Surface-Plasmon-Assisted Growth, Reshaping and Transformation of Nanomaterials
Abstract
:1. Introduction
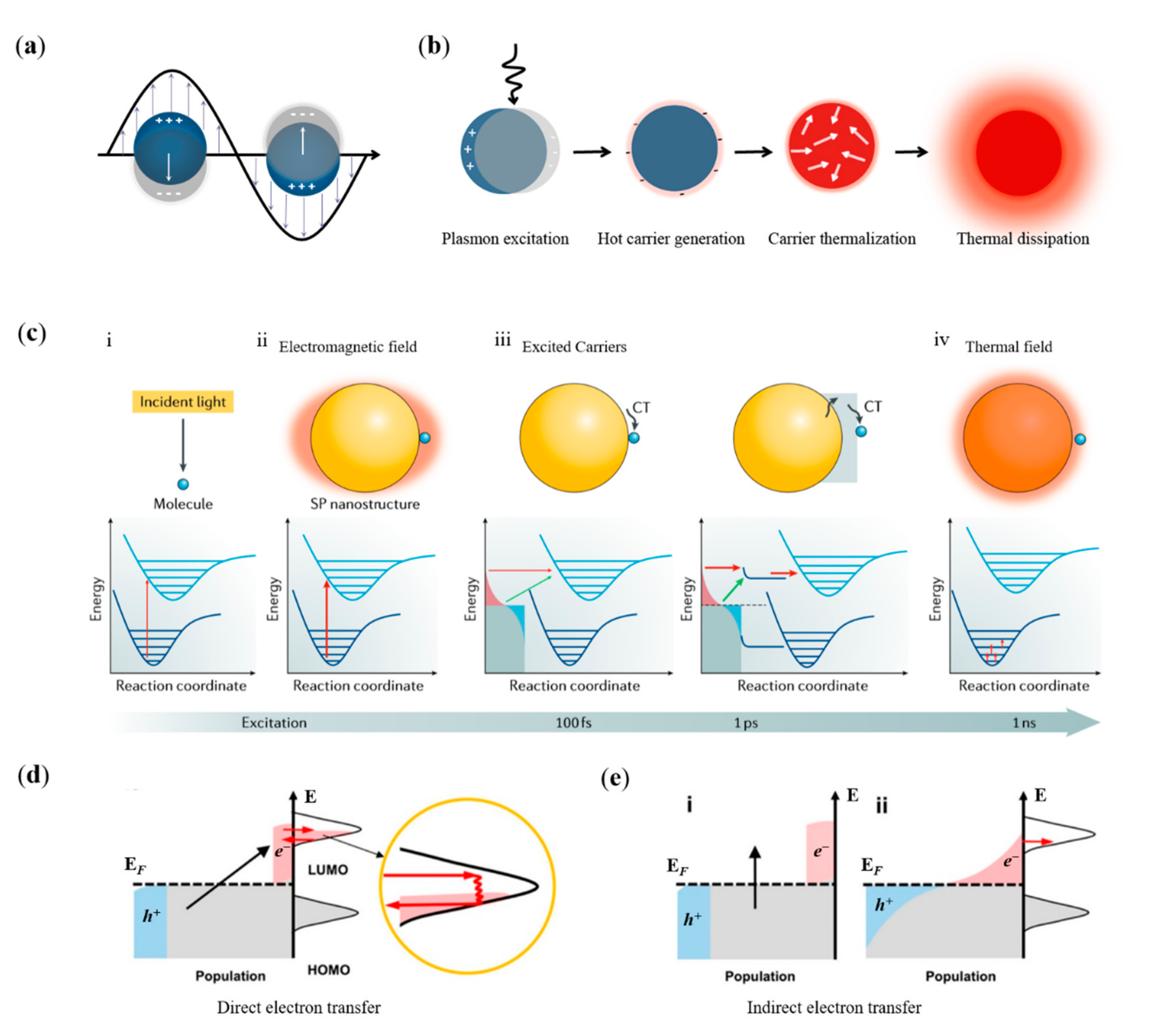
2. Mechanisms of Surface-Plasmon-Modulated Chemical Reaction
2.1. The Catalytic Effect of Plasmonic EM Field
2.2. The Catalytic Effect of Hot Carriers
2.3. The Catalytic Effect of Plasmonic Thermal Field
3. Self-Modulatation of Plasmonic Nanostructure
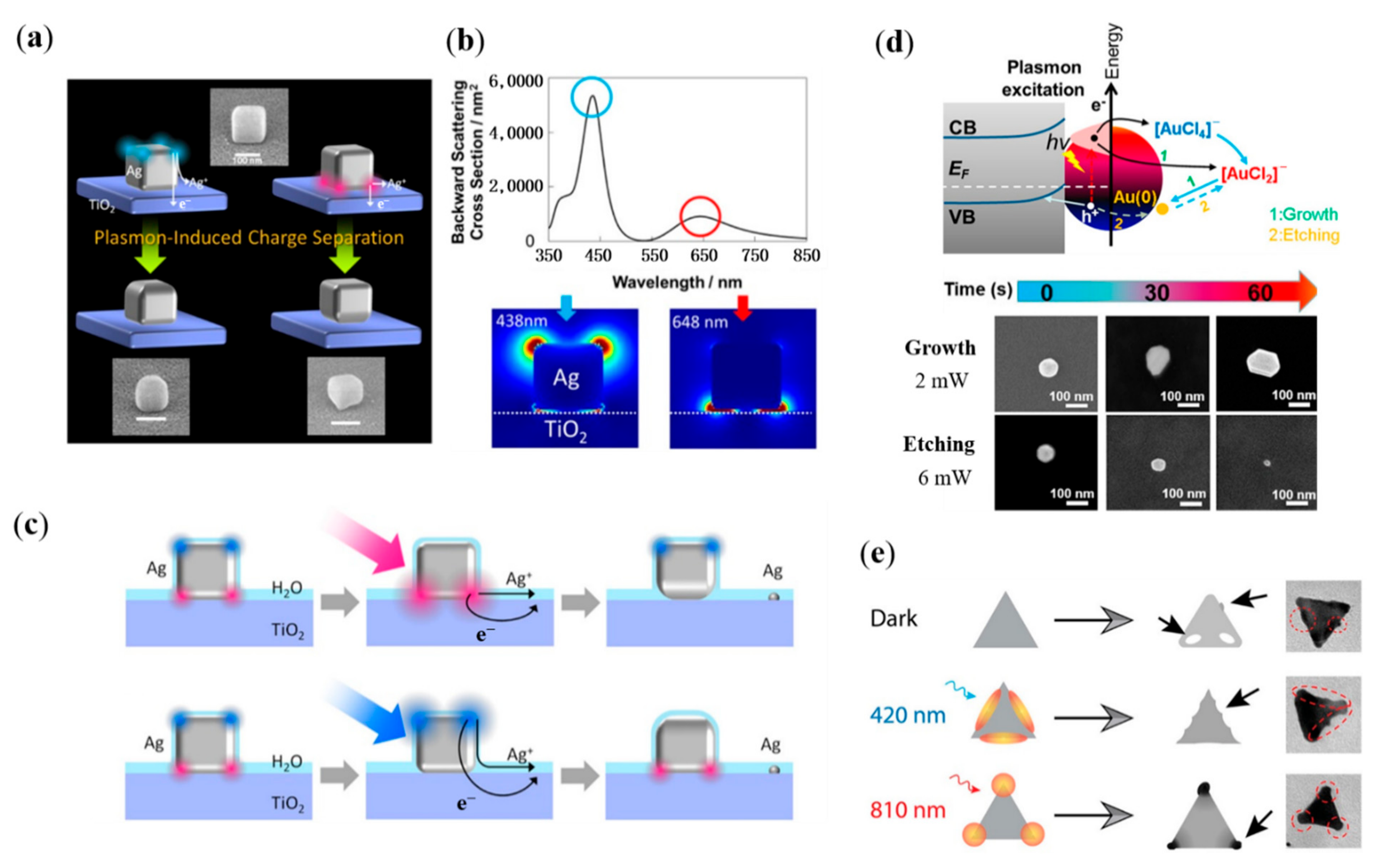
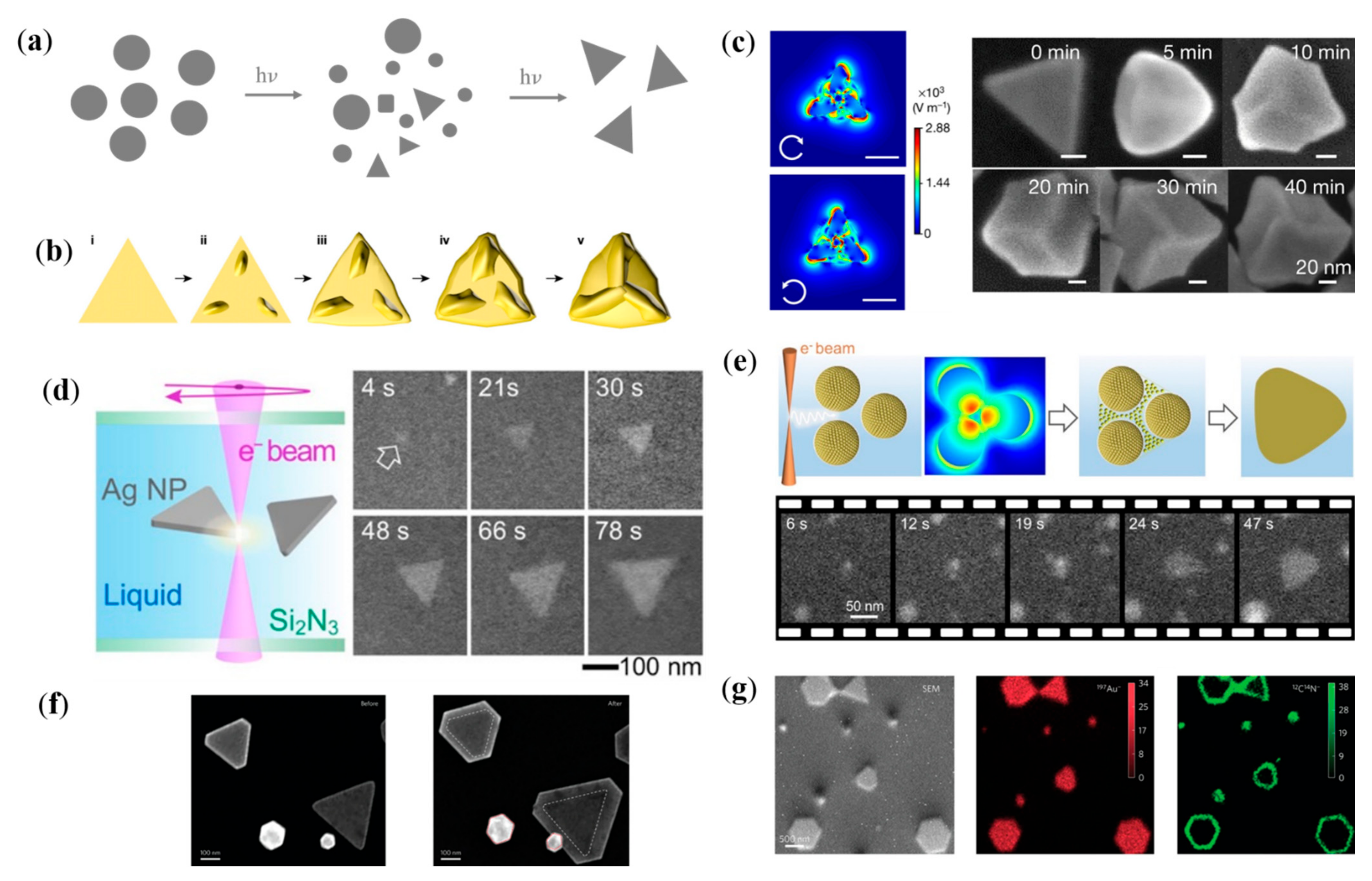
3.1. Plasmonic Thermal-Field-Assisted Reshaping of Plasmonic Nanostructure
3.2. Hot-Electron-Assisted Self-Growth/Etching of Plasmonic Nanostructure
4. Plasmon-Assisted Selective Deposition of Non-Plasmonic Nanomaterials
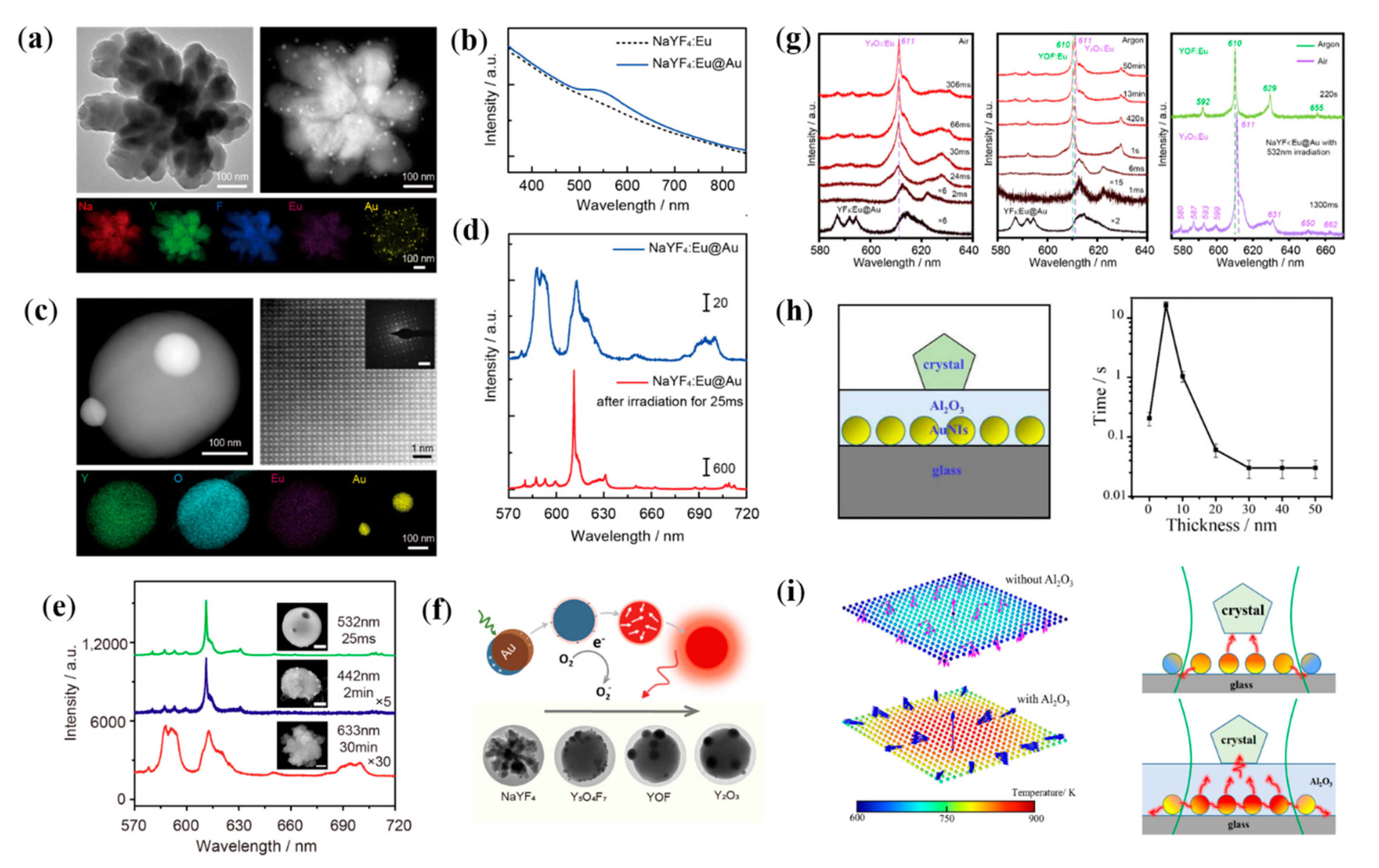
5. Plasmon-Assisted Transformation of Luminescent Nanocrystal
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2017, 118, 2927–2954. [Google Scholar] [CrossRef]
- Misewich, J.A.; Heinz, T.F.; Newns, D.M. Desorption induced by multiple electronic transitions. Phys. Rev. Lett. 1992, 68, 3737–3740. [Google Scholar] [CrossRef]
- Dong, J.; Cao, Y.; Han, Q.; Wang, Y.; Qi, M.; Zhang, W.; Qiao, L.; Qi, J.; Gao, W. Plasmon-exciton coupling for nanophotonic sensing on chip. Opt. Express 2020, 28, 20817–20829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fang, Y.; Wang, W.; Chen, L.; Sun, M. Propagating Surface Plasmon Polaritons: Towards Applications for Remote-Excitation Surface Catalytic Reactions. Adv. Sci. 2016, 3, 1500215. [Google Scholar] [CrossRef] [PubMed]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, X.; Zhou, Q.; Zhu, H. Infrared driven hot electron generation and transfer from non-noble metal plasmonic nanocrystals. Nat. Commun. 2020, 11, 2944. [Google Scholar] [CrossRef]
- Govorov, A.O.; Richardson, H.H. Generating heat with metal nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; Javier Garcia de Abajo, F. Nanoscale Control of Optical Heating in Complex Plasmonic Systems. ACS Nano 2010, 4, 709–716. [Google Scholar] [CrossRef]
- Marcos, M.; Alvarez, J.T.K.; Schaaff, T.G.; Marat, N.; Shafigullin, I.V.; Robert, L. Whetten Optical Absorption Spectra of Nanocrystal Gold Molecules. J. Phys. Chem. B 1997, 101, 3706–3712. [Google Scholar]
- Ding, S.; Yi, J.; Li, J.; Ren, B.; Wu, D.; Panneerselvam, R.; Tian, Z. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Z.; Zheng, H.; Sun, M. Recent Progress on Plasmon-Enhanced Fluorescence. Nanophotonics 2015, 4, 472–490. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhang, M.; Zhao, L.B.; Feng, J.M.; Wu, D.Y.; Ren, B.; Tian, Z.Q. Activation of oxygen on gold and silver nanoparticles assisted by surface plasmon resonances. Angew. Chem. Int. Ed. Engl. 2014, 53, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Aslam, U.; Chavez, S.; Linic, S. Controlling energy flow in multimetallic nanostructures for plasmonic catalysis. Nat. Nanotechnol. 2017, 12, 1000–1005. [Google Scholar] [CrossRef]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct Photocatalysis by Plasmonic Nanostructures. ACS Catal. 2013, 4, 116–128. [Google Scholar] [CrossRef]
- Shirhatti, P.R.; Rahinov, I.; Golibrzuch, K.; Werdecker, J.; Geweke, J.; Altschaffel, J.; Kumar, S.; Auerbach, D.J.; Bartels, C.; Wodtke, A.M. Observation of the adsorption and desorption of vibrationally excited molecules on a metal surface. Nat. Chem. 2018, 10, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Schlucker, S. Hot electron-induced reduction of small molecules on photorecycling metal surfaces. Nat. Commun. 2015, 6, 7570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Reish, M.E.; Zhang, D.; Su, N.Q.; Gutierrez, Y.; Moreno, F.; Yang, W.; Everitt, H.O.; Liu, J. Plasmon-Enhanced Catalysis: Distinguishing Thermal and Nonthermal Effects. Nano Lett. 2018, 18, 1714–1723. [Google Scholar] [CrossRef]
- Zhan, C.; Chen, X.-J.; Yi, J.; Li, J.-F.; Wu, D.-Y.; Tian, Z.-Q. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2018, 2, 216–230. [Google Scholar] [CrossRef]
- Wang, S.; Ding, T. Photothermal-Assisted Optical Stretching of Gold Nanoparticles. ACS Nano 2019, 13, 32–37. [Google Scholar] [CrossRef]
- Novo, C.; Funston, A.M.; Mulvaney, P. Direct observation of chemical reactions on single gold nanocrystals using surface plasmon spectroscopy. Nat. Nanotechnol. 2008, 3, 598–602. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot electrons do the impossible: Plasmon-induced dissociation of H2 on Au. Nano Lett. 2013, 13, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Koichi, A.; Makoto, F.; Carsten, R.; Junji, T.; Hirotaka, M.; Yoshimichi, O.; Naoya, Y.; Toshiya, W. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar]
- Liu, Z.W.; Hou, W.B.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon Resonant Enhancement of Photocatalytic Water Splitting Under Visible Illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef]
- Tesema, T.E.; Kafle, B.; Tadesse, M.G.; Habteyes, T.G. Plasmon-Enhanced Resonant Excitation and Demethylation of Methylene Blue. J. Phys. Chem. C 2017, 121, 7421–7428. [Google Scholar] [CrossRef]
- Kazuma, E.; Jung, J.; Ueba, H.; Trenary, M.; Kim, Y. Direct Pathway to Molecular Photodissociation on Metal Surfaces Using Visible Light. J. Am. Chem. Soc. 2017, 139, 3115–3121. [Google Scholar] [CrossRef]
- Zhang, Z.; Deckert-Gaudig, T.; Deckert, V. Label-free monitoring of plasmonic catalysis on the nanoscale. Analyst 2015, 140, 4325–4335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Richard-Lacroix, M.; Deckert, V. Plasmon induced polymerization using a TERS approach: A platform for nanostructured 2D/1D material production. Faraday Discuss. 2017, 205, 213–226. [Google Scholar] [CrossRef]
- Foerster, B.; Joplin, A.; Kaefer, K.; Celiksoy, S.; Link, S.; Sonnichsen, C. Chemical Interface Damping Depends on Electrons Reaching the Surface. ACS Nano 2017, 11, 2886–2893. [Google Scholar] [CrossRef]
- Cortés, E. Activating plasmonic chemistry. Science 2018, 362, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Chavez, S.; Elias, R. Flow and extraction of energy and charge carriers in hybrid plasmonic nanostructures. Nat. Mater. 2021, 20, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Najmaei, S.; Liu, Z.; Bao, Y.J.; Wang, Y.M.; Zhu, X.; Halas, N.J.; Nordlander, P.; Ajayan, P.M.; Lou, J.; et al. Plasmonic Hot Electron Induced Structural Phase Transition in a MoS2 Monolayer. Adv. Mater. 2014, 26, 6467–6471. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.J.; Christopher, P. Plasmons at the interface. Science 2015, 349, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Chen, J.; McBride, J.R.; Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [Green Version]
- Foerster, B.; Spata, V.A.; Carter, E.A.; Sönnichsen, C.; Link, S.J.S.A. Plasmon damping depends on the chemical nature of the nanoparticle interface. Sci. Adv. 2019, 5, eaav0704. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Hsu, Y.K.; Popescu, R.; Gerthsen, D.; Lin, Y.G.; Feldmann, C. Au@Nb@H x K1-xNbO3 nanopeapods with near-infrared active plasmonic hot-electron injection for water splitting. Nat. Commun. 2018, 9, 232. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, S.; Sun, Y. Quantum-Sized Metal Catalysts for Hot-Electron-Driven Chemical Transformation. Adv. Mater. 2018, 30, e1802082. [Google Scholar] [CrossRef]
- Kim, Y.; Smith, J.G.; Jain, P.K. Harvesting multiple electron-hole pairs generated through plasmonic excitation of Au nanoparticles. Nat. Chem. 2018, 10, 763–769. [Google Scholar] [CrossRef]
- Manjavacas, A.; Liu, J.G.; Kulkarni, V.; Nordlander, P. Plasmon-Induced Hot Carriers in Metallic Nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef]
- Tagliabue, G.; DuChene, J.S.; Abdellah, M.; Habib, A.; Gosztola, D.J.; Hattori, Y.; Cheng, W.H.; Zheng, K.; Canton, S.E.; Sundararaman, R.; et al. Ultrafast hot-hole injection modifies hot-electron dynamics in Au/p-GaN heterostructures. Nat. Mater. 2020, 19, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Cullen, D.A.; Xie, Z.; Lian, T. Efficient Hot Electron Transfer from Small Au Nanoparticles. Nano Lett. 2020, 20, 4322–4329. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Zhou, L.; Goodman, A.M.; Large, N.; Ayala-Orozco, C.; Zhang, Y.; Nordlander, P.; Halas, N.J. Hot-electron-induced dissociation of H2 on gold nanoparticles supported on SiO2. J. Am. Chem. Soc. 2014, 136, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, H.; Schoen, D.; Brongersma, M.L. Hot-electron photodetection with a plasmonic nanostripe antenna. Nano Lett. 2014, 14, 1374–1380. [Google Scholar] [CrossRef]
- Heilpern, T.; Manjare, M.; Govorov, A.O.; Wiederrecht, G.P.; Gray, S.K.; Harutyunyan, H. Determination of hot carrier energy distributions from inversion of ultrafast pump-probe reflectivity measurements. Nat. Commun. 2018, 9, 1853. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Rossi, T.P.; Erhart, P.; Kuisma, M. Hot-Carrier Generation in Plasmonic Nanoparticles: The Importance of Atomic Structure. ACS Nano 2020, 14, 9963–9971. [Google Scholar] [CrossRef]
- Takami, A.; Kurita, H.; Koda, S. Laser-Induced Size Reduction of Noble Metal Particles. J. Phys. Chem. B 1999, 103, 1226–1232. [Google Scholar] [CrossRef]
- Boyer, D.; Tamarat, P.; Maali, A.; Lounis, B.; Orrit, M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science 2002, 297, 1160–1163. [Google Scholar] [CrossRef]
- Wang, Z.; Horseman, T.; Straub, A.P.; Yip, N.Y.; Li, D.; Elimelech, M.; Lin, S. Pathways and challenges for efficient solar-thermal desalination. Sci. Adv. 2019, 5, eaax0763. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhao, F.; Guo, Y.; Rosenberger, B.; Yu, G. Architecting highly hydratable polymer networks to tune the water state for solar water purification. Sci. Adv. 2019, 5, eaaw5484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Swearer, D.F.; Zhang, C.; Robatjazi, H.; Zhao, H.; Henderson, L.; Dong, L.; Christopher, P.; Carter, E.A.; Nordlander, P. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 2018, 362, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Baffou, G.; Quidant, R. Thermo-plasmonics: Using metallic nanostructures as nano-sources of heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Neumann, O.; Feronti, C.; Neumann, A.D.; Dong, A.; Schell, K.; Lu, B.; Kim, E.; Quinn, M.; Thompson, S.; Grady, N.; et al. Compact solar autoclave based on steam generation using broadband light-harvesting nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 11677–11681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Sundaresan, V.; Willets, K.A. Hot Carriers versus Thermal Effects: Resolving the Enhancement Mechanisms for Plasmon-Mediated Photoelectrochemical Reactions. J. Phys. Chem. C 2018, 122, 5040–5048. [Google Scholar] [CrossRef]
- Aibara, I.; Mukai, S.; Hashimoto, S. Plasmonic-Heating-Induced Nanoscale Phase Separation of Free Poly(N-isopropylacrylamide) Molecules. J. Phys. Chem. C 2016, 120, 17745–17752. [Google Scholar] [CrossRef] [Green Version]
- Ou, W.; Zhou, B.; Shen, J.; Lo, T.W.; Lei, D.; Li, S.; Zhong, J.; Li, Y.Y.; Lu, J. Thermal and Nonthermal Effects in Plasmon-Mediated Electrochemistry at Nanostructured Ag Electrodes. Angew. Chem.-Int. Ed. 2020, 59, 6790–6793. [Google Scholar] [CrossRef]
- Zhan, C.; Liu, B.W.; Huang, Y.F.; Hu, S.; Ren, B.; Moskovits, M.; Tian, Z.Q. Disentangling charge carrier from photothermal effects in plasmonic metal nanostructures. Nat. Commun. 2019, 10, 2671. [Google Scholar] [CrossRef] [Green Version]
- Golubev, A.A.; Khlebtsov, B.N.; Rodriguez, R.D.; Chen, Y.; Zahn, D.R.T. Plasmonic Heating Plays a Dominant Role in the Plasmon-Induced Photocatalytic Reduction of 4-Nitrobenzenethiol. J. Phys. Chem. C 2018, 122, 5657–5663. [Google Scholar] [CrossRef]
- Kamarudheen, R.; Castellanos, G.W.; Kamp, L.P.J.; Clercx, H.J.H.; Baldi, A. Quantifying Photothermal and Hot Charge Carrier Effects in Plasmon-Driven Nanoparticle Syntheses. ACS Nano 2018, 12, 8447–8455. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xia, Q.; Cao, Y.; Min, Q.; Zhang, J.; Chen, Z.; Chen, H.Y.; Zhu, J.J. Imaging the transient heat generation of individual nanostructures with a mechanoresponsive polymer. Nat. Commun. 2017, 8, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, M.; Tang, F.; Zhang, H.; Fu, Y.; Liu, D.; Song, X. Transient Electronic Depletion and Lattice Expansion Induced Ultrafast Bandedge Plasmons. Adv. Sci. 2019, 7, 1902408. [Google Scholar] [CrossRef] [PubMed]
- González-Rubio, G.; Díaz-Núñez, P.; Rivera, A.; Prada, A.; Tardajos, G.; González-Izquierdo, J.; Bañares, L.; Llombart, P.; Macdowell, L.G.; Alcolea, P.M.J.S. Femtosecond laser reshaping yields gold nanorods with ultranarrow surface plasmon resonances. Science 2017, 358, 640. [Google Scholar] [CrossRef] [PubMed]
- Babynina, A.; Fedoruk, M.; Kuehler, P.; Meledin, A.; Doeblinger, M.; Lohmueller, T. Bending Gold Nanorods with Light. Nano Lett. 2016, 16, 6485–6490. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, L.O.; Valev, V.K.; Tserkezis, C.; Barnard, J.S.; Kasera, S.; Scherman, O.A.; Aizpurua, J.; Baumberg, J.J. Threading plasmonic nanoparticle strings with light. Nat. Commun. 2014, 5, 4568. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Rubio, G.; Gonzalez-Izquierdo, J.; Banares, L.; Tardajos, G.; Rivera, A.; Altantzis, T.; Bals, S.; Pena-Rodriguez, O.; Guerrero-Martinez, A.; Liz-Marzan, L.M. Femtosecond Laser-Controlled Tip-to-Tip Assembly and Welding of Gold Nanorods. Nano Lett. 2015, 15, 8282–8288. [Google Scholar] [CrossRef]
- Zijlstra, P.; Chon, J.W.; Gu, M. Five-dimensional optical recording mediated by surface plasmons in gold nanorods. Nature 2009, 459, 410–413. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, F.; Li, Z.; Huang, X.; Lu, G. Plasmon-generated hot holes for chemical reactions. Nano Res. 2020, 13, 3183–3197. [Google Scholar] [CrossRef]
- Ma, X.C.; Dai, Y.; Yu, L.; Huang, B.B. Energy transfer in plasmonic photocatalytic composites. Light Sci. Appl. 2016, 5, e16017. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kazuma, E. Mechanistic studies of plasmon chemistry on metal catalysts. Angew. Chem. Int. Ed. Engl. 2018, 58, 4800–4808. [Google Scholar]
- Saito, K.; Tanabe, I.; Tatsuma, T. Site-Selective Plasmonic Etching of Silver Nanocubes. J. Phys. Chem. Lett. 2016, 7, 4363–4368. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, S.; Wang, Y.; Deng, F.; Ding, T. Light-Directed Growth/Etching of Gold Nanoparticles via Plasmonic Hot Carriers. J. Phys. Chem. C 2020, 124, 19212–19218. [Google Scholar] [CrossRef]
- Bhanushali, S.; Mahasivam, S.; Ramanathan, R.; Singh, M.; Harrop Mayes, E.L.; Murdoch, B.J.; Bansal, V.; Sastry, M. Photomodulated Spatially Confined Chemical Reactivity in a Single Silver Nanoprism. ACS Nano 2020, 14, 11100–11109. [Google Scholar] [CrossRef]
- Jin, R.C.; Cao, Y.W.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Personick, M.L.; Langille, M.R.; Zhang, J.; Wu, J.; Li, S.; Mirkin, C.A. Plasmon-mediated synthesis of silver cubes with unusual twinning structures using short wavelength excitation. Small 2013, 9, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Wang, W.; Sun, M.; Choi, W.J.; Kim, J.Y.; Hao, C.; Li, S.; Qu, A.; Lu, M.; et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 2022, 601, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Sutter, P.; Li, Y.; Argyropoulos, C.; Sutter, E. In Situ Electron Microscopy of Plasmon-Mediated Nanocrystal Synthesis. J. Am. Chem. Soc. 2017, 139, 6771–6776. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Li, Y.; Zhang, B.; Argyropoulos, C.; Sutter, P.; Sutter, E. Plasmonic Effects on the Growth of Ag Nanocrystals in Solution. Langmuir 2020, 36, 2044–2051. [Google Scholar] [CrossRef]
- Zhai, Y.; DuChene, J.S.; Wang, Y.C.; Qiu, J.; Johnston-Peck, A.C.; You, B.; Guo, W.; DiCiaccio, B.; Qian, K.; Zhao, E.W.; et al. Polyvinylpyrrolidone-induced anisotropic growth of gold nanoprisms in plasmon-driven synthesis. Nat. Mater. 2016, 15, 889–895. [Google Scholar] [CrossRef]
- Cao, L.; Barsic, D.N.; Guichard, A.R.; Brongersma, M.L. Plasmon-assisted local temperature control to pattern individual semiconductor nanowires and carbon nanotubes. Nano Lett. 2007, 7, 3523–3527. [Google Scholar] [CrossRef]
- Boyd, D.A.; Greengard, L.; Brongersma, M.; El-Naggar, M.Y.; Goodwin, D.G. Plasmon-Assisted Chemical Vapor Deposition. Nano Lett. 2006, 6, 2592–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, H.M.L.; Kundrat, F.; Bermudez-Urena, E.; Rigneault, H.; Monneret, S.; Quidant, R.; Polleux, J.; Baffou, G. Light-Assisted Solvothermal Chemistry Using Plasmonic Nanoparticles. ACS Omega 2016, 1, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Suzuki, T.; Pin, C.; Sasaki, K. Localized ZnO Growth on a Gold Nanoantenna by Plasmon-Assisted Hydrothermal Synthesis. Nano Lett. 2020, 20, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Forcherio, G.T.; Baker, D.R.; Boltersdorf, J.; Leff, A.C.; McClure, J.P.; Grew, K.N.; Lundgren, C.A. Targeted Deposition of Platinum onto Gold Nanorods by Plasmonic Hot Electrons. J. Phys. Chem. C 2018, 122, 28901–28909. [Google Scholar] [CrossRef]
- Robatjazi, H.; Lou, M.; Clark, B.D.; Jacobson, C.R.; Swearer, D.F.; Nordlander, P.; Halas, N.J. Site-Selective Nanoreactor Deposition on Photocatalytic Al Nanocubes. Nano Lett. 2020, 20, 4550–4557. [Google Scholar] [CrossRef]
- Habib, A.; King, M.E.; Etemad, L.L.; Distler, M.E.; Morrissey, K.H.; Personick, M.L. Plasmon-Mediated Synthesis of Hybrid Silver–Platinum Nanostructures. J. Phys. Chem. C 2020, 124, 6853–6860. [Google Scholar] [CrossRef]
- Dong, J.; Gao, W.; Han, Q.; Wang, Y.; Qi, J.; Yan, X.; Sun, M. Plasmon-enhanced upconversion photoluminescence: Mechanism and application. Rev. Phys. 2019, 4, 100026. [Google Scholar] [CrossRef]
- Chen, H.; Sun, M.; Ma, J.; Zhang, B.; Wang, C.; Guo, L.; Ding, T.; Zhang, Z.; Zheng, H.; Xu, H. Multiplasmons-Pumped Excited-State Absorption and Energy Transfer Upconversion of Rare-Earth-Doped Luminescence beyond the Diffraction Limit. ACS Photonics 2021, 8, 1335–1343. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Jin, N.; Dong, L.; Fu, Z.; Zhang, Z.; Zheng, H. Plasmon-Driven Rapid In Situ Formation of Luminescence Single Crystal Nanoparticle. Small 2019, 15, 1901286. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, T.; Fu, Z.; Zhang, Z.; Zheng, H. Hot electron and thermal effects in plasmonic catalysis of nanocrystal transformation. Nanoscale 2020, 12, 8768–8774. [Google Scholar] [CrossRef]
- Kong, T.; Zhang, C.; Lu, J.; Kang, B.; Fu, Z.; Li, J.; Yan, L.; Zhang, Z.; Zheng, H.; Xu, H. An enhanced plasmonic photothermal effect for crystal transformation by a heat-trapping structure. Nanoscale 2021, 13, 4585–4591. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, C.; Yan, L.; Zhang, B.; Chen, H.; Mi, X.; Fu, Z.; Zhang, Z.; Zheng, H. Quantifying plasmon resonance and interband transition contributions in photocatalysis of gold nanoparticle. Chin. Phys. B 2021, 30, 077301. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Qi, J.; Li, Y.; Han, Q.; Gao, W.; Wang, Y.; Dong, J. Surface-Plasmon-Assisted Growth, Reshaping and Transformation of Nanomaterials. Nanomaterials 2022, 12, 1329. https://doi.org/10.3390/nano12081329
Zhang C, Qi J, Li Y, Han Q, Gao W, Wang Y, Dong J. Surface-Plasmon-Assisted Growth, Reshaping and Transformation of Nanomaterials. Nanomaterials. 2022; 12(8):1329. https://doi.org/10.3390/nano12081329
Chicago/Turabian StyleZhang, Chengyun, Jianxia Qi, Yangyang Li, Qingyan Han, Wei Gao, Yongkai Wang, and Jun Dong. 2022. "Surface-Plasmon-Assisted Growth, Reshaping and Transformation of Nanomaterials" Nanomaterials 12, no. 8: 1329. https://doi.org/10.3390/nano12081329





