Improving the Chemical Stability of Al Alloy through the Densification of the Alumina Layer Assisted by SiF62− Anion Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formation of the Oxide Layers via PE
2.2. Microstructural and Compositional Analysis
2.3. Electrochemical and Quantum Chemical Computational Analysis
3. Results and Discussion
3.1. Morphology and Composition of the Oxide Layers
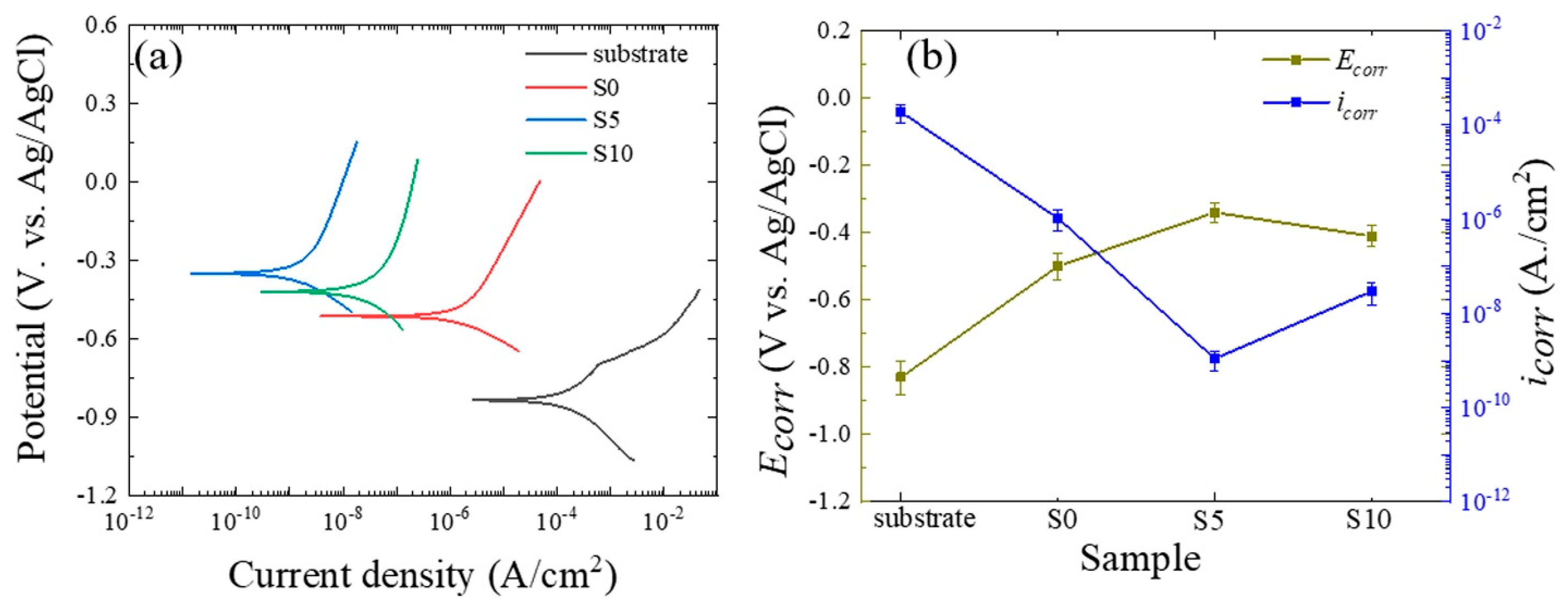
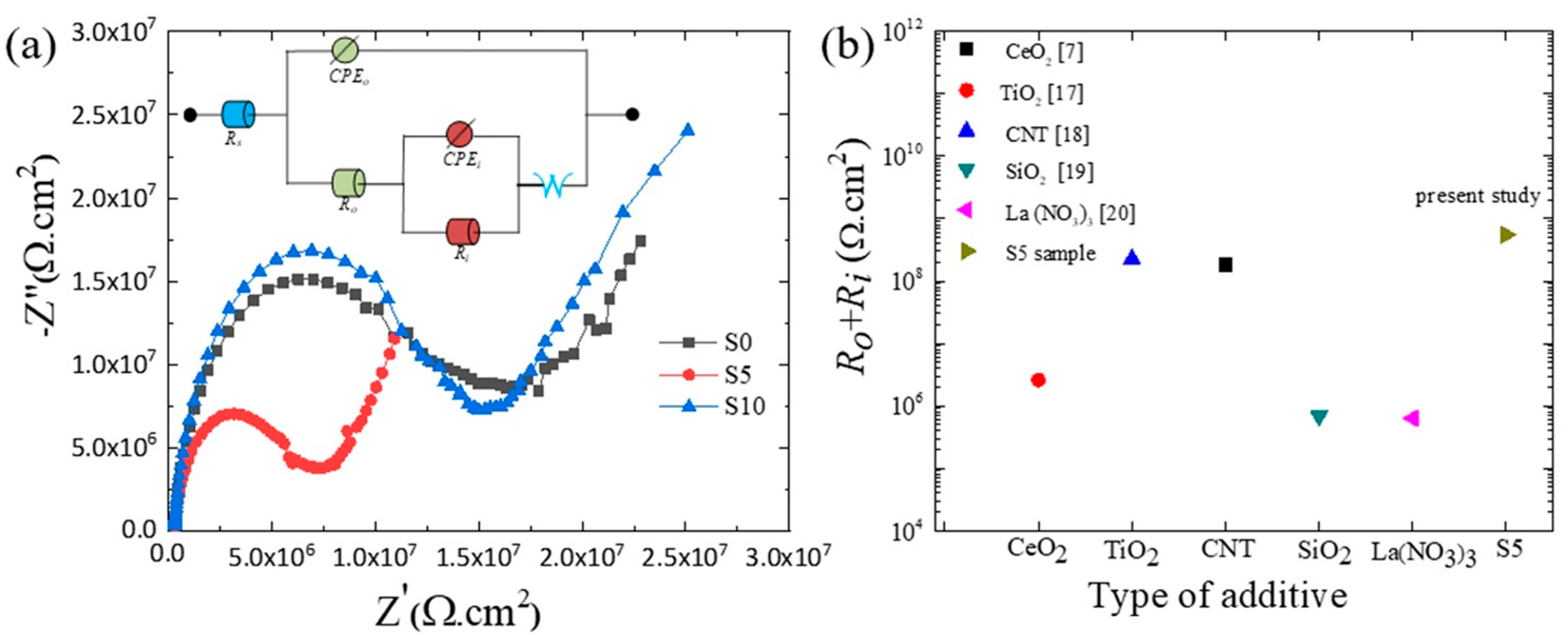
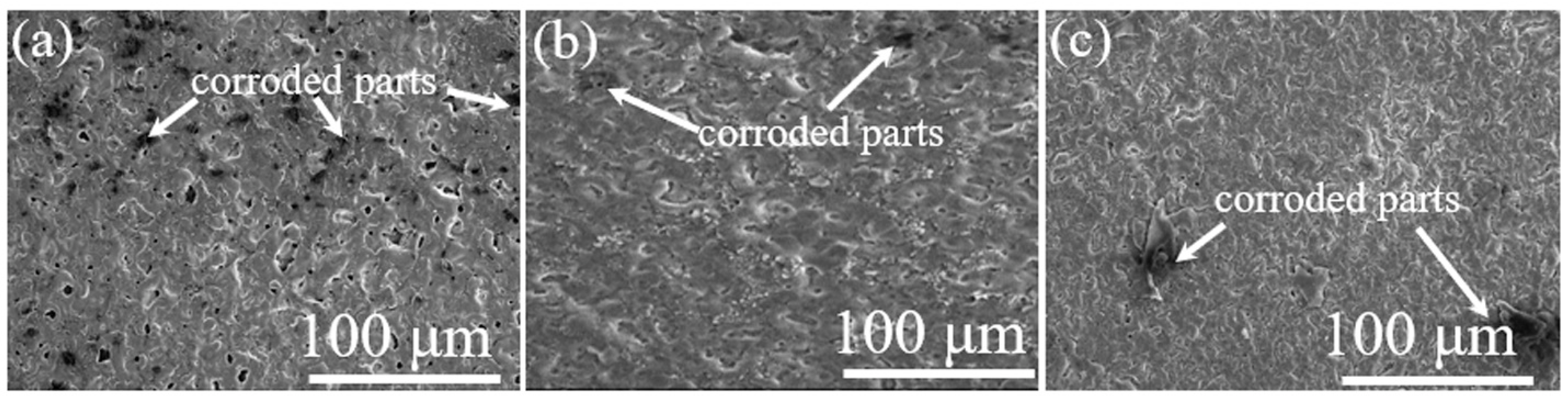
3.2. Chemical Performance of the Oxide Layers
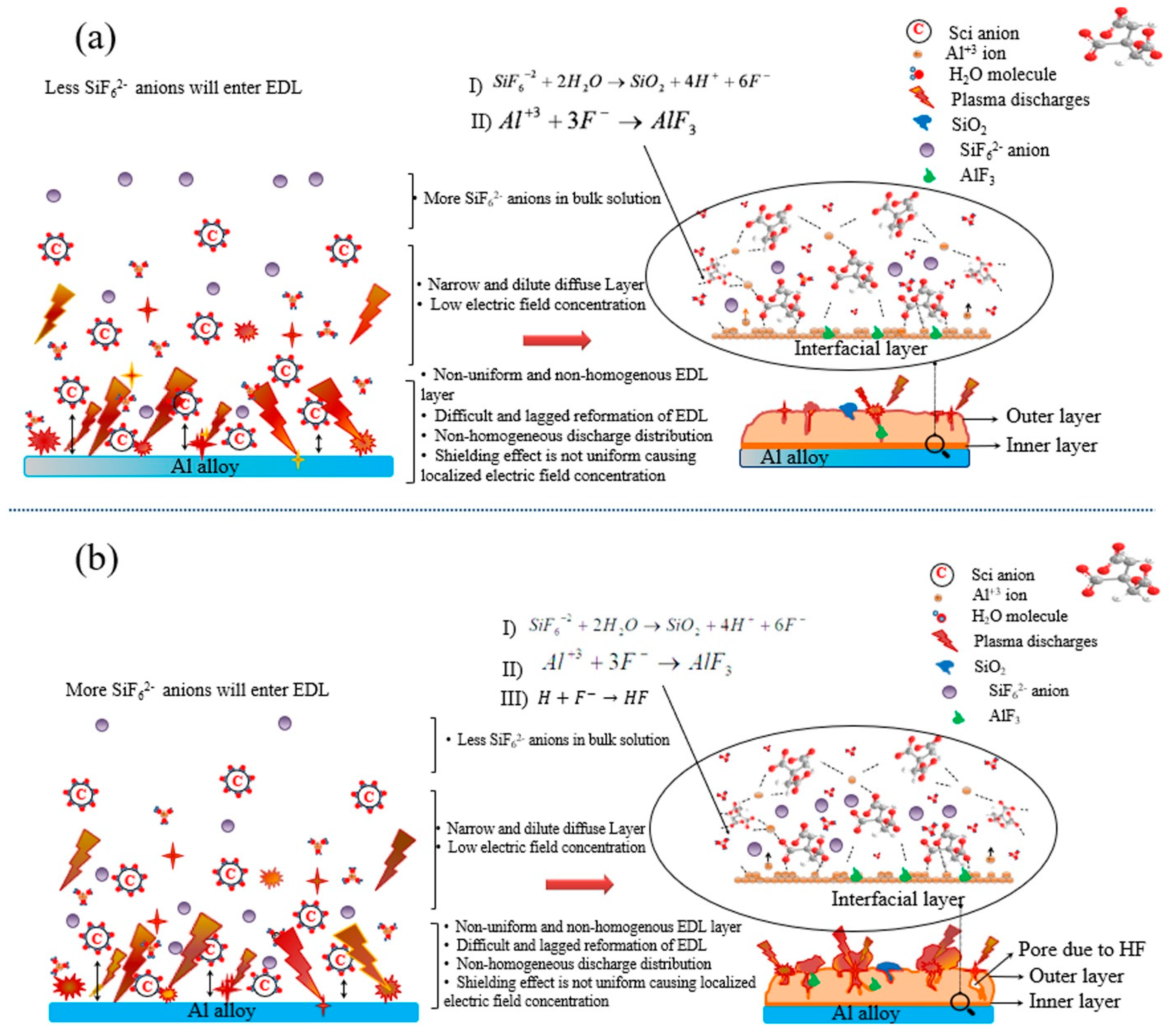
3.3. Mechanism Underlying the Promotion of SiF62− Anion Hydrolysis
4. Conclusions
- (1)
- PE treatment in solutions containing SCi gave rise to relatively compact structures with minimum sizes of micropores.
- (2)
- A thick EDL tended to be formed on the substrate surface during the initial stage of the PE process conducted in a solution containing SCi additive.
- (3)
- The hydrolysis of SiF62− anions transferred through the EDL was found to be affected by the thickness of the EDL.
- (4)
- A controlled hydrolysis of SiF62− anions was found in the case of the S5 sample treated in a solution containing 5 g/L of SCi, which resulted in the formation of a very dense oxide layer on its surface.
- (5)
- The S5 sample exhibited the lowest value of icorr, the highest value of Ecorr, and the highest impedance. Thus, the chemical stability of this sample in 3.5 wt.% NaCl solution was superior to the other samples.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. Porosity in plasma electrolytic oxide coatings. Acta Mater. 2006, 54, 1985–1993. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Liang, J.; Blawert, C.; Störmer, M.; Dietzel, W. Effect of current density on the microstructure and corrosion behavior of plasma electrolytic oxidation treated AM50 magnesium alloy. Appl. Surf. Sci. 2009, 255, 4212–4218. [Google Scholar] [CrossRef] [Green Version]
- Arunnellaiappan, T.; Ashfaq, M.; Krishna, L.R.; Rameshbabu, N. Fabrication of corrosion-resistant Al2O3–CeO2 composite coating on AA7075 via plasma electrolytic oxidation coupled with electrophoretic deposition. Ceram. Int. 2016, 42, 5897–5905. [Google Scholar] [CrossRef]
- Hussain, T.; Kaseem, M.; Ko, Y.G. Hard acid–hard base interactions responsible for densification of alumina layer for superior electrochemical performance. Corros. Sci. 2020, 170, 108663. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, Y.; Dong, S.; Chen, X.-B.; Dong, J. Effects of fluoride ions as electrolyte additives for a PEO/Ni-P composite coating onto Mg alloy AZ31B. Surf. Coat. Technol. 2021, 417, 126883. [Google Scholar] [CrossRef]
- Kaseem, M.; Yang, H.W.; Ko, Y.G. Toward a nearly defect-free coating via high-energy plasma sparks. Sci. Rep. 2017, 7, 2378. [Google Scholar] [CrossRef]
- Narayanan, T.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Ryu, H.S.; Hong, S.-H. Effects of KF, NaOH, and KOH Electrolytes on Properties of Microarc-Oxidized Coatings on AZ91D Magnesium Alloy. J. Electrochem. Soc. 2009, 156, C298–C303. [Google Scholar] [CrossRef]
- Kazanski, B.; Kossenko, A.; Zinigrad, M.; Lugovskoy, A. Fluoride ions as modifiers of the oxide layer produced by plasma electrolytic oxidation on AZ91D magnesium alloy. Appl. Surf. Sci. 2013, 287, 461–466. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Lyubimov, V.V.; Ashitkov, R.V. Phase formation in ceramic coatings during plasma electrolytic oxidation of aluminum alloys. Ceram. Int. 1998, 24, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Cai, W.; Shan, A.; Jiang, Z. Effects of fluoride on the structure and properties of microarc oxidation coating on aluminium alloy. J. Alloys Compd. 2010, 505, 188–193. [Google Scholar] [CrossRef]
- Kamil, M.P.; Kaseem, M.; Lee, Y.H.; Ko, Y.G. Microstructural characteristics of oxide layer formed by plasma electrolytic oxi-dation: Nanocrystalline and amorphous structures. J. Alloys Compd. 2017, 707, 167–171. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxi-dation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Koo, B.H. Combined Effect of Long Processing Time and Na2SiF6 on the Properties of PEO Coatings Formed on AZ91D. J. Mater. Eng. Perform. 2016, 25, 3531–3537. [Google Scholar] [CrossRef]
- Kaseem, M.; Choi, K.; Ko, Y.G. A highly compact coating responsible for enhancing corrosion properties of Al-Mg-Si alloy. Mater. Lett. 2017, 196, 316–319. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Morphological modification and corrosion response of MgO and Mg3(PO4)2 composite formed on magnesium alloy. Compos. Part B Eng. 2019, 176, 107225. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, Z.; Xin, S.; Sun, X.; Wu, X. Electrochemical impedance spectroscopy of ceramic coatings on Ti–6Al–4V by mi-cro-plasma oxidation. Electrochim. Acta 2005, 50, 3273–3279. [Google Scholar] [CrossRef]
- He, D.; Li, G.; Shen, D.; Guo, C.; Ma, H.; Cai, J. Effect mechanism of ultrasound on growth of micro-arc oxidation coatings on A96061 aluminum alloy. Vacuum 2014, 107, 99–102. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Golozar, M.A.; Lu, X.; Blawert, C.; Zheludkevich, M.L. The effect of pulse waveforms on surface morphology, composition and corrosion behavior of Al2O3 and Al2O3/TiO2 nano-composite PEO coatings on 7075 aluminum alloy. Surf. Coat. Technol. 2017, 324, 208–221. [Google Scholar] [CrossRef]
- Lee, K.M.; Ko, Y.G.; Shin, D.H. Incorporation of multi-walled carbon nanotubes into the oxide layer on a 7075 Al alloy coated by plasma electrolytic oxidation: Coating structure and corrosion properties. Curr. Appl. Phys. 2011, 11, S55–S59. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, D.; Li, C.; Zhang, X.; Yang, Z.; Lei, M. Effect of Shot Peening and Plasma Electrolytic Oxidation on the Intergranular Corrosion Behavior of 7A85 Aluminum Alloy. Acta Met. Sin. 2014, 27, 705–713. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Dabalà, M. Corrosion properties of plasma electrolytic oxidation coated AA7075 treated using an electrolyte containing lanthanum-salts. Surf. Interface Anal. 2016, 48, 729–738. [Google Scholar] [CrossRef]
- Wei, Z.; Tian, P.; Liu, X.; Zhou, B. In vitrodegradation, hemolysis, and cytocompatibility of PEO/PLLA composite coating on biodegradable AZ31 alloy. J. Biomed. Mater. Res. 2015, 103, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Arslan, T.; Kandemirli, F.; Ebenso, E.; Love, I.; Alemu, H. Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros. Sci. 2009, 51, 35–47. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.; Obot, I.; El-Khaiary, M.I. Quantum chemical investigation and statistical analysis of the relationship between corrosion inhibition efficiency and molecular structure of xanthene and its derivatives on mild steel in sulphuric acid. J. Mol. Struct. 2011, 1002, 86–96. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Zeeshan, U.; Yang, H.; Dikici, B.; Ko, Y. Fabrication of functionalized coating with a unique flowery-flake structure for an effective corrosion performance and catalytic degradation. Chem. Eng. J. 2021, 420, 129737. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Santos, J.S.; Rodrigues, A.; Simon, A.P.; Ferreira, C.H.; Santos, V.A.Q.; Sikora, M.S.; Cruz, N.C.; Mambrini, G.P.; Trivinho-Strixino, F. One-Step Synthesis of Antibacterial Coatings by Plasma Electrolytic Oxidation of Aluminum. Adv. Eng. Mater. 2019, 21, 1900119. [Google Scholar] [CrossRef]
- Wang, S.; Liu, P. The technology of preparing green coating by conducting micro-arc oxidation on AZ91D magnesium alloy. Pol. J. Chem. Technol. 2016, 18, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Fatimah, S.; Kamil, M.P.; Kwon, J.H.; Kaseem, M.; Ko, Y.G. Dual incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloys Compd. 2017, 707, 358–364. [Google Scholar] [CrossRef]
- Kaseem, M.; Min, J.H.; Ko, Y.G. Corrosion behavior of Al-1wt% Mg-0.85wt%Si alloy coated by micro-arc-oxidation using TiO2 and Na2MoO4 additives: Role of current density. J. Alloys Compd. 2017, 723, 448–455. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. A novel composite system composed of zirconia and LDHs film grown on plasma electrolysis coating: Toward a stable smart coating. Ultrason. Sonochem. 2018, 49, 316–324. [Google Scholar] [CrossRef]
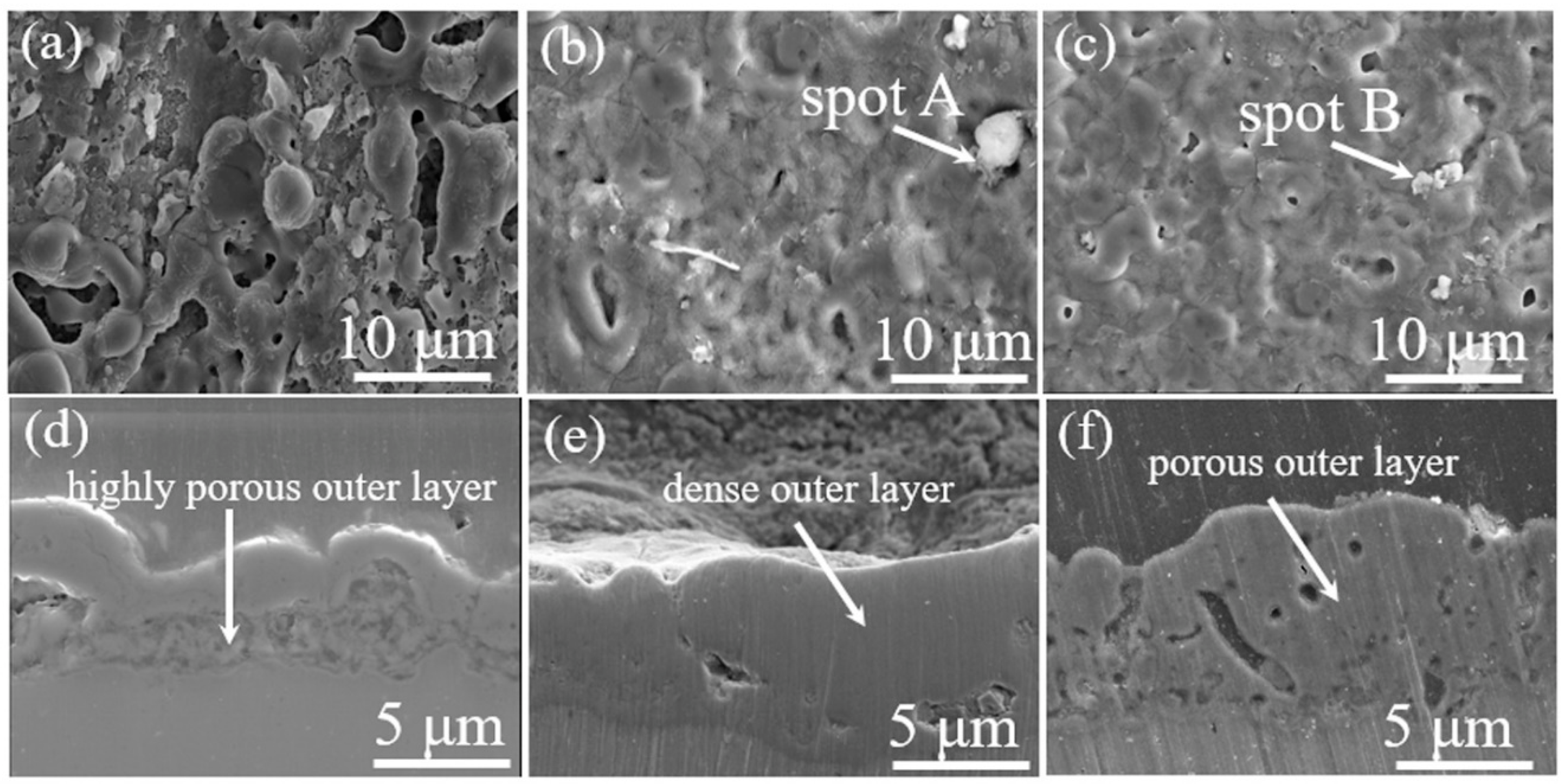

| Sample | Al (wt.%) | O (wt.%) | Si (wt.%) | F (wt.%) | C (wt.%) |
|---|---|---|---|---|---|
| S0 | 47.05 | 45.27 | 1.16 | 6.52 | - |
| S5 | 27.72 | 38.41 | 6.50 | 15.94 | 11.43 |
| S10 | 27.20 | 25.96 | 8.98 | 20.15 | 17.71 |
| Sample | Rs × 10−3 (Ωcm2) | Ro (MΩcm2) | CPE-no | CPE-Yo × 10−12 (S.sn.cm−2) | Ri (MΩcm2) | CPE-ni | CPE-Yi × 10−9 (S.sn.cm−2) | W × 10−6 (S*S1/2) |
|---|---|---|---|---|---|---|---|---|
| S0 | 1.34 ± 1.05 | 0.273 ± 0.03 | 0.78 ± 0.03 | 18.4 ± 2.77 | 7.77 ± 0.97 | 0.48 ± 0.12 | 6.52 ± 3.64 | 0.326 ± 0.08 |
| S5 | 3.58 ± 2.22 | 4.57 ± 1.13 | 0.91 ± 0.02 | 1.90 ± 0.77 | 550 ± 38.0 | 0.72 ± 0.17 | 0.172 ± 0.08 | 9.72 ± 1.43 |
| S10 | 7.60 ± 3.48 | 0.822 ± 0.02 | 0.83 ± 0.01 | 5.25 ± 2.19 | 21.2 ± 3.42 | 0.56 ± 0.14 | 6.03 ± 4.11 | 3.98 ± 0.93 |
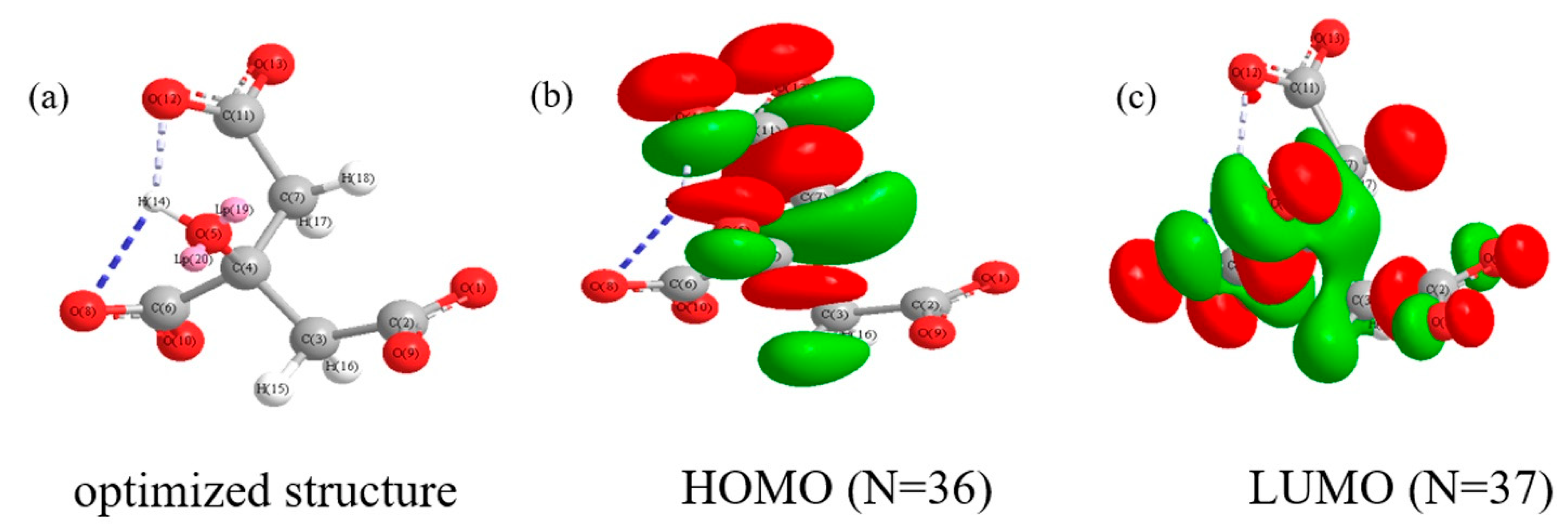
| Anion | EHOMO (eV) | ELUMO (eV) | ∆E (eV) | A | I | χ | η | ∆N | |
|---|---|---|---|---|---|---|---|---|---|
| SCi | −12.356 | −10.807 | 1.549 | 10.807 | 12.356 | 11.581 | 0.774 | −1.178 | 1.291 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaseem, M.; Dikici, B.; Liu, H. Improving the Chemical Stability of Al Alloy through the Densification of the Alumina Layer Assisted by SiF62− Anion Hydrolysis. Nanomaterials 2022, 12, 1354. https://doi.org/10.3390/nano12081354
Kaseem M, Dikici B, Liu H. Improving the Chemical Stability of Al Alloy through the Densification of the Alumina Layer Assisted by SiF62− Anion Hydrolysis. Nanomaterials. 2022; 12(8):1354. https://doi.org/10.3390/nano12081354
Chicago/Turabian StyleKaseem, Mosab, Burak Dikici, and Hongfei Liu. 2022. "Improving the Chemical Stability of Al Alloy through the Densification of the Alumina Layer Assisted by SiF62− Anion Hydrolysis" Nanomaterials 12, no. 8: 1354. https://doi.org/10.3390/nano12081354








