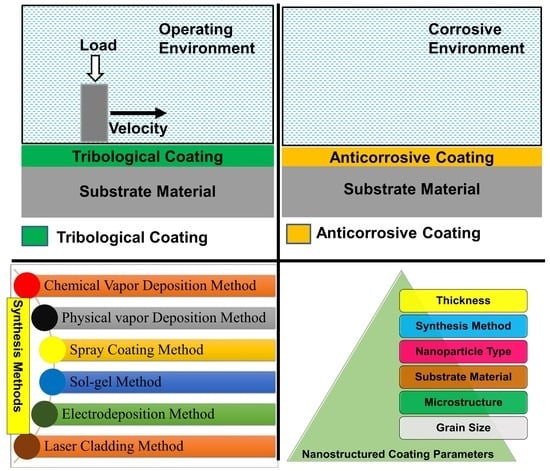
Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance
Abstract
:1. Introduction
2. Synthesising Methods
2.1. Chemical Vapor Deposition
2.2. Physical Vapor Deposition
2.3. Spray Coating
2.4. Sol-Gel Process
2.5. Electrodeposition
2.6. Laser Cladding
3. Corrosion Behaviour
3.1. Ceramic Nanostructured Coatings
3.1.1. Alumina (Al2O3) Nanostructured Coatings
3.1.2. Titanium Oxide (TiO2) Nanostructured Coatings
3.1.3. Tantalum Pentoxide (Ta2O5) Nanostructured Coatings
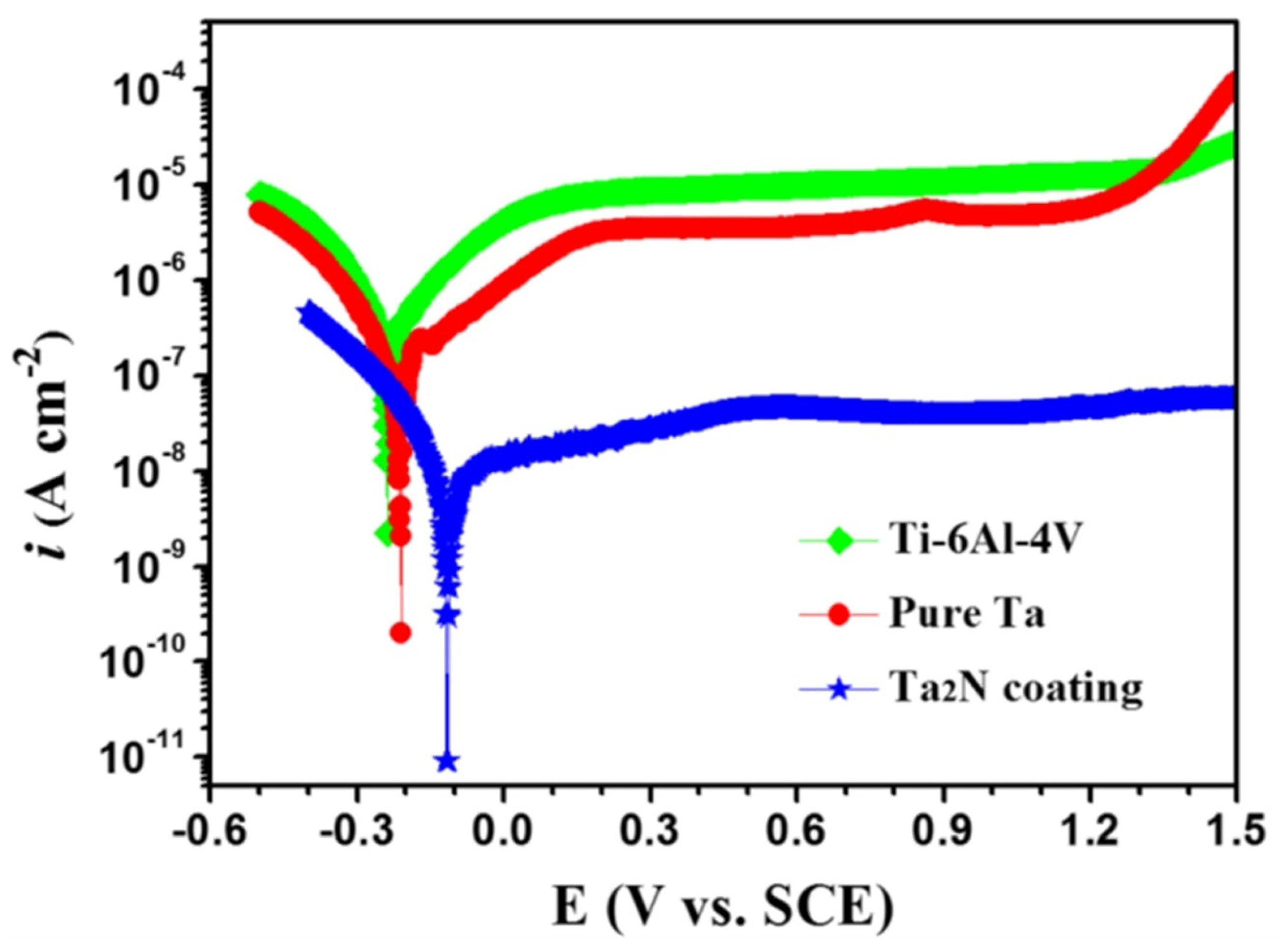
3.1.4. Tantalum Nitride (Ta2N) Nanostructured Coatings
3.2. Metallic Nanostructured Coatings
3.2.1. Grain Size
3.2.2. Composition
3.2.3. Synthesis Method
3.2.4. Environment
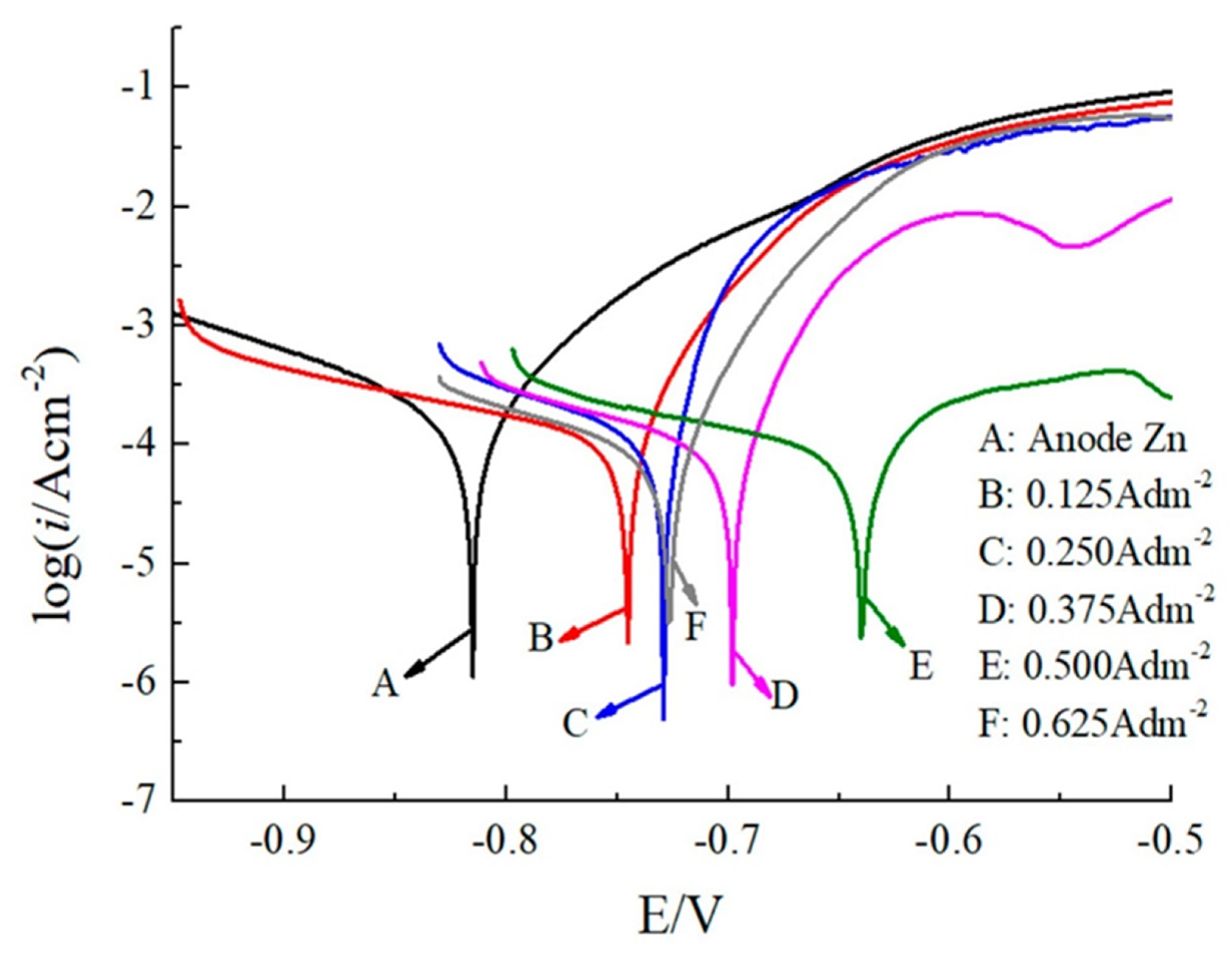
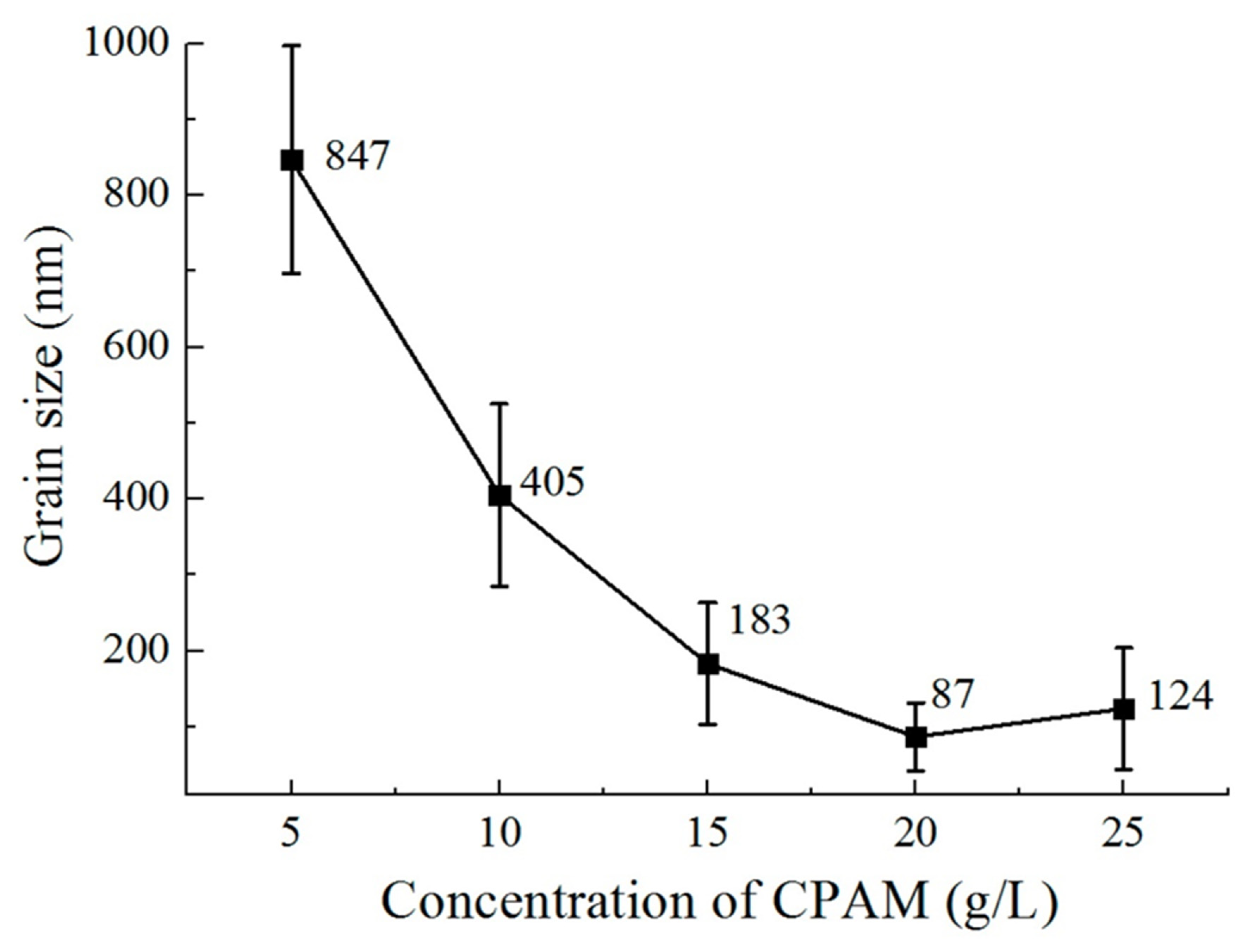
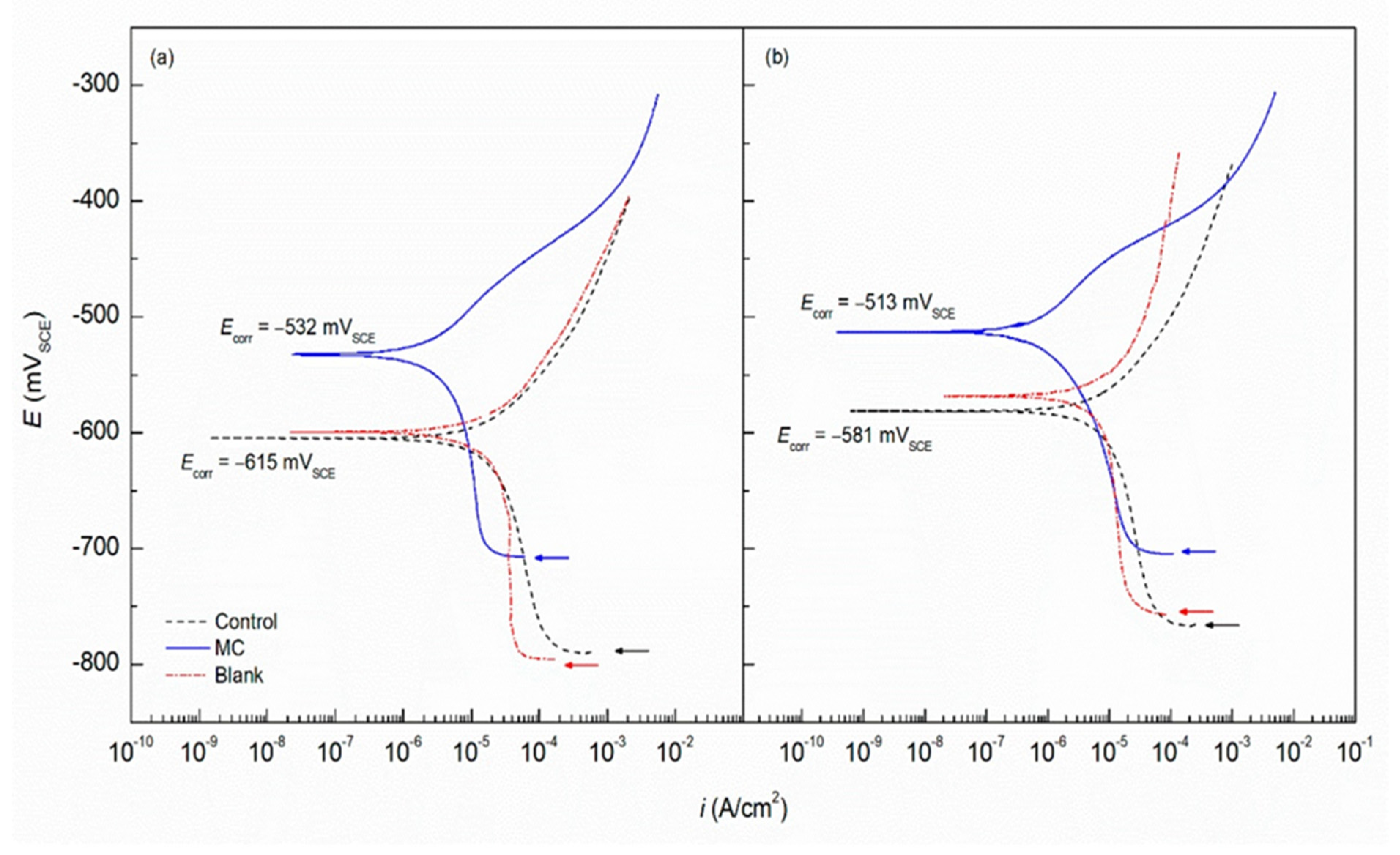
3.2.5. Additives
3.3. Nanocomposite Coatings
3.3.1. Polymer Matrix Nanocomposite Coatings
3.3.2. Waterborne Polymer Nanocomposite Coatings
3.3.3. Metallic Matrix Nanocomposite Coatings
4. Tribological Performance
4.1. Ceramic Nanostructured Coatings
4.1.1. Zirconia (ZrO2)-Based Nanostructured Coatings
4.1.2. Alumina (Al2O3)-Based Nanostructured Coatings
4.1.3. Chromia (Cr2O3)-Based Nanostructured Coatings
4.1.4. Other Ceramic Nanostructured Coatings
4.2. Metallic Nanostructured Coatings
4.2.1. Coating Method
4.2.2. Composition
4.2.3. Grain Size
4.3. Nanocomposite Coatings
4.3.1. Metallic Matrix Nanocomposite Coatings
4.3.2. Polymer Matrix Nanocomposite Coatings
4.3.3. Ceramic Matrix Nanocomposite Coatings
5. Biomimetic Approaches
5.1. Nature Inspired Anticorrosion Coatings
5.2. Nature Inspired Tribological Coatings
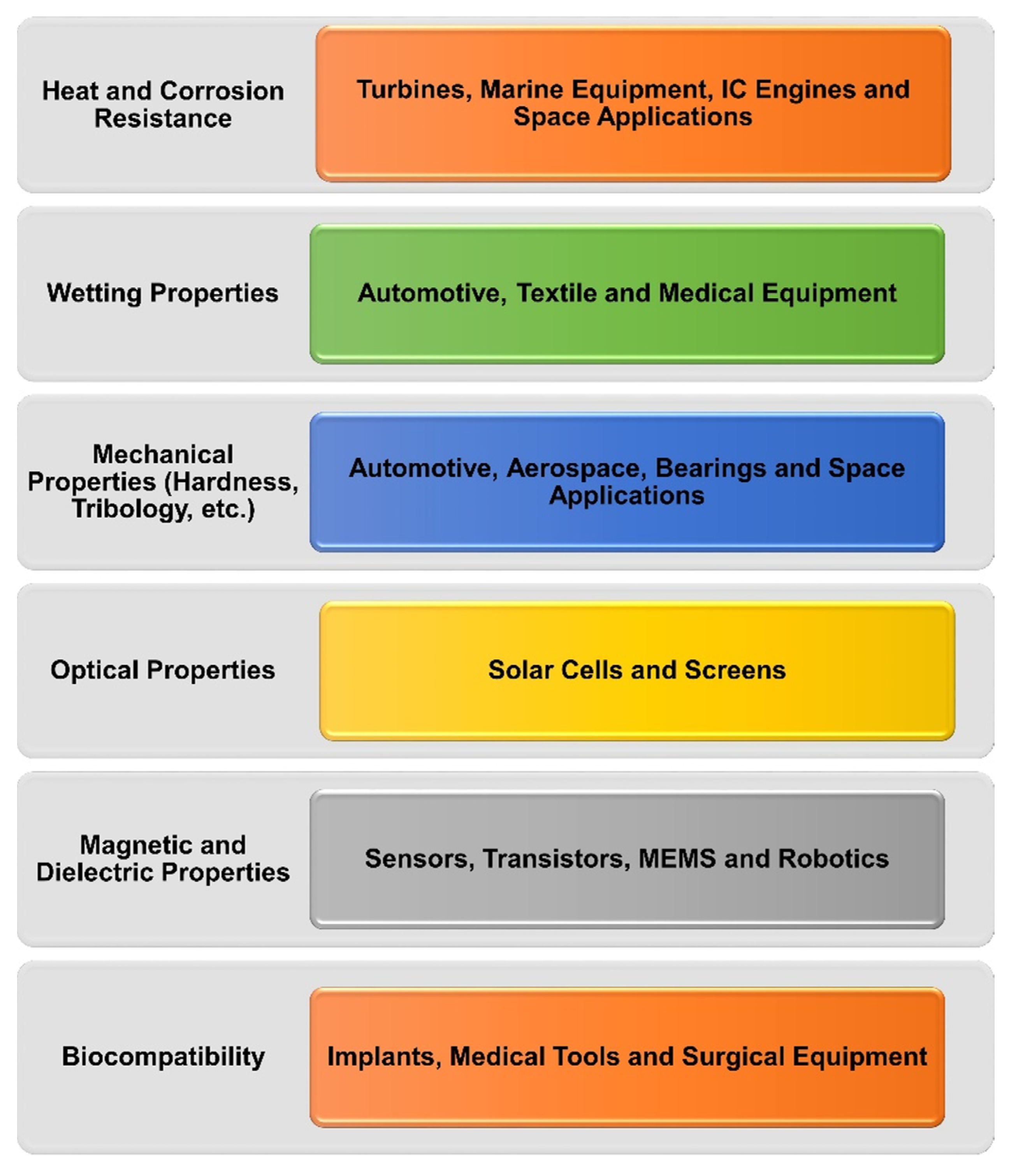
6. Application Areas
7. Challenges in Developing Nanostructured Coatings
8. Summary and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, A.; Tripathi, R.M.; Zafar, F.; Singh, P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater. Lett. 2012, 67, 91–94. [Google Scholar] [CrossRef]
- Joshi, M.; Bhattacharyya, A. Nanotechnology—A new route to high-performance functional textiles. Text. Prog. 2011, 43, 155–233. [Google Scholar] [CrossRef]
- Gleiter, H. Nanocrystalline materials. Prog. Mater. Sci. 1989, 33, 223–315. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.M.; Chung, S.J. Biogenic nanomaterials: Synthesis, characterization, growth mechanism, and biomedical applications. J. Microbiol. Methods 2019, 157, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Bharmoria, P.; Ventura, S.P.M. Optical Applications of Nanomaterials. Adv. Struct. Mater. 2019, 118, 1–29. [Google Scholar]
- Volz, S. Thermal Nanosystems and Nanomaterials; Springer Science & Business Media: Amsterdam, The Netherlands, 2009; Volume 118. [Google Scholar]
- Carpenter, M.A.; Mathur, S.; Kolmakov, A. Metal Oxide Nanomaterials for Chemical Sensors; Springer Science & Business Media: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wu, Q.; Miao, W.; Gao, H.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R. Magnetic Nanomaterials; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Gajanan, K.; Tijare, S.N. Applications of nanomaterials. Mater. Today Proc. 2018, 5, 1093–1096. [Google Scholar] [CrossRef]
- Abdeen, D.H.; el Hachach, M.; Koc, M.; Atieh, M.A. A review on the corrosion behaviour of nanocoatings on metallic substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef] [Green Version]
- Tzanakis, I.; Hadfield, M.; Thomas, B.; Noya, S.M.; Henshaw, I.; Austen, S. Future perspectives on sustainable tribology. Renew. Sustain. Energy Rev. 2012, 16, 4126–4140. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Hogmark, S.; Jacobson, S.; Larsson, M. Design and evaluation of tribological coatings. Wear 2000, 246, 20–33. [Google Scholar] [CrossRef]
- Hirvonen, J.P.; Koskinen, J.; Jervis, J.R.; Nastasi, M. Present progress in the development of low friction coatings. Surf. Coat. Technol. 1996, 80, 139–150. [Google Scholar] [CrossRef]
- Khan, Z.A.; Latif, J.; Hammad Nazir, A.S.; Stokes, K. Predictive and prognostic modelling and simulation of coatings subject to corrosion and mechanical failures. Mater. Charact. 2018, 6, 487–498. [Google Scholar]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Stokes, K. Modeling the Effect of Residual and Diffusion-Induced Stresses on Corrosion at the Interface of Coating and Substrate. Corrosion 2016, 72, 500–517. [Google Scholar]
- Safakish, G.R. Temperature Estimation in the Combustion Chamber of an Internal Combustion Engine. Adv. Mech. Eng. 2012, 4, 931584. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Nicholls, J.R. Advances in Coating Design for High-Performance Gas Turbines. MRS Bull. 2003, 28, 659–670. [Google Scholar] [CrossRef]
- Voevodin, A.A.; O’Neill, J.P.; Zabinski, J.S. Nanocomposite tribological coatings for aerospace applications. Surf. Coat. Technol. 1999, 116–119, 36–45. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A. A Novel Non-Destructive Sensing Technology for On-Site Corrosion Failure Evaluation of Coatings. IEEE Access 2017, 6, 1042–1054. [Google Scholar] [CrossRef]
- Gu, Y.; Xia, K.; Wu, D.; Mou, J.; Zheng, S. Technical Characteristics and Wear-Resistant Mechanism of Nano Coatings: A Review. Coatings 2020, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Aliofkhazraei, M. Nanocoatings: Size Effect in Nanostructured Films; Springer Science & Business Media: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Aliofkhazraei, M. Synthesis, Processing and Application of Nanostructured Coatings Nanocoatings; Springer: Amsterdam, The Netherlands, 2011; pp. 1–28. [Google Scholar]
- Makhlouf, A.S.H. Current and advanced coating technologies for industrial applications. In Nanocoatings and Ultra-Thin Films; Woodhead Publishing: Sawston, UK, 2011; pp. 3–23. [Google Scholar]
- Behera, A.; Mallick, P.; Mohapatra, S.S. Chapter 13—Nanocoatings for Anticorrosion: An introduction Micro and Nano Technologies; Rajendran, S., Nguyen, T.A., Kakooei, S., Yeganeh, M., Li, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 227–243. [Google Scholar]
- Shishkovsky, I.V.; Lebedev, P.N. Chemical and physical vapor deposition methods for nanocoatings. In Nanocoatings and Ultra-Thin Films; Woodhead Publishing: Sawston, UK, 2011; pp. 55–77. [Google Scholar]
- van Lente, H.; van Til, J.I. Articulation of sustainability in the emerging field of nanocoatings. J. Clean. Prod. 2008, 16, 967–976. [Google Scholar] [CrossRef]
- Baptista, A.; Silva FJ, G.; Porteiro, J.; Míguez, J.L.; Pinto, G.; Fernandes, L. On the Physical Vapour Deposition (PVD): Evolution of Magnetron Sputtering Processes for Industrial Applications. Procedia Manuf. 2018, 17, 746–757. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, T.A.; Carriere, P.; Ngo Xuan, C. Nanocomposite Coatings: Preparation, Characterization, Properties, and Applications. Int. J. Corros. 2018, 2018, 4749501. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Goyenola, C.; Schmidt, S.; Hultman, L. CFx: A first-principles study of structural patterns arising during synthetic growth. Chem. Phys. Lett. 2011, 516, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Hellgren, N.; Berlind, T.; Gueorguiev, G.K.; Johansson, M.P.; Stafström, S.; Hultman, L. Fullerene-like BCN thin films: A computational and experimental study. Mater. Sci. Eng. B 2004, 113, 242–247. [Google Scholar] [CrossRef]
- Broitman, E.; Gueorguiev, G.K.; Furlan, A.; Son, N.T.; Gellman, A.J.; Stafström, S.; Hultman, L. Water adsorption on fullerene-like carbon nitride overcoats. Thin Solid Film. 2008, 517, 1106–1110. [Google Scholar] [CrossRef]
- Chu, K.; Shum, P.W.; Shen, Y.G. Substrate bias effects on mechanical and tribological properties of substitutional solid solution (Ti, Al)N films prepared by reactive magnetron sputtering. Mater. Sci. Eng. B 2006, 131, 62–71. [Google Scholar] [CrossRef]
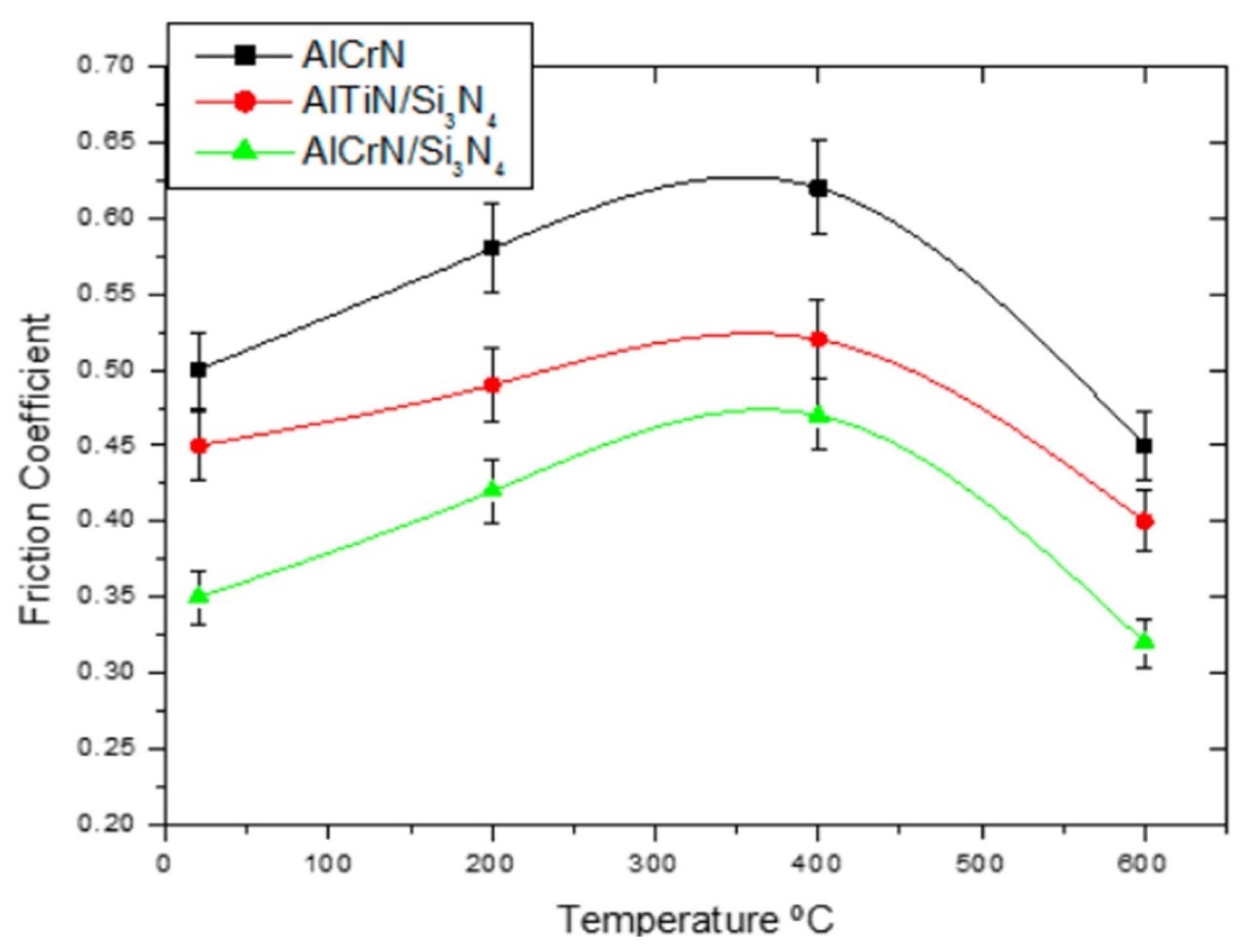
- Avila, P.R.T.; Apolinário, R.C.; Rodrigues, A.M.; Fernandes, J.V.; Menezes, R.R.; Neves, G.D.A.; Pinto, H.C. On Improving Wear Resistance of Cr-Al-N Coatings Using Dynamic Glancing Angle DC Magnetron Sputtering. Nanomaterials 2021, 11, 2187. [Google Scholar] [CrossRef]
- Yilmaz, O.; Yorgancioglu, A. Nanocoatings: Preparation, Properties, and Biomedical Applications. In Polymeric Nanomaterials in Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–331. [Google Scholar]
- Wang, X. Preparation, Synthesis and Application of Sol-Gel Method; Vidyasirimedhi Institute of Science and Technology: Rayong, Thailand, 2020. [Google Scholar]
- Lavollee, C.; Gressier, M.; Garcia, J.; Sobrino, J.M.; Reby, J.; Menu, M.J.; Rossi, S.; Fedel, M. New architectured hybrid sol-gel coatings for wear and corrosion protection of low-carbon steel. Prog. Org. Coat. 2016, 99, 337–345. [Google Scholar]
- Jiang, Y.; Shi, K.; Tang, H.; Wang, Y. Enhanced wettability and wear resistance on TiO2/PDA thin films prepared by sol-gel dip coating. Surf. Coat. Technol. 2019, 375, 334–340. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. Modern Electroplating; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 55. [Google Scholar]
- Basile, F.; Benito, P.; Fornasari, G.; Monti, M.; Scavetta, E.; Tonelli, D.; Vaccari, A. A novel electrochemical route for the catalytic coating of metallic supports. Stud. Surf. Sci. Catal. 2010, 175, 51–58. [Google Scholar]
- Toyserkani, E.; Khajepour, A.; Corbin, S.F. Laser Cladding; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Gu, Y.; Yu, S.; Mou, J.; Wu, D.; Zheng, S. Research Progress on the Collaborative Drag Reduction Effect of Polymers and Surfactants. Materials 2020, 13, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman Rashid, R.A.; Nazari, K.A.; Barr, C.; Palanisamy, S.; Orchowski, N.; Matthews, N.; Dargusch, M.S. Effect of laser reheat post-treatment on the microstructural characteristics of laser-cladded ultra-high strength steel. Surf. Coat. Technol. 2019, 372, 93–102. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, B.; Cui, H.; Lu, F.; Xu, P. Microstructure, wear, and oxidation resistance of nanostructured carbide-strengthened cobalt-based composite coatings on Invar alloys by laser cladding. Surf. Coat. Technol. 2020, 381, 125188. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Electroless Ni-P-PTFE-Al2O3 dispersion nanocomposite coating for corrosion and wear resistance. J. Mater. Eng. Perform. 2014, 23, 142–151. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Wang, L.; Hao, G.; Sun, X.; Shan, F.; Zou, Z. Nanocomposite Lanthanum Zirconate Thermal Barrier Coating Deposited by Suspension Plasma Spray Process. J. Therm. Spray Technol. 2014, 23, 1030–1036. [Google Scholar] [CrossRef]
- Tettey, K.E.; Yee, M.Q.; Lee, D. Photocatalytic and conductive MWCNT/TiO2 nanocomposite thin films. ACS Appl. Mater. Interfaces 2010, 2, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Electrochemical Corrosion; ASM International: Almere, The Netherlands, 2000. [Google Scholar]
- Baena, L.M.; Gómez, M.; Calderón, J.A. Aggressiveness of a 20% bioethanol–80% gasoline mixture on autoparts: I behavior of metallic materials and evaluation of their electrochemical properties. Fuel 2012, 95, 320–328. [Google Scholar] [CrossRef]
- Davis, J.R. The Effects and Economic Impact of Corrosion. In Corrosion: Understanding the Basics; ASM International: Almere, The Netherlands, 2000. [Google Scholar]
- Koch, G.H.; Varney, J.; Neil, T.; Oliver, M.; Gould, M.; Payer, J. International Measures of Prevention, Application and Economics of Corrosion Technologies Study. NACE Int. 2016, 216, 3. [Google Scholar]
- Nazir, M.H.; Khan, Z.A. A review of theoretical analysis techniques for cracking and corrosive degradation of film-substrate systems. Eng. Fail. Anal. 2017, 72, 80–113. [Google Scholar] [CrossRef]
- Grumezescu, V.; Negut, I. Nanocoatings and Thin Films Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 463–477. [Google Scholar]
- Cui, J.; Ren, S.; Lin, T.; Feng, Y.; Jia, S. Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic Fe3O4@ silica core shell nanosphere. Chem. Eng. J. 2018, 343, 629–637. [Google Scholar] [CrossRef]
- Zeng, A.; Yao, X.; Gui, Y.; Li, Y.; Jones, K.J.; Yu, L. Inhibiting surface crystallization and improving dissolution of amorphous loratadine by dextran sulfate nanocoating. J. Pharm. Sci. 2019, 108, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Callister, W.D., Jr.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering: An Integrated Approach; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Piwoński, I.; Soliwoda, K. The effect of ceramic nanoparticles on tribological properties of alumina sol–gel thin coatings. Ceram. Int. 2010, 36, 47–54. [Google Scholar] [CrossRef]
- Li, H.; Cui, Y.; Li, Z.; Zhu, Y.; Wang, H. Fabrication of microcapsules containing dual-functional tung oil and properties suitable for self-healing and self-lubricating coatings. Prog. Org. Coat. 2018, 115, 164–171. [Google Scholar] [CrossRef]
- Mersagh Dezfuli, S.; Sabzi, M. A study on the effect of presence of CeO2 and benzotriazole on activation of self-healing mechanism in ZrO2 ceramic-based coating. Int. J. Appl. Ceram. Technol. 2018, 15, 1248–1260. [Google Scholar] [CrossRef]
- Potts, S.E.; Schmalz, L.; Fenker, M.; Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Radnóczi, G.; Tóth, L. Ultra-thin aluminium oxide films deposited by plasma-enhanced atomic layer deposition for corrosion protection. J. Electrochem. Soc. 2011, 158, C132. [Google Scholar] [CrossRef]
- Härkönen, E.; Potts, S.E.; Kessels, W.M.M.; Díaz, B.; Seyeux, A.; Światowska, J.; Maurice, V.; Marcus, P.; Radnóczi, G.; Tóth, L.; et al. Hydrogen–argon plasma pre-treatment for improving the anti-corrosion properties of thin Al2O3 films deposited using atomic layer deposition on steel. Thin Solid Film. 2013, 534, 384–393. [Google Scholar] [CrossRef]
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Klein, L.H.; Salmi, E.; Ritala, M.; Marcus, P. The role of surface preparation in corrosion protection of copper with nanometer-thick ALD alumina coatings. Appl. Surf. Sci. 2016, 387, 1054–1061. [Google Scholar] [CrossRef]
- Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Normand, B.; Härkönen, E.; Ritala, M.; Marcus, P. Electrochemical and time-of-flight secondary ion mass spectrometry analysis of ultra-thin metal oxide (Al2O3 and Ta2O5) coatings deposited by atomic layer deposition on stainless steel. Electrochim. Acta 2011, 56, 10516–10523. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Ritala, M. Low-temperature atomic layer deposition of Al2O3 thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2011, 53, 2168–2175. [Google Scholar] [CrossRef]
- Shen, G.X.; Chen, Y.C.; Lin, L.; Lin, C.J.; Scantlebury, D. Study on a hydrophobic nano-TiO2 coating and its properties for corrosion protection of metals. Electrochim. Acta 2005, 50, 5083–5089. [Google Scholar] [CrossRef]
- Lorencik, S.; Yu, Q.L.; Brouwers, H.J.H. Photocatalytic coating for indoor air purification: Synergetic effect of photocatalyst dosage and silica modification. Chem. Eng. J. 2016, 306, 942–952. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Giolando, D.M. Transparent self-cleaning coating applicable to solar energy consisting of nano-crystals of titanium dioxide in fluorine doped tin dioxide. Sol. Energy 2016, 124, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Deyab, M.A.; Keera, S.T. Effect of nano-TiO2 particles size on the corrosion resistance of alkyd coating. Mater. Chem. Phys. 2014, 146, 406–411. [Google Scholar] [CrossRef]
- Ammar, A.U.; Shahid, M.; Ahmed, M.K.; Khan, M.; Khalid, A.; Khan, Z.A. Electrochemical Study of Polymer and Ceramic-Based Nanocomposite Coatings for Corrosion Protection of Cast Iron Pipeline. Materials 2018, 11, 332. [Google Scholar] [CrossRef] [Green Version]
- De Falco, G.; de Filippis, G.; Scudieri, C.; Vitale, L.; Commodo, M.; Minutolo, P.; D’Anna, A.; Ciambelli, P. Nano-TiO2 Coating Layers with Improved Anticorrosive Properties by Aerosol Flame Synthesis and Thermophoretic Deposition on Aluminium Surfaces. Materials 2021, 14, 2918. [Google Scholar] [CrossRef]
- Fairbrother, F. The Chemistry of Niobium and Tantalum; Elsevier: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Rahmati, B.; Sarhan AA, D.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D.; Abas, W. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480. [Google Scholar] [CrossRef]
- Díaz, B.; Światowska, J.; Maurice, V.; Pisarek, M.; Seyeux, A.; Zanna, S.; Tervakangas, S.; Kolehmainen, J.; Marcus, P. Chromium and tantalum oxide nanocoatings prepared by filtered cathodic arc deposition for corrosion protection of carbon steel. Surf. Coat. Technol. 2012, 206, 3903–3910. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; Fan, X.; Wang, Y.; Liu, G. Growth and dielectric properties of Ta2O5 single crystal by the floating zone method. Cryst. Res. Technol. 2012, 47, 903–908. [Google Scholar] [CrossRef]
- Habibi, M.H.; Mokhtari, R. Novel sulfur-doped niobium pentoxide nanoparticles: Fabrication, characterization, visible light sensitization and redox charge transfer study. J. Sol-Gel Sci. Technol. 2011, 59, 352–357. [Google Scholar] [CrossRef]
- Chaluvaraju, B.V.; Ganiger, S.K.; Murugendrappa, M.V. Synthesis, Characterization and DC Conductivity Studies of Polypyrrole/Tantalum Pentoxide Composites. Int. J. Latest Technol. Eng. 2014, 3, 33–36. [Google Scholar]
- Hu, W.; Xu, J.; Lu, X.; Hu, D.; Tao, H.; Munroe, P.; Xie, Z.-H. Corrosion and wear behaviours of a reactive-sputter-deposited Ta2O5 nanoceramic coating. Appl. Surf. Sci. 2016, 368, 177–190. [Google Scholar] [CrossRef]
- Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Härkönen, E.; Ritala, M.; Tervakangas, S.; Kolehmainen, J.; Marcus, P. Tantalum oxide nanocoatings prepared by atomic layer and filtered cathodic arc deposition for corrosion protection of steel: Comparative surface and electrochemical analysis. Electrochim. Acta 2013, 90, 232–245. [Google Scholar] [CrossRef]
- Ma, J.J.; Xu, J.; Jiang, S.; Munroe, P.; Xie, Z.-H. Effects of pH value and temperature on the corrosion behavior of a Ta2N nanoceramic coating in simulated polymer electrolyte membrane fuel cell environment. Ceram. Int. 2016, 42, 16833–16851. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, J.; Liu, L.L.; Jiang, S. Electrochemical Corrosion Behavior of Ta2N Nanoceramic Coating in Simulated Body Fluid. Materials 2016, 9, 772. [Google Scholar] [CrossRef]
- Hirpara, J.; Chawla, V.; Chandra, R. Anticorrosive Behavior Enhancement of Stainless Steel 304 through Tantalum-Based Coatings: Role of Coating Morphology. J. Mater. Eng. Perform. 2021, 30, 1895–1905. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Shao, Y.; Zhang, T.; Wang, Y.; Wang, F.; Cheng, X.; Dong, C.; Li, X. Effect of Microstructures on Corrosion Behavior of Nickel Coatings: (I) Abnormal Grain Size Effect on Corrosion Behavior. J. Mater. Sci. Technol. 2015, 31, 1186–1192. [Google Scholar] [CrossRef]
- Chianpairot, A.; Lothongkum, G.; Schuh, C.A.; Boonyongmaneerat, Y. Corrosion of nanocrystalline Ni–W alloys in alkaline and acidic 3.5wt.% NaCl solutions. Corros. Sci. 2011, 53, 1066–1071. [Google Scholar] [CrossRef]
- Cheng, W.; Ge, W.; Yang, Q.; Qu, X. Study on the corrosion properties of nanocrystalline nickel electrodepositied by reverse pulse current. Appl. Surf. Sci. 2013, 276, 604–608. [Google Scholar] [CrossRef]
- Hyie, K.M.; Resali, N.A.; Abdullah WN, R.; Chong, W.T. Synthesis and Characterization of Nanocrystalline Pure Cobalt Coating: Effect of pH. Procedia Eng. 2012, 41, 1627–1633. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Bakolas, V.; Braun, W.; Bajwa, R.; Rafique, S. Analyzing and Modelling the Corrosion Behavior of Ni/Al2O3, Ni/SiC, Ni/ZrO2 and Ni/Graphene Nanocomposite Coatings. Materials 2017, 10, 1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulaeto, S.B.; Pancrecious, J.K.; Ajekwene, K.K.; Mathew, G.M.; Rajan, T.P.D. Advanced nanocoatings for anticorrosion. In Corrosion Protection at the Nanoscale; Elsevier: Amsterdam, The Netherlands, 2020; pp. 499–510. [Google Scholar]
- Feng, Z.; Li, Q.; Zhang, J.; Yang, P.; Song, H.; An, M. Electrodeposition of nanocrystalline Zn–Ni coatings with single gamma phase from an alkaline bath. Surf. Coat. Technol. 2015, 270, 47–56. [Google Scholar] [CrossRef]
- Mosavat, S.H.; Shariat, M.H.; Bahrololoom, M.E. Study of corrosion performance of electrodeposited nanocrystalline Zn–Ni alloy coatings. Corros. Sci. 2012, 59, 81–87. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, W.H.; Pei, Z.L.; Gong, J.; Sun, C. Investigations on the structure and properties of nanocrystalline Ni-Mo alloy coatings. Mater. Charact. 2020, 167, 110532. [Google Scholar] [CrossRef]
- Laszczyńska, A.; Tylus, W.; Winiarski, J.; Szczygieł, I. Evolution of corrosion resistance and passive film properties of Ni-Mo alloy coatings during exposure to 0.5 M NaCl solution. Surf. Coat. Technol. 2017, 317, 26–37. [Google Scholar] [CrossRef]
- Bigos, A.; Beltowska-Lehman, E.; Kot, M. Studies on electrochemical deposition and physicochemical properties of nanocrystalline Ni-Mo alloys. Surf. Coat. Technol. 2017, 317, 103–109. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A. Nanocrystalline Ni–Co alloy coatings: Electrodeposition using horizontal electrodes and corrosion resistance. J. Coat. Technol. Res. 2013, 10, 285–295. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Li, K.; Zhong, Q.; Bui, Q.B. Preparation of Ni–W–SiO2 nanocomposite coating and evaluation of its hardness and corrosion resistance. Ceram. Int. 2015, 41, 79–84. [Google Scholar] [CrossRef]
- Liu, J.H.; Pei, Z.L.; Shi, W.B.; Liu, Y.D.; Gong, J.; Sun, C. Studies on preparation, microstructure, mechanical properties and corrosion resistance of NiMo/micron-sized diamond composite coatings. Surf. Coat. Technol. 2020, 385, 125451. [Google Scholar] [CrossRef]
- Selvi, V.E.; Seenivasan, H.; Rajam, K.S. Electrochemical corrosion behavior of pulse and DC electrodeposited Co–P coatings. Surf. Coat. Technol. 2012, 206, 2199–2206. [Google Scholar] [CrossRef]
- Chandrasekar, M.S.; Shanmugasigamani Malathy, P. Synergetic effects of pulse constraints and additives in electrodeposition of nanocrystalline zinc: Corrosion, structural and textural characterization. Mater. Chem. Phys. 2010, 124, 516–528. [Google Scholar] [CrossRef]
- Li, Q.; Lu, H.; Cui, J.; An, M.; Li, D. Electrodeposition of nanocrystalline zinc on steel for enhanced resistance to corrosive wear. Surf. Coat. Technol. 2016, 304, 567–573. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhu, J.; Cai, T.; Guo, Z. Investigation on the Electrochemical Deposition of Nanocrystalline Zinc with Cationic Polyacrylamide (CPAM)-ZnSO4 Electrolyte. Micromachines 2021, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Kasar, A.K.; Bhutta, M.U.; Khan, Z.A.; Menezes, P.L. Corrosion performance of nanocomposite coatings in moist SO2 environment. Int. J. Adv. Manuf. Technol. 2020, 106, 4769–4776. [Google Scholar] [CrossRef]
- Pramod Kumar, U.; Kennady, C.J.; Zhou, Q. Effect of salicylaldehyde on microstructure and corrosion resistance of electrodeposited nanocrystalline Ni–W alloy coatings. Surf. Coat. Technol. 2015, 283, 148–155. [Google Scholar] [CrossRef]
- Meng, G.; Sun, F.; Shaoa, Y.; Zhang, T.; Wang, F.; Dong, C.; Li, X. Effect of phytic acid on the microstructure and corrosion resistance of Ni coating. Electrochim. Acta 2010, 55, 5990–5995. [Google Scholar] [CrossRef]
- Danişman, M. The corrosion behavior of nanocrystalline nickel based thin films. Mater. Chem. Phys. 2016, 171, 276–280. [Google Scholar] [CrossRef]
- Rathod, V.T.; Kumar, J.S.; Jain, A. Polymer and ceramic nanocomposites for aerospace applications. Appl. Nanosci. 2017, 7, 519–548. [Google Scholar] [CrossRef]
- Asmatulu, R. 14-Nanocoatings for corrosion protection of aerospace alloys. In Metals and Surface Engineering; Saji, V.S., Cook, R., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 357–374. [Google Scholar]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Chandra, A.K.; Kumar, N.R. Polymer Nanocomposites for Automobile Engineering Applications. In Properties and Applications of Polymer Nanocomposites: Clay and Carbon Based Polymer Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2017; pp. 139–172. [Google Scholar]
- Xue, L.; Wang, W.; Guo, Y.; Liu, G.; Wan, P. Flexible polyaniline/carbon nanotube nanocomposite film-based electronic gas sensors. Sens. Actuators B Chem. 2017, 244, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Penkov, O.V.; Pukha, V.E.; Starikova, S.L.; Khadem, M.; Starikov, V.V.; Maleev, M.V.; Kim, D.E. Highly wear-resistant and biocompatible carbon nanocomposite coatings for dental implants. Biomaterials 2016, 102, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Mehdikhani-Nahrkhalaji, M.; Fathi, M.H.; Mortazavi, V.; Mousavi, S.B.; Hashemi-Beni, B.; Razavi, S.M. Novel nanocomposite coating for dental implant applications in vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2011, 23, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.E.; Bayer, I.S.; Palumbo, J.M.; Carroll, P.J.; Megaridis, C.M. Extremely stretchable and conductive water-repellent coatings for low-cost ultra-flexible electronics. Nat. Commun. 2015, 6, 8874. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Ali, N.; Ochsner, A. Nanocomposite Coatings and Nanocomposite Materials; Trans Tech Publications: Zurich, Switzerland, 2009. [Google Scholar]
- Fayyad, E.M.; Sadasivuni, K.K.; Ponnamma, D.; Al-Maadeed, M.A.A. Oleic acid-grafted chitosan/graphene oxide composite coating for corrosion protection of carbon steel. Carbohydr. Polym. 2016, 151, 871–878. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. New approach for simultaneous enhancement of anticorrosive and mechanical properties of coatings: Application of water repellent nano CaCO3–PANI emulsion nanocomposite in alkyd resin. Chem. Eng. J. 2010, 156, 177–183. [Google Scholar] [CrossRef]
- Fernando, R.H. Nanocomposite and Nanostructured Coatings: Recent Advancements; ACS Publications: Washington, DC, USA, 2009. [Google Scholar]
- John, S.; Joseph, A.; Jose, A.J.; Narayana, B. Enhancement of corrosion protection of mild steel by chitosan/ZnO nanoparticle composite membranes. Prog. Org. Coat. 2015, 84, 28–34. [Google Scholar] [CrossRef]
- Aliyu, I.K.; Mohammed, A.S. Wear and corrosion resistance performance of UHMWPE/GNPs nanocomposite coatings on AA2028 Al alloys. Prog. Org. Coat. 2021, 151, 106072. [Google Scholar] [CrossRef]
- Mahato, N.; Cho, M.H. Graphene integrated polyaniline nanostructured composite coating for protecting steels from corrosion: Synthesis, characterization, and protection mechanism of the coating material in acidic environment. Constr. Build. Mater. 2016, 115, 618–633. [Google Scholar] [CrossRef]
- Jafari, Y.; Ghoreishi, S.M.; Shabani-Nooshabadi, M. Polyaniline/Graphene nanocomposite coatings on copper: Electropolymerization, characterization, and evaluation of corrosion protection performance. Synth. Met. 2016, 217, 220–230. [Google Scholar] [CrossRef]
- Chen, L.; Song, R.G.; Li, X.W.; Guo, Y.Q.; Wang, C.; Jiang, Y. The improvement of corrosion resistance of fluoropolymer coatings by SiO2/poly(styrene-co-butyl acrylate) nanocomposite particles. Appl. Surf. Sci. 2015, 353, 254–262. [Google Scholar] [CrossRef]
- Rahman, O.U.; Kashif, M.; Ahmad, S. Nanoferrite dispersed waterborne epoxy-acrylate: Anticorrosive nanocomposite coatings. Prog. Org. Coat. 2015, 80, 77–86. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J. Painting the future green. Eur. Coat. J. 2010, 4, 20–25. [Google Scholar]
- Ress, J.; Martin, U.; Bastidas, D.M. Improved Corrosion Protection of Acrylic Waterborne Coating by Doping with Microencapsulated Corrosion Inhibitors. Coatings 2021, 11, 1134. [Google Scholar] [CrossRef]
- Dehgahi, S.; Amini, R.; Alizadeh, M. Corrosion, passivation and wear behaviors of electrodeposited Ni–Al2O3–SiC nano-composite coatings. Surf. Coat. Technol. 2016, 304, 502–511. [Google Scholar] [CrossRef]
- Cai, C.; Zhu, X.B.; Zheng, G.Q.; Yuan, Y.N.; Huang, X.Q.; Cao, F.H.; Yang, J.F.; Zhang, B. Electrodeposition and characterization of nano-structured Ni–SiC composite films. Surf. Coat. Technol. 2011, 205, 3448–3454. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Allahkaram, S.R. Corrosion resistance enhancement of Ni–P electroless coatings by incorporation of nano-SiO2 particles. Mater. Des. 2011, 32, 133–138. [Google Scholar] [CrossRef]
- Makkar, P.; Agarwala, R.C.; Agarwala, V. Wear and corrosion characteristics of alumina dispersed Ni–P nanocomposite coating developed by electroless technique. J. Mater. Sci. 2015, 50, 2813–2823. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Electroless Ni-P and Ni-P-Al2O3 nanocomposite coatings and their corrosion and wear resistance. J. Mater. Eng. Perform. 2013, 22, 176–183. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Aminikia, H.; Bagheri, R. Electroless Deposition of Ni-Cu-P Coatings Containing Nano-Al2O3 Particles and Study of Its Corrosion Protective Behaviour in 0.5 M H2SO4. Int. J. Corros. 2014, 2014, 391502. [Google Scholar] [CrossRef] [Green Version]
- Jie, X.; Hong-Wen, Z.; Xiu-Qing, F.; Jin-Ran, L.; Shuang-Lu, D.; Mo-Qi, S. Effect of CeO2 on Electrochemical Corrosion Resistance of Ni–P–CeO2 Composite Coatings Prepared by Submerged Jet Electrodeposition. Int. J. Electrochem. Sci. 2020, 15, 7559–7574. [Google Scholar] [CrossRef]
- Alishahi, M.; Monirvaghefi, S.M.; Saatchi, A.; Hosseini, S.M. The effect of carbon nanotubes on the corrosion and tribological behavior of electroless Ni–P–CNT composite coating. Appl. Surf. Sci. 2012, 258, 2439–2446. [Google Scholar] [CrossRef]
- Vlasa, A.; Varvara, S.; Pop, A.; Bulea, C.; Muresan, L.M. Electrodeposited Zn–TiO2 nanocomposite coatings and their corrosion behavior. J. Appl. Electrochem. 2010, 40, 1519–1527. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Lee, P.M.; Carpick, R. Tribological opportunities for enhancing America’s energy efficiency. In Proceedings of the Report to the Advanced Research Projects Agency-Energy (ARPA-E) at the US Department of Energy, Washington, DC, USA, 14 February 2017. [Google Scholar]
- Kim, G.E.; Brochu, M. 19-Thermal Spray Nanostructured Ceramic and Metal-Matrix Composite Coatings; Aliofkhazraei, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 481–511. [Google Scholar]
- Mel’nikova, I.P.; Lyasnikova, A.V.; Lyasnikov, V.N. Physical Bases of Formation of Nanostructured Biocompatible Coatings on Medical Implants. Russ. Phys. J. 2014, 56, 1190–1197. [Google Scholar] [CrossRef]
- Sathish, S.; Geetha, M.; Aruna, S.T.; Balaji, N.; Rajam, K.S.; Asokamani, R. Sliding wear behavior of plasma sprayed nanoceramic coatings for biomedical applications. Wear 2011, 271, 934–941. [Google Scholar] [CrossRef]
- Jevnikar, P.; Golobič, M.; Kocjan, A.; Kosmač, T. The effect of nano-structured alumina coating on the bond strength of resin-modified glass ionomer cements to zirconia ceramics. J. Eur. Ceram. Soc. 2012, 32, 2641–2645. [Google Scholar] [CrossRef]
- McIntyre, R.A. Common nano-materials and their use in real world applications. Sci. Prog. 2012, 95, 1–22. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Salman, S.R.; Wasna’a, M.A. Structural, Optical, Electrical and Gas Sensor Properties of ZrO2 Thin Films prepared by Sol-Gel Technique. Neuroquantology 2020, 18, 22. [Google Scholar] [CrossRef]
- Sathyaseelan, B.; Manikandan, E.; Baskaran, I.; Senthilnathan, K.; Sivakumar, K.; Moodley, M.K.; Ladchumananandasivam, R.; Maaza, M. Studies on structural and optical properties of ZrO2 nanopowder for opto-electronic applications. J. Alloy. Compd. 2017, 694, 556–559. [Google Scholar] [CrossRef]
- Zalnezhad, E. Effect of structural evolution on mechanical properties of ZrO2 coated Ti–6Al–7Nb-biomedical application. Appl. Surf. Sci. 2016, 370, 32–39. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, G.; Bu, A.; Zhang, B. Corrosion and Tribological Behavior of ZrO2 Films Prepared on Stainless Steel Surface by the Sol–Gel Method. ACS Appl. Mater. Interfaces 2015, 7, 28264–28272. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, S.P.; de Silva, N.; Kaupuge, T.K.; Weerasekera, T.S.; Senarath Yapa, M.D.; Karunaratne, V.; Amaratunga, G.A.J. Oxidation protection of carbon fiber by sol-gel derived boron doped yttria stabilized zirconia coatings. Mater. Sci. Eng. B 2018, 229, 59–64. [Google Scholar] [CrossRef]
- Hao, S.J.; Wang, C.; Liu, T.L.; Mao, Z.M.; Mao, Z.Q.; Wang, J.L. Fabrication of nanoscale yttria stabilized zirconia for solid oxide fuel cell. Int. J. Hydrog. Energy 2017, 42, 29949–29959. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, G.; Shi, Z. Tribological properties of ZrO2 nanofilms coated on stainless steel in a 5% NaCl solution, distilled water and a dry environment. Surf. Coat. Technol. 2018, 350, 128–135. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, G.; Liao, H.L.; Coddet, C.; Ding, C.X. Structure and tribological performance of nanostructured ZrO 2–3 mol% Y2O3 coatings deposited by air plasma spraying. J. Therm. Spray Technol. 2010, 19, 1163–1170. [Google Scholar]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Camposilvan, E.; Marro, F.G.; Mestra, A.; Anglada, M. Enhanced reliability of yttria-stabilized zirconia for dental applications. Acta Biomater. 2015, 17, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef]
- Lamuta, C.; di Girolamo, G.; Pagnotta, L. Microstructural, mechanical and tribological properties of nanostructured YSZ coatings produced with different APS process parameters. Ceram. Int. 2015, 41, 8904–8914. [Google Scholar] [CrossRef]
- Soltani, R.; Coyle, T.W.; Mostaghimi, J. Wear resistance of nanostructured thermal barrier coatings. In Proceedings of the International Thermal Spray Conference 2003: Advancing the Science and Applying the Technology, Orlando, FL, USA, 5–8 May 2003; pp. 1535–1540. [Google Scholar]
- Tao, S.; Liang, B.; Ding, C.; Liao, H.; Coddet, C. Wear characteristics of plasma-sprayed nanostructured yttria partially stabilized zirconia coatings. J. Therm. Spray Technol. 2005, 14, 518–523. [Google Scholar] [CrossRef]
- Singh, V.P.; Sil, A.; Jayaganthan, R. A study on sliding and erosive wear behaviour of atmospheric plasma sprayed conventional and nanostructured alumina coatings. Mater. Des. 2011, 32, 584–591. [Google Scholar] [CrossRef]
- Singh, V.P.; Sil, A.; Jayaganthan, R. Tribological behaviour of nanostructured Al2O3 coatings. Surf. Eng. 2013, 28, 277–284. [Google Scholar] [CrossRef]
- Żórawski, W.; Góral, A.; Bokuvka, O.; Lityńska-Dobrzyńska, L.; Berent, K. Microstructure and tribological properties of nanostructured and conventional plasma sprayed alumina–titania coatings. Surf. Coat. Technol. 2015, 268, 190–197. [Google Scholar]
- Richard, C.; Kowandy, C.; Landoulsi, J.; Geetha, M.; Ramasawmy, H. Corrosion and wear behavior of thermally sprayed nano ceramic coatings on commercially pure Titanium and Ti–13Nb–13Zr substrates International. J. Refract. Met. Hard Mater. 2010, 28, 115–123. [Google Scholar] [CrossRef]
- Rico, A.; Poza, P.; Rodríguez, J. High temperature tribological behavior of nanostructured and conventional plasma sprayed alumina-titania coatings. Vacuum 2013, 88, 149–154. [Google Scholar] [CrossRef]
- Zamani, P.; Valefi, Z. Microstructure, phase composition and mechanical properties of plasma sprayed Al2O3, Cr2O3 and Cr2O3-Al2O3 composite coatings. Surf. Coat. Technol. 2017, 316, 138–145. [Google Scholar] [CrossRef]
- Sathish, S.; Geetha, M. Comparative study on corrosion behavior of plasma sprayed Al2O3, ZrO2, Al2O3/ZrO2 and ZrO2/Al2O3 coatings. Trans. Nonferrous Met. Soc. China 2016, 26, 1336–1344. [Google Scholar] [CrossRef]
- Monticelli, C.; Balbo, A.; Zucchi, F. Corrosion and tribocorrosion behaviour of thermally sprayed ceramic coatings on steel. Surf. Coat. Technol. 2011, 205, 3683–3691. [Google Scholar] [CrossRef]
- Tao, S.; Yin, Z.; Zhou, X.; Ding, C. Sliding wear characteristics of plasma-sprayed Al2O3 and Cr2O3 coatings against copper alloy under severe conditions. Tribol. Int. 2010, 43, 69–75. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, J.; Zhang, H.; Wang, J.; Sun, J.; Dong, S.; Jiang, J.; Deng, L.; Zhou, X.; Cao, X. Correlation between microstructure, chemical components and tribological properties of plasma-sprayed Cr2O3-based coatings. Ceram. Int. 2018, 44, 10154–10168. [Google Scholar] [CrossRef]
- Singh, V.P.; Sil, A.; Jayaganthan, R. Wear of plasma sprayed conventional and nanostructured Al2O3 and Cr2O3, based coatings. Trans. Indian Inst. Met. 2012, 65, 1–12. [Google Scholar] [CrossRef]
- Vernhes, L.; Bekins, C.; Lourdel, N.; Poirier, D.; Lima, R.S.; Li, D.; Klemberg-Sapieha, J.E. Nanostructured and Conventional Cr2O3, TiO2, and TiO2-Cr2O3 Thermal-Sprayed Coatings for Metal-Seated Ball Valve Applications in Hydrometallurgy. J. Therm. Spray Technol. 2016, 25, 1068–1078. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Parvin, N.; Valefi, Z. Effect of microstructure and mechanical properties on wear behavior of plasma-sprayed Cr2O3-YSZ-SiC coatings. Ceram. Int. 2019, 45, 5284–5296. [Google Scholar] [CrossRef]
- Singh, V.P.; Sil, A.; Jayaganthan, R. Tribological behavior of plasma sprayed Cr2O3–3%TiO2 coatings. Wear 2011, 272, 149–158. [Google Scholar] [CrossRef]
- Xu, J.; Hu, W.; Xie, Z.-H.; Munroe, P. Reactive-sputter-deposited β-Ta2O5 and TaON nanoceramic coatings on Ti–6Al–4V alloy against wear and corrosion damage. Surf. Coat. Technol. 2016, 296, 171–184. [Google Scholar] [CrossRef]
- Nik Rozlin, N.M.; Alfantazi, A.M. Nanocrystalline cobalt–iron alloy: Synthesis and characterization. Mater. Sci. Eng. A 2012, 550, 388–394. [Google Scholar] [CrossRef]
- Farabi Khaneghahi, S.; Sharafi, S. Magnetic and structural properties of nanostructured (Fe65Co35)100−xCrx (x = 0, 10) powders prepared by mechanical alloying process. Adv. Powder Technol. 2014, 25, 211–218. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Strauss, H.W.; Brahimi, S.; Chromik, R.R.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Tribological behavior of electrodeposited Zn, Zn–Ni, Cd and Cd–Ti coatings on low carbon steel substrates. Tribol. Int. 2012, 56, 107–120. [Google Scholar] [CrossRef]
- Enayati, M.H.; Karimzadeh, F.; Jafari, M.; Markazi, A.; Tahvilian, A. Microstructural and wear characteristics of HVOF-sprayed nanocrystalline NiAl coating. Wear 2014, 309, 192–199. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Chen, J.; Zhou, H.; Guo, C.; Wang, L.; Yang, L. Preparation, microstructure and tribological behavior of laser cladding NiAl intermetallic compound coatings. Wear 2012, 274–275, 298–305. [Google Scholar] [CrossRef]
- Yousefi, E.; Sharafi, S.; Irannejad, A. The structural, magnetic, and tribological properties of nanocrystalline Fe-Ni permalloy and Fe-Ni-TiO2 composite coatings produced by pulse electro co-deposition. J. Alloys Compd. 2018, 753, 308–319. [Google Scholar] [CrossRef]
- Su, F.H.; Huang, P. Microstructure and tribological property of nanocrystalline Co–W alloy coating produced by dual-pulse electrodeposition. Mater. Chem. Phys. 2012, 134, 350–359. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Liu, L.; Sun, J.; Qu, Y.; Li, F.; An, M. Research on the tribological behavior of a nanocrystalline zinc coating prepared by pulse reverse electrodeposition. RSC Adv. 2015, 5, 12025–12033. [Google Scholar] [CrossRef]
- Geetha, M.; Kumar, N.; Panda, K.; Dhara, S.; Dash, S.; Panigrahi, B.K.; Tyagi, A.K.; Jayavel, R.; Kamaraj, V. Tribological and electrical properties of nanocrystalline Cu films deposited by DC magnetron sputtering with varying temperature. Tribol. Int. 2013, 58, 79–84. [Google Scholar] [CrossRef]
- Rupert, T.J.; Schuh, C.A. Sliding wear of nanocrystalline Ni–W: Structural evolution and the apparent breakdown of Archard scaling. Acta Mater. 2010, 58, 4137–4148. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 2004, 24, 981. [Google Scholar] [CrossRef]
- Su, F.; Liu, C.; Huang, P. Friction and wear of nanocrystalline Co and Co–W alloy coatings produced by pulse reverse electrodeposition. Wear 2013, 300, 114–125. [Google Scholar] [CrossRef]
- George, E.D. Mechanical Metallurgy; Tata McGraw-Hill: Noida, India, 2017. [Google Scholar]
- Wasekar, N.P.; Haridoss, P.; Seshadri, S.K.; Sundararajan, G. Sliding wear behavior of nanocrystalline nickel coatings: Influence of grain size. Wear 2012, 296, 536–546. [Google Scholar] [CrossRef]
- Jeng, Y.R.; Tsai, P.C.; Chiang, S.H. Effects of grain size and orientation on mechanical and tribological characterizations of nanocrystalline nickel films. Wear 2013, 303, 262–268. [Google Scholar] [CrossRef]
- Vardanyan, V.; Poaty, B.; Landry, V.; Chauve, G.; Galstian, T.; Riedl, B. 8-Wear Resistance of Nanocomposite Coatings; Aliofkhazraei, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 201–223. [Google Scholar]
- Wu, Y.S.; Zeng, D.C.; Liu, Z.W.; Qiu, W.Q.; Zhong, X.C.; Yu, H.Y.; Li, S.Z. Microstructure and sliding wear behavior of nanostructured Ni60–TiB2 composite coating sprayed by HVOF technique. Surf. Coat. Technol. 2011, 206, 1102–1108. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, K.; Yao, C.; Nie, P.; Huang, J.; Li, Z. Microstructure and tribological properties of laser cladded self-lubricating nickel-base composite coatings containing nano-Cu and h-BN solid lubricants. Surf. Coat. Technol. 2019, 359, 485–494. [Google Scholar] [CrossRef]
- Lu, X.L.; Liu, X.B.; Yu, P.C.; Zhai, Y.J.; Qiao, S.J.; Wang, M.D.; Wang, Y.G.; Chen, Y. Effects of heat treatment on microstructure and mechanical properties of Ni60/h-BN self-lubricating anti-wear composite coatings on 304 stainless steel by laser cladding. Appl. Surf. Sci. 2015, 355, 350–358. [Google Scholar] [CrossRef]
- Mirzamohammadi, S.; Khorsand, H.; Aliofkhazraei, M.; Shtansky, D.V. Effect of carbamide concentration on electrodeposition and tribological properties of Al2O3 nanoparticle reinforced nickel nanocomposite coatings. Tribol. Int. 2018, 117, 68–77. [Google Scholar] [CrossRef]
- Adamiak, S.; Bochnowski, W.; Dziedzic, A.; Szyller, Ł.; Adamiak, D. Characteristics of the Structure, Mechanical, and Tribological Properties of a Mo-Mo2N Nanocomposite Coating Deposited on the Ti6Al4V Alloy by Magnetron Sputtering. Materials 2021, 14, 6819. [Google Scholar] [CrossRef]
- Klekotka, M.; Zielińska, K.; Stankiewicz, A.; Kuciej, M. Tribological and Anticorrosion Performance of Electroplated Zinc Based Nanocomposite Coatings. Coatings 2020, 10, 594. [Google Scholar] [CrossRef]
- Konovalova, O.; Suchanek, J. Significance of Polymer Nanocomposites in Tribo engineering Systems. In Proceedings of the 4th International Conference NANOCON, Brno, Czech Republic, 23–25 October 2012. [Google Scholar]
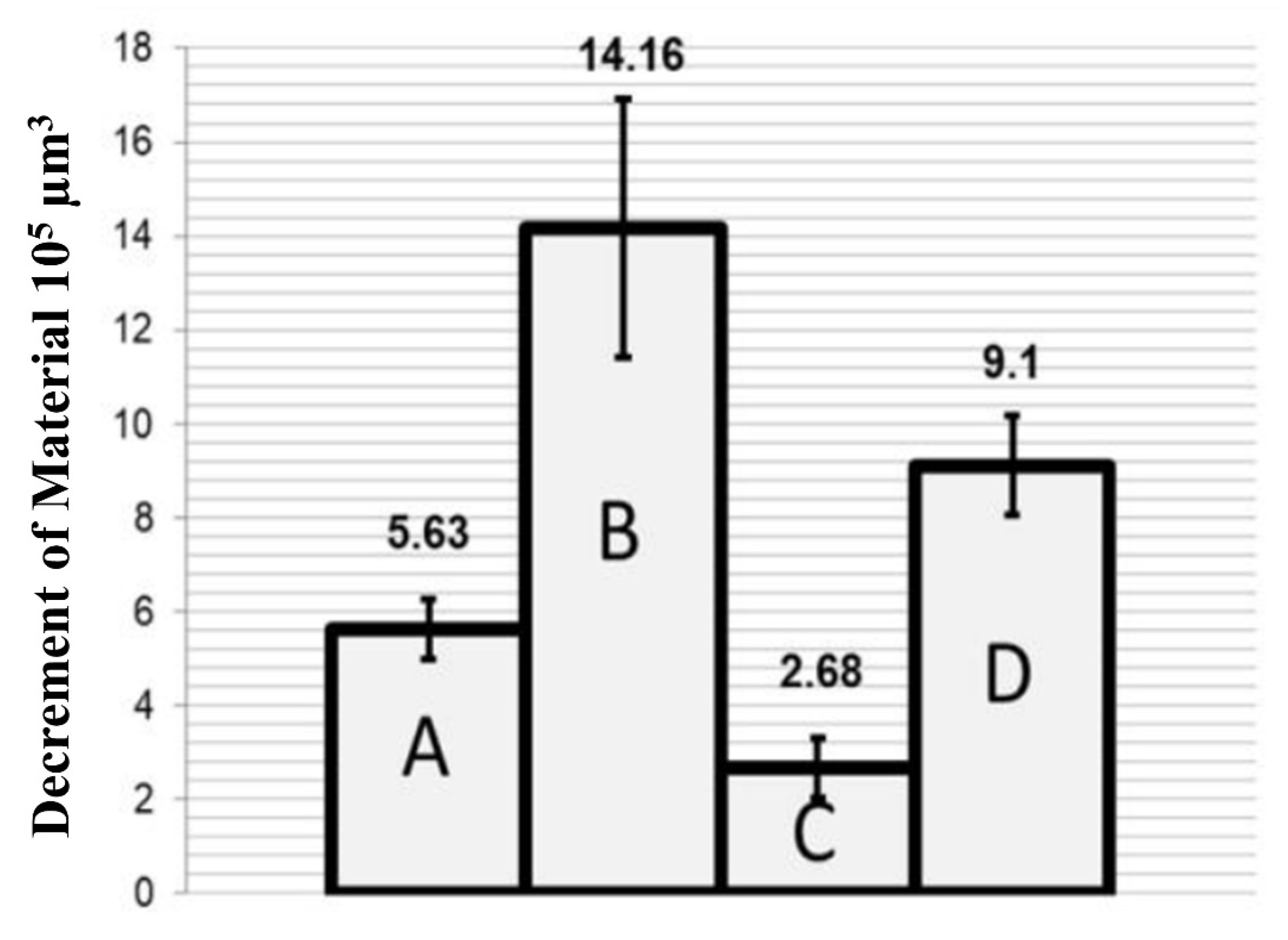
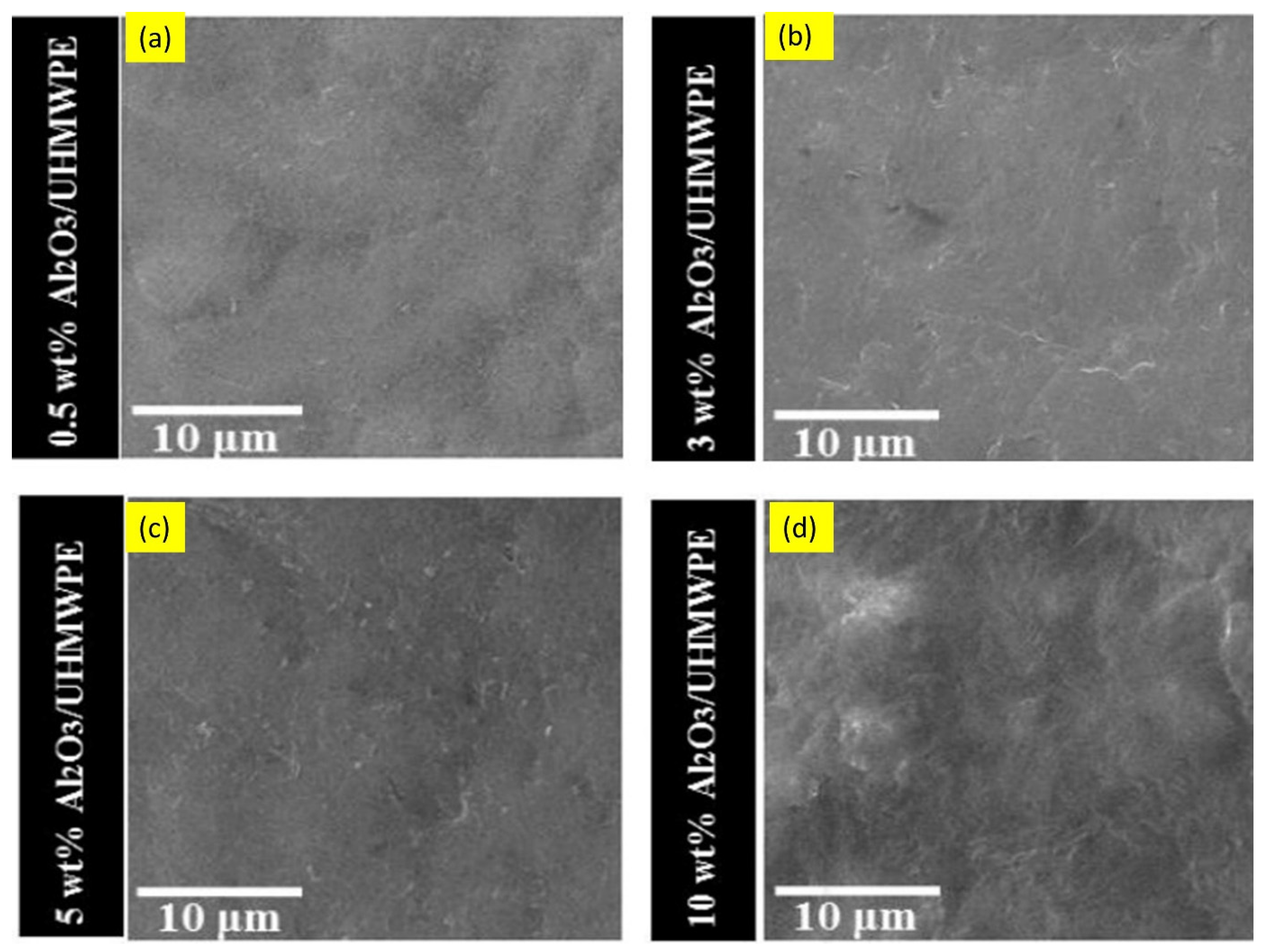
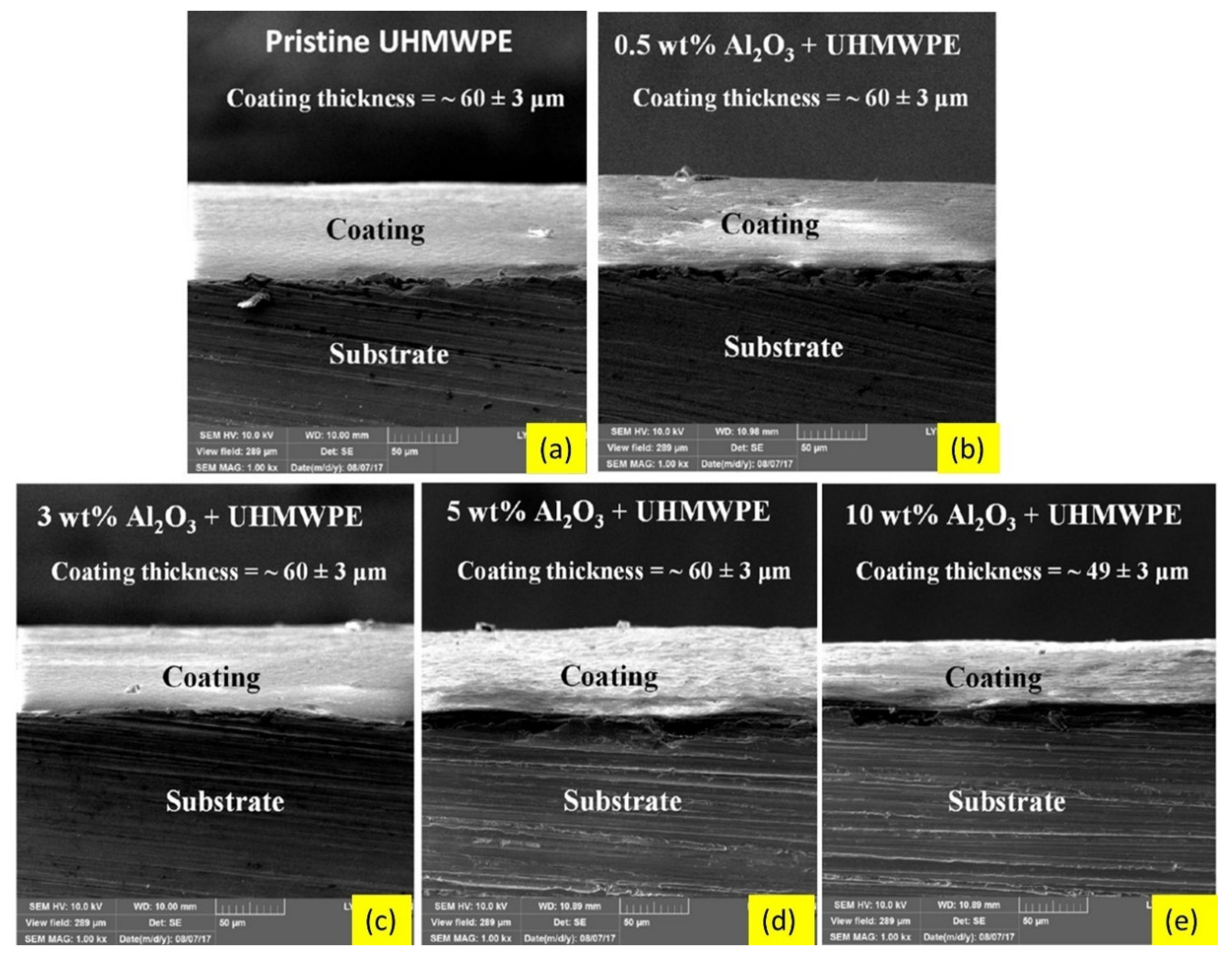
- Mohammed, A.S. UHMWPE Nanocomposite Coatings Reinforced with Alumina (Al2O3) Nanoparticles for Tribological Applications. Coatings 2018, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Devaneyan, S.P.; Pushpanathan, D.P.; Senthilvelan, T.; Ganesh, R. Enhanced Corrosion and Wear Behavior of Nano Titanium Carbide Reinforced Polyurethane PMC Coating on Aluminium 7075. Mater. Today Proc. 2018, 5, 11491–11497. [Google Scholar] [CrossRef]
- Dutta Majumdar, J.; Manna, I. Mechanical properties of a laser-surface-alloyed magnesium-based alloy (AZ91) with nickel. Scr. Mater. 2010, 62, 579–581. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ganesh, P.; Palai, R.; Wu, J.A.; Kaul, R.; Dutta Majumdar, J.; Roy Choudhury, A. Effect of h-BN addition on the properties of nanostructured Al2O3–TiB2–TiN based coatings developed by combined SHS and laser surface alloying. Surf. Coat. Technol. 2010, 204, 1702–1709. [Google Scholar] [CrossRef]
- Chatterjee, S.; Majumdar, J.D.; Singaiah, K.; Shariff, S.M.; Padmanabham, G.; Choudhury, A.R. Performance evaluation of laser surface alloyed hard nanostructured Al2O3–TiB2–TiN composite coatings with in-situ and ex-situ reinforcements. Surf. Coat. Technol. 2011, 205, 3478–3484. [Google Scholar] [CrossRef]
- Wang, L.; Yan, D.; Dong, Y.; Zhang, J.; Chen, X. Nanostructured ceramic composite coating prepared by reactive plasma spraying micro-sized Al–Fe2O3 composite powders. Ceram. Int. 2013, 39, 2437–2442. [Google Scholar] [CrossRef]
- Higdon, C.; Cook, B.; Harringa, J.; Russell, A.; Goldsmith, J.; Qu, J.; Blau, P. Friction and wear mechanisms in AlMgB14-TiB2 nanocoatings. Wear 2011, 271, 2111–2115. [Google Scholar] [CrossRef]
- Muneer, S.M.; Nadeera, M. Wear characterization and microstructure evaluation of silicon carbide based nano composite coating using plasma spraying. Mater. Today Proc. 2018, 5, 23834–23843. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H. Effects of Current Frequency on the Microstructure and Wear Resistance of Ceramic Coatings Embedded with SiC Nano-particles Produced by Micro-arc Oxidation on AZ91D Magnesium Alloy. J. Mater. Sci. Technol. 2010, 26, 865–871. [Google Scholar] [CrossRef]
- Atapour, M.; Blawert, C.; Zheludkevich, M.L. The wear characteristics of CeO2 containing nanocomposite coating made by aluminate-based PEO on AM 50 magnesium alloy. Surf. Coat. Technol. 2019, 357, 626–637. [Google Scholar] [CrossRef]
- Paiva, J.M.; Fox-Rabinovich, G.; Junior, E.L.; Stolf, P.; Ahmed, Y.S.; Martins, M.M.; Bork, C.; Veldhuis, S. Tribological and Wear Performance of Nanocomposite PVD Hard Coatings Deposited on Aluminum Die Casting Tool. Materials 2018, 11, 358. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B. Biomimetics: Lessons from nature—An overview. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, E.; Kim, D.-H.; Lee, L.P. Handbook of Biomimetics and Bioinspiration: Biologically-Driven Engineering of Materials, Processes, Devices, and Systems; World Scientific: Singapore, 2014. [Google Scholar]
- Samaha, M.A.; Gad-el-Hak, M. Polymeric slippery coatings: Nature and applications. Polymers 2014, 6, 1266–1311. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.B.; Hamed, A.M.; Gorumlu, S.; Doosttalab, A.; Aksak, B.; Chamorro, L.P.; Castillo, L. Engineered bio-inspired coating for passive flow control. Proc. Natl. Acad. Sci. USA 2018, 115, 1210–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.M.; Resnick, M.; Flynn, K.; Dwivedi, G.; Sampath, S. Nature inspired, multi-functional, damage tolerant thermal spray coatings. Surf. Coat. Technol. 2016, 297, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Achazi, K.; Liebe, H.; Schulz, A.; Noeske, P.M.; Grunwald, I.; Haag, R. Mussel-inspired dendritic polymers as universal multifunctional coatings. Angew. Chem. Int. Ed. 2014, 53, 11650–11655. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.; Gao, L.; Liu, Y.; Hu, H. Bio-Inspired Icephobic Coatings for Aircraft Icing Mitigation: A Critical Review. Prog. Adhes. Adhes. 2021, 6, 171–201. [Google Scholar]
- Plamadeala, C.; Gosain, S.R.; Hischen, F.; Buchroithner, B.; Puthukodan, S.; Jacak, J.; Bocchino, A.; Whelan, D.; O’Mahony, C.; Baumgartner, W. Bio-inspired microneedle design for efficient drug/vaccine coating. Biomed. Microdevices 2020, 22, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.A.; Siyuan, L.; Satyanarayana, N.; Kustandi, T.S.; Sinha, S.K. Bio-inspired polymeric patterns with enhanced wear durability for microsystem applications. Mater. Sci. Eng. C 2011, 31, 1577–1583. [Google Scholar] [CrossRef]
- Xu, J.; Cai, Q.; Lian, Z.; Yu, Z.; Ren, W.; Yu, H. Research progress on corrosion resistance of magnesium alloys with bio-inspired water-repellent properties: A review. J. Bionic Eng. 2021, 18, 735–763. [Google Scholar] [CrossRef]
- Yang, X.; Tian, L.; Wang, W.; Fan, Y.; Sun, J.; Zhao, J.; Ren, L. Bio-inspired superhydrophobic self-healing surfaces with synergistic anticorrosion performance. J. Bionic Eng. 2020, 17, 1196–1208. [Google Scholar] [CrossRef]
- Chang, K.-C.; Ji, W.-F.; Lai, M.-C.; Hsiao, Y.-R.; Hsu, C.-H.; Chuang, T.-L.; Wei, Y.; Yeh, J.-M.; Liu, W.-R. Synergistic effects of hydrophobicity and gas barrier properties on the anticorrosion property of PMMA nanocomposite coatings embedded with graphene nanosheets. Polym. Chem. 2013, 5, 1049–1056. [Google Scholar] [CrossRef]
- Xiang, T.; Zheng, S.; Zhang, M.; Sadig, H.R.; Li, C. Bioinspired slippery zinc phosphate coating for sustainable corrosion protection. ACS Sustain. Chem. Eng. 2018, 6, 10960–10968. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, H.; Wang, L.; Xue, Q. Bioinspired ultrathin graphene nanosheets sandwiched between epoxy layers for high performance of anticorrosion coatings. Chem. Eng. J. 2021, 410, 128301. [Google Scholar] [CrossRef]
- Cui, M.; Wang, P.-Y.; Wang, Z.; Wang, B. Mangrove inspired anti-corrosion coatings. Coatings 2019, 9, 725. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.K.; Behmer, S.T.; Marquess, R.; Yegin, C.; Scholar, E.A.; Akbulut, M. Structural, tribological, and mechanical properties of the hind leg joint of a jumping insect: Using katydids to inform bioinspired lubrication systems. Acta Biomater. 2017, 62, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.M.; Waldhauser, W.; Major, L.; Teichert, C.; Hartmann, P. Tribology of bio-inspired nanowrinkled films on ultrasoft substrates. Comput. Struct. Biotechnol. J. 2013, 6, e201303002. [Google Scholar] [CrossRef] [Green Version]
- Global Nanocoatings Market by Type (Anti-fingerprint, Antimicrobial, Easy-to-Clean & Anti-Fouling, Self-Cleaning {Bionic & Photocatalytic}, Anti-Icing & Deicing, Anticorrosion, Conductive, UV, Abrasion, & Wear Resistant), by Application (Electronics, Energy, Food & Packaging, Construction, Marine Industry, Military & Defense, Automotive, Aerospace, Healthcare)-Global Opportunity Analysis and Industry Forecast, 2020–2030. Available online: https://www.vantagemarketresearch.com/industry-report/nanocoatings-market-1096 (accessed on 28 January 2022).
- Abdel-Karim, R.; Waheed, A.F. Nanocoatings Modern Surface Engineering Treatments; InTech: London, UK, 2013. [Google Scholar]
- Berger, M. Nanotechnology and 3D-Printings 2016. Available online: https://www.nanowerk.com/spotlight/spotid=37541.php (accessed on 28 January 2022).






| No. | Coating Preparation Method | Process Parameters | Coating Material |
|---|---|---|---|
| 1. | CVD
|
| Metallic, Ceramic |
| 2. | PVD
|
| Nanocomposite, Metallic |
| 3. | Spray Coating High-Velocity Oxy-Fuel (HVOF) |
| Ceramic, Metallic Nanocomposite |
| 4. | Sol-Gel Process |
| Polymer Matrix Nanocomposite |
| 5. | Electrodeposition
|
| Metallic, Metallic Matrix Nanocomposite |
| 6. | Laser Cladding |
| Metallic, Ceramic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, S.A.; Raina, A.; Mohan, S.; Arvind Singh, R.; Jayalakshmi, S.; Irfan Ul Haq, M. Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance. Nanomaterials 2022, 12, 1323. https://doi.org/10.3390/nano12081323
Farooq SA, Raina A, Mohan S, Arvind Singh R, Jayalakshmi S, Irfan Ul Haq M. Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance. Nanomaterials. 2022; 12(8):1323. https://doi.org/10.3390/nano12081323
Chicago/Turabian StyleFarooq, Sheikh Aamir, Ankush Raina, Sanjay Mohan, Ramachandra Arvind Singh, Subramanian Jayalakshmi, and Mir Irfan Ul Haq. 2022. "Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance" Nanomaterials 12, no. 8: 1323. https://doi.org/10.3390/nano12081323
APA StyleFarooq, S. A., Raina, A., Mohan, S., Arvind Singh, R., Jayalakshmi, S., & Irfan Ul Haq, M. (2022). Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance. Nanomaterials, 12(8), 1323. https://doi.org/10.3390/nano12081323