Preparation, Characterization of ZnTiO3/ZnO Composite Materials and Their Photocatalytic Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Preparation
2.2. Characterization
2.3. Photocatalysis Experiment
3. Results and Discussion
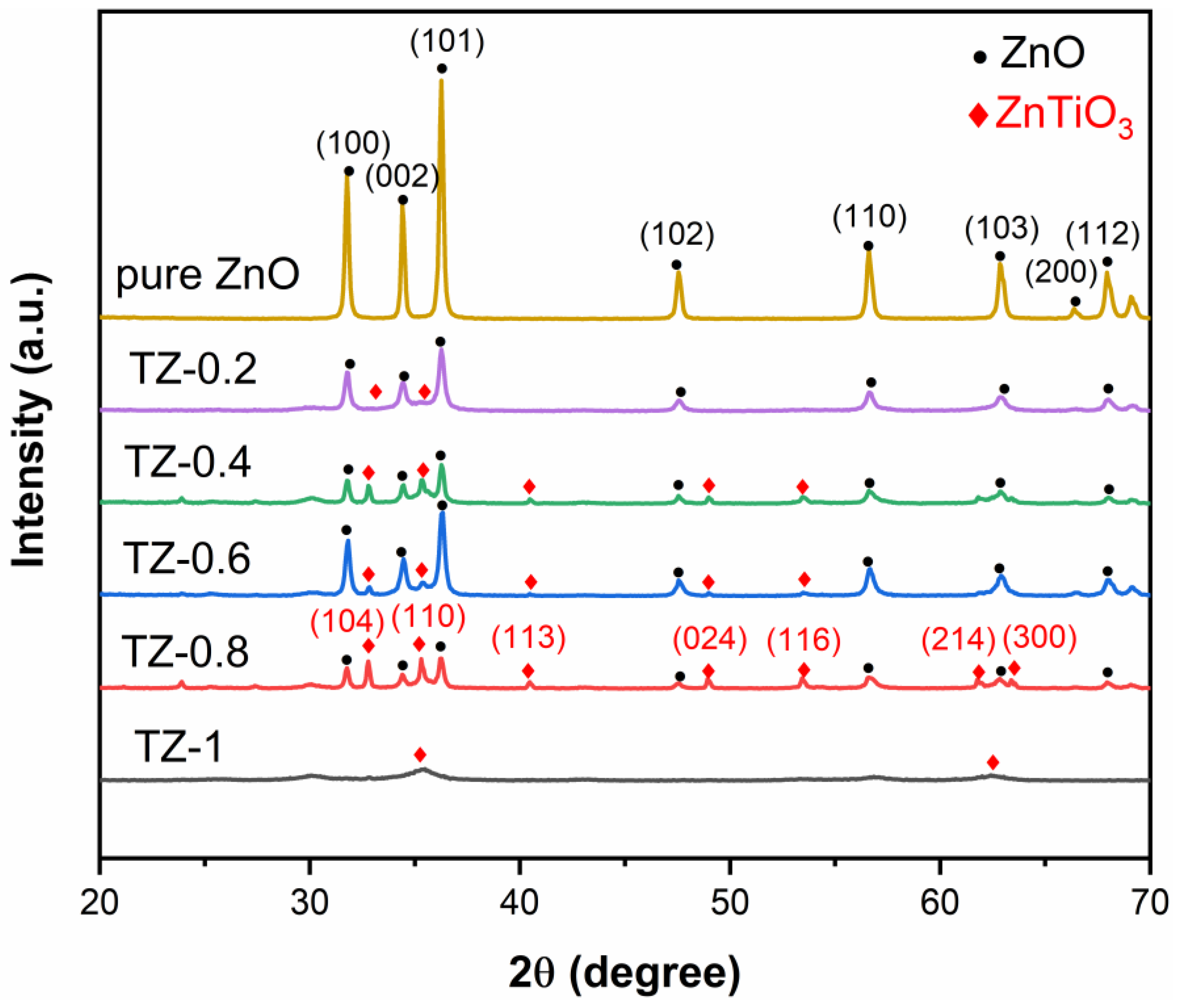
3.1. XRD Analysis
3.2. SEM and TEM Analyses
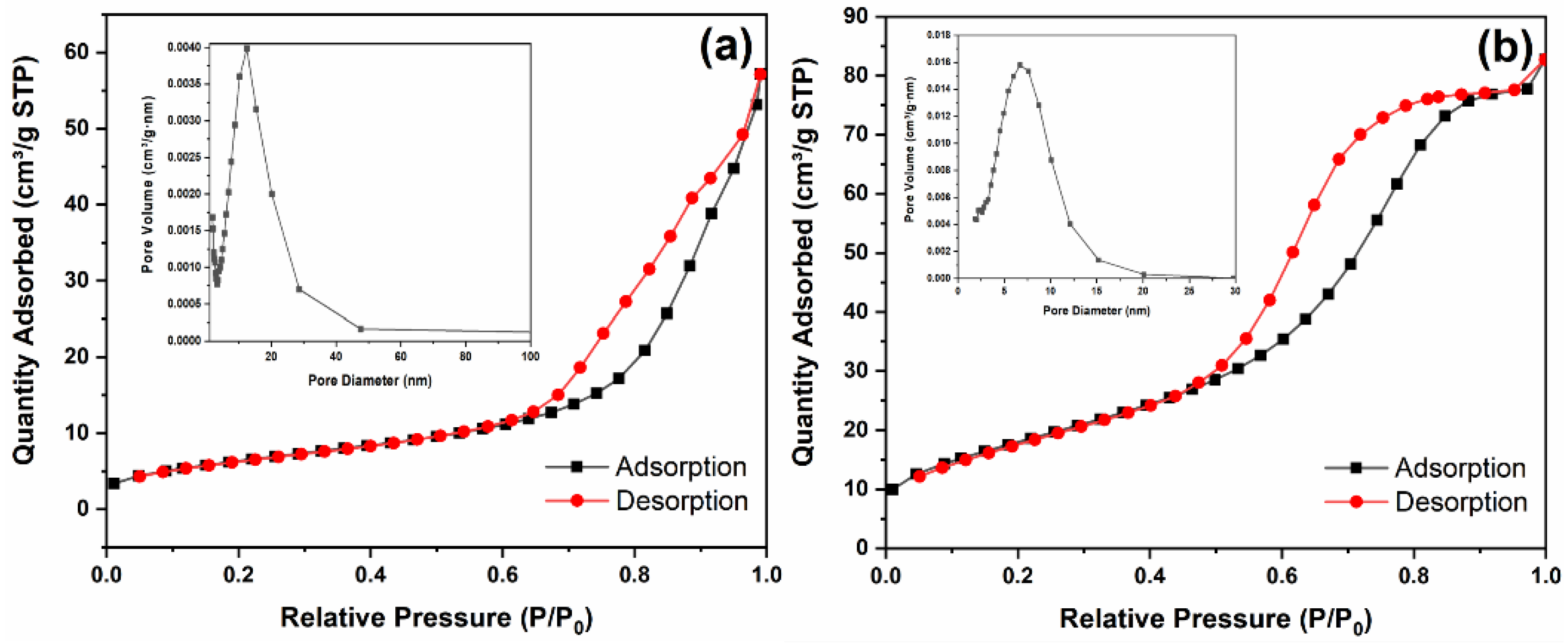
3.3. BET Analysis
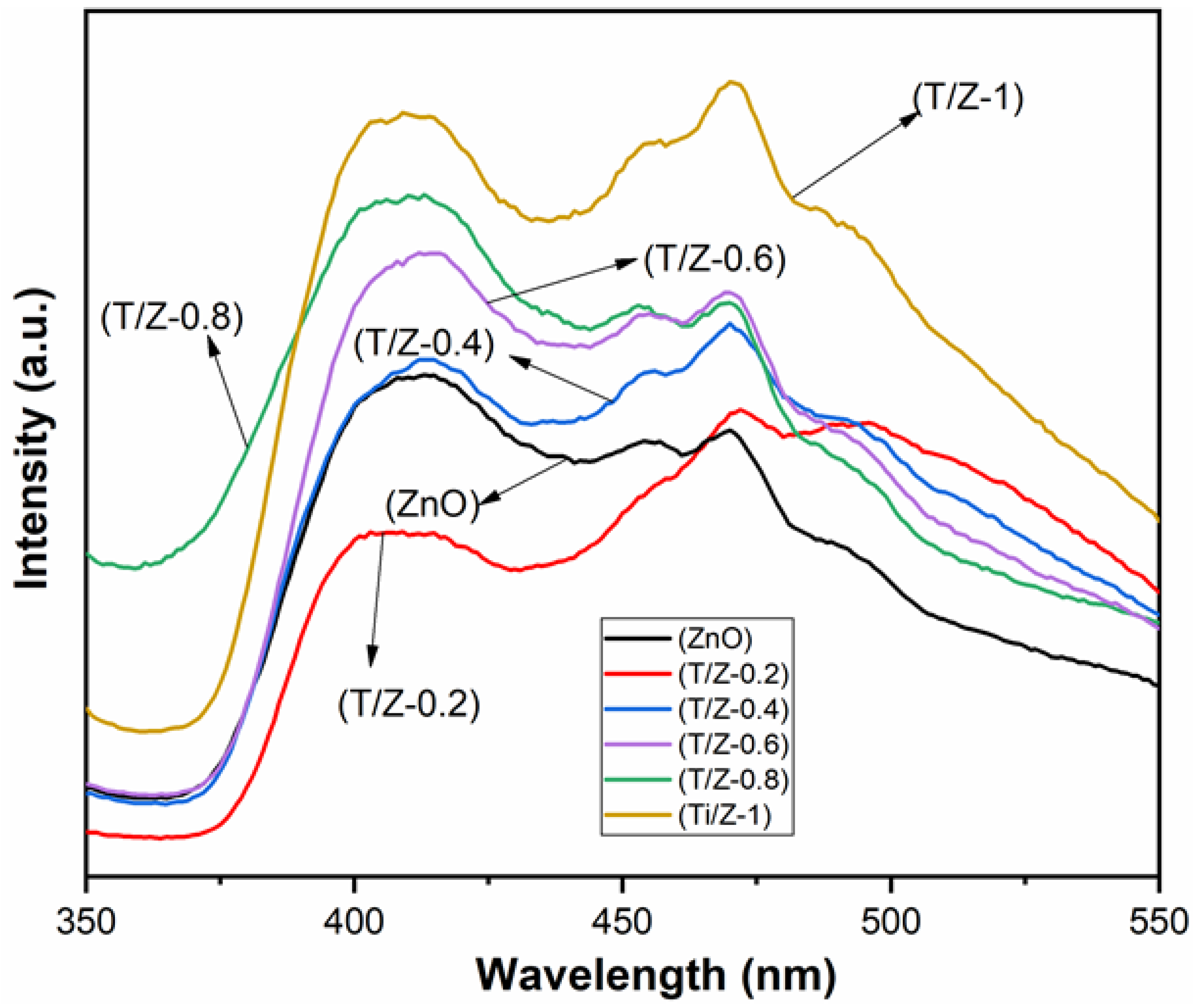
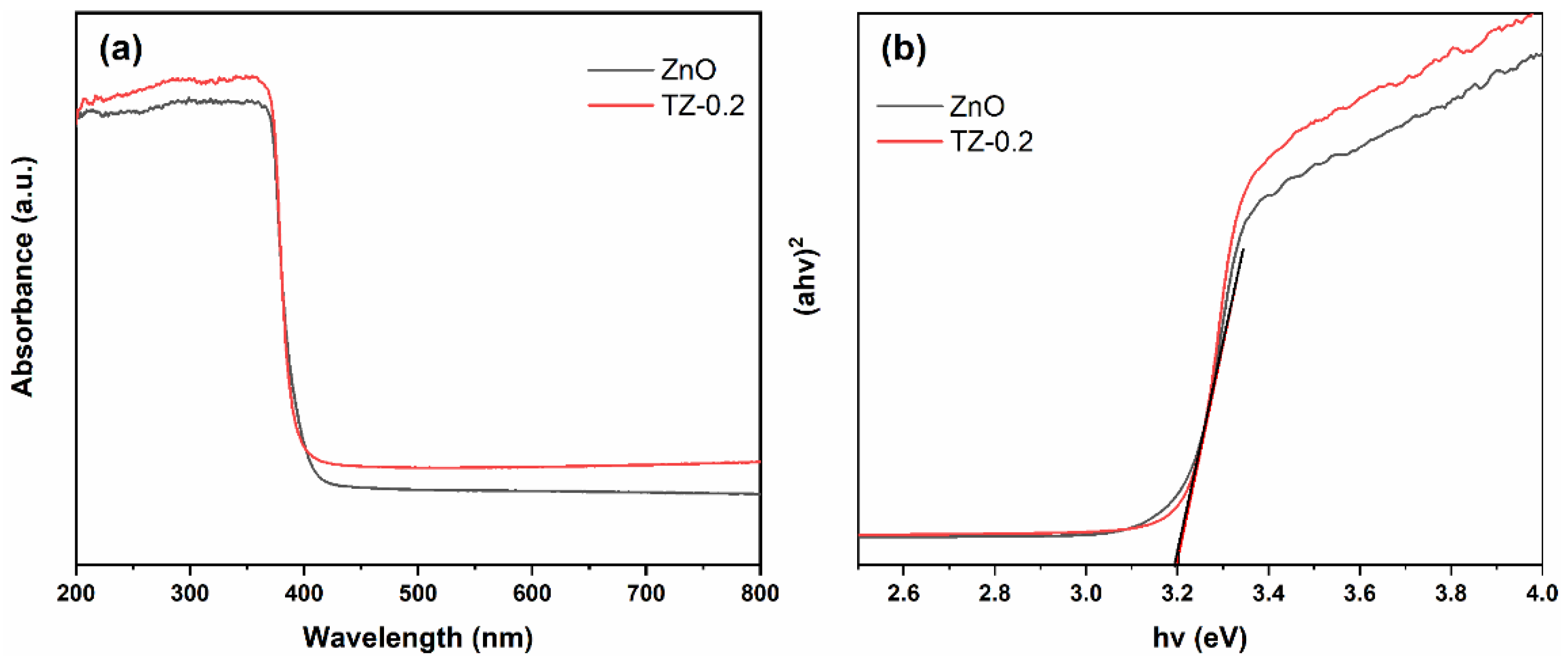
3.4. Optical Property
3.5. Element Composition and State
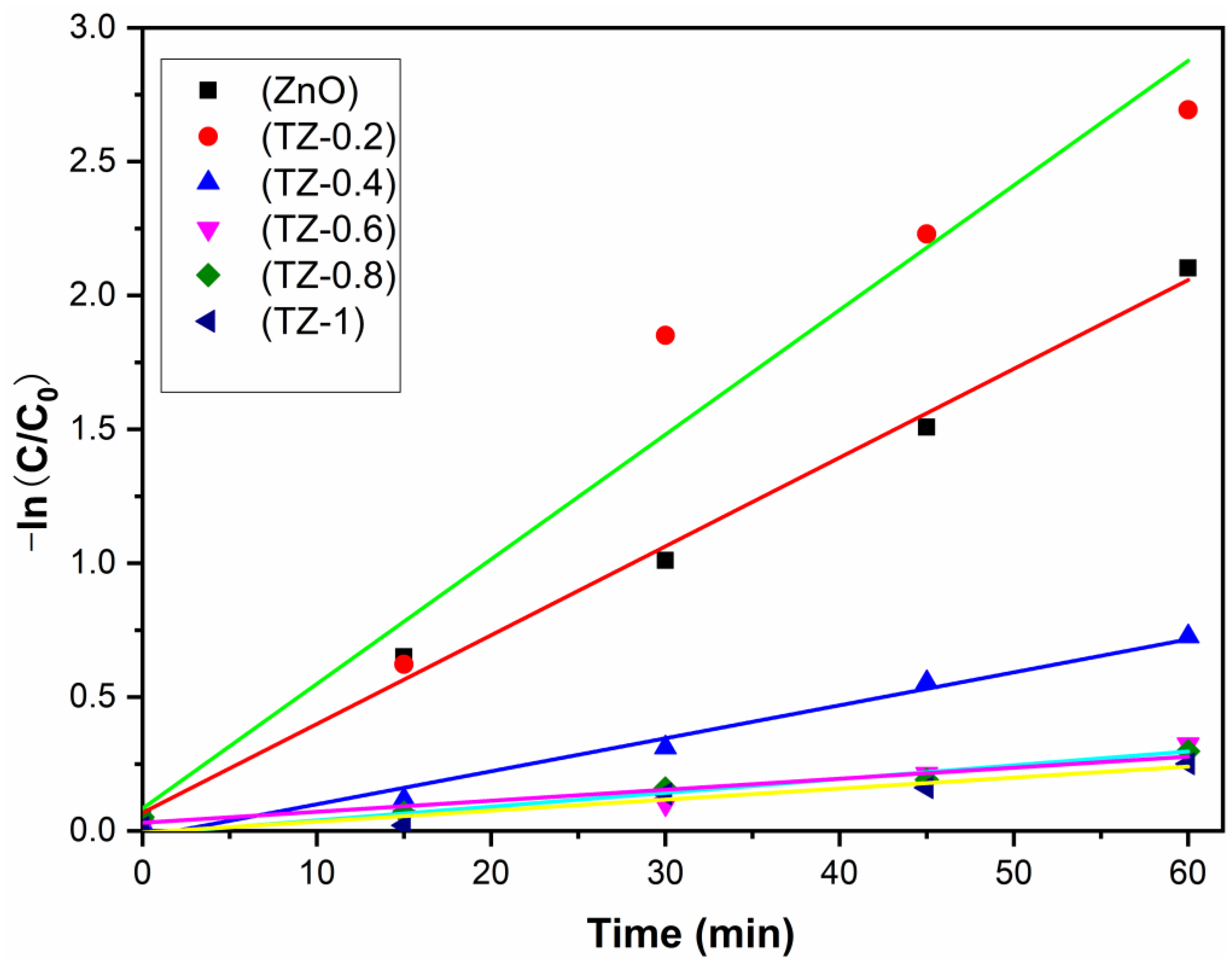
3.6. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Gao, Y.; Zhao, B.S.; Xu, S.; Luo, C.H.; Zhao, Q. One-step hydrothermal preparation and characterization of ZnO-TiO2 nanocomposites for photocatalytic activity. Mater. Res. Express. 2020, 7, 085010. [Google Scholar] [CrossRef]
- Liu, Z.J.; Chen, K.Y.; Jia, Y.F.; Zhu, G.Q.; Wang, Q.Z. A novel magnetically separable CoFe2O4-BiO2−x heterojunction with enhanced photocatalytic activity under near-infrared light irradiation. Mater. Lett. 2021, 303, 130497. [Google Scholar] [CrossRef]
- Zhu, X.D.; Xu, H.Y.; Yao, Y.; Liu, H.; Wang, J.; Pu, Y.; Feng, W.; Chen, S.H. Effects of Ag0-modifification and Fe3+-doping on the structural, optical and photocatalytic properties of TiO2. RSC Adv. 2019, 9, 40003–40012. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, T.; Khan, M.A.; Mahmood, H. Facile synthesis of ZnO nanosheets: Structural, antibacterial and photocatalytic studies. Mater. Lett. 2018, 224, 59–63. [Google Scholar] [CrossRef]
- Hu, C.C.; Lu, L.; Zhu, Y.J.; Li, R.; Xing, Y.J. Morphological controlled preparation and photocatalytic activity of zinc oxide. Mater. Chem. Phys. 2018, 217, 182–191. [Google Scholar] [CrossRef]
- Seo, H.K.; Shin, H.S. Study on photocatalytic activity of ZnO nanodisks for the degradation of Rhodamine B dye. Mater. Lett. 2015, 159, 265–268. [Google Scholar] [CrossRef]
- Chang, Y.C.; Guo, J.Y.; Chen, C.M. Double-sided plasmonic Au nanoparticles on Cu-doped ZnO/ZnO heterostructures with enhanced photocatalytic activity. Mater. Lett. 2017, 209, 60–63. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Deng, Y.J.; Liu, J.K.; Lu, Y.; Yang, X.H. Mass preparation and novel visible light photocatalytic activity of C and Ag Co-modified ZnO nanocrystals. J. Colloid Interface Sci. 2015, 459, 1–9. [Google Scholar] [CrossRef]
- Li, X.F.; Hu, Z.Z.; Liu, J.W.; Li, D.Z.; Zhang, X.Y.; Chen, J.; Fang, J.L. Ga doped ZnO photonic crystals with enhanced photocatalytic activity and its reaction mechanism. Appl. Catal. B-Environ. 2016, 195, 29–38. [Google Scholar] [CrossRef]
- Beura, R.; Pachaiappan, R.; Thangadurai, P. A detailed study on Sn4+ doped ZnO for enhanced photocatalytic degradation. Appl. Surf. Sci. 2018, 433, 887–898. [Google Scholar] [CrossRef]
- Ma, L.; Ding, S.J. Synthesis of thermostable Au@ZnO core-shell nanorods with efficient visible-light photocatalytic activity. Mater. Lett. 2018, 217, 255–258. [Google Scholar] [CrossRef]
- Zhu, X.D.; Wang, J.; Yang, D.X.; Liu, J.W.; He, L.L.; Tang, M.; Feng, W.; Wu, X.Q. Fabrication, characterization and high photocatalytic activity of Ag-ZnO heterojunctions under UV–visible light. RSC Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef]
- Reddy, C.V.; Babu, B.; Shim, J. Synthesis, optical properties and efficient photocatalytic activity of CdO/ZnO hybrid nanocomposite. J. Phys. Chem. Solids 2018, 112, 20–28. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.; Duan, L.B.; Liu, R.D.; Luo, C.H.; Li, Q.; Zhang, Q.; Zhao, X.R. Enhanced photocatalytic activity and synthesis of ZnO nanorods/MoS2 composites. Superlattices Microstruct. 2018, 117, 336–341. [Google Scholar] [CrossRef]
- Taghavi, M.; Tabatabaee, M.; Ehrampoush, M.H.; Ghaneian, M.T.; Afsharnia, M.; Alami, A.; Mardaneh, J. Synthesis, characterization and photocatalytic activity of TiO2/ZnO-supported phosphomolybdic acid nanocomposites. J. Mol. Liq. 2018, 249, 546–553. [Google Scholar] [CrossRef]
- Chang, Y.C. Complex ZnO/ZnS nanocable and nanotube arrays with high performance photocatalytic activity. J. Alloys Compd. 2016, 664, 538–546. [Google Scholar] [CrossRef]
- He, Z.M.; Xia, Y.M.; Tang, B.; Jiang, X.F.; Su, J.B. Fabrication and photocatalytic property of ZnO/Cu2O core-shell nanocomposites. Mater. Lett. 2016, 184, 148–151. [Google Scholar] [CrossRef]
- Cui, Y.F.; Wang, C.; Liu, G.; Yang, H.C.; Wu, S.J.; Wang, T.M. Fabrication and photocatalytic property of ZnO nanorod arrays on Cu2O thin film. Mater. Lett. 2011, 65, 2284–2286. [Google Scholar] [CrossRef]
- Liu, Y.J.; Sun, L.; Wu, J.G.; Fang, T.; Cai, R.; Wei, A. Preparation and photocatalytic activity of ZnO/Fe2O3 nanotube composites. Mater. Sci. Eng. B. 2015, 194, 9–13. [Google Scholar] [CrossRef]
- Zgura, I.; Preda, N.; Socol, G.; Ghica, C.; Ghica, D.; Enculescu, M.; Negut, I.; Nedelcu, L.; Frunza, L.; Ganea, C.P.; et al. Wet chemical synthesis of ZnO-CdS composites and their photocatalytic activity. Mater. Res. Bull. 2018, 99, 174–181. [Google Scholar] [CrossRef]
- Kotresh, M.G.; Patil, M.K.; Inamdar, S.R. Reaction temperature based synthesis of ZnO nanoparticles using co-precipitation method: Detailed structural and optical characterization. Optik 2021, 243, 167506. [Google Scholar] [CrossRef]
- Lang, J.H.; Wang, J.Y.; Zhang, Q.; Li, X.Y.; Han, Q.; Wei, M.B.; Sui, Y.G.; Wang, D.D.; Yang, J.H. Chemical precipitation synthesis and significant enhancement in photocatalytic activity of Ce-doped ZnO nanoparticles. Ceram. Int. 2016, 42, 14175–14181. [Google Scholar] [CrossRef]
- Zhu, X.D.; Han, S.H.; Feng, W.; Kong, Q.Q.; Dong, Z.H.; Wang, C.X.; Lei, J.H.; Yi, Q. The effect of heat treatment on the anatase–rutile phase transformation and photocatalytic activity of Sn-doped TiO2 nanomaterials. RSC Adv. 2018, 8, 14249–14257. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.Y.; Zhang, J.C.; Yang, X.Y.; Zhu, H.; Cao, W.L. Synthesis and photoelectric property of poly (3-octylthiophene)/zinc oxide complexes. Sol. Energy Mater. Sol. C 2010, 94, 194–200. [Google Scholar] [CrossRef]
- Khataee, A.; Soltani, R.D.C.; Hanifehpour, Y.; Safarpour, M.; Ranjbar, H.G.; Joo, S.W. Synthesis and characterization of dysprosium-doped ZnO nanoparticles for photocatalysis of a textile dye under visible light irradiation. Ind. Eng. Chem. Res. 2014, 53, 1924–1932. [Google Scholar] [CrossRef]
- Jung, H.J.; Koutavarapu, R.; Lee, S.; Kim, J.H.; Choi, H.C.; Choi, M.Y. Enhanced photocatalytic activity of Au-doped Au@ZnO core-shell flower-like nanocomposites. J. Alloys Compd. 2018, 735, 2058–2066. [Google Scholar] [CrossRef]
- Huang, N.; Shu, J.X.; Wang, Z.H.; Chen, M.; Ren, C.G.; Zhang, W. One-step pyrolytic synthesis of ZnO nanorods with enhanced photocatalytic activity and high photostability under visible light and UV light irradiation. J. Alloys Compd. 2015, 648, 919–929. [Google Scholar] [CrossRef]
- Zhu, X.D.; Pei, L.X.; Zhu, R.R.; Jiao, Y.; Tang, R.Y.; Feng, W. Preparation and characterization of Sn/La co-doped TiO2 nanomaterials and their phase transformation and photocatalytic activity. Sci. Rep. 2018, 8, 12387. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.D.; Zhou, Q.; Xia, Y.W.; Chen, H.J.; Xu, Q.; Liu, J.W.; Feng, W.; Chen, S.H. Preparation and characterization of Cu-doped TiO2 nanomaterials with anatase/rutile/brookite triphasic structure and their photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 21511–21524. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.X.; Sun, T.M.; Tang, Y.F.; Jiang, G.Q.; Shi, Y.J. Facile photochemical synthesis of hierarchical cake-like ZnO/Ag composites with enhanced visible-light photocatalytic activities. Mater. Lett. 2018, 219, 236–239. [Google Scholar] [CrossRef]
- Kannadasan, N.; Shanmugam, N.; Cholan, S.; Sathishkumar, K.; Viruthagiri, G.; Poonguzhali, R. The effect of Ce4+ incorporation on structural, morphological and photocatalytic characters of ZnO nanoparticles. Mater. Charact. 2014, 97, 37–46. [Google Scholar] [CrossRef]
- Falak, P.; Hassanzadeh-Tabrizi, S.A.; Saffar-Teluri, A. Synthesis, characterization, and magnetic properties of ZnO-ZnFe2O4 nanoparticles with high photocatalytic activity. J. Magn. Magn. Mater. 2017, 441, 98–104. [Google Scholar] [CrossRef]
- Jia, T.K.; Liu, M.; Zheng, C.Y.; Long, F.; Min, Z.Y.; Fu, F.; Yu, D.S.; Li, J.L.; Lee, J.H.; Kim, N.H. One-pot hydrothermal synthesis of La-doped ZnIn2S4 microspheres with improved visible-light photocatalytic performance. Nanomaterials 2020, 10, 2026. [Google Scholar] [CrossRef]
- Einert, M.; Hartmann, P.; Smarsly, B.; Brezesinski, T. Quasi-homogenous photocatalysis of quantum-sized Fe-doped TiO2 in optically transparent aqueous dispersions. Sci. Rep. 2021, 11, 17687. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Q.H. Effects of amorphous contents and particle size on the photocatalytic properties of TiO2 nanoparticles. Scr. Mater. 2001, 44, 1195–1198. [Google Scholar] [CrossRef]
- Wang, F.X.; Liang, L.; Shi, L.; Liu, M.S.; Sun, J.M. CO2-assisted synthesis of mesoporous carbon/C-doped ZnO composites for enhanced photocatalytic performance under visible light. Dalton Trans. 2014, 43, 16441. [Google Scholar] [CrossRef]
- Jia, C.C.; Chen, H.; Yang, P. Construction of hollow waxberry-like rutile-/anatase-TiO2/SnO2 towards enhanced photocatalysis. J. Ind. Eng. Chem. 2018, 58, 278–289. [Google Scholar] [CrossRef]
- Zhu, X.D.; Zhu, R.R.; Pei, L.X.; Liu, H.; Wang, J.; Feng, W.; Jiao, Y.; Zhang, W.M. Fabrication, characterization, and photocatalytic activity of anatase/rutile/ SnO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 21210–21218. [Google Scholar] [CrossRef]
- Mirikaram, N.; Pérez-Molina, A.; Morales-Torres, S.; Salemi, A.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Photocatalytic perfomance of ZnO-graphene oxide composites towards the degradation of vanillic acid under solar radiation and visible-LED. Nanomaterials 2021, 11, 1576. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Enhancing the photocatalytic oxidation of dibenzothiophene using visible light responsive Fe and N co-doped TiO2 nanoparticles. Ceram. Int. 2017, 43, 973–981. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, C.H.; Zhang, X.X.; Liu, Y.N.; Yan, J.H. Preparation and photocatalytic performance of ZnO/ZnGa2O4 composite microspheres. J. Cent. South Univ. 2016, 23, 3092–3099. [Google Scholar] [CrossRef]


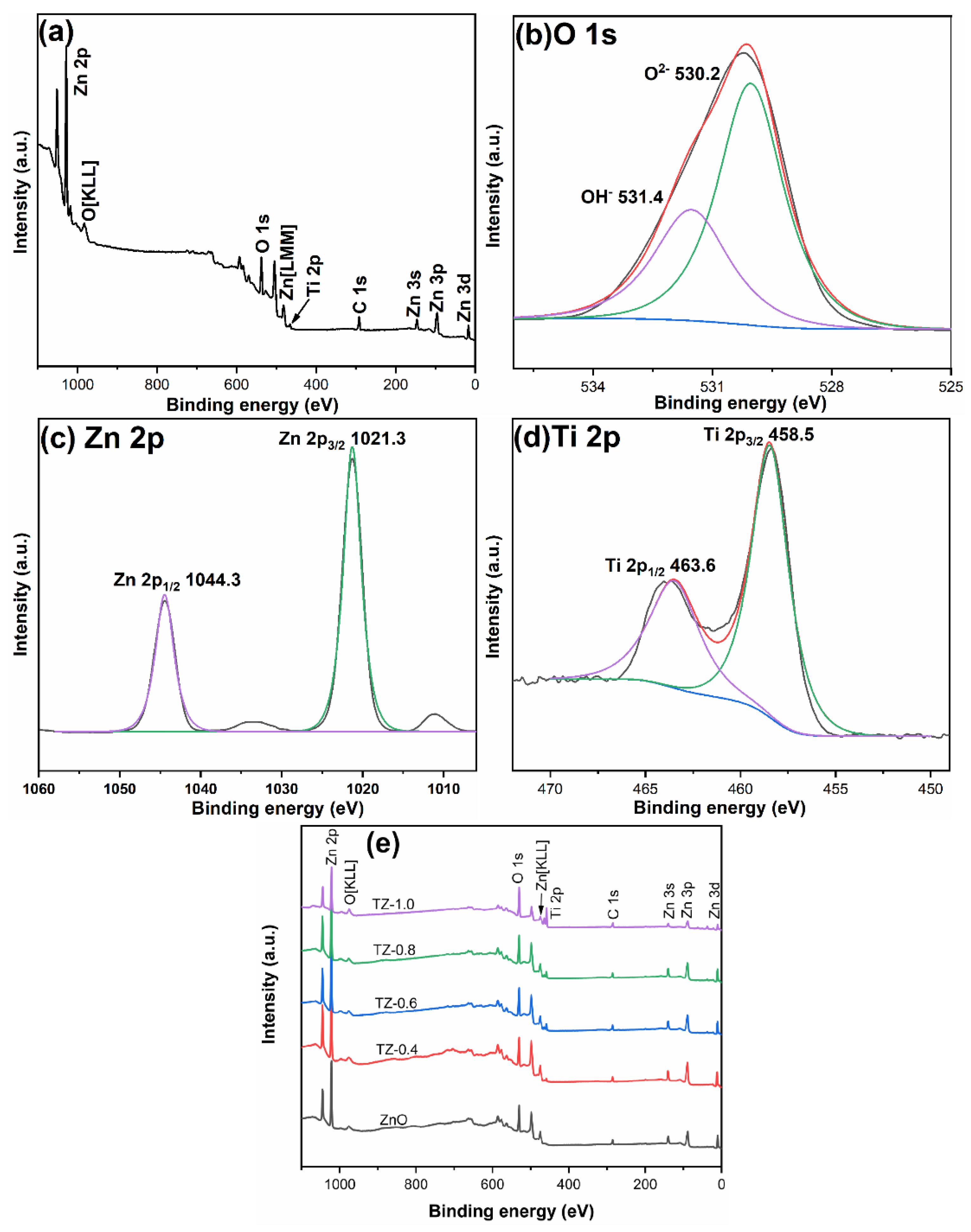

| Zn | O | Ti | ||||
|---|---|---|---|---|---|---|
| Sample | 2p1/2 | 2p3/2 | O2− | OH− | 2p1/2 | 2p3/2 |
| ZnO | 1044.9 | 1021.8 | 530.2 | 531.8 | ||
| Ti-0.2 | 1044.3 | 1021.3 | 530.2 | 531.4 | 463.6 | 458.5 |
| Ti-0.4 | 1044.5 | 1021.4 | 530.1 | 531.9 | 463.9 | 458.5 |
| Ti-0.6 | 1044.5 | 1021.4 | 530.0 | 531.6 | 464.0 | 458.4 |
| Ti-0.8 | 1044.5 | 1021.4 | 529.9 | 531.0 | 463.1 | 458.3 |
| Ti-1.0 | 1044.3 | 1021.2 | 529.6 | 530.9 | 463.8 | 458.0 |
| Sample | O (at%) | Ti (at%) | Zn (at%) | [Ti]/[Zn] | [O]/([Ti] + [Zn]) |
|---|---|---|---|---|---|
| pure ZnO | 53.78 | 0 | 46.22 | 0 | 1.16 |
| TZ-0.2 | 55.42 | 5.64 | 38.94 | 0.14 | 1.24 |
| TZ-0.4 | 58.47 | 9.46 | 32.07 | 0.29 | 1.40 |
| TZ-0.6 | 49.20 | 13.37 | 37.43 | 0.36 | 0.97 |
| TZ-0.8 | 57.36 | 14.14 | 28.50 | 0.50 | 1.34 |
| TZ-1 | 54.38 | 20.45 | 25.17 | 0.81 | 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Lu, S.; He, L.; Zhu, X.; Feng, W.; Zhang, W. Preparation, Characterization of ZnTiO3/ZnO Composite Materials and Their Photocatalytic Performance. Nanomaterials 2022, 12, 1345. https://doi.org/10.3390/nano12081345
Tang M, Lu S, He L, Zhu X, Feng W, Zhang W. Preparation, Characterization of ZnTiO3/ZnO Composite Materials and Their Photocatalytic Performance. Nanomaterials. 2022; 12(8):1345. https://doi.org/10.3390/nano12081345
Chicago/Turabian StyleTang, Mao, Shiji Lu, Lili He, Xiaodong Zhu, Wei Feng, and Wanming Zhang. 2022. "Preparation, Characterization of ZnTiO3/ZnO Composite Materials and Their Photocatalytic Performance" Nanomaterials 12, no. 8: 1345. https://doi.org/10.3390/nano12081345
APA StyleTang, M., Lu, S., He, L., Zhu, X., Feng, W., & Zhang, W. (2022). Preparation, Characterization of ZnTiO3/ZnO Composite Materials and Their Photocatalytic Performance. Nanomaterials, 12(8), 1345. https://doi.org/10.3390/nano12081345






