Gold Nanoparticle-Mediated Lateral Flow Assays for Detection of Host Antibodies and COVID-19 Proteins
Abstract
:1. Introduction
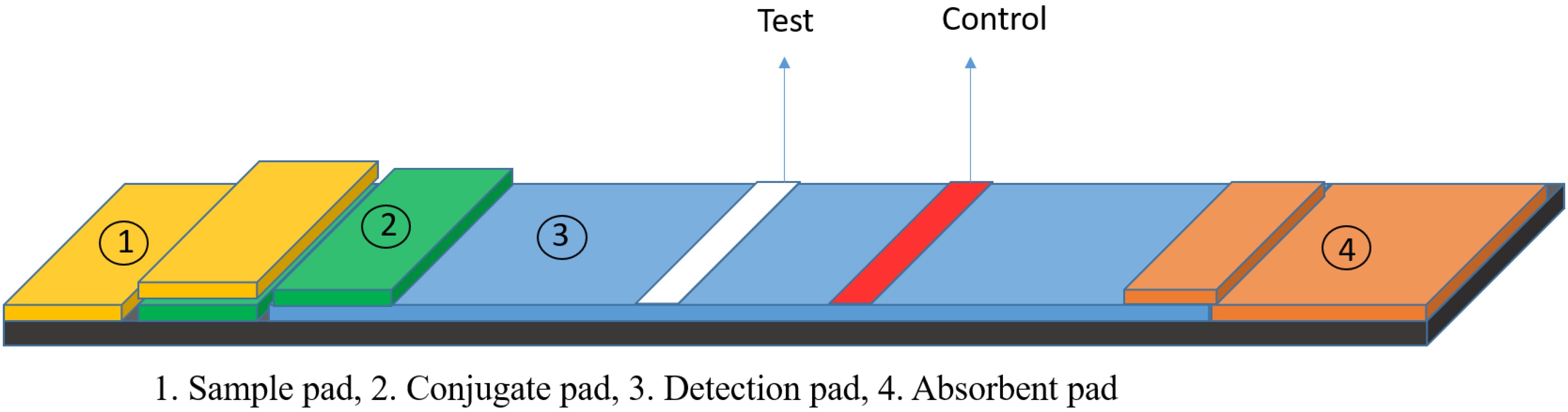
2. Lateral Flow Assays
3. GNP-Based LFAs
4. Gold-Based LFA for COVID-19 Diagnosis
4.1. Detection of Host Antibody
4.2. Detection of Antigen
5. Limitations and Solutions
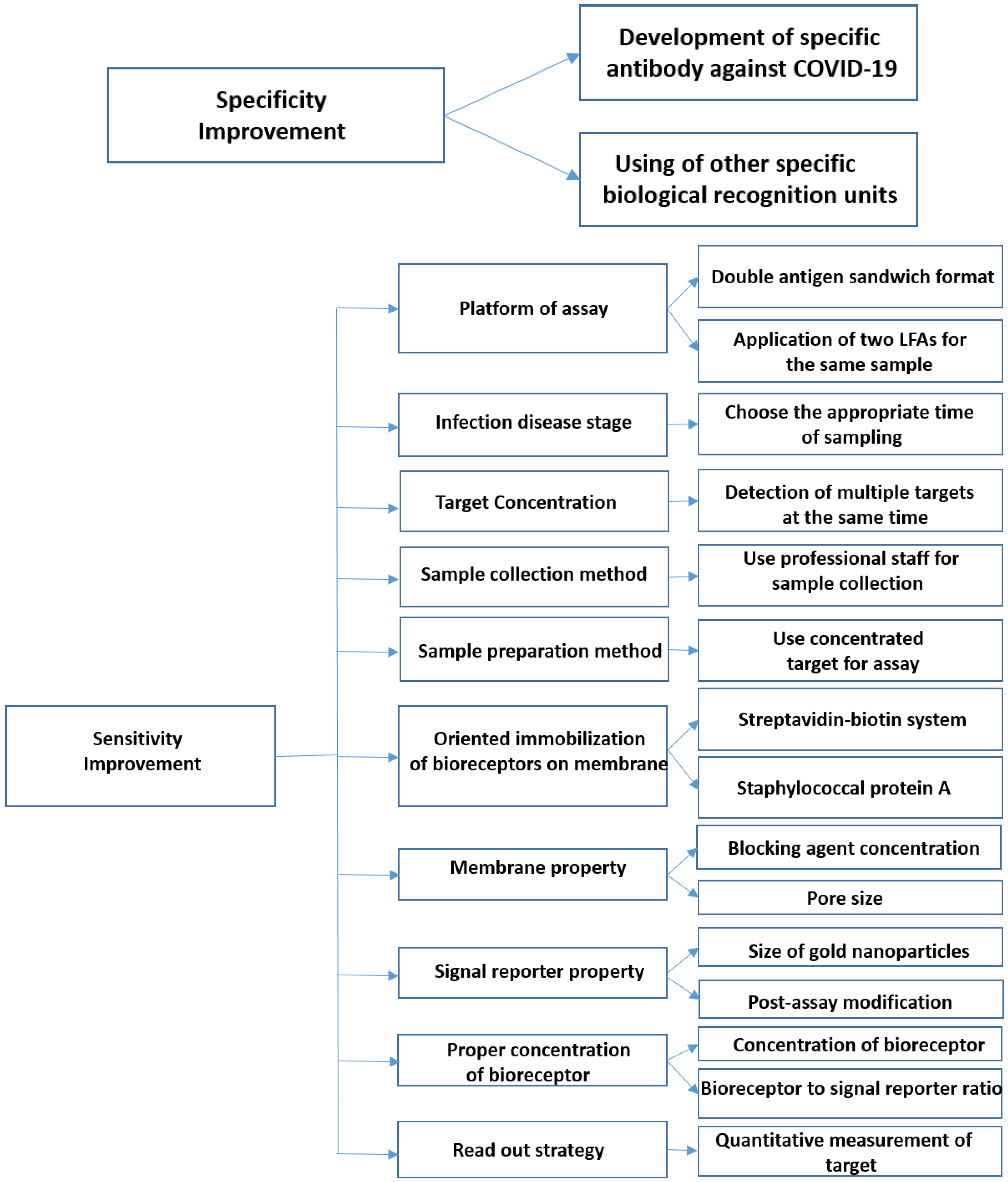
5.1. How Can the Specificity of LFAs Be Improved?
5.1.1. Development of Specific Antibodies against SARS-CoV-2
5.1.2. Deployment of Other Specific Bio-Recognition Elements
5.2. How Can the Sensitivity of LFA Be Improved?
5.2.1. Platform of Assay
5.2.2. Infection Disease Stage
5.2.3. Target Concentration
5.2.4. Sample Collection Method
5.2.5. Sample Preparation Method
5.2.6. Proper Orientation of Bioreceptors
5.2.7. Proper Concentration of the Bioreceptor
5.2.8. Membrane Properties
5.2.9. Signal Reporter Properties
5.2.10. Read-Out Strategy
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, D.; Wu, F.; Cen, Y.; Ye, L.; Shi, X.; Huang, Y.; Fang, S.; Ma, L. Comparative research on nucleocapsid and spike glycoprotein as the rapid immunodetection targets of COVID-19 and establishment of immunoassay strips. Mol. Immunol. 2021, 131, 6–12. [Google Scholar] [CrossRef]
- Hussein, H.A.; Hassan, R.Y.; Chino, M.; Febbraio, F. Point-of-Care Diagnostics of COVID-19: From Current Work to Future Perspectives. Sensors 2020, 20, 4289. [Google Scholar] [CrossRef]
- Ventura, B.D.; Cennamo, M.; Minopoli, A.; Campanile, R.; Censi, S.B.; Terracciano, D.; Portella, G.; Velotta, R. Colorimetric Test for Fast Detection of SARS-CoV-2 in Nasal and Throat Swabs. ACS Sens. 2020, 5, 3043–3048. [Google Scholar] [CrossRef]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef]
- Adrover-Jaume, C.; Alba-Patiño, A.; Clemente, A.; Santopolo, G.; Vaquer, A.; Russell, S.M.; Barón, E.; Del Campo, M.D.M.G.; Ferrer, J.M.; Berman-Riu, M. Paper biosensors for detecting elevated IL-6 levels in blood and respiratory samples from COVID-19 patients. Sens. Actuators B Chem. 2021, 330, 129333. [Google Scholar] [CrossRef]
- Shaw, A.M.; Hyde, C.; Merrick, B.; James-Pemberton, P.; Squires, B.K.; Olkhov, R.V.; Batra, R.; Patel, A.; Bisnauthsing, K.; Nebbia, G. Real-world evaluation of a novel technology for quantitative simultaneous antibody detection against multiple SARS-CoV-2 antigens in a cohort of patients presenting with COVID-19 syndrome. Analyst 2020, 145, 5638–5646. [Google Scholar] [CrossRef]
- Mavrikou, S.; Moschopoulou, G.; Tsekouras, V.; Kintzios, S. Development of a portable, ultra-rapid and ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 spike protein antigen. Sensors 2020, 20, 3121. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wei, X.; Zheng, S.W.; Lenhart, B.J.; Xu, P.; Li, J.; Pan, J.; Albrecht, H.; Liu, C. Multiplex quantitative detection of SARS-CoV-2 specific IgG and IgM antibodies based on DNA-assisted nanopore sensing. BioSens. Bioelectron. 2021, 181, 113134. [Google Scholar] [CrossRef]
- Shao, W.; Shurin, M.R.; Wheeler, S.E.; He, X.; Star, A. Rapid Detection of SARS-CoV-2 Antigens Using High-Purity Semiconducting Single-Walled Carbon Nanotube-Based Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 10321–10327. [Google Scholar] [CrossRef]
- Song, F.; Shen, Y.; Wei, Y.; Yang, C.; Ge, X.; Wang, A.; Li, C.; Wan, Y.; Li, J. Botulinum toxin as an ultrasensitive reporter for bacterial and SARS-CoV-2 nucleic acid diagnostics. BioSens. Bioelectron. 2021, 176, 112953. [Google Scholar] [CrossRef]
- Lin, Q.; Wen, D.; Wu, J.; Liu, L.; Wu, W.; Fang, X.; Kong, J. Microfluidic immunoassays for sensitive and simultaneous detection of IgG/IgM/antigen of SARS-CoV-2 within 15 min. Anal. Chem. 2020, 92, 9454–9458. [Google Scholar] [CrossRef]
- Nag, P.; Sadani, K.; Mukherji, S. Optical fiber sensors for rapid screening of COVID-19. Trans. Indian Natl. Acad. Eng. 2020, 5, 233–236. [Google Scholar] [CrossRef]
- Zhong, J.; Rösch, E.L.; Viereck, T.; Schilling, M.; Ludwig, F. Toward rapid and sensitive detection of SARS-CoV-2 with functionalized magnetic nanoparticles. ACS Sens. 2021, 6, 976–984. [Google Scholar] [CrossRef]
- Soler, M.; Estevez, M.C.; Cardenosa-Rubio, M.; Astua, A.; Lechuga, L.M. How nanophotonic label-free biosensors can contribute to rapid and massive diagnostics of respiratory virus infections: COVID-19 case. ACS Sens. 2020, 5, 2663–2678. [Google Scholar] [CrossRef]
- Mojsoska, B.; Larsen, S.; Olsen, D.A.; Madsen, J.S.; Brandslund, I.; Alatraktchi, F.A. Rapid SARS-CoV-2 Detection Using Electrochemical Immunosensor. Sensors 2021, 21, 390. [Google Scholar] [CrossRef]
- Pramanik, A.; Gao, Y.; Patibandla, S.; Mitra, D.; McCandless, M.G.; Fassero, L.A.; Gates, K.; Tandon, R.; Ray, P.C. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv. 2021, 3, 1588–1596. [Google Scholar] [CrossRef]
- Sharma, S.; Zapatero-Rodríguez, J.; Estrela, P.; O’Kennedy, R. Point-of-care diagnostics in low resource settings: Present status and future role of microfluidics. Biosensors 2015, 5, 577–601. [Google Scholar] [CrossRef] [Green Version]
- Hristov, D.R.; Rodriguez-Quijada, C.; Gomez-Marquez, J.; Hamad-Schifferli, K. Designing paper-based immunoassays for biomedical applications. Sensors 2019, 19, 554. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Jaiswal, A. Recent advances in nanoparticle-based lateral flow immunoassay as a point-of-care diagnostic tool for infectious agents and diseases. Analyst 2018, 143, 1970–1996. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; He, S.; Wang, X.; Yan, Y.; Liu, J.; Wu, S.; Liu, S.; Lei, Y.; Chen, M.; Li, L. Rapid lateral flow immunoassay for the fluorescence detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020, 4, 1150–1158. [Google Scholar] [CrossRef]
- Yu, S.; Nimse, S.B.; Kim, J.; Song, K.-S.; Kim, T. Development of a Lateral Flow Strip Membrane Assay for Rapid and Sensitive Detection of the SARS-CoV-2. Anal. Chem. 2020, 92, 14139–14144. [Google Scholar] [CrossRef]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Singh, J.; Streithorst, J.; Granados, A.; Sotomayor-Gonzalez, A.; Zorn, K.; Gopez, A. Rapid detection of 2019 novel coronavirus SARS-CoV-2 using a CRISPR-based DETECTR lateral flow assay. medRxiv, 2020; Preprint. [Google Scholar]
- Xiong, E.; Jiang, L.; Tian, T.; Hu, M.; Yue, H.; Huang, M.; Lin, W.; Jiang, Y.; Zhu, D.; Zhou, X. Simultaneous dual-gene diagnosis of SARS-CoV-2 based on CRISPR/Cas9-mediated lateral flow assay. Angew. Chem. 2021, 133, 5367–5375. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Hu, H.; Zhou, Q.; Liu, W.; Li, X.; Liu, Z.; Wang, Y.; Ma, Y. A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab Chip 2020, 20, 4255–4261. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, M.; Jung, Y.; Lee, S.K.; Lee, C.-S.; Kim, J.; Kim, J.; Kim, N.H.; Kim, B.-T.; Kim, H.G. A novel rapid detection for SARS-CoV-2 spike 1 antigens using human angiotensin converting enzyme 2 (ACE2). Biosens. Bioelectron. 2021, 171, 112715. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, J.-H.; Kim, M.J.; Park, S.C.; Choi, M.; Lee, W.; Ku, K.B.; Kim, B.T.; Park, E.C.; Kim, H.G. Development of a SARS-CoV-2-specific biosensor for antigen detection using scFv-Fc fusion proteins. Biosens. Bioelectron. 2021, 175, 112868. [Google Scholar] [CrossRef]
- Syedmoradi, L.; Daneshpour, M.; Alvandipour, M.; Gomez, F.A.; Hajghassem, H.; Omidfar, K. Point of care testing: The impact of nanotechnology. Biosens. Bioelectron. 2017, 87, 373–387. [Google Scholar] [CrossRef]
- Nielsen, K.; Yu, W.; Lin, M.; Davis, S.N.; Elmgren, C.; Mackenzie, R.; Tanha, J.; Li, S.; Dubuc, G.; Brown, E. Prototype single step lateral flow technology for detection of avian influenza virus and chicken antibody to avian influenza virus. J. Immunoass. Immunochem. 2007, 28, 307–318. [Google Scholar] [CrossRef]
- Jung, B.Y.; Jung, S.C.; Kweon, C.H. Development of a rapid immunochromatographic strip for detection of Escherichia coli O157. J. Food Protect. 2005, 68, 2140–2143. [Google Scholar] [CrossRef]
- Rong-Hwa, S.; Shiao-Shek, T.; Der-Jiang, C.; Yao-Wen, H. Gold nanoparticle-based lateral flow assay for detection of staphylococcal enterotoxin B. Food Chem. 2010, 118, 462–466. [Google Scholar] [CrossRef]
- Jung, Y.; Heo, Y.; Lee, J.J.; Deering, A.; Bae, E. Smartphone-based lateral flow imaging system for detection of food-borne bacteria E. coli O157: H7. J. Microbiol. Methods 2020, 168, 105800. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Zhang, Y.; Wang, R.; Ji, Y.; Sun, J.; Zhang, D.; Wang, J. Functional nanozyme mediated multi-readout and label-free lateral flow immunoassay for rapid detection of Escherichia coli O157: H7. Food Chem. 2020, 329, 127224. [Google Scholar] [CrossRef]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A. 91 CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Smith, B.M.; Jain, P.K. 92 Enhancement of trans-cleavage activity of Cas12a with engineered crRNA enables amplified nucleic acid detection. Nat. Commun. 2020, 11, 4906. [Google Scholar]
- Arizti-Sanz, J.; A’Doriann Bradley, Y.B.Z.; Boehm, C.K.; Freije, C.A.; Grunberg, M.E.; Kosoko-Thoroddsen, T.-S.F.; Welch, N.L.; Pillai, P.P.; Mantena, S.; Kim, G. Equipment-free detection of SARS-CoV-2 and Variants of Concern using Cas13. medrxiv, 2021; Preprint. [Google Scholar]
- Gupta, R.; Sagar, P.; Priyadarshi, N.; Kaul, S.; Sandhir, R.; Rishi, V.; Singhal, N.K. Nanotechnology-based approaches for the detection of SARS-CoV-2. Front. Nanotechnol. 2020, 2, 589832. [Google Scholar] [CrossRef]
- Younes, N.; Al-Sadeq, D.W.; Al-Jighefee, H.; Younes, S.; Al-Jamal, O.; Daas, H.I.; Yassine, H.; Nasrallah, G.K. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses 2020, 12, 582. [Google Scholar] [CrossRef]
- Suleman, S.; Shukla, S.K.; Malhotra, N.; Bukkitgar, S.D.; Shetti, N.P.; Pilloton, R.; Narang, J.; Tan, Y.N.; Aminabhavi, T.M. Point of care detection of COVID-19: Advancement in biosensing and diagnostic methods. Chem. Eng. J. 2021, 414, 128759. [Google Scholar] [CrossRef]
- Wang, J.; Drelich, A.J.; Hopkins, C.M.; Mecozzi, S.; Li, L.; Kwon, G.; Hong, S. Gold nanoparticles in virus detection: Recent advances and potential considerations for SARS-CoV-2 testing development. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1754. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Ding, L.; Huang, X.; Xiong, Y. Point-of-care COVID-19 diagnostics powered by lateral flow assay. TrAC Trends Anal. Chem. 2021, 145, 116452. [Google Scholar] [CrossRef]
- Mahmoudinobar, F.; Britton, D.; Montclare, J.K. Protein-based lateral flow assays for COVID-19 detection. Protein Eng. Design Sel. 2021, 34, gzab010. [Google Scholar] [CrossRef]
- Hsiao, W.W.-W.; Le, T.-N.; Pham, D.M.; Ko, H.-H.; Chang, H.-C.; Lee, C.-C.; Sharma, N.; Lee, C.-K.; Chiang, W.-H. Recent advances in novel lateral flow technologies for detection of COVID-19. Biosensors 2021, 11, 295. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Cavalera, S.; Colitti, B.; Rosati, S.; Ferrara, G.; Bertolotti, L.; Nogarol, C.; Guiotto, C.; Cagnazzo, C.; Denina, M.; Fagioli, F. A multi-target lateral flow immunoassay enabling the specific and sensitive detection of total antibodies to SARS CoV-2. Talanta 2021, 223, 121737. [Google Scholar] [CrossRef]
- Liu, G.; Rusling, J.F. COVID-19 antibody tests and their limitations. ACS Sens. 2021, 6, 593–612. [Google Scholar] [CrossRef]
- Huang, C.; Wen, T.; Shi, F.-J.; Zeng, X.-Y.; Jiao, Y.-J. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 2020, 5, 12550–12556. [Google Scholar] [CrossRef]
- Wen, T.; Huang, C.; Shi, F.-J.; Zeng, X.-Y.; Lu, T.; Ding, S.-N.; Jiao, Y.-J. Development of a lateral flow immunoassay strip for rapid detection of IgG antibody against SARS-CoV-2 virus. Analyst 2020, 145, 5345–5352. [Google Scholar] [CrossRef]
- Peng, T.; Sui, Z.; Huang, Z.; Xie, J.; Wen, K.; Zhang, Y.; Huang, W.; Mi, W.; Peng, K.; Dai, X. Point-of-care test system for detection of immunoglobulin-G and-M against nucleocapsid protein and spike glycoprotein of SARS-CoV-2. Sens. Actuators B Chem. 2021, 331, 129415. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Martinez, V.; Chen, X.; Li, Y.; He, L.; Chen, S.; Guo, X.; Shen, X.; Bao, X. A facile assay for rapid detection of COVID-19 antibodies. RSC Adv. 2020, 10, 28041–28048. [Google Scholar] [CrossRef]
- Roda, A.; Cavalera, S.; Di Nardo, F.; Calabria, D.; Rosati, S.; Simoni, P.; Colitti, B.; Baggiani, C.; Roda, M.; Anfossi, L. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens. Bioelectron. 2021, 172, 112765. [Google Scholar] [CrossRef]
- Zeng, L.; Li, Y.; Liu, J.; Guo, L.; Wang, Z.; Xu, X.; Song, S.; Hao, C.; Liu, L.; Xin, M. Rapid, ultrasensitive and highly specific biosensor for the diagnosis of SARS-CoV-2 in clinical blood samples. Mater. Chem. Front. 2020, 4, 2000–2005. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Gu, B.; Liu, H.; Zhou, Z.; Shi, L.; Cheng, X.; Wang, S. Sensitive and Simultaneous Detection of SARS-CoV-2-Specific IgM/IgG Using Lateral Flow Immunoassay Based on Dual-Mode Quantum Dot Nanobeads. Anal. Chem. 2020, 92, 15542–15549. [Google Scholar] [CrossRef]
- Tian, J.; Kan, W.; Yanlei, L.; Hui, L.; Xueling, L.; Daxiang, C. Development and Head-to-Head Comparison of Two Colloidal Gold Based Serologic Lateral Flow Assays for SARS-CoV-2 Antibody Tests. Nano Biomed. Eng 2020, 12, 306–310. [Google Scholar] [CrossRef]
- Fulford, T.S.; Van, H.; Gherardin, N.A.; Zheng, S.; Ciula, M.; Drummer, H.E.; Redmond, S.; Tan, H.-X.; Boo, I.; Center, R.J. A point-of-care lateral flow assay for neutralising antibodies against SARS-CoV-2. EBioMedicine 2021, 74, 103729. [Google Scholar] [CrossRef]
- Pavlova, I.P.; Nair, S.S.; Kyprianou, N.; Tewari, A.K. The rapid coronavirus antibody test: Can we improve accuracy? Front. Med. 2020, 7, 569. [Google Scholar] [CrossRef]
- EUA (Ed.) A Common List of COVID-19 Rapid Antigen Tests; EUA: Brussels, Belgium, 2022.
- WHO. WHO Emergency Use Listing for In Vitro Diagn.s (IVDs) Detecting SARS-CoV-2; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Mertens, P.; De Vos, N.; Martiny, D.; Jassoy, C.; Mirazimi, A.; Cuypers, L.; Van den Wijngaert, S.; Monteil, V.; Melin, P.; Stoffels, K. Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Front. Med. 2020, 7, 225. [Google Scholar] [CrossRef]
- Baker, A.N.; Richards, S.-J.; Guy, C.S.; Congdon, T.R.; Hasan, M.; Zwetsloot, A.J.; Gallo, A.; Lewandowski, J.R.; Stansfeld, P.J.; Straube, A. The SARS-CoV-2 spike protein binds sialic acids and enables rapid detection in a lateral flow point of care diagnostic device. ACS Cent. Sci. 2020, 6, 2046–2052. [Google Scholar] [CrossRef]
- Khairat, S.M.; Guindy, N.E.; Motaleb, M.; Soliman, N.S. Evaluation of two rapid antigen tests for detection of SARS-CoV-2 virus. Internatl. J. Microbiol. Biotechnol. 2020, 5, 131–134. [Google Scholar] [CrossRef]
- Li, G.; Wang, A.; Chen, Y.; Sun, Y.; Du, Y.; Wang, X.; Ding, P.; Jia, R.; Wang, Y.; Zhang, G. 88 Development of a Colloidal Gold-Based Immunochromatographic Strip for Rapid Detection of Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein. Front. Immunol. 2021, 12, 560. [Google Scholar]
- Zhu, N.; Wong, P.K. Advances in Viral Diagnostic Technologies for Combating COVID-19 and Future Pandemics. SLAS TECHNOLOGY Transl. Life Sci. Innov. 2020, 25, 513–521. [Google Scholar] [CrossRef]
- Shen, Y.; Anwar, T.B.; Mulchandani, A. Current status, advances, challenges and perspectives on biosensors for COVID-19 diagnosis in resource-limited settings. Sens. Actuators Rep. 2021, 3, 100025. [Google Scholar] [CrossRef]
- Grant, B.D.; Anderson, C.E.; Williford, J.R.; Alonzo, L.F.; Glukhova, V.A.; Boyle, D.S.; Weigl, B.H.; Nichols, K.P. SARS-CoV-2 coronavirus nucleocapsid antigen-detecting half-strip lateral flow assay toward the development of point of care tests using commercially available reagents. Anal. Chem. 2020, 92, 11305–11309. [Google Scholar] [CrossRef]
- Hristov, D.; Rijal, H.; Gomez-Marquez, J.; Hamad, K. Developing a Paper-Based Antigen Assay to Differentiate Between Coronaviruses and SARS-CoV-2 Spike Variants. Anal Chem. 2021, 93, 7825. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Gualerzi, G.; Ranzieri, S.; Henry, B.M.; Said, Y.B.; Pyatigorskaya, N.V.; Nevolina, E.; Wu, J.; Bragazzi, N.L. Point-of-care diagnostic tests for detecting SARS-CoV-2 antibodies: A systematic review and meta-analysis of real-world data. J. Clin. Med. 2020, 9, 1515. [Google Scholar] [CrossRef]
- Dörschug, A.; Schwanbeck, J.; Hahn, A.; Hillebrecht, A.; Blaschke, S.; Mese, K.; Groß, U.; Dierks, S.; Frickmann, H.; Zautner, A.E. Comparison of Five Serological Assays for the Detection of SARS-CoV-2 Antibodies. Diagnostics 2021, 11, 78. [Google Scholar] [CrossRef]
- Guglielmi, G. Rapid coronavirus tests: A guide for the perplexed. Nature 2021, 590, 202–205. [Google Scholar] [CrossRef]
- Brümmer, L.E.; Katzenschlager, S.; Gaeddert, M.; Erdmann, C.; Schmitz, S.; Bota, M.; Grilli, M.; Larmann, J.; Weigand, M.A.; Pollock, N.R. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLoS Med. 2021, 18, e1003735. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Lateral Flow Serodiagnosis in the Double-Antigen Sandwich Format: Theoretical Consideration and Confirmation of Advantages. Sensors 2021, 21, 39. [Google Scholar] [CrossRef]
- Hoste, A.C.; Venteo, A.; Fresco-Taboada, A.; Tapia, I.; Monedero, A.; López, L.; Jebbink, M.F.; Pérez-Ramírez, E.; Jimenez-Clavero, M.A.; Almonacid, M. Two serological approaches for detection of antibodies to SARS-CoV-2 in different scenarios: A screening tool and a point-of-care test. Diagn. Microbiol. Infect. Dis. 2020, 98, 115167. [Google Scholar] [CrossRef]
- Daoud, Z.; McLeod, J.; Stockman, D.L. Higher Sensitivity Provided by the Combination of Two Lateral Flow Immunoassay Tests for the Detection of COVID-19 Immunoglobulins. Front. Cell. Infect. Microbiol. 2020, 10, 479. [Google Scholar] [CrossRef]
- Fischer, P.U.; Fischer, K.; Curtis, K.C.; Huang, Y.; Fetcho, N.; Goss, C.W.; Weil, G.J. Evaluation of commercial rapid lateral flow tests, alone or in combination, for SARS-CoV-2 antibody testing. Am. J. Trop. Med. Hyg. 2021, 105, 378. [Google Scholar] [CrossRef]
- Lindner, A.K.; Nikolai, O.; Kausch, F.; Wintel, M.; Hommes, F.; Gertler, M.; Krüger, L.J.; Gaeddert, M.; Tobian, F.; Lainati, F. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur. Respir. J. 2021, 57, 2003961. [Google Scholar] [CrossRef]
- Cerofolini, L.; Fragai, M.; Luchinat, C.; Ravera, E. Orientation of immobilized antigens on common surfaces by a simple computational model: Exposition of SARS-CoV-2 spike protein RBD epitopes. Biophys. Chem. 2020, 265, 106441. [Google Scholar] [CrossRef]
- Zhan, L.; Guo, S.-z.; Song, F.; Gong, Y.; Xu, F.; Boulware, D.R.; McAlpine, M.C.; Chan, W.C.; Bischof, J.C. 90 The role of nanoparticle design in determining analytical performance of lateral flow immunoassays. Nano Lett. 2017, 17, 7207–7212. [Google Scholar] [CrossRef] [Green Version]
- Panferov, V.G.; Byzova, N.A.; Biketov, S.F.; Zherdev, A.V.; Dzantiev, B.B. Comparative study of in situ techniques to enlarge gold nanoparticles for highly sensitive lateral flow immunoassay of SARS-CoV-2. Biosensors 2021, 11, 229. [Google Scholar] [CrossRef]
- Peng, T.; Liu, X.; Adams, L.G.; Agarwal, G.; Akey, B.; Cirillo, J.; Deckert, V.; Delfan, S.; Fry, E.; Han, Z. Enhancing sensitivity of lateral flow assay with application to SARS-CoV-2. Appl. Phys. Lett. 2020, 117, 120601. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Ko, C.-H.; Wang, C.J.; Chen, C.-W.; Chiu, W.-H.; Hong, C.; Cheng, H.-M.; Wang, I.-J. The early detection of immunoglobulins via optical-based lateral flow immunoassay platform in COVID-19 pandemic. PLoS ONE 2021, 16, e0254486. [Google Scholar] [CrossRef]
- Chiu, R.Y.; Thach, A.V.; Wu, C.M.; Wu, B.M.; Kamei, D.T. An aqueous two-phase system for the concentration and extraction of proteins from the interface for detection using the lateral-flow immunoassay. PLoS ONE 2015, 10, e0142654. [Google Scholar] [CrossRef]
- Tang, R.; Yang, H.; Choi, J.R.; Gong, Y.; Hu, J.; Feng, S.; Pingguan-Murphy, B.; Mei, Q.; Xu, F. Improved sensitivity of lateral flow assay using paper-based sample concentration technique. Talanta 2016, 152, 269–276. [Google Scholar] [CrossRef]
- Moghadam, B.Y.; Connelly, K.T.; Posner, J.D. Two orders of magnitude improvement in detection limit of lateral flow assays using isotachophoresis. Anal. Chem. 2015, 87, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Schepens, B.; van Schie, L.; Nerinckx, W.; Roose, K.; Van Breedam, W.; Fijalkowska, D.; Devos, S.; Weyts, W.; De Cae, S.; Vanmarcke, S. An affinity-enhanced, broadly neutralizing heavy chain–only antibody protects against SARS-CoV-2 infection in animal models. Sci. Transl. Med. 2021, 13, eabi7826. [Google Scholar] [CrossRef] [PubMed]
- Schoof, M.; Faust, B.; Saunders, R.A.; Sangwan, S.; Rezelj, V.; Hoppe, N.; Boone, M.; Billesbølle, C.B.; Zimanyi, M.; Deshpande, I. An ultra-high affinity synthetic nanobody blocks SARS-CoV-2 infection by locking Spike into an inactive conformation. BioRxiv, 2020; Preprint. [Google Scholar]
- Nambulli, S.; Xiang, Y.; Tilston-Lunel, N.L.; Rennick, L.J.; Sang, Z.; Klimstra, W.B.; Reed, D.S.; Crossland, N.A.; Shi, Y.; Duprex, W.P. Inhalable Nanobody (PiN-21) prevents and treats SARS-CoV-2 infections in Syrian hamsters at ultra-low doses. Sci. Adv. 2021, 7, eabh0319. [Google Scholar] [CrossRef] [PubMed]
| Biosensors for COVID-19 Diagnosis | |||
|---|---|---|---|
| Targets | Methods | Ref. | |
| RNA | Colorimetric-based assay | [4] | |
| Fluorescence-based assay | [10] | ||
| Immunological reaction | IgG, IgM, IgA | Colorimetric-based assay | [6] |
| Electrical-based sensor | [8] | ||
| Fluorescence-based assay | [11] | ||
| Optical fiber sensor | [12] | ||
| IL-6 | Colorimetric-based assay | [5] | |
| Viral proteins | Colorimetric-based assay | [3] | |
| Electrical-based sensor | [7,9] | ||
| Optical fiber sensor | [12] | ||
| Magnetic nanoparticle-based biosensor | [13] | ||
| Surface Plasmon Resonance-based sensor | [14] | ||
| Electrochemical immunosensor | [15] | ||
| Colorimetric-based assay | [16] | ||
| Target | Bioreceptor on Test Line | Gold-Labeled Reporter | Specificity | Sensitivity | Sample | Ref. |
|---|---|---|---|---|---|---|
| Anti-N IgM | N Protein | Anti-human IgM | 93.3% | 100% | Serum | [47] |
| Anti-N IgG | N Protein | Anti-human IgG | 100% | 69.1% | Serum | [48] |
| Anti-N IgG | Anti-human IgG | N Protein | 96% | 96% | Serum | [49] |
| Anti-S IgG | Anti-human IgG | S-RBD protein | 96.1% | 95.9% | Serum | [49] |
| Anti-S IgM | Anti-human IgM | S-RBD protein | 100% | 96.6% | Serum | [49] |
| Anti-N IgG and IgM | Anti-human IgG and IgM | N protein | 97.47% | 95.85% | Serum | [50] |
| Anti-N IgG/IgM/IgA | N protein | N protein | 94.6% | 100% | Serum | [45] |
| Anti-N IgA | N protein | Anti-human IgA | - | - | Saliva | [51] |
| Anti-S IgG | Anti-human IgG | S Protein | - | 61.76% | Serum | [52] |
| Anti-S IgM | Anti-human IgM | S Protein | - | 82.35% | Serum | [52] |
| Anti-S IgG/IgM/IgA | S Protein | S Protein | 100% | 90% | Serum | [54] |
| Neutralizing Antibodies | ACE-2 | RBD | 100% | 96% | Whole blood | [55] |
| N protein | Anti-COVID-19 antibody | Anti-COVID-19 antibody | 99.5% | 57.6% | Nasopharyngeal sample | [59] |
| S protein | N-acetyl neuraminic acid | N-acetyl neuraminic acid | - | - | - | [60] |
| N protein | Anti-COVID-19 antibody | Anti-COVID-19 antibody | - | - | Nasopharyngeal and oropharyngeal samples | [1] |
| S protein | Anti-COVID-19 antibody | Anti-COVID-19 antibody | - | - | Recombinant S protein | [62] |
| Company | Product | Sample Source | Target | Storage Condition |
|---|---|---|---|---|
| Jiangsu Well Biotech Co., Ltd. (Changzhou, China) | Orawell IgM/IgG Rapid Test | Serum | IgM and IgG | 2–8 ℃ |
| Xiamen Biotime Biotech-nology Co., Ltd. (Xiamen, China) | BIOTIME SARS-CoV-2 IgG/IgM Rapid Qualitative Test | Serum | IgM and IgG | - |
| Megna Health, Inc. (Exton, PA, USA) | Rapid COVID-19 IgM/IgG Combo Test Kit | Serum | IgM and IgG | 4 to 30 °C |
| Access Bio, Inc. (Somerset, NJ, USA) | CareStart COVID-19 Antigen Test | Nasopharyngeal swab (NPS) | Nucleocapsid Antigen | 1 to 30 °C |
| Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. (Beijing, China) | Rapid test for SARS-CoV-2 Antigen | Nasopharyngeal swab (NPS) | Nucleocapsid Antigen | - |
| Zhuhai Livzon Diagnostics (Guangdong, China) | The Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) | serum, plasma, venous whole blood | IgM and IgG | 2 to 30 °C |
| Zhuhai Livzon Diagnostics (Guangdong, China) | Livzon Rapid Test for SARS-CoV-2 Antigen | Nasopharyngeal swabs, oropharyngeal swabs | - | 2 to 30 °C |
| Assure Tech Co. (Hangzhou, China) | Assure COVID-19 IgG/IgM Rapid Test Device | serum, plasma, venous whole blood | IgG and IgM | - |
| Beijing Diagreat Biotechnologies (Beijing, China) | 2019-nCoV IgG/IgM Antibody Rapid Test Kit | Blood | IgG and IgM | - |
| Biolidics (Singapore) | Rapid test kit for COVID-19-IgG/IgM Antibody Detection Kit | venous whole blood/serum/plasma | IgG and IgM | 2–8 °C |
| Sugentech (Daejeon, Korea) | SGTi-flex COVID-19 IgM/IgG | venous whole blood/serum/plasma | IgG and IgM | - |
| SD Biosensor (Gyeonggi-do, Korea) | STANDARD Q COVID-19 IgM/IgG Duo | venous whole blood/serum/plasma | IgG and IgM | 2 to 30 °C |
| SD Biosensor (Gyeonggi-do, Korea) | STANDARD Q COVID-19 Ag Test | Nasopharyngeal swab | - | 2 to 30 °C |
| BioVendor R&D (Brno, Czech Republic) | BIOCREDIT COVID-19 Ag Detection Kit | Nasopharyngeal swabs | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardekani, L.S.; Thulstrup, P.W. Gold Nanoparticle-Mediated Lateral Flow Assays for Detection of Host Antibodies and COVID-19 Proteins. Nanomaterials 2022, 12, 1456. https://doi.org/10.3390/nano12091456
Ardekani LS, Thulstrup PW. Gold Nanoparticle-Mediated Lateral Flow Assays for Detection of Host Antibodies and COVID-19 Proteins. Nanomaterials. 2022; 12(9):1456. https://doi.org/10.3390/nano12091456
Chicago/Turabian StyleArdekani, Leila Safaee, and Peter Waaben Thulstrup. 2022. "Gold Nanoparticle-Mediated Lateral Flow Assays for Detection of Host Antibodies and COVID-19 Proteins" Nanomaterials 12, no. 9: 1456. https://doi.org/10.3390/nano12091456
APA StyleArdekani, L. S., & Thulstrup, P. W. (2022). Gold Nanoparticle-Mediated Lateral Flow Assays for Detection of Host Antibodies and COVID-19 Proteins. Nanomaterials, 12(9), 1456. https://doi.org/10.3390/nano12091456








