. Trukhanov, Siarhei I. Latushka, Daria I. Tishkevich, and et al. 2022. "Creation and Magnetic Study of Ferrites with Magnetoplumbite Structure Multisubstituted by Al3+, Cr3+, Ga3+, and In3+ Cations" Nanomaterials 12, no. 8: 1306. https://doi.org/10.3390/nano12081306







