Thy-AuNP-AgNP Hybrid Systems for Colorimetric Determination of Copper (II) Ions Using UV-Vis Spectroscopy and Smartphone-Based Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus and Measurements
2.3. Detection of Cu2+
2.4. Smartphone Application for Cu2+ Detection
2.5. Real Water Analysis
3. Results and Discussion
3.1. Principle for Cu2+ Detection
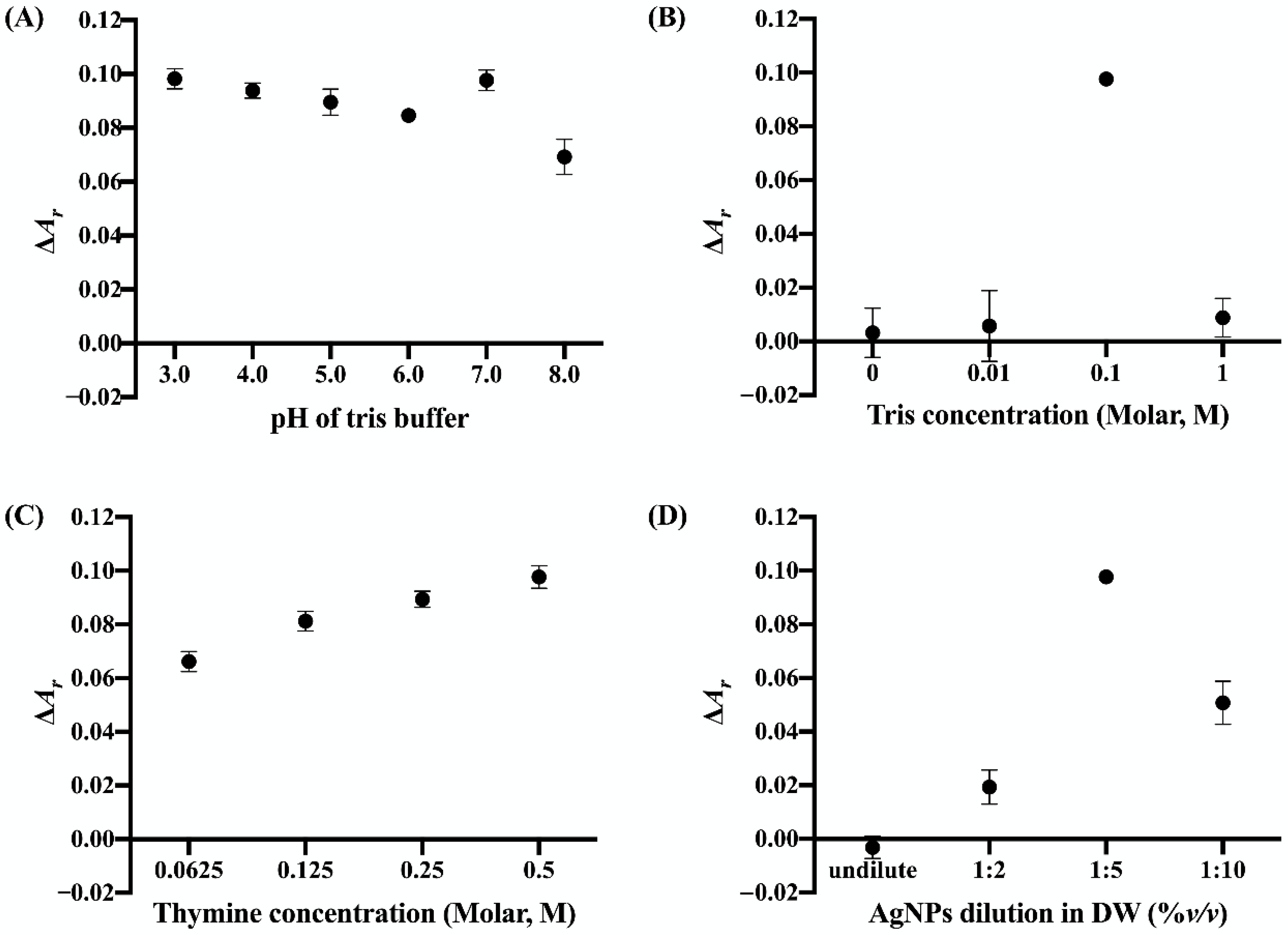
3.2. Optimization of Cu2+ Sensing Conditions
3.2.1. pH of Tris Buffer
3.2.2. Tris Buffer Concentration
3.2.3. Thymine Concentration
3.2.4. AgNP Dilution
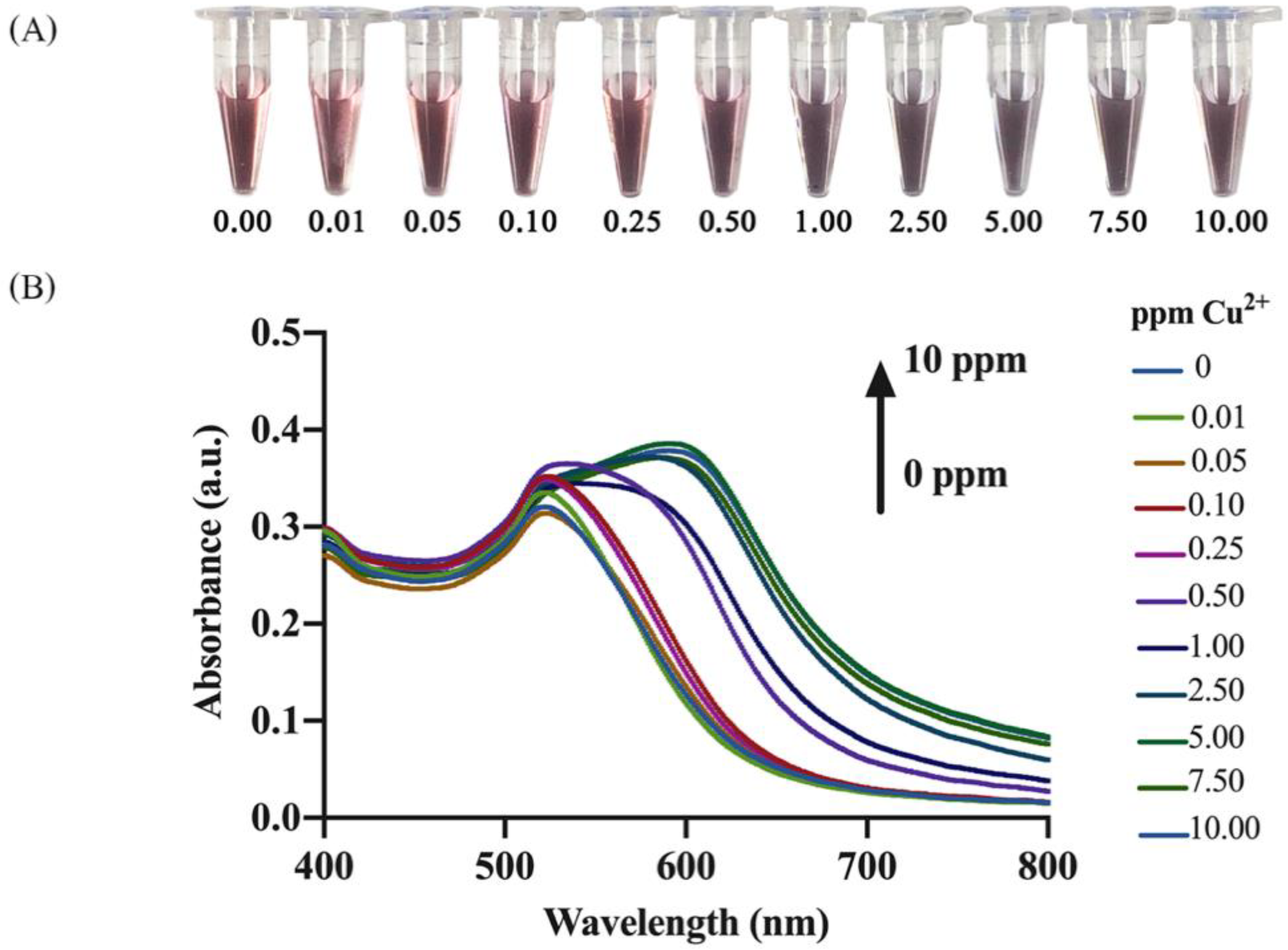
3.3. Analysis of Cu2+
3.3.1. Naked Eye Detection and Spectrophotometric Quantification
3.3.2. Smartphone-Based Detection
3.4. Selectivity Test
3.5. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latorre, M.; Troncoso, R.; Uauy, R. Biological Aspects of Copper. In Clinical and Translational Perspectives on Wilson Disease; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 4; pp. 25–31. [Google Scholar]
- Olivares, M.; Uauy, R. Copper as an essential nutrient. Am. J. Clin. Nutr. 1996, 63, 791S–796S. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R. Copper. In Present Knowledge in Nutrition; Wiley: Hoboken, NJ, USA, 2012; pp. 540–553. [Google Scholar]
- Siotto, M.; Squitti, R. Copper imbalance in Alzheimer’s disease: Overview of the exchangeable copper component in plasma and the intriguing role albumin plays. Coord. Chem. Rev. 2018, 371, 86–95. [Google Scholar] [CrossRef]
- Borchard, S.; Bork, F.; Rieder, T.; Eberhagen, C.; Popper, B.; Lichtmannegger, J.; Schmitt, S.; Adamski, J.; Klingenspor, M.; Weiss, K.H.; et al. The exceptional sensitivity of brain mitochondria to copper. Toxicol. In Vitro 2018, 51, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chang, S.; Li, Z.; Zhang, X.; Hu, X.; Zhang, H. Gamma-radiation assisted preparation of Au/Fe3O4/poly (styrene-sodium styrene sulphonate) magnetic composite microspheres for catalysis. J. Radioanal. Nucl. Chem. 2020, 325, 453–462. [Google Scholar] [CrossRef]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments-A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef]
- Meseldzija, S.; Petrovic, J.; Onjia, A.; Volkov-Husovic, T.; Nesic, A.; Vukelic, N. Utilization of agro-industrial waste for removal of copper ions from aqueous solutions and mining-wastewater. J. Ind. Eng. Chem. 2019, 75, 246–252. [Google Scholar] [CrossRef]
- Olaniyi, I.; Raphael, O.; Nwadiogbu, J.O. Effect of Industrial Effluent on the Surrounding Environment. Arch. Appl. Sci. Res. 2012, 4, 406–413. [Google Scholar]
- English.Arnika.org. Available online: https://english.arnika.org/press-releases/thailand-high-concentration-of-heavy-metals-found-in-water (accessed on 15 October 2021).
- Yolcubal, I.; Demiray, A.D.; Çiftçi, E.; Sanğu, E. Environmental impact of mining activities on surface water and sediment qualities around Murgul copper mine, Northeastern Turkey. Environ. Earth Sci. 2016, 75, 1415. [Google Scholar] [CrossRef]
- Mingkhwan, R.; Worakhunpiset, S. Heavy Metal Contamination Near Industrial Estate Areas in Phra Nakhon Si Ayutthaya Province, Thailand and Human Health Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 1890. [Google Scholar] [CrossRef] [Green Version]
- Thongra-ar, W.; Musika, C.; Wongsudawan, W.; Munhapon, A. Heavy Metals Contamination in Sediments along the Eastern Coast of the Gulf of Thailand. Environmentasia 2008, 1, 37–45. [Google Scholar]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp132.pdf (accessed on 15 October 2021).
- Zaitsev, N.; Stanev, D.; Stancheva, K.; Trifonova, V.; Shivacheva, V.; Jecheva, H. Simultaneous Determination of Copper and Lead in Environmental Samples by Anodic Stripping Voltammetry. Donnish J. Pure Appl. Chem. 2017, 3, 001–006. [Google Scholar]
- Kitte, S.A.; Li, S.; Nsabimana, A.; Gao, W.; Lai, J.; Liu, Z.; Xu, G. Stainless steel electrode for simultaneous stripping analysis of Cd(II), Pb(II), Cu(II) and Hg(II). Talanta 2019, 191, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Shi, B.; Liao, S.; Qin, Y.; Zhang, L.; Huang, M.; Zhao, S. Facile preparation of fluorescent polydihydroxyphenylalanine nanoparticles for label-free detection of copper ions. Sens. Actuators B Chem. 2016, 225, 334–339. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.; Pu, S.; Ding, H.; Li, G. A highly selective fluorescent chemosensor for Cu2+ based on a new diarylethene with triazole-linked fluorescein. Tetrahedron 2016, 72, 985–991. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, S.Y.; Ahn, H.M.; Kim, C. Sequential detection of copper(II) and cyanide by a simple colorimetric chemosensor. Inorg. Chem. Commun. 2016, 74, 62–65. [Google Scholar] [CrossRef]
- Shen, Q.; Li, W.; Tang, S.; Hu, Y.; Nie, Z.; Huang, Y.; Yao, S. A simple “clickable” biosensor for colorimetric detection of copper(II) ions based on unmodified gold nanoparticles. Biosens. Bioelectron. 2013, 41, 663–668. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, Y.; Liu, C.; Zhou, X.; Shi, H. Label-free colorimetric detection of Cu2+ on the basis of Fenton reaction-assisted signal amplification with unmodified gold nanoparticles as indicator. Sens. Actuators B Chem. 2015, 215, 561–567. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors—A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Noah, N. Green synthesis: Characterization and application of silver and gold nanoparticles. In Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 6; pp. 111–135. [Google Scholar]
- Diamai, S.; Negi, D.P.S. Cysteine-stabilized silver nanoparticles as a colorimetric probe for the selective detection of cysteamine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 215, 203–208. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, W.; Sun, M.; Liu, X.; Zhang, Q.; Zong, C.; Qu, J.; Gai, H. Colorimetric detection of Cu2+ by surface coordination complexes of polyethyleneimine-capped Au nanoparticles. Sens. Actuators B Chem. 2016, 223, 411–416. [Google Scholar] [CrossRef]
- Hormozi-Nezhad, M.R.; Abbasi-Moayed, S. A sensitive and selective colorimetric method for detection of copper ions based on anti-aggregation of unmodified gold nanoparticles. Talanta 2014, 129, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Niu, H.; Cai, Y. Colorimetric detection of copper ions in tap water during the synthesis of silver/dopamine nanoparticles. Chem. Commun. 2011, 47, 12643–12645. [Google Scholar] [CrossRef] [PubMed]
- Ghodake, G.S.; Shinde, S.K.; Saratale, R.G.; Kadam, A.A.; Saratale, G.D.; Syed, A.; Ameen, F.; Kim, D.Y. Colorimetric detection of Cu2+ based on the formation of peptide–copper complexes on silver nanoparticle surfaces. Beilstein J. Nanotechnol. 2018, 9, 1414–1422. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, W.; Hillerbrand, M.; Klenert, M. Charge-transfer interactions between nucleobases and metal ions: ESR and optical absorption studies. Z. Naturforsch. C Biosci. 1974, 29, 667–671. [Google Scholar] [CrossRef] [Green Version]
- Russo, N.; Toscano, M.; Grand, A. Gas-phase theoretical prediction of the metal affinity of copper(I) ion for DNA and RNA bases. J. Mass Spectrom. 2003, 38, 265–270. [Google Scholar] [CrossRef]
- Fattahi, A.; Jahromi, M.S.F. DFT Study of the Interaction of Thymine with Cu+ and Zn2+. Sci. Iran. 2009, 16, 75–80. [Google Scholar]
- Salcedo, A.R.M.; Sevilla, F.B., III. Citrate-capped gold nanoparticles as colorimetric reagent for copper (II) ions. Philipp. Sci. Lett. 2013, 6, 90–96. [Google Scholar]
- Wagers, K.; Chui, T.; Adem, S. Effect of pH on the stability of gold nanoparticles and their application for melamine detection in infant formula. IOSR J. Appl. Chem. 2014, 7, 15–20. [Google Scholar] [CrossRef]
- Sivaraman, S.K.; Kumar, S.; Santhanam, V. Monodisperse sub-10 nm gold nanoparticles by reversing the order of addition in Turkevich method–The role of chloroauric acid. J. Colloid Interface Sci. 2011, 361, 543–547. [Google Scholar] [CrossRef]
- Franco-Ulloa, S.; Tatulli, G.; Bore, S.L.; Moglianetti, M.; Pompa, P.P.; Cascella, M.; De Vivo, M. Dispersion state phase diagram of citrate-coated metallic nanoparticles in saline solutions. Nat. Commun. 2020, 11, 5422. [Google Scholar] [CrossRef] [PubMed]
- Hammud, H.H.; El-Dakdouki, M.H.; Sonji, N.; Sonji, G.; Bouhadir, K.H. Interactions of Some Divalent Metal Ions with Thymine and Uracil Thiosemicarbazide Derivatives. Nucleosides Nucleotides Nucleic Acids 2016, 35, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.H.; Kraatz, H.B. Interactions of Metal Ions with DNA and Some Applications. J. Inorg. Organomet. Polym. Mater. 2012, 23, 4–23. [Google Scholar] [CrossRef]
- Deng, H.H.; Li, G.W.; Liu, A.L.; Chen, W.; Lin, X.H.; Xia, X.H. Thermally treated bare gold nanoparticles for colorimetric sensing of copper ions. Microchim. Acta 2014, 181, 911–916. [Google Scholar] [CrossRef]
- Fernando, I.; Zhou, Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere 2019, 216, 297–305. [Google Scholar] [CrossRef]
- EPA. Available online: https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants#Secondary (accessed on 10 October 2021).
- Fuller, M.; Köper, I. Polyelectrolyte-coated gold nanoparticles: The effect of salt and polyelectrolyte concentration on colloidal stability. Polymers 2018, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 10 October 2021).
- Ye, Y.; Guo, Y.; Yue, Y.; Huang, H.; Zhao, L.; Gao, Y.; Zhang, Y. Colorimetric sensing of copper ions based on the anti-aggregation of unmodified silver nanoparticles in the presence of 1, 4-dithiothreitol. Anal. Methods 2015, 7, 566–572. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Zhang, K.; Jiang, X. Visual detection of copper (II) by azide-and alkyne-functionalized gold nanoparticles using click chemistry. Angew. Chem. 2008, 120, 7564–7566. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Colorimetric Cu2+ detection with a ligation DNAzyme and nanoparticles. Chem. Commun. 2007, 46, 4872–4874. [Google Scholar] [CrossRef]
- Maiti, S.; Barman, G.; Laha, J.K. Detection of heavy metals (Cu+2, Hg+2) by biosynthesized silver nanoparticles. Appl. Nanosci. 2016, 6, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Kwon, M.; Lee, J.Y.; Won, W.Y.; Park, J.W.; Min, J.A.; Hahn, C.; Gu, X.; Choi, J.H.; Kim, D.J. Development and validation of a smartphone addiction scale (SAS). PLoS ONE 2013, 8, e56936. [Google Scholar] [CrossRef] [PubMed]
- Samaha, M.; Hawi, N.S. Relationships among smartphone addiction, stress, academic performance, and satisfaction with life. Comput. Hum. Behav. 2016, 57, 321–325. [Google Scholar] [CrossRef]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Puri, A. A review of permissible limits of drinking water. Indian J. Occup. Environ. Med. 2012, 16, 40–44. [Google Scholar] [PubMed] [Green Version]
- Purushotham, D.; Rashid, M.; Lone, M.A.; Rao, A.N.; Ahmed, S.; Nagaiah, E.; Dar, F.A. Environmental impact assessment of air and heavy metal concentration in groundwater of Maheshwaram watershed, Ranga Reddy district, Andhra Pradesh. J. Geol. Soc. India 2013, 81, 385–396. [Google Scholar] [CrossRef]
- Fao.org. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B90-2017%252FCXG_090e.pdf (accessed on 15 October 2021).






| Detection Probe | LOD (ppm) | Detection Time (min) | Reference |
|---|---|---|---|
| AuNPs | 320 | 6 | [33] |
| Alkyne-AuNPs | 3.2 | N/A | [45] |
| DNA-functionalized AgNPs | 0.32 | N/A | [46] |
| AgNPs | 0.0064 | 30 | [44] |
| Casein peptide- functionalized AgNPs | 0.01 | 20 | [29] |
| MPD-AgNPs | 1.28 | 10 | [47] |
| Thy-AuNPs-AgNPs | 0.03 (UV-vis spectrometry) 0.09 (Smartphone) | 15 | This work |
| Samples | Final Conc. (ppm) | UV-Vis Spectrophotometer | RGB Application | ||||
|---|---|---|---|---|---|---|---|
| Detection Value (ppm) | RSD (%) | Recovery (%) | Detection Value (ppm) | RSD (%) | Recovery (%) | ||
| 1 | 0 | ND | ND | ND | ND | ND | ND |
| 2 | 0.2 | 0.140 | 3.21 | 70.14 | 0.203 | 9.49 | 101.74 |
| 3 | 0.4 | 0.336 | 17.63 | 84.01 | 0.361 | 8.71 | 90.32 |
| 4 | 1.0 | 0.835 | 14.51 | 83.60 | 1.036 | 6.26 | 103.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongkam, T.; Apilux, A.; Tusai, T.; Parnklang, T.; Kladsomboon, S. Thy-AuNP-AgNP Hybrid Systems for Colorimetric Determination of Copper (II) Ions Using UV-Vis Spectroscopy and Smartphone-Based Detection. Nanomaterials 2022, 12, 1449. https://doi.org/10.3390/nano12091449
Thongkam T, Apilux A, Tusai T, Parnklang T, Kladsomboon S. Thy-AuNP-AgNP Hybrid Systems for Colorimetric Determination of Copper (II) Ions Using UV-Vis Spectroscopy and Smartphone-Based Detection. Nanomaterials. 2022; 12(9):1449. https://doi.org/10.3390/nano12091449
Chicago/Turabian StyleThongkam, Thitiporn, Amara Apilux, Thitaporn Tusai, Tewarak Parnklang, and Sumana Kladsomboon. 2022. "Thy-AuNP-AgNP Hybrid Systems for Colorimetric Determination of Copper (II) Ions Using UV-Vis Spectroscopy and Smartphone-Based Detection" Nanomaterials 12, no. 9: 1449. https://doi.org/10.3390/nano12091449








