Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application
Abstract
:1. Introduction
2. Nanoparticles and Surface Functionalization
2.1. Interactions Involved in Surface Functionalization
2.2. General Protocols and Material Required for Surface Functionalization
3. Mechanism of Surface Functionalization of NPs
3.1. Surface Modification with Organic Molecules
3.2. Surface Modification with Inorganic Molecules
3.3. Stabilization of Nanoparticles
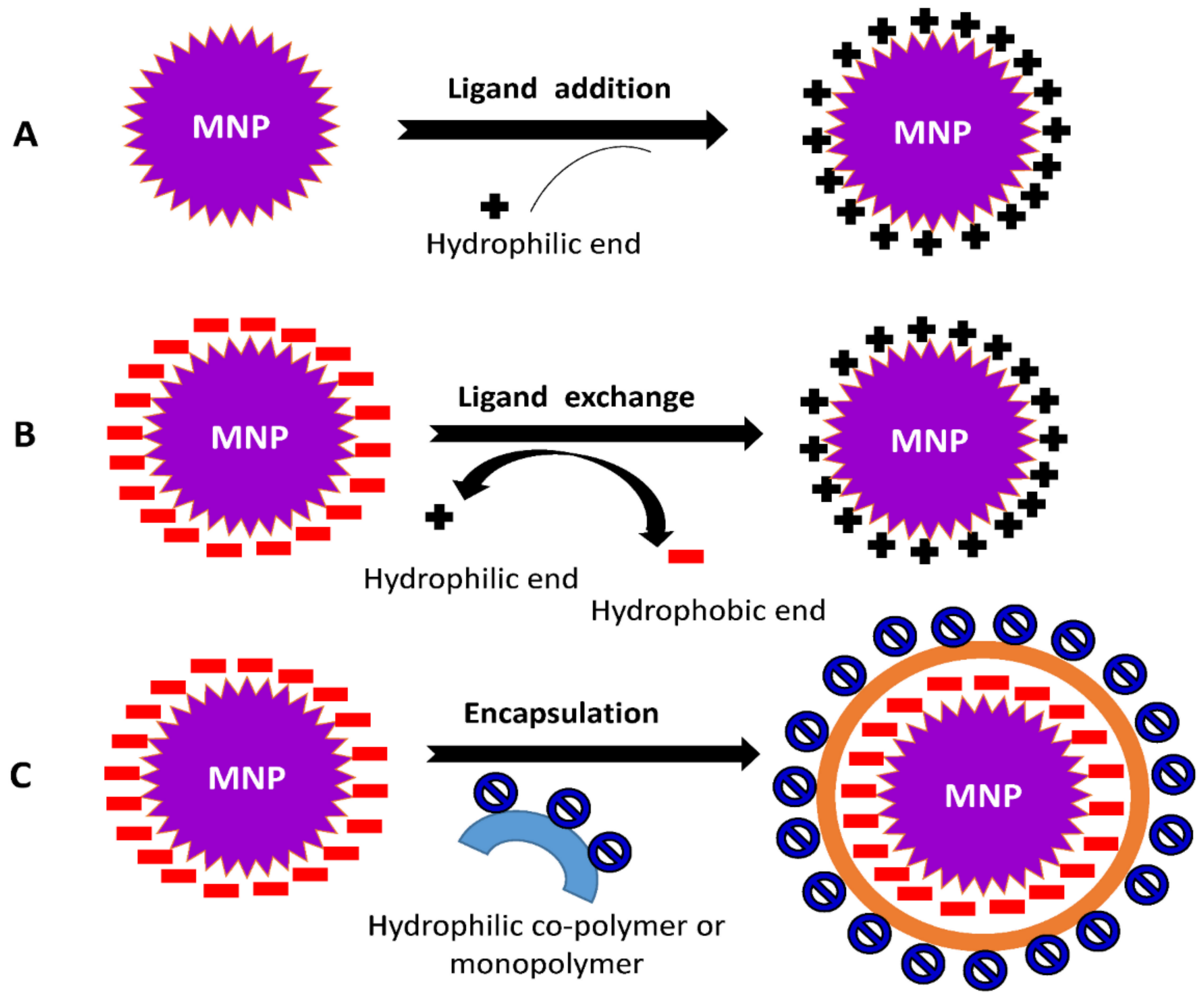
3.4. Ligand Addition
3.5. Ligand Exchange
3.6. Encapsulation
4. A Brief Note on the Unique Properties and Importance of Some Nanoparticles
4.1. Iron Oxide Nanoparticles (IONPs)
4.2. Gold Nanoparticles (AuNPs)
4.3. Platinum Nanoparticles (PtNPs)
4.4. Silver Nanoparticles (AgNPs)
4.5. Silica-Coated Nanoparticle
5. Toxicity of Surface-Functionalized Nanoparticles
6. Application of Surface-Functionalized NPs in Biological Sciences
6.1. Nano-Based Imaging
6.2. Gene Delivery
6.3. Drug Loading
6.4. Immunoassay
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulzer, F.; Orrit, M. Single-Molecule Optics. Annu. Rev. Phys. Chem. 2004, 55, 585–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarafdar, J.C.; Sharma, S.; Raliya, R. Nanotechnology: Interdisciplinary science of applications. Afr. J. Biotechnol. 2013, 12, 219–226. [Google Scholar]
- What Does Nano Mean? Swiss Nanoscience Institute. Available online: https://nanoscience.ch/en/about-us/nanosciences/what-does-nano-mean/ (accessed on 9 February 2022).
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.T.; Kim, K.S. Functionalization of magnetic nanoparticles for biomedical applications. Korean J. Chem. Eng. 2014, 31, 1289–1305. [Google Scholar] [CrossRef]
- Sun, S.N.; Wei, C.; Zhu, Z.Z.; Hou, Y.L.; Venkatraman, S.S.; Xu, Z.C. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 37503. [Google Scholar] [CrossRef]
- Neouze, M.A.; Schubert, U. Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands. Mon. Für Chem. Chem. Mon. 2008, 139, 183–195. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Hirani, N.D.; Natarajan, C. Nanotechnology-Based Medical and Biomedical Imaging for Diagnostics. Emerg. Nanotechnologies Diagn. Drug Deliv. Med. Devices 2017, 355–374. [Google Scholar]
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent trends of the bio-inspired nanoparticles in cancer theranostics. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef]
- Malik, P.; Gupta, R.; Malik, V.; Ameta, R.K. Emerging nanomaterials for improved biosensing. Meas. Sens. 2021, 16, 100050. [Google Scholar] [CrossRef]
- Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Nanotechnology: A New Beginning to Mitigate the Effect of Plant-Parasitic Nematodes. In Innovative Approaches in Diagnosis and Management of Crop Diseases, 1st ed; Singh, R.K., Gopala, Eds.; Apple Academic Press: New York, NY, USA, 2021; Volume 3, pp. 19–43. [Google Scholar]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Tao, Y.; Xue, R.; Song, C.; Wu, Q.; Ren, Y. Continuous-Flow Nanoparticle Trapping Driven by Hybrid Electrokinetics in Microfluidics. Electrophoresis 2021, 42, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Farah, F.; Farah, F.H. Nanocarriers as delivery systems for therapeutics agents. Int. J. Pharm. Sci. Res. 2019, 10, 3487. [Google Scholar]
- Zhu, X.; Vo, C.; Taylor, M.; Smith, B.R. Non-spherical micro- and nanoparticles in nanomedicine. Mater. Horiz. 2019, 6, 1094–1121. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Mironov, V.; Trusk, T.; Kasyanov, V.; Little, S.; Swaja, R.; Markwald, R. Biofabrication: A 21st century manufacturing paradigm. Biofabrication 2009, 1, 22001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.Q.; Courchesne, N.M.D.; Duraj-Thatte, A.; Praveschotinunt, P.; Joshi, N.S. Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials. Adv. Mater. 2018, 30, 1704847. [Google Scholar] [CrossRef] [PubMed]
- Holy, J.; Perkins, E.; Yu, X. Adhesion, proliferation and differentiation of pluripotent stem cells on multi-walled carbon nanotubes. IET Nanobiotechnology 2011, 5, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Pagels, R.F.; Pinkerton, N.M.; York, A.W.; Prud’homme, R.K. Synthesis of Heterobifunctional Thiol-poly(lactic acid)-b-poly(ethylene glycol)-hydroxyl for Nanoparticle Drug Delivery Applications. Macromol. Chem. Phys. 2020, 221, 1900396. [Google Scholar] [CrossRef]
- Nobs, L.; Buchegger, F.; Gurny, R.; Allémann, E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J. Pharm. Sci. 2004, 93, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.C.; Tshikhudo, T.R.; Brust, M.; Fernig, D.G. Extremely Stable Water-Soluble Ag Nanoparticles. Chem. Mater. 2005, 17, 4630–4635. [Google Scholar] [CrossRef]
- Moon, J.H.; Shul, Y.G.; Han, H.S.; Hong, S.Y.; Choi, Y.S.; Kim, H.T. A study on UV-curable adhesives for optical pick-up: I. Photo-initiator effects. Int. J. Adhes. Adhes. 2005, 25, 301–312. [Google Scholar] [CrossRef]
- Kumar, S.; Aziz, S.K.T.; Girshevitz, O.; Nessim, G.D. One-Step Synthesis of N-Doped Graphene Quantum Dots from Chitosan as a Sole Precursor Using Chemical Vapor Deposition. J. Phys. Chem. C 2018, 122, 2343–2349. [Google Scholar] [CrossRef]
- Bteich, J.; McManus, S.A.; Ernsting, M.J.; Mohammed, M.Z.; Prud’Homme, R.K.; Sokoll, K.K. Using Flash Nanoprecipitation to Produce Highly Potent and Stable Cellax Nanoparticles from Amphiphilic Polymers Derived from Carboxymethyl Cellulose, Polyethylene Glycol, and Cabazitaxel. Mol. Pharm. 2017, 14, 3998–4007. [Google Scholar] [CrossRef]
- Li, H.; Lu, Z.; Cheng, G.; Rong, K.; Chen, F.; Chen, R. HEPES-involved hydrothermal synthesis of Fe3O4 nanoparticles and their biological application. RSC Adv. 2015, 5, 5059–5067. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, C.; Liu, C.; Zuo, M.; Qin, L.; Yan, X.; Xing, Y.; Li, H.; Si, R.; Zhou, S.; et al. Electrochemical deposition as a universal route for fabricating single-atom catalysts. Nat. Commun. 2020, 11, 1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmen Bautista, M.; Bomati-Miguel, O.; Del Puerto Morales, M.; Serna, C.J.; Veintemillas-Verdaguer, S. Surface characterisation of dextran-coated iron oxide nanoparticles prepared by laser pyrolysis and coprecipitation. J. Magn. Magn. Mater. 2005, 293, 20–27. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Olvera, M.D.L.L. Synthesis of ZnO nanoparticles from water-in-oil (w/o) microemulsions. Mater. Chem. Phys. 2018, 203, 141–147. [Google Scholar] [CrossRef]
- Ooi, F.; Duchene, J.S.; Qiu, J.; Graham, J.O.; Engelhard, M.H.; Cao, G.; Gai, Z.; Wei, W.D. A Facile Solvothermal Synthesis of Octahedral Fe3O4 Nanoparticles. Small 2015, 11, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, Y.; Ziemann, E.; Be’Er, A.; Bashouti, M.Y.; Elimelech, M.; Bernstein, R. One-step sonochemical synthesis of a reduced graphene oxide–ZnO nanocomposite with antibacterial and antibiofouling properties. Environ. Sci. Nano 2019, 6, 3080–3090. [Google Scholar] [CrossRef]
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V. A Microwave-Assisted Synthesis of Zinc Oxide Nanocrystals Finely Tuned for Biological Applications. Nanomaterials 2019, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Strobel, R.; Pratsinis, S.E. Flame aerosol synthesis of smart nanostructured materials. J. Mater. Chem. 2007, 17, 4743–4756. [Google Scholar] [CrossRef]
- Sibeaud, M.; Croutxé-Barghorn, C.; Rigolet, S.; Michelin, L.; Josien, L.; Vidal, L.; Lebeau, B.; Wörner, M.; Chemtob, A. UV aerosol synthesis: A one-step route to silica, organic-silica and surfactant/silica nanostructured materials. RSC Adv. 2016, 6, 65047–65054. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2009, 110, 2574. [Google Scholar] [CrossRef]
- Ali, N.; Zhang, B.; Zhang, H.; Zaman, W.; Li, W.; Zhang, Q. Key synthesis of magnetic Janus nanoparticles using a modified facile method. Particuology 2014, 17, 59–65. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Sci. Rep. 2020, 10, 10793. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, E.H.; Shao, H.; Kwak, B.K. Synthesis of SPIO-chitosan microspheres for MRI-detectable embolotherapy. J. Magn. Magn. Mater. 2005, 293, 102–105. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Lin, P.C.; Chou, P.H.; Chen, S.H.; Liao, H.K.; Wang, K.Y.; Chen, Y.J.; Lin, C.C.; Lin, P.C.; Chou, P.H.; Chen, S.H.; et al. Ethylene Glycol-Protected Magnetic Nanoparticles for a Multiplexed Immunoassay in Human Plasma. Small 2006, 2, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhou, H.; Wang, Y.; Liu, D.; Li, J.; Deng, H.; Qi, X.; Chen, T.; Zhang, L.M.; Li, G. One-pot synthesis of dextran-coated iron oxide nanoclusters for real-time regional lymph node mapping. Int. J. Nanomed. 2017, 12, 3365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, J.B.; Shao, H.H.; Jing, G.L.; Huang, F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: Preparation, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2013, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Moraes Silva, S.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed Core−Shell Fe3O4@Au Nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef]
- Cheng, S.H.; Lee, C.H.; Chen, M.C.; Souris, J.S.; Tseng, F.G.; Yang, C.S.; Mou, C.Y.; Chen, C.T.; Lo, L.W. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics—The trio of imaging, targeting and therapy. J. Mater. Chem. 2010, 20, 6149–6157. [Google Scholar] [CrossRef]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474–1504. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Chu, X.; Yu, J.; Hou, Y.-L. Surface modification of magnetic nanoparticles in biomedicine. Chin. Phys. B 2015, 24, 14704. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Ovejero, J.G.; Spizzo, F.; Morales, M.P.; Del Bianco, L. Nanoparticles for Magnetic Heating: When Two (or More) Is Better Than One. Materials 2021, 14, 6416. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.W.; Holoman, T.R.; Kofinas, P.; Bentley, W.E. Towards oriented assembly of proteins onto magnetic nanoparticles. Biochem. Eng. J. 2008, 38, 164–170. [Google Scholar] [CrossRef]
- Shivashankarappa, A.; Sanjay, K.R. Escherichia coli-based synthesis of cadmium sulfide nanoparticles, characterization, antimicrobial and cytotoxicity studies. Braz. J. Microbiol. 2020, 51, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Shoudri, R.A. Titanium Oxide (TiO2) Nanoparticles for Treatment of Wound Infection. J. Pure Appl. Microbiol. 2020, 15, 437–451. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Saeri, M.R.; Azizi, M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicol. Environ. Saf. 2015, 120, 400–408. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Farha, O.K.; Hupp, J.T.; Pohl, E.; Yeh, J.I.; Rosi, N.L. Metal-adeninate vertices for the construction of an exceptionally porous metal-organic framework. Nat. Commun. 2012, 3, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annamalai, J.; Nallamuthu, T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Attarde, S.S.; Pandit, S.V. Anticancer potential of nanogold conjugated toxin GNP-NN-32 from Naja naja venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, 20190047. [Google Scholar] [CrossRef] [PubMed]
- Aygun, A.; Gülbagca, F.; Ozer, L.Y.; Ustaoglu, B.; Altunoglu, Y.C.; Baloglu, M.C.; Atalar, M.N.; Alma, M.H.; Sen, F. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J. Pharm. Biomed. Anal. 2020, 179, 112961. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, B.; Muthukumarasamy, A.; Chidambaram, S.; Sugumaran, A.; Ramachandran, K.; Manimuthu, T.R. Cytotoxic potentials of biologically fabricated platinum nanoparticles from Streptomyces sp. on MCF-7 breast cancer cells. IET Nanobiotechnology 2017, 11, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Wang, T.; Zhang, L.; Li, L.; Wang, C. A combination of tri-modal cancer imaging and in vivo drug delivery by metal–organic framework based composite nanoparticles. Biomater. Sci. 2015, 3, 1270–1278. [Google Scholar] [CrossRef]
- Cheng, Z.; Dai, Y.; Kang, X.; Li, C.; Huang, S.; Lian, H.; Hou, Z.; Ma, P.; Lin, J. Gelatin-encapsulated iron oxide nanoparticles for platinum (IV) prodrug delivery, enzyme-stimulated release and MRI. Biomaterials 2014, 35, 6359–6368. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Scheffler, L.; Ebenau, A.; Lyer, S.; Alexiou, C.; Goppelt-Struebe, M. Mitoxantrone-loaded superparamagnetic iron oxide nanoparticles as drug carriers for cancer therapy: Uptake and toxicity in primary human tubular epithelial cells. Nanotoxicology 2016, 10, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold nanoparticles as contrast agents in x-ray imaging and computed tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Zhao, X.; Liu, Y.; Chen, B.; Ding, X.; Zhao, N.; Xu, F.-J.; Dai, X.; Zhao, X.; Liu, Y.; et al. Controlled Synthesis and Surface Engineering of Janus Chitosan-Gold Nanoparticles for Photoacoustic Imaging-Guided Synergistic Gene/Photothermal Therapy. Small 2021, 17, 2006004. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Godugu, D.; Beedu, S.R. Biopolymer-mediated synthesis and characterisation of platinum nanocomposite and its anti-fungal activity against a. parasiticus and a. flavus. Micro Nano Lett. 2018, 13, 1491–1496. [Google Scholar] [CrossRef]
- Elbeshehy, E.K.F.; Elazzazy, A.M.; Aggelis, G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against Bean Yellow Mosaic Virus and human pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.U.R. Biomedical Potential of Plant-Based Selenium Nanoparticles: A Comprehensive Review on Therapeutic and Mechanistic Aspects. Int. J. Nanomed. 2021, 16, 249. [Google Scholar] [CrossRef]
- Jameii, F.; Dalimi Asl, A.; Karimi, M.; Ghaffarifar, F. Healing Effect Comparison of Selenium and Silver Nanoparticles on Skin Leishmanial Lesions in Mice. Avicenna J. Clin. Med. 2015, 22, 217–223. [Google Scholar]
- Javanbakht, T.; Laurent, S.; Stanicki, D.; Wilkinson, K.J. Relating the Surface Properties of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) to Their Bactericidal Effect towards a Biofilm of Streptococcus mutans. PLoS ONE 2016, 11, e0154445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, B.; Ikram, M.; Farooq, F.; Sultana, T.; Mashwani, Z.U.R.; Raja, N.I. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: A mechanistic overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Jebril, S.; Khanfir Ben Jenana, R.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Jin, S.E.; Jin, H.E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Characteristics to Pharmacological Aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Kesse, S.; Boakye-Yiadom, K.O.; Ochete, B.O.; Opoku-Damoah, Y.; Akhtar, F.; Filli, M.S.; Farooq, M.A.; Aquib, M.; Mily, B.J.M.; Murtaza, G.; et al. Mesoporous Silica Nanomaterials: Versatile Nanocarriers for Cancer Theranostics and Drug and Gene Delivery. Pharmaceutics 2019, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- LewisOscar, F.; Nithya, C.; Vismaya, S.; Arunkumar, M.; Pugazhendhi, A.; Nguyen-Tri, P.; Alharbi, S.A.; Alharbi, N.S.; Thajuddin, N. In vitro analysis of green fabricated silver nanoparticles (AgNPs) against Pseudomonas aeruginosa PA14 biofilm formation, their application on urinary catheter. Prog. Org. Coat. 2021, 151, 106058. [Google Scholar] [CrossRef]
- Li, J.; Shen, S.; Kong, F.; Jiang, T.; Tang, C.; Yin, C. Effects of pore size on in vitro and in vivo anticancer efficacies of mesoporous silica nanoparticles. RSC Adv. 2018, 8, 24633–24640. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.L.; Wang, J.P.; Liu, W.C.; Zhuang, X.Y.; Liu, J.Q.; Fan, G.L.; Li, B.H.; Lin, W.N.; Man, J.H. A new (4,8)-connected topological MOF as potential drug delivery. Inorg. Chem. Commun. 2015, 55, 8–10. [Google Scholar] [CrossRef]
- Loan, T.T.; Do, L.T.; Yoo, H. Platinum Nanoparticles Induce Apoptosis on Raw 264.7 Macrophage Cells. J. Nanosci. Nanotechnol. 2017, 18, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Luo, L.; Song, Y.; Zhu, C.; Fu, S.; Shi, Q.; Sun, Y.M.; Jia, B.; Du, D.; Xu, Z.L.; Lin, Y. Fluorescent silicon nanoparticles-based ratiometric fluorescence immunoassay for sensitive detection of ethyl carbamate in red wine. Sens. Actuators B Chem. 2018, 255, 2742–2749. [Google Scholar] [CrossRef]
- Murugan, K.; Benelli, G.; Panneerselvam, C.; Subramaniam, J.; Jeyalalitha, T.; Dinesh, D.; Nicoletti, M.; Hwang, J.S.; Suresh, U.; Madhiyazhagan, P. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp. Parasitol. 2015, 153, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Naseer, A.; Ali, A.; Ali, S.; Mahmood, A.; Kusuma, H.S.; Nazir, A.; Yaseen, M.; Khan, M.I.; Ghaffar, A.; Abbas, M.; et al. Biogenic and eco-benign synthesis of platinum nanoparticles (Pt NPs) using plants aqueous extracts and biological derivatives: Environmental, biological and catalytic applications. J. Mater. Res. Technol. 2020, 9, 9093–9107. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Davaran, S.; Danafar, H.; Manjili, H.K. Methotrexate-conjugated L-lysine coated iron oxide magnetic nanoparticles for inhibition of MCF-7 breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.V.; Huynh, T.C.; Manivasagan, P.; Mondal, S.; Oh, J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poopathi, S.; De Britto, L.J.; Praba, V.L.; Mani, C.; Praveen, M. Synthesis of silver nanoparticles from Azadirachta indica--a most effective method for mosquito control. Environ. Sci. Pollut. Res. Int. 2015, 22, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, A.J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.T.; Yehye, W.A.; Saad, O.; Simarani, K.; Chowdhury, Z.Z.; Alhadi, A.A.; Al-Ani, L.A. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of Silver Nanoparticles and their Biomedical Applications-A Comprehensive Review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Singh, H.; Wang, C.; Hwang, K.H.; Farh, M.E.A.; Yang, D.C. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int. J. Nanomed. 2015, 10, 2567. [Google Scholar]
- Sun, J.; Fan, Y.; Zhang, P.; Zhang, X.; Zhou, Q.; Zhao, J.; Ren, L. Self-enriched mesoporous silica nanoparticle composite membrane with remarkable photodynamic antimicrobial performances. J. Colloid Interface Sci. 2020, 559, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Okinaga, T.; Iwanaga, K.; Matsuo, K.; Toyono, T.; Sasaguri, M.; Ariyoshi, W.; Tominaga, K.; Enomoto, Y.; Matsumura, Y.; et al. Anticancer effect of novel platinum nanocomposite beads on oral squamous cell carcinoma cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Tomah, A.A.; Alamer, I.S.A.; Li, B.; Zhang, J.Z. Mycosynthesis of Silver Nanoparticles Using Screened Trichoderma Isolates and Their Antifungal Activity against Sclerotinia sclerotiorum. Nanomaterials 2020, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, H.; Bao, G. Magnetic iron oxide nanoparticles for disease detection and therapy. Mater. Today 2019, 31, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J. Alloys Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Xu, H.L.; Mao, K.L.; Huang, Y.P.; Yang, J.J.; Xu, J.; Chen, P.P.; Fan, Z.L.; Zou, S.; Gao, Z.Z.; Yin, J.Y.; et al. Glioma-targeted superparamagnetic iron oxide nanoparticles as drug-carrying vehicles for theranostic effects. Nanoscale 2016, 8, 14222–14236. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, Y.; He, Y.; Chen, F.; Gong, Y.; Chen, S.; Xu, Y.; Su, Y.; Wang, C.; Wang, J. Succinylated casein-coated peptide-mesoporous silica nanoparticles as an antibiotic against intestinal bacterial infection. Biomater. Sci. 2019, 7, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Banasiuk, R.; Krychowiak, M.; Swigon, D.; Tomaszewicz, W.; Michalak, A.; Chylewska, A.; Ziabka, M.; Lapinski, M.; Koscielska, B.; Narajczyk, M.; et al. Carnivorous plants used for green synthesis of silver nanoparticles with broad-spectrum antimicrobial activity. Arab. J. Chem. 2020, 13, 1415–1428. [Google Scholar] [CrossRef]
- Zeng, H.J.; Zhao, R.L.; Wang, D.S.; Li, C.X.; Liu, Y.Y. Optical Analysis of the Interaction of Mercaptan Derivatives of Nanogold Particles with Carcinoembryonic Antigen. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 478–481. [Google Scholar]
- Acunto, M.D.; Moroni, D.; Salvetti, O. Nanoscale Biomolecular Detection Limit for Gold Nanoparticles Based on Near-Infrared Response. Adv. Opt. Technol. 2012, 2012, 278194. [Google Scholar]
- DeLong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A.; Ullah, H. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Aghaie, T.; Avan, A.; Vatankhah, A.; Ghaffari, M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens. Bio-Sens. Res. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 1449. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, Y.; Wang, H.; Wilson, R.; Hui, Y.; Yu, L.; Wibowo, D.; Zhang, C.; Whittaker, A.K.; Middelberg, A.P.J.; et al. Bioinspired Core–Shell Nanoparticles for Hydrophobic Drug Delivery. Angew. Chem. Int. Ed. 2019, 58, 14357–14364. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Abedi, A.; Akbarzadeh, A.; Mokhtari, M.J.; Shahmabadi, H.E.; Mehrabi, M.R.; Javadian, S.; Chiani, M. Evaluation of synthesized platinum nanoparticles on the MCF-7 and HepG-2 cancer cell lines. Int. Nano Lett. 2013, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Sawosz, E.; Chwalibog, A.; Szeliga, J.; Sawosz, F.; Grodzik, M.; Rupiewicz, M.; Niemiec, T.; Kacprzyk, K. Visualization of gold and platinum nanoparticles interacting with Salmonella Enteritidis and Listeria monocytogenes. Int. J. Nanomed. 2010, 5, 631. [Google Scholar]
- Hikosaka, K.; Kim, J.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Platinum nanoparticles have an activity similar to mitochondrial NADH:ubiquinone oxidoreductase. Colloids Surf. B Biointerfaces 2008, 66, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kajita, M.; Kim, J.; Kanayama, A.; Takahashi, K.; Mashino, T.; Miyamoto, Y. In vitro free radical scavenging activity of platinum nanoparticles. Nanotechnology 2009, 20, 455105. [Google Scholar] [CrossRef]
- Menshikov, V.S.; Novomlinsky, I.N.; Belenov, S.V.; Alekseenko, A.A.; Safronenko, O.I.; Guterman, V.E. Methanol, Ethanol, and Formic Acid Oxidation on New Platinum-Containing Catalysts. Catalysts 2021, 11, 158. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Zhong, L.W. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Shenton, W.; Davis, S.; Mann, S. Directed self-assembly of nanoparticles into macroscopic materials using antibody-antigen recognition. Adv. Mater. 1999, 11, 449–452. [Google Scholar] [CrossRef]
- Claussen, J.C.; Hengenius, J.B.; Wickner, M.M.; Fisher, T.S.; Umulis, D.M.; Porterfield, D.M. Effects of carbon nanotube-tethered nanosphere density on amperometric biosensing: Simulation and experiment. J. Phys. Chem. C 2011, 115, 20896–20904. [Google Scholar] [CrossRef]
- Yao, H.; Yi, C.; Tzang, C.H.; Zhu, J.; Yang, M. DNA-directed self-assembly of gold nanoparticles into binary and ternary nanostructures. Nanotechnology 2006, 18, 15102. [Google Scholar] [CrossRef]
- Berry, V.; Saraf, R.F.; Berry, V.; Saraf, R.F. Self-Assembly of Nanoparticles on Live Bacterium: An Avenue to Fabricate Electronic Devices. Angew. Chem. Int. Ed. 2005, 44, 6668–6673. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Siddiqui, M.A.; Babalola, O.O.; Wu, H.F. Biofunctionalization of nanoparticle assisted mass spectrometry as biosensors for rapid detection of plant associated bacteria. Biosens. Bioelectron. 2012, 35, 235–242. [Google Scholar] [CrossRef]
- Lin, H.C.; Wang, I.L.; Lin, H.P.; Chang, T.C.; Lin, Y.C. Enhancement of an immunoassay using platinum nanoparticles and an optical detection. Sensors Actuators B Chem. 2011, 154, 185–190. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2002, 107, 668–677. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skewis, L.R.; Reinhard, B.M. Control of Colloid Surface Chemistry through Matrix Confinement: Facile Preparation of Stable Antibody Functionalized Silver Nanoparticles. ACS Appl. Mater. Interfaces 2009, 2, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wei, X.; Yuan, Y. Synthesis of magnetic nanoparticles composed by Prussian blue and glucose oxidase for preparing highly sensitive and selective glucose biosensor. Sensors Actuators B Chem. 2009, 139, 400–406. [Google Scholar] [CrossRef]
- Kudelski, A.; Michota, A.; Bukowska, J. Monolayers of sulfur-containing molecules at metal surfaces as studied using SERS: 3, 3′-thiodipropionic acid and 3-mercaptopropionic acid adsorbed on silver and copper. J. Raman Spectrosc. 2005, 36, 709–714. [Google Scholar] [CrossRef]
- Gui, J.Y.; Stern, D.A.; Frank, D.G.; Lu, F.; Zapien, D.C.; Hubbard, A.T. Adsorption and surface structural chemistry of thiophenol, benzyl mercaptan, and alkyl mercaptans. Comparative studies at silver(111) and platinum(111) electrodes by means of Auger spectroscopy, electron energy loss spectroscopy, low energy electron diffraction and electrochemistry. Langmuir 2002, 7, 955–963. [Google Scholar]
- Jana, N.R.; Earhart, C.; Ying, J.Y. Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem. Mater. 2007, 19, 5074–5082. [Google Scholar] [CrossRef]
- Larsen, E.K.U.; Nielsen, T.; Wittenborn, T.; Birkedal, H.; Vorup-Jensen, T.; Jakobsen, M.H.; Ostergaard, L.; Horsman, M.R.; Besenbacher, F.; Howard, K.A.; et al. Size-dependent accumulation of pegylated silane-coated magnetic iron oxide nanoparticles in murine tumors. ACS Nano 2009, 3, 1947–1951. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, M.; Xiang, D.; Dai, N.; Qing, Y.; Wang, D.; Tang, D. Signal amplification of electrochemical immunosensor for the detection of human serum IgG using double-codified nanosilica particles as labels. Biosens. Bioelectron. 2009, 24, 2246–2249. [Google Scholar] [CrossRef]
- Koole, R.; Van Schooneveld, M.M.; Hilhorst, J.; Castermans, K.; Cormode, D.P.; Strijkers, G.J.; Donegá, C.D.M.; Vanmaekelbergh, D.; Griffioen, A.W.; Nicolay, K.; et al. Paramagnetic Lipid-Coated Silica Nanoparticles with a Fluorescent Quantum Dot Core: A New Contrast Agent Platform for Multimodality Imaging. Bioconjugate Chem. 2008, 19, 2471–2479. [Google Scholar] [CrossRef] [Green Version]
- Senarath-Yapa, M.D.; Phimphivong, S.; Coym, J.W.; Wirth, M.J.; Aspinwall, C.A.; Saavedra, S.S. Preparation and characterization of poly(lipid)-coated, fluorophore-doped silica nanoparticles for biolabeling and cellular imaging. Langmuir 2007, 23, 12624–12633. [Google Scholar] [CrossRef]
- Wang, L.S.; Wu, L.C.; Lu, S.Y.; Chang, L.L.; Teng, I.T.; Yang, C.M.; Ho, J.A.A. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: Improved water suspensibility and decreased nonspecific protein binding. ACS Nano 2010, 4, 4371–4379. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Gnach, A.; Lipinski, T.; Bednarkiewicz, A.; Rybka, J.; Capobianco, J.A. Upconverting nanoparticles: Assessing the toxicity. Chem. Soc. Rev. 2015, 44, 1561–1584. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 16. [Google Scholar] [CrossRef] [Green Version]
- Mosayebi, J.; Kiyasatfar, M.; Laurent, S. Synthesis, Functionalization, and Design of Magnetic Nanoparticles for Theranostic Applications. Adv. Healthc. Mater. 2017, 6, 1700306. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent Advances in Nanomaterials for Gene Delivery—A Review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, R.M.; Stankowska, D.L.; Maliwal, B.P.; Sørensen, T.J.; Laursen, B.W.; Krishnamoorthy, R.R.; Gryczynski, Z.; Borejdo, J.; Gryczynski, I.; Fudala, R. Elimination of autofluorescence background from fluorescence tissue images by use of time-gated detection and the AzaDiOxaTriAngulenium (ADOTA) fluorophore. Anal. Bioanal. Chem. 2013, 405, 2065–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Mikulic, M. Top Therapeutic Classes Global Pharmaceutical Sales 2018 Estimates. Available online: https://www.statista.com/statistics/279916/top-10-therapeutic-classes-by-global-pharmaceutical-sales/ (accessed on 10 February 2022).
- Wang, Y.X.J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015, 21, 13400. [Google Scholar] [CrossRef] [PubMed]
- Tipirneni, K.E.; Warram, J.M.; Moore, L.S.; Prince, A.C.; De Boer, E.; Jani, A.H.; Wapnir, I.L.; Liao, J.C.; Bouvet, M.; Behnke, N.K.; et al. Oncologic Procedures Amenable to Fluorescence-guided Surgery. Ann. Surg. 2017, 266, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef]
- Neumaier, C.E.; Baio, G.; Ferrini, S.; Corte, G.; Daga, A. MR and iron magnetic nanoparticles. Imaging opportunities in preclinical and translational research. Tumori 2008, 94, 226–233. [Google Scholar] [CrossRef]
- Matson, J.B.; Grubbs, R.H. Synthesis of Fluorine-18 Functionalized Nanoparticles for Use as in Vivo Molecular Imaging Agents. NATO Sci. Peace Secur. Ser. A Chem. Biol. 2009, 237–247. [Google Scholar] [CrossRef]
- Radiology, E.S. of Medical imaging in personalised medicine: A white paper of the research committee of the European Society of Radiology (ESR). Insights Imaging 2011, 2, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Mirón-Barroso, S.; Domènech, E.B.; Trigueros, S. Nanotechnology-Based Strategies to Overcome Current Barriers in Gene Delivery. Int. J. Mol. Sci. 2021, 22, 8537. [Google Scholar] [CrossRef]
- Kim, J.; Hu, C.; El Achkar, C.M.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K. Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. N. Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef]
- Liu, D.Z.; Cheng, Y.; Cai, R.Q.; Wang, W.W.; Cui, H.; Liu, M.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. The enhancement of siPLK1 penetration across BBB and its anti glioblastoma activity in vivo by magnet and transferrin co-modified nanoparticle. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 991–1003. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Pereira, A.E.S.; De Oliveira, J.L.; Carvalho, L.B.; Guilger-Casagrande, M.; De Lima, R.; Fraceto, L.F. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnol. 2020, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Chen, J.Y.; Chen, H.W.; Jack Hu, C.M. Nanoparticle Vaccines Adopting Virus-like Features for Enhanced Immune Potentiation. Nanotheranostics 2017, 1, 244–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.S.; Xu, Q.; Boylan, N.J.; Chisholm, J.; Tang, B.C.; Schuster, B.S.; Henning, A.; Ensign, L.M.; Lee, E.; Adstamongkonkul, P.; et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv. 2017, 3, e1601556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.Y.; Ro, K.E.; McPheron, B.A. Stability of Gold Nanoparticle-Bound DNA toward Biological, Physical, and Chemical Agents. Chem. Biol. Drug Des. 2006, 67, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.E.; Zugates, G.T.; Ferreira, L.S.; Ow, H.S.; Nguyen, N.N.; Wiesner, U.B.; Langer, R.S. Intracellular delivery of core–shell fluorescent silica nanoparticles. Biomaterials 2008, 29, 1526–1532. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Baby, T.; Tengjisi; Chen, D.; Weitz, D.A.; Zhao, C.X. Stable Polymer Nanoparticles with Exceptionally High Drug Loading by Sequential Nanoprecipitation. Angew. Chem. Int. Ed. 2020, 59, 4720–4728. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085. [Google Scholar] [CrossRef] [Green Version]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul. 1996, 13, 245–255. [Google Scholar] [CrossRef]
- Croissant, J.G.; Zhang, D.; Alsaiari, S.; Lu, J.; Deng, L.; Tamanoi, F.; Almalik, A.M.; Zink, J.I.; Khashab, N.M. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J. Control. Release 2016, 229, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Z.; Zhang, S.; Saltzman, W.M. Poly(ω-pentadecalactone-co-butylene-co-succinate) nanoparticles as biodegradable carriers for camptothecin delivery. Biomaterials 2009, 30, 5707–5719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.; Bhattacharya, J.; Mukherjee, A.; Ghosh, A.K.; Santra, C.R.; Dasgupta, A.K.; Karmakar, P. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett. 2007, 2, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Vasir, J.K.; Reddy, M.K.; Labhasetwar, V.D. Nanosystems in Drug Targeting: Opportunities and Challenges. Curr. Nanosci. 2005, 1, 47–64. [Google Scholar] [CrossRef]
- Bonamico, M.; Tiberti, C.; Picarelli, A.; Mariani, P.; Rossi, D.; Cipolletta, E.; Greco, M.; Di Tola, M.; Sabbatella, L.; Carabba, B.; et al. Radioimmunoassay to detect antitransglutaminase autoantibodies is the most sensitive and specific screening method for celiac disease. Am. J. Gastroenterol. 2001, 96, 1536–1540. [Google Scholar] [CrossRef]
- Starodub, V.M.; Fedorenko, L.L.; Starodub, N.F. Optical immune sensors for the monitoring protein substances in the air. Sens. Actuators B Chem. 2000, 68, 40–47. [Google Scholar] [CrossRef]
- Tanaka, T.; Matsunaga, T. Fully Automated Chemiluminescence Immunoassay of Insulin Using Antibody−Protein A−Bacterial Magnetic Particle Complexes. Anal. Chem. 2000, 72, 3518–3522. [Google Scholar] [CrossRef]
- Di Pasqua, A.J.; Mishler, R.E.; Ship, Y.L.; Dabrowiak, J.C.; Asefa, T. Preparation of antibody-conjugated gold nanoparticles. Mater. Lett. 2009, 63, 1876–1879. [Google Scholar] [CrossRef]
- Brindle, K. New approaches for imaging tumour responses to treatment. Nat. Rev. Cancer 2008, 8, 94–107. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, K.S.; Kim, C.J.; Hahn, S.K.; Jo, M.H. Electrical detection of VEGFs for cancer diagnoses using anti-vascular endotherial growth factor aptamer-modified Si nanowire FETs. Biosens. Bioelectron. 2009, 24, 1801–1805. [Google Scholar] [CrossRef]
- Bangar, M.A.; Shirale, D.J.; Chen, W.; Myung, N.V.; Mulchandani, A. Single Conducting Polymer Nanowire Chemiresistive Label-Free Immunosensor for Cancer Biomarker. Anal. Chem. 2009, 81, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.N.; Chang, H.K.; Curreli, M.; Liao, H.I.; Olson, C.A.; Chen, P.C.; Zhang, R.; Roberts, R.W.; Sun, R.; Cote, R.J.; et al. Label-free, electrical detection of the SARS virus n-protein with nanowire biosensors utilizing antibody mimics as capture probes. ACS Nano 2009, 3, 1219–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Wu, J.; Ju, H.X. Clinical Immunoassays and Immunosensing. Compr. Sampl. Sample Prep. 2012, 3, 143–167. [Google Scholar]
- Park, S.J.; Taton, T.A.; Mirkin, C.A. Array-based electrical detection of DNA with nanoparticle probes. Science 2002, 295, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Qriouet, Z.; Cherrah, Y.; Sefrioui, H.; Qmichou, Z. Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection. Molecules 2021, 26, 1058. [Google Scholar] [CrossRef] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; Van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, S.; Alnasser, F.; Polo, E.; Garry, D.; Lo Giudice, M.C.; Hristov, D.R.; Rocks, L.; Salvati, A.; Yan, Y.; Dawson, K.A. Identification of Receptor Binding to the Biomolecular Corona of Nanoparticles. ACS Nano 2017, 11, 1884–1893. [Google Scholar] [CrossRef] [PubMed]



| Nanoparticle | Bio-Application | Reference |
|---|---|---|
| Cadmium sulfide | Antimicrobial | [65] |
| Titanium oxide | Treatment of wound infection | [66] |
| Silver | Cytotoxicity | [67] |
| Zinc | Drug loading | [68] |
| Gold | Capping agent and medical field | [69] |
| Gold | Anticancer | [70] |
| Platinum | Antimicrobial and anticancer | [71] |
| Platinum | Cytotoxicity | [72] |
| Zinc | MRI and CT bioimaging | [73] |
| Titanium oxide | Drug delivery | [74] |
| Iron oxide | Drug carriers for cancer therapy | [75] |
| Gold | Ultrasound | [76] |
| Chitosan-gold | Synergistic gene/photothermal therapy | [77] |
| Gold | X-ray/CT scan | [78] |
| Platinum | Anti-fungal activity against A. parasiticus and A. flavus | [79] |
| Silver | Antiviral and antibacterial | [80] |
| Selenium | Biomedical applications | [81] |
| Silver | Showed antileishmanial effect in vivo | [82] |
| Iron oxide | Bactericidal against Streptococcus | [83] |
| Silver | Treat cancer, diabetes, and microbial infections | [84] |
| Silver | Antifungal | [85] |
| Zinc oxide | Antimicrobial activity | [86] |
| Silica | Nanocarriers for drug and gene delivery | [87] |
| Silver | Activity against Pseudomonas aeruginosa | [88] |
| Silica | In vitro and in vivo anticancer activity | [89] |
| Zinc | Drug delivery | [90] |
| Platinum | Induce apoptosis | [91] |
| Iron oxide | MRI imaging | [92] |
| Silicon | Ratiometric fluorescence Immunoassay | [93] |
| Gold | Mosquitocidal | [94] |
| Platinum | Environmental, biological, and catalytic applications | [95] |
| Iron oxide | Inhibition of MCF-7 breast cancer cells | [96] |
| Palladium | Biomedical applications | [97] |
| Silver | Biolarvicidal | [98] |
| Iron | Drug loading | [48] |
| Silver and Gold | Biomedical applications | [99] |
| Iron oxide | Biological applications | [100] |
| Silica oxide | Production of thermal and electric insulators gene delivery, drug carriers | [101] |
| Iron oxide | Antioxidant and antimicrobial | [102] |
| Silver oxide | Drug delivery, gene therapies, imaging | [103] |
| Silver and Gold | Antimicrobial | [104] |
| Silica | Antimicrobial | [105] |
| Platinum | Anticancer | [106] |
| Silver | Antifungal | [107] |
| Iron oxide | Drug delivery | [108] |
| Zinc oxide | Biomedical applications | [109] |
| Iron oxide | Drug-carrying vehicles | [110] |
| Silica | Drug loading | [111] |
| Silica | Activity as an antibiotic against intestinal bacterial infection | [112] |
| Silver | Antimicrobial | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. https://doi.org/10.3390/nano12081333
Ahmad F, Salem-Bekhit MM, Khan F, Alshehri S, Khan A, Ghoneim MM, Wu H-F, Taha EI, Elbagory I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials. 2022; 12(8):1333. https://doi.org/10.3390/nano12081333
Chicago/Turabian StyleAhmad, Faheem, Mounir M. Salem-Bekhit, Faryad Khan, Sultan Alshehri, Amir Khan, Mohammed M. Ghoneim, Hui-Fen Wu, Ehab I. Taha, and Ibrahim Elbagory. 2022. "Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application" Nanomaterials 12, no. 8: 1333. https://doi.org/10.3390/nano12081333







