When Super-Resolution Localization Microscopy Meets Carbon Nanotubes
Abstract
:1. Introduction/Background
2. Discussion
2.1. A Brief Overview of SRM Techniques
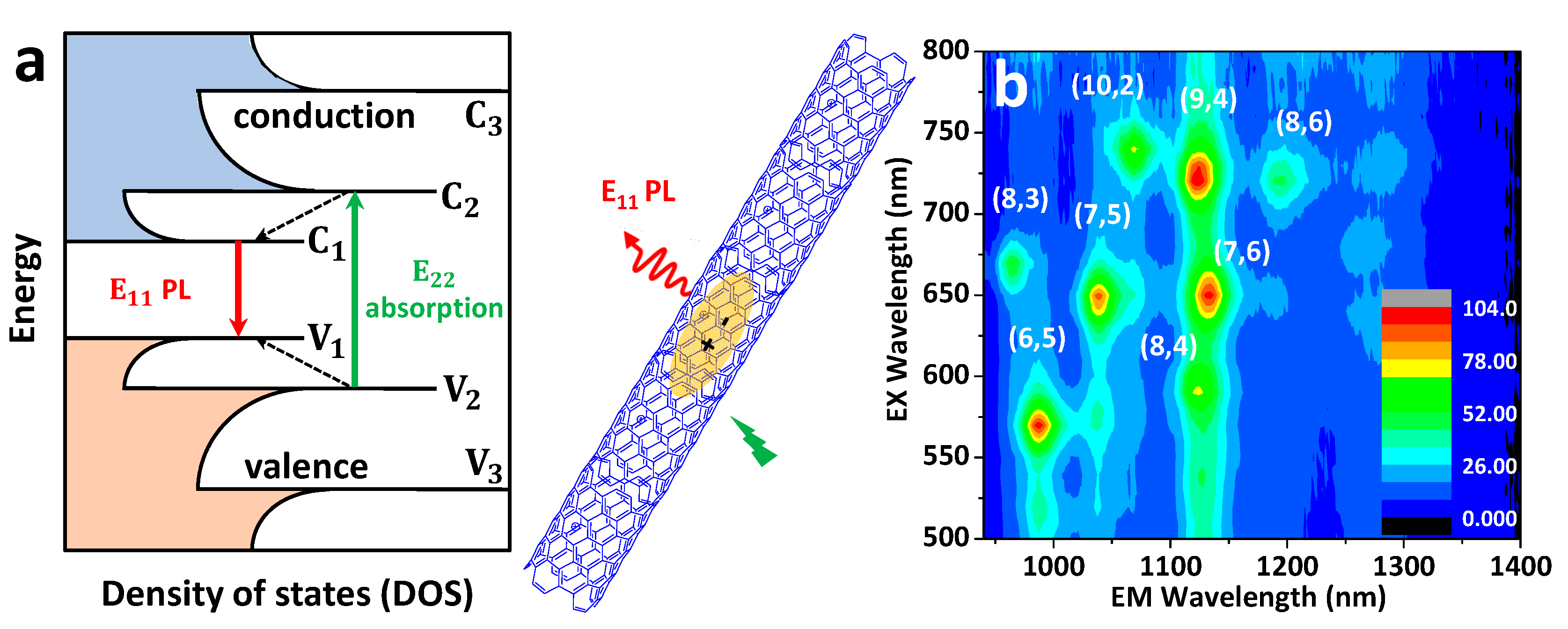
2.2. SWCNTs: Structure and Photophysical Properties
2.2.1. Pristine SWCNTs
2.2.2. Sp3 Defect-Functionalized SWCNTs
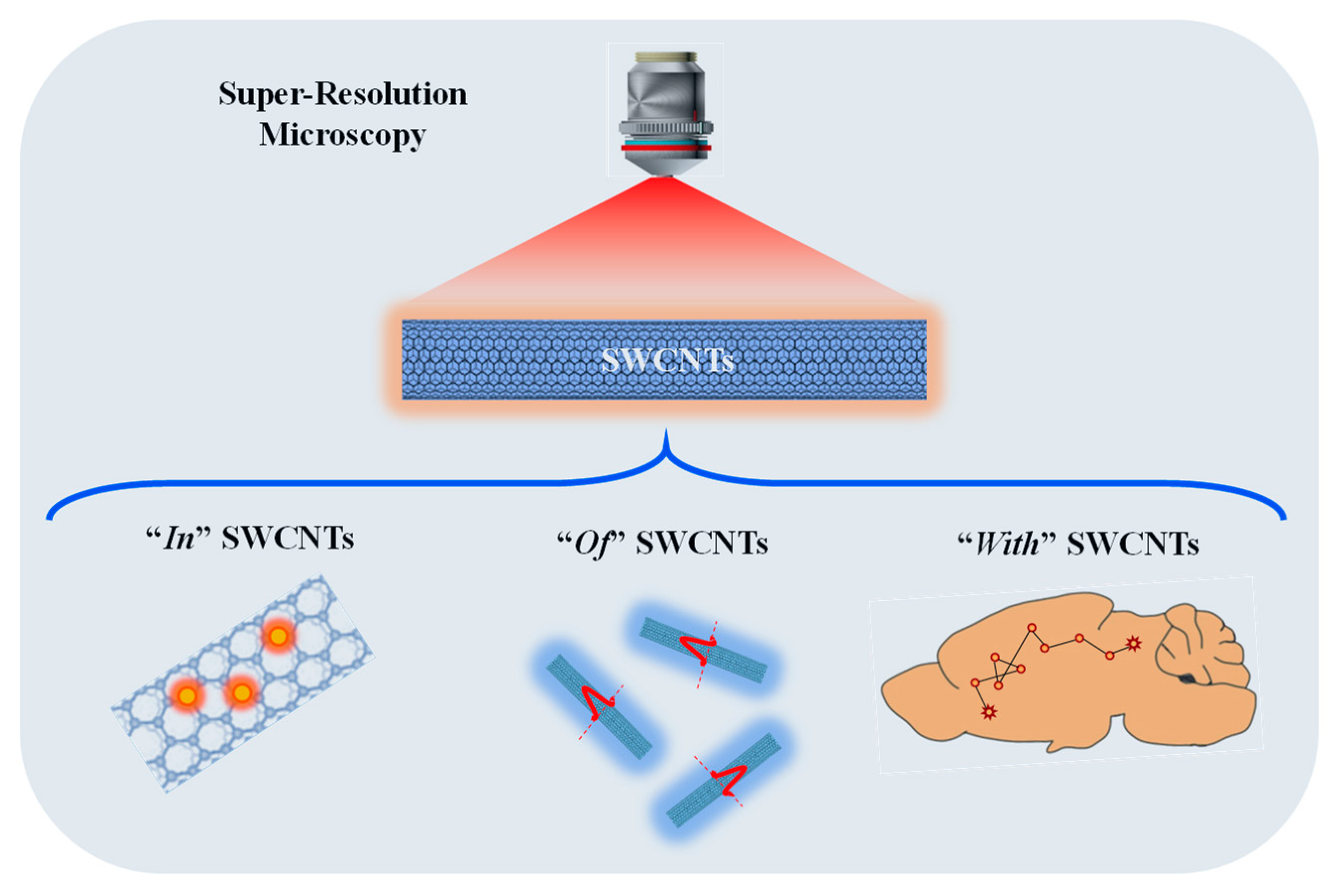
2.3. Super-Resolution Imaging “of” and “in” SWCNTs: Unveiling the Intra-Nanotube Features with Subwavelength Resolution
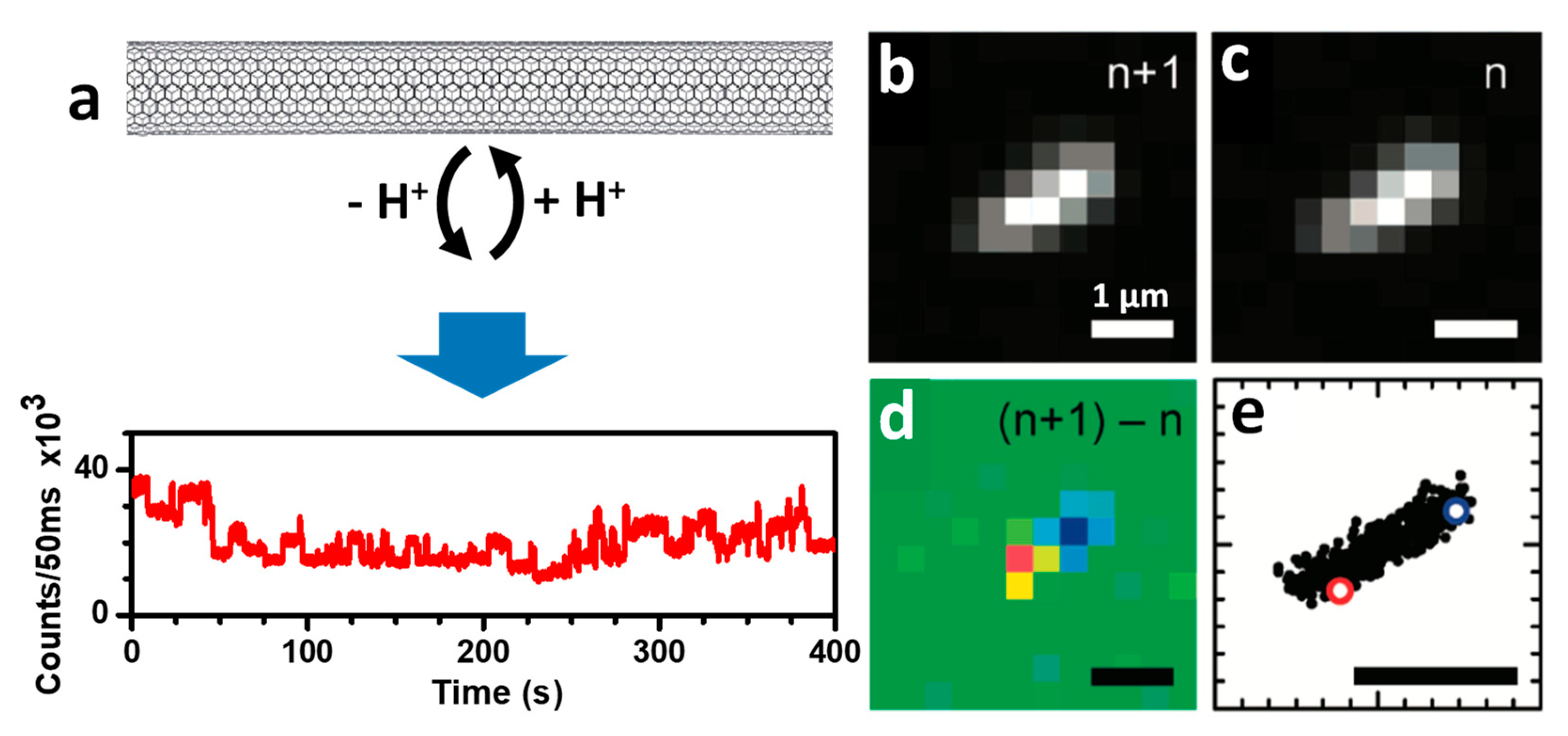
2.3.1. Localization of Bright Excitons in Pristine and Sp3 Defect-Functionalized SWCNTs
2.3.2. Super-Resolution Imaging of Individual SWCNTs Using Photocontrolled Luminescence Intermittency
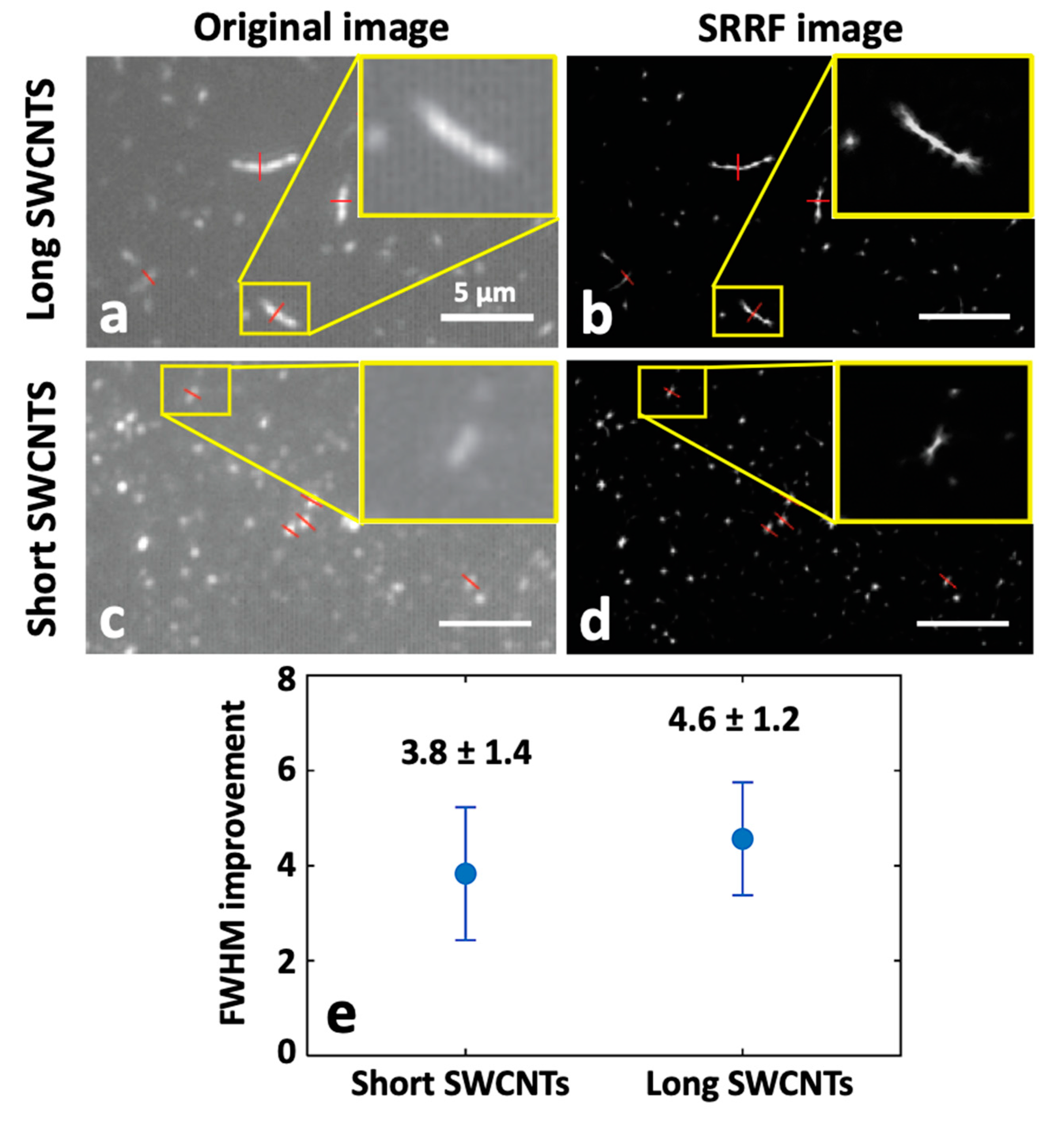
2.3.3. Super-Resolution Radial Fluctuation (SRRF) Nanoscopy of SWCNTs
2.4. Super Resolution “with” Carbon Nanotubes: Single-Particle Tracking (SPT) Provides a Nanoscale Map of Complex Architecture
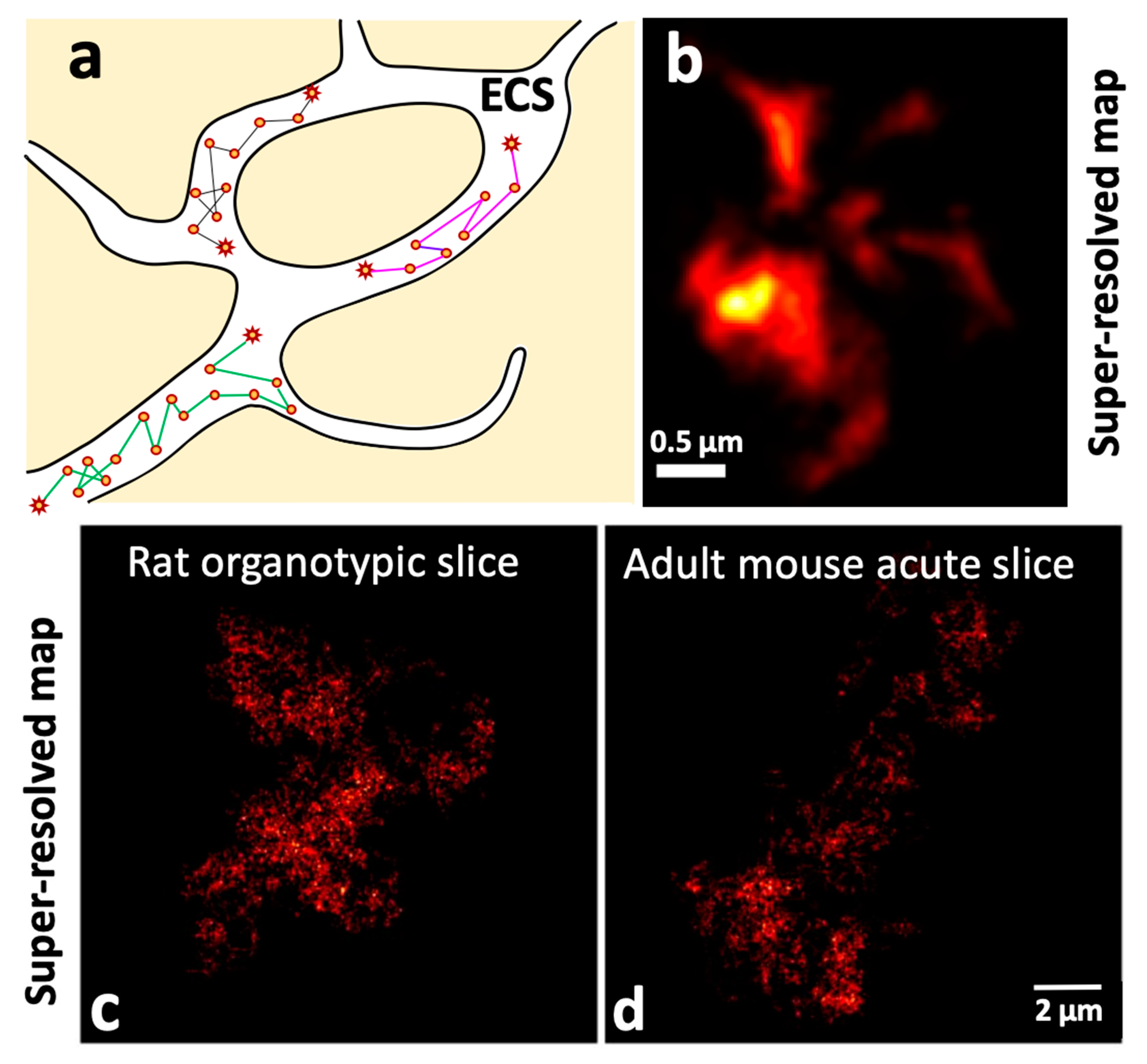
2.4.1. Unveiling the Extracellular Space (ECS) Features of the Brain at the Nanoscale Using Single Carbon Nanotube Diffusion
3. Conclusions and Future Outlook
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betzig, E. Nobel Lecture: Single Molecules, Cells, and Super-Resolution Optics. Rev. Mod. Phys. 2015, 87, 1153–1168. [Google Scholar] [CrossRef]
- Hell, S.W. Nobel Lecture: Nanoscopy with Freely Propagating Light. Rev. Mod. Phys. 2015, 87, 1169–1181. [Google Scholar] [CrossRef]
- Moerner, W.E. Nobel Lecture: Single-Molecule Spectroscopy, Imaging, and Photocontrol: Foundations for Super-Resolution Microscopy. Rev. Mod. Phys. 2015, 87, 1183–1212. [Google Scholar] [CrossRef] [Green Version]
- Sauer, M.; Heilemann, M. Single-Molecule Localization Microscopy in Eukaryotes. Chem. Rev. 2017, 117, 7478–7509. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Bates, M.; Zhuang, X. Super-Resolution Fluorescence Microscopy. Annu. Rev. Biochem. 2009, 78, 993–1016. [Google Scholar] [CrossRef] [Green Version]
- Godin, A.G.; Lounis, B.; Cognet, L. Super-Resolution Microscopy Approaches for Live Cell Imaging. Biophys. J. 2014, 107, 1777–1784. [Google Scholar] [CrossRef] [Green Version]
- Pujals, S.; Feiner-Gracia, N.; Delcanale, P.; Voets, I.; Albertazzi, L. Super-Resolution Microscopy as a Powerful Tool to Study Complex Synthetic Materials. Nat. Rev. Chem. 2019, 3, 68–84. [Google Scholar] [CrossRef] [Green Version]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G.P.C. Super-Resolution Microscopy Demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef]
- Sahl, S.J.; Hell, S.W.; Jakobs, S. Fluorescence Nanoscopy in Cell Biology. Nat. Rev. Mol. Cell Biol. 2017, 18, 685–701. [Google Scholar] [CrossRef]
- Feng, J.; Deschout, H.; Caneva, S.; Hofmann, S.; Lončarić, I.; Lazić, P.; Radenovic, A. Imaging of Optically Active Defects with Nanometer Resolution. Nano Lett. 2018, 18, 1739–1744. [Google Scholar] [CrossRef]
- Delcanale, P.; Miret-Ontiveros, B.; Arista-Romero, M.; Pujals, S.; Albertazzi, L. Nanoscale Mapping Functional Sites on Nanoparticles by Points Accumulation for Imaging in Nanoscale Topography (PAINT). ACS Nano 2018, 12, 7629–7637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lihter, M.; Chen, T.-H.; Macha, M.; Rayabharam, A.; Banjac, K.; Zhao, Y.; Wang, Z.; Zhang, J.; Comtet, J.; et al. Super-Resolved Optical Mapping of Reactive Sulfur-Vacancies in Two-Dimensional Transition Metal Dichalcogenides. ACS Nano 2021, 15, 7168–7178. [Google Scholar] [CrossRef] [PubMed]
- Cognet, L.; Tsyboulski, D.A.; Weisman, R.B. Subdiffraction Far-Field Imaging of Luminescent Single-Walled Carbon Nanotubes. Nano Lett. 2008, 8, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, C.; Yan, S.; Chen, T.; Fang, H.; Yuan, X. Wide-Field Super-Resolved Raman Imaging of Carbon Materials. ACS Photonics 2021, 8, 1801–1809. [Google Scholar] [CrossRef]
- Gustafsson, M.G.L. Surpassing the Lateral Resolution Limit by a Factor of Two Using Structured Illumination Microscopy. J. Microsc. 2000, 198, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Klar, T.A.; Jakobs, S.; Dyba, M.; Egner, A.; Hell, S.W. Fluorescence Microscopy with Diffraction Resolution Barrier Broken by Stimulated Emission. Proc. Natl. Acad. Sci. USA 2000, 97, 8206–8210. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, M.; Eggeling, C.; Jakobs, S.; Hell, S.W. Breaking the Diffraction Barrier in Fluorescence Microscopy at Low Light Intensities by Using Reversibly Photoswitchable Proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 17565–17569. [Google Scholar] [CrossRef] [Green Version]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-Diffraction-Limit Imaging by Stochastic Optical Reconstruction Microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Heilemann, M.; van de Linde, S.; Schüttpelz, M.; Kasper, R.; Seefeldt, B.; Mukherjee, A.; Tinnefeld, P.; Sauer, M. Subdiffraction-Resolution Fluorescence Imaging with Conventional Fluorescent Probes. Angew. Chem. Int. Ed. 2008, 47, 6172–6176. [Google Scholar] [CrossRef]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [Green Version]
- Hess, S.T.; Girirajan, T.P.K.; Mason, M.D. Ultra-High Resolution Imaging by Fluorescence Photoactivation Localization Microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharonov, A.; Hochstrasser, R.M. Wide-Field Subdiffraction Imaging by Accumulated Binding of Diffusing Probes. Proc. Natl. Acad. Sci. USA 2006, 103, 18911–18916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, M.G.L. Nonlinear Structured-Illumination Microscopy: Wide-Field Fluorescence Imaging with Theoretically Unlimited Resolution. Proc. Natl. Acad. Sci. USA 2005, 102, 13081–13086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- York, A.G.; Chandris, P.; Nogare, D.D.; Head, J.; Wawrzusin, P.; Fischer, R.S.; Chitnis, A.; Shroff, H. Instant Super-Resolution Imaging in Live Cells and Embryos via Analog Image Processing. Nat. Methods 2013, 10, 1122–1126. [Google Scholar] [CrossRef]
- Dyba, M.; Hell, S.W. Focal Spots of Size λ/23 Open Up Far-Field Florescence Microscopy at 33 Nm Axial Resolution. Phys. Rev. Lett. 2002, 88, 163901. [Google Scholar] [CrossRef] [Green Version]
- Jungmann, R.; Steinhauer, C.; Scheible, M.; Kuzyk, A.; Tinnefeld, P.; Simmel, F.C. Single-Molecule Kinetics and Super-Resolution Microscopy by Fluorescence Imaging of Transient Binding on DNA Origami. Nano Lett. 2010, 10, 4756–4761. [Google Scholar] [CrossRef]
- Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E.H.K. Optical Sectioning Deep Inside Live Embryos by Selective Plane Illumination Microscopy. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef] [Green Version]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer Resolution Imaging and Tracking of Fluorescent Molecules with Minimal Photon Fluxes. Science 2017, 355, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Dertinger, T.; Colyer, R.; Iyer, G.; Weiss, S.; Enderlein, J. Fast, Background-Free, 3D Super-Resolution Optical Fluctuation Imaging (SOFI). Proc. Natl. Acad. Sci. USA 2009, 106, 22287–22292. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, N.; Culley, S.; Ashdown, G.; Owen, D.M.; Pereira, P.M.; Henriques, R. Fast Live-Cell Conventional Fluorophore Nanoscopy with ImageJ through Super-Resolution Radial Fluctuations. Nat. Commun. 2016, 7, 12471. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Zhang, D.; Yang, J.; Zheng, M.; Li, Y. Chirality Pure Carbon Nanotubes: Growth, Sorting, and Characterization. Chem. Rev. 2020, 120, 2693–2758. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.H.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, J.; Metternich, J.T.; Herbertz, S.; Kruss, S. Biosensing with Fluorescent Carbon Nanotubes. Angew. Chemie Int. Ed. 2022, 61, e202112372. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef]
- He, X.; Htoon, H.; Doorn, S.K.; Pernice, W.H.P.; Pyatkov, F.; Krupke, R.; Jeantet, A.; Chassagneux, Y.; Voisin, C. Carbon Nanotubes as Emerging Quantum-Light Sources. Nat. Mater. 2018, 17, 663–670. [Google Scholar] [CrossRef]
- Lüer, L.; Hoseinkhani, S.; Polli, D.; Crochet, J.; Hertel, T.; Lanzani, G. Size and Mobility of Excitons in (6, 5) Carbon nanotubes. Nat. Phys. 2009, 5, 54–58. [Google Scholar] [CrossRef]
- Cognet, L.; Tsyboulski, D.A.; Rocha, J.-D.R.; Doyle, C.D.; Tour, J.M.; Weisman, R.B. Stepwise Quenching of Exciton Fluorescence in Carbon Nanotubes by Single-Molecule Reactions. Science 2007, 316, 1465–1468. [Google Scholar] [CrossRef] [Green Version]
- Siitonen, A.J.; Tsyboulski, D.A.; Bachilo, S.M.; Weisman, R.B. Surfactant-Dependent Exciton Mobility in Single-Walled Carbon Nanotubes Studied by Single-Molecule Reactions. Nano Lett. 2010, 10, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Spataru, C.D.; Ismail-Beigi, S.; Capaz, R.B.; Louie, S.G. Theory and Ab Initio Calculation of Radiative Lifetime of Excitons in Semiconducting Carbon Nanotubes. Phys. Rev. Lett. 2005, 95, 247402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisman, R.B.; Bachilo, S.M. Dependence of Optical Transition Energies on Structure for Single-Walled Carbon Nanotubes in Aqueous Suspension: An Empirical Kataura Plot. Nano Lett. 2003, 3, 1235–1238. [Google Scholar] [CrossRef]
- Tu, X.; Manohar, S.; Jagota, A.; Zheng, M. DNA Sequence Motifs for Structure-Specific Recognition and Separation of Carbon Nanotubes. Nature 2009, 460, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Crochet, J.; Clemens, M.; Hertel, T. Quantum Yield Heterogeneities of Aqueous Single-Wall Carbon Nanotube Suspensions. J. Am. Chem. Soc. 2007, 129, 8058–8059. [Google Scholar] [CrossRef] [PubMed]
- Berciaud, S.; Cognet, L.; Lounis, B. Luminescence Decay and the Absorption Cross Section of Individual Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2008, 101, 15–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholes, G.D.; Tretiak, S.; McDonald, T.J.; Metzger, W.K.; Engtrakul, C.; Rumbles, G.; Heben, M.J. Low-Lying Exciton States Determine the Photophysics of Semiconducting Single Wall Carbon Nanotubes. J. Phys. Chem. C 2007, 111, 11139–11149. [Google Scholar] [CrossRef]
- Perebeinos, V.; Avouris, P. Phonon and Electronic Nonradiative Decay Mechanisms of Excitons in Carbon Nanotubes. Phys. Rev. Lett. 2008, 101, 057401. [Google Scholar] [CrossRef] [Green Version]
- Crochet, J.J.; Duque, J.G.; Werner, J.H.; Lounis, B.; Cognet, L.; Doorn, S.K. Disorder Limited Exciton Transport in Colloidal Single-Wall Carbon Nanotubes. Nano Lett. 2012, 12, 5091–5096. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Danné, N.; Godin, A.G.; Lounis, B.; Cognet, L. Evaluation of Different Single-Walled Carbon Nanotube Surface Coatings for Single-Particle Tracking Applications in Biological Environments. Nanomaterials 2017, 7, 393. [Google Scholar] [CrossRef] [Green Version]
- Piao, Y.; Meany, B.; Powell, L.R.; Valley, N.; Kwon, H.; Schatz, G.C.; Wang, Y. Brightening of Carbon Nanotube Photoluminescence through the Incorporation of Sp3 Defects. Nat. Chem. 2013, 5, 840–845. [Google Scholar] [CrossRef]
- Kwon, H.; Furmanchuk, A.; Kim, M.; Meany, B.; Guo, Y.; Schatz, G.C.; Wang, Y. Molecularly Tunable Fluorescent Quantum Defects. J. Am. Chem. Soc. 2016, 138, 6878–6885. [Google Scholar] [CrossRef] [PubMed]
- Danné, N.; Kim, M.; Godin, A.G.; Kwon, H.; Gao, Z.; Wu, X.; Hartmann, N.F.; Doorn, S.K.; Lounis, B.; Wang, Y.; et al. Ultrashort Carbon Nanotubes That Fluoresce Brightly in the Near-Infrared. ACS Nano 2018, 12, 6059–6065. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Kim, M.; Fortner, J.; Qu, H.; Wang, Y. Fluorescent Ultrashort Nanotubes from Defect-Induced Chemical Cutting. Chem. Mater. 2019, 31, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zaric, S.; Daranciang, D.; Welsher, K.; Lu, Y.; Li, X.; Dai, H. Optical Properties of Ultrashort Semiconducting Single-Walled Carbon Nanotube Capsules Down to Sub-10 Nm. J. Am. Chem. Soc. 2008, 130, 6551–6555. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Oudjedi, L.; Faes, R.; Moroté, F.; Jaillet, C.; Poulin, P.; Lounis, B.; Cognet, L. Optical Detection of Individual Ultra-Short Carbon Nanotubes Enables Their Length Characterization down to 10 Nm. Sci. Rep. 2015, 5, 17093. [Google Scholar] [CrossRef] [Green Version]
- Dukovic, G.; White, B.E.; Zhou, Z.; Wang, F.; Jockusch, S.; Steigerwald, M.L.; Heinz, T.F.; Friesner, R.A.; Turro, N.J.; Brus, L.E. Reversible Surface Oxidation and Efficient Luminescence Quenching in Semiconductor Single-Wall Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 15269–15276. [Google Scholar] [CrossRef]
- Giannone, G.; Hosy, E.; Levet, F.; Constals, A.; Schulze, K.; Sobolevsky, A.I.; Rosconi, M.P.; Gouaux, E.; Tampé, R.; Choquet, D.; et al. Dynamic Superresolution Imaging of Endogenous Proteins on Living Cells at Ultra-High Density. Biophys. J. 2010, 99, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Schnitzbauer, J.; Strauss, M.T.; Schlichthaerle, T.; Schueder, F.; Jungmann, R. Super-Resolution Microscopy with DNA-PAINT. Nat. Protoc. 2017, 12, 1198–1228. [Google Scholar] [CrossRef]
- Kim, M.; Adamska, L.; Hartmann, N.F.; Kwon, H.; Liu, J.; Velizhanin, K.A.; Piao, Y.; Powell, L.R.; Meany, B.; Doorn, S.K.; et al. Fluorescent Carbon Nanotube Defects Manifest Substantial Vibrational Reorganization. J. Phys. Chem. C 2016, 120, 11268–11276. [Google Scholar] [CrossRef]
- Hartmann, N.F.; Velizhanin, K.A.; Haroz, E.H.; Kim, M.; Ma, X.; Wang, Y.; Htoon, H.; Doorn, S.K. Photoluminescence Dynamics of Aryl Sp3 Defect States in Single-Walled Carbon Nanotubes. ACS Nano 2016, 10, 8355–8365. [Google Scholar] [CrossRef]
- Wu, X.; Kim, M.; Qu, H.; Wang, Y.H. Single-Defect Spectroscopy in the Shortwave Infrared. Nat. Commun. 2019, 10, 2672. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, C.; Claude, T.; Borel, A.; Amara, M.R.; Graf, A.; Zaumseil, J.; Lauret, J.S.; Chassagneux, Y.; Voisin, C. Superlocalization of Excitons in Carbon Nanotubes at Cryogenic Temperature. Nano Lett. 2019, 19, 7210–7216. [Google Scholar] [CrossRef] [PubMed]
- Oijen, A.M.V.; Köhler, J.; Schmidt, J.; Müller, M.; Brakenhoff, G.J. 3-Dimensional super-resolution by spectrally selective imaging. Chem. Phys. Lett. 1998, 292, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Godin, A.G.; Setaro, A.; Gandil, M.; Haag, R.; Adeli, M.; Reich, S.; Cognet, L. Photoswitchable Single-Walled Carbon Nanotubes for Super-Resolution Microscopy in the near-Infrared. Sci. Adv. 2019, 5, eaax1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setaro, A.; Bluemmel, P.; Maity, C.; Hecht, S.; Reich, S. Non-Covalent Functionalization of Individual Nanotubes with Spiropyran-Based Molecular Switches. Adv. Func. Mat. 2012, 22, 2425–2431. [Google Scholar] [CrossRef]
- Chung, S.H.; Kennedy, R.A. Forward-Backward Non-Linear Filtering Technique for Extracting Small Biological Signals from Noise. J. Neurosc. Methods 1991, 40, 71–86. [Google Scholar] [CrossRef]
- Culley, S.; Tosheva, K.L.; Matos Pereira, P.; Henriques, R. SRRF: Universal Live-Cell Super-Resolution Microscopy. Int. J. Biochem. Cell Biol. 2018, 101, 74–79. [Google Scholar] [CrossRef]
- Ehrlich, R.; Wulf, V.; Hendler-Neumark, A.; Kagan, B.; Bisker, G. Super-Resolution Radial Fluctuations (SRRF) Nanoscopy in the near Infrared. Opt. Express 2022, 30, 1130. [Google Scholar] [CrossRef]
- Fakhri, N.; MacKintosh, F.C.; Lounis, B.; Cognet, L.; Pasquali, M. Brownian Motion of Stiff Filaments in a Crowded Environment. Science 2010, 330, 1804–1808. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Tauzin, L.J.; Baiyasi, R.; Wang, W.; Moringo, N.; Shuang, B.; Landes, C.F. Single Particle Tracking: From Theory to Biophysical Applications. Chem. Rev. 2017, 117, 7331–7376. [Google Scholar] [CrossRef]
- Nandi, S.; Parui, S.; Jana, B.; Bhattacharyya, K. Local Environment of Organic Dyes in an Ionic Liquid-Water Mixture: FCS and MD Simulation. J. Chem. Phys. 2018, 149, 054501. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Medina, S.; Chen, S.; Blankenburg, J.; Swanglap, P.; Landes, C.F.; Link, S. Measuring the Hydrodynamic Size of Nanoparticles Using Fluctuation Correlation Spectroscopy. Annu. Rev. Phys. Chem. 2016, 67, 489–514. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.J.; Reits, E.A.J. From Fixed to FRAP: Measuring Protein Mobility and Activity in Living Cells. Nat. Cell Biol. 2001, 3, E145–E147. [Google Scholar]
- Schütz, G.J.; Schindler, H.; Schmidt, T. Single-Molecule Microscopy on Model Membranes Reveals Anomalous Diffusion. Biophys. J. 1997, 73, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Peters, I.M.; de Grooth, B.G.; Schins, J.M.; Figdor, C.G.; Greve, J. Three Dimensional Single-Particle Tracking with Nanometer Resolution. Rev. Sci. Instrum. 1998, 69, 2762–2766. [Google Scholar] [CrossRef]
- Mortensen, K.I.; Churchman, L.S.; Spudich, J.A.; Flyvbjerg, H. Optimized Localization Analysis for Single-Molecule Tracking and Super-Resolution Microscopy. Nat. Methods 2010, 7, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Tsekouras, K.; Calderon, C.; Bustamante, C.; Pressé, S. Unraveling the Thousand Word Picture: An Introduction to Super-Resolution Data Analysis. Chem. Rev. 2017, 117, 7276–7330. [Google Scholar] [CrossRef] [Green Version]
- Werley, C.A.; Moerner, W.E. Single-Molecule Nanoprobes Explore Defects in Spin-Grown Crystal. J. Phys. Chem. B 2006, 110, 18939–18944. [Google Scholar] [CrossRef]
- Zürner, A.; Kirstein, J.; Döblinger, M.; Bräuchle, C.; Bein, T. Visualizing Single-Molecule Diffusion in Mesoporous Materials. Nature 2007, 450, 705–708. [Google Scholar] [CrossRef]
- Kirstein, J.; Platschek, B.; Jung, C.; Brown, R.; Bein, T.; Bräuchle, C. Exploration of Nanostructured Channel Systems with Single-Molecule Probes. Nat. Mater. 2007, 6, 303–310. [Google Scholar] [CrossRef]
- Roeffaers, M.B.J.; Sels, B.F.; Uji-i, H.; De Schryver, F.C.; Jacobs, P.A.; De Vos, D.E.; Hofkens, J. Spatially Resolved Observation of Crystal-Face-Dependent Catalysis by Single Turnover Counting. Nature 2006, 439, 572–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhri, N.; Wessel, A.D.; Willms, C.; Pasquali, M.; Klopfenstein, D.R.; MacKintosh, F.C.; Schmidt, C.F. High-Resolution Mapping of Intracellular Fluctuations Using Carbon Nanotubes. Science 2014, 344, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Godin, A.G.; Varela, J.A.; Gao, Z.; Danné, N.; Dupuis, J.P.; Lounis, B.; Groc, L.; Cognet, L. Single-Nanotube Tracking Reveals the Nanoscale Organization of the Extracellular Space in the Live Brain. Nat. Nanotechnol. 2017, 12, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrabetova, S.; Cognet, L.; Rusakov, D.A.; Nägerl, U.V. Unveiling the Extracellular Space of the Brain: From Super-Resolved Microstructure to in Vivo Function. J. Neurosc. 2018, 38, 9355–9363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paviolo, C.; Soria, F.N.; Ferreira, J.S.; Lee, A.; Groc, L.; Bezard, E.; Cognet, L. Nanoscale Exploration of the Extracellular Space in the Live Brain by Combining Single Carbon Nanotube Tracking and Super-Resolution Imaging Analysis. Methods 2020, 174, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.N.; Paviolo, C.; Doudnikoff, E.; Arotcarena, M.L.; Lee, A.; Danné, N.; Mandal, A.K.; Gosset, P.; Dehay, B.; Groc, L.; et al. Synucleinopathy Alters Nanoscale Organization and Diffusion in the Brain Extracellular Space through Hyaluronan Remodeling. Nat. Commun. 2020, 11, 3440. [Google Scholar] [CrossRef]
- Paviolo, C.; Cognet, L. Near-Infrared Nanoscopy with Carbon-Based Nanoparticles for the Exploration of the Brain Extracellular Space. Neurobiol. Dis. 2021, 153, 105328. [Google Scholar] [CrossRef]
- Bourdenx, M.; Nioche, A.; Dovero, S.; Arotcarena, M.L.; Camus, S.; Porras, G.; Thiolat, M.L.; Rougier, N.P.; Prigent, A.; Aubert, P.; et al. Identification of Distinct Pathological Signatures Induced by Patient-Derived α-Synuclein Structures in Nonhuman Primates. Sci. Adv. 2020, 6, eaaz9165. [Google Scholar] [CrossRef]
- Dong, B.; Soetikno, B.T.; Chen, X.; Backman, V.; Sun, C.; Zhang, H.F. Parallel Three-Dimensional Tracking of Quantum Rods Using Polarization-Sensitive Spectroscopic Photon Localization Microscopy. ACS Photonics 2017, 4, 1747–1752. [Google Scholar] [CrossRef]
- Kim, D.; Zhang, Z.; Xu, K. Spectrally Resolved Super-Resolution Microscopy Unveils Multipath Reaction Pathways of Single Spiropyran Molecules. J. Am. Chem. Soc. 2017, 139, 9447–9450. [Google Scholar] [CrossRef]
- Comtet, J.; Glushkov, E.; Navikas, V.; Feng, J.; Babenko, V.; Hofmann, S.; Watanabe, K.; Taniguchi, T.; Radenovic, A. Wide-Field Spectral Super-Resolution Mapping of Optically Active Defects in Hexagonal Boron Nitride. Nano Lett. 2019, 19, 2516–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bon, P.; Linarès-Loyez, J.; Feyeux, M.; Alessandri, K.; Lounis, B.; Nassoy, P.; Cognet, L. Self-Interference 3D Super-Resolution Microscopy for Deep Tissue Investigations. Nat. Methods 2018, 15, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Von Diezmann, L.; Shechtman, Y.; Moerner, W.E. Three-Dimensional Localization of Single Molecules for Super-Resolution Imaging and Single-Particle Tracking. Chem. Rev. 2017, 117, 7244–7275. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandi, S.; Caicedo, K.; Cognet, L. When Super-Resolution Localization Microscopy Meets Carbon Nanotubes. Nanomaterials 2022, 12, 1433. https://doi.org/10.3390/nano12091433
Nandi S, Caicedo K, Cognet L. When Super-Resolution Localization Microscopy Meets Carbon Nanotubes. Nanomaterials. 2022; 12(9):1433. https://doi.org/10.3390/nano12091433
Chicago/Turabian StyleNandi, Somen, Karen Caicedo, and Laurent Cognet. 2022. "When Super-Resolution Localization Microscopy Meets Carbon Nanotubes" Nanomaterials 12, no. 9: 1433. https://doi.org/10.3390/nano12091433
APA StyleNandi, S., Caicedo, K., & Cognet, L. (2022). When Super-Resolution Localization Microscopy Meets Carbon Nanotubes. Nanomaterials, 12(9), 1433. https://doi.org/10.3390/nano12091433