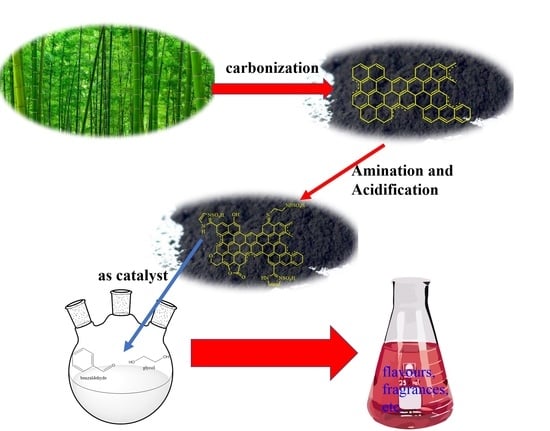
Sulfuric Acid Immobilized on Activated Carbon Aminated with Ethylenediamine: An Efficient Reusable Catalyst for the Synthesis of Acetals (Ketals)
Abstract
:1. Introduction
2. Materials and Methods
2.1. AC-N-SO4H Preparation
2.2. Sample Characterization
2.3. AC-N-SO4H Catalytic Properties on Synthesis of Acetals (Ketals)
3. Results and Discussion
3.1. AC-N-SO4H Preparation
3.2. AC-N-SO4H Structural Analysis
3.2.1. Specific Surface Area
3.2.2. FT-IR
3.2.3. TG-DTG
3.3. AC-N-SO4H Catalytic Properties in Synthesis of Acetals (Ketals)
3.3.1. Effects of Reaction Conditions on Benzaldehyde Conversion
3.3.2. Performance of Reusability
3.3.3. Comparison of Catalytic Efficiency with Reported Solid Acid Catalysts
3.3.4. Substrate Suitability
4. Conclusions
- (1)
- With activated carbon as the raw material, a strong and stable carbon-based solid acid catalyst with hydrogen sulfate AC-N-SO4H with a surface acid content of 0.85 mmol/g was prepared after oxidation with HNO3, amination with ethylenediamine, and acidification with dilute aqueous sulfuric acid. The structural analysis showed that the specific surface area of AC-N-SO4H was almost the same as that of AC-N while preserving surface-active functional groups. The N-containing structure on AC-N surface was not damaged after impregnation with aqueous sulfuric acid. However, the thermal stability of the activated carbon surface structure was slightly lower than that of aminated activated carbon AC-N after sulfuric acid impregnation for introduction of the electrophilic group -SO4H.
- (2)
- As a catalyst, AC-N-SO4H demonstrated excellent performance in synthesis of acetals (ketals) reactions. In the catalytic condensation of benzaldehyde with ethylene glycol, the conversion of benzaldehyde and the selectivity of benzaldehyde glycol acetal were both above 99%. The performance of the catalyst showed no significant reduction even after ten successive runs, still achieving a 99% benzaldehyde conversion yield and 99% benzaldehyde glycol acetal selectivity. At the same time, AC-N-SO4H showed excellent catalytic properties in the study of substrate applicability for the condensation reaction of ethylene glycol, propylene glycol, and butylene glycol with different chain and cyclic aldehydes (ketones), which indicated the excellent application prospects of AC-N-SO4H as a solid acid catalyst.
- (3)
- The excellent catalytic properties of AC-N-SO4H in synthesis of acetals (ketals) can be attributed to its strong acidic functional groups and good stability. This provides a novel method for preparing carbon materials with stable strong acidic functional groups on surface. The detailed structure of the modified activated carbon surface and its catalytic mechanism still need to be further explored.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maki, Y.; Nomura, K.; Okamoto, R.; Izumi, M.; Mizutani, Y.; Kajihara, Y. Acceleration and deceleration factors on the hydrolysis reaction of 4, 6-O-Benzylidene acetal group. J. Org. Chem. 2020, 85, 15849–15856. [Google Scholar] [CrossRef] [PubMed]
- Sartori, G.; Ballini, R.; Bigi, F.; Bosica, G.; Maggi, R.; Righi, P. Protection (and deprotection) of functional groups in organic synthesis by heterogeneous catalysis. Chem. Rev. 2004, 104, 199–250. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.T.; Broaders, K.E. Spirocyclic acetal-modified dextran as a flexible ph-sensitive solubility-switching material. Biomacromolecules 2019, 20, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, Y.; Sakurai, S.; Sakamoto, R.; Matsumoto, A.; Maruoka, K. Iron-catalyzed radical cleavage/C−C bond formation of acetal-derived alkylsilyl peroxides. Chem.-Asian J. 2020, 15, 573–576. [Google Scholar] [CrossRef] [Green Version]
- Moity, L.; Benazzouz, A.; Molinier, V.; Nardello-Rataj, V.; Elmkaddem, M.K.; De Caro, P.; Thiébaud-Roux, S.; Gerbaud, V.; Marion, P.; Aubry, J.M. Glycerol acetals and ketals as bio-based solvents: Positioning in Hansen and COSMO-RS spaces, volatility and stability towards hydrolysis and autoxidation. Green Chem. 2015, 17, 1779–1792. [Google Scholar] [CrossRef] [Green Version]
- Rigo, D.; Calmanti, R.; Perosa, A.; Selva, M. A transesterification–acetalization catalytic tandem process for the functionalization of glycerol: The pivotal role of isopropenyl acetate. Green Chem. 2020, 22, 5487–5496. [Google Scholar] [CrossRef]
- Han, X.; Cai, J.; Mao, X.; Yang, X.; Qiu, L.; Li, F.; Tang, X.; Wang, Y.; Liu, S. Highly active solid oxide acid catalyst for the synthesis of benzaldehyde glycol acetal. Appl. Catal. A Gen. 2021, 618, 118136. [Google Scholar] [CrossRef]
- Dong, J.L.; Yu, L.S.H.; Xie, J.W. A simple and versatile method for the formation of acetals/ketals using trace conventional acids. ACS Omega 2018, 3, 4974–4985. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Velty, A.; Susarte, M. Zeolites for the production of fine chemicals: Synthesis of fructone fragrancy. J. Catal. 2000, 196, 345–351. [Google Scholar] [CrossRef]
- Khaef, S.; Zolfigol, M.A.; Taherpour, A.A.; Yarie, M. Catalytic application of sulfamic acid-functionalized magnetic Fe3O4 nanoparticles (SA-MNPs) for protection of aromatic carbonyl compounds and alcohols: Experimental and theoretical studies. RSC Adv. 2020, 10, 44946–44957. [Google Scholar] [CrossRef]
- Han, X.; Yan, W.; Chen, K.; Hung, C.T.; Liu, L.L.; Wu, P.H.; Huang, S.J.; Liu, S.B. Heteropoly acid-based ionic liquids as effective catalysts for the synthesis of benzaldehyde glycol acetal. Appl. Catal. A Gen. 2014, 485, 149–156. [Google Scholar] [CrossRef]
- Zhou, B.; Song, F.; Ma, X.; Wang, L. Batch and continuous-flow preparation of biomass-derived furfural acetals over a TiO2 nanoparticle-exfoliated montmorillonite composite catalyst. ChemSusChem 2021, 14, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Luan, Q.J.; Lu, J.; Lv, D.M.; Duan, W.Z.; Wang, X.; Gong, S.W. 8-Hydroxy-2-methylquinoline-modified H4SiW12O40: A reusable heterogeneous catalyst for acetal/ketal formation. RSC Adv. 2018, 8, 26180–26187. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Zhao, X.; Li, D.; Chen, M.; Wei, X.; Fang, J.; Cui, K.; Ma, Y.; Hou, Z. Synthesis of bio-additive fuels from glycerol acetalization over a heterogeneous Ta/W mixed addenda heteropolyacid catalyst. Fuel Process. Technol. 2021, 214, 106705. [Google Scholar] [CrossRef]
- Xu, M.; Richard, F.; Corbet, M.; Marion, P.; Clacens, J.M. Pickering emulsions assisted synthesis of fatty acetal over phenyl sulfonic groups grafted on activated charcoal. Appl. Catal. A Gen. 2020, 597, 117543. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.F.; Dezaneti, L.M.; Garcia, F.A.; de Macedo, J.L.; Dias, J.A.; Dias, S.C.; Alvim, K.S. Esterification of oleic acid with ethanol by 12-tungstophosphoric acid supported on zirconia. Appl. Catal. A Gen. 2010, 372, 153–161. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Y.; Yuan, X.; Shen, J.; Kim, J. Direct synthesis, characterization and catalytic application of SBA-15 containing heteropolyacid H3PW12O40. J. Mol. Catal. A Chem. 2005, 229, 199–205. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- KOLUR, N.A.; Sharifian, S.; Kaghazchi, T. Investigation of sulfuric acid-treated activated carbon properties. Turk. J. Chem. 2019, 43, 663–675. [Google Scholar] [CrossRef]
- Ternero-Hidalgo, J.J.; Rosas, J.M.; Palomo, J.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Functionalization of activated carbons by HNO3 treatment: Influence of phosphorus surface groups. Carbon 2016, 101, 409–419. [Google Scholar] [CrossRef]
- Ang, T.N.; Young, B.R.; Taylor, M.; Burrell, R.; Aroua, M.K.; Chen, W.H.; Baroutian, S. Enrichment of surface oxygen functionalities on activated carbon for adsorptive removal of sevoflurane. Chemosphere 2020, 260, 127496. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Mikkola, J.P. SO3H-containing functional carbon materials: Synthesis, structure, and acid catalysis. Chem. Rev. 2019, 119, 11576–11630. [Google Scholar] [CrossRef]
- Watanabe, H.; Asano, S.; Fujita, S.I.; Yoshida, H.; Arai, M. Nitrogen-doped, metal-free activated carbon catalysts for aerobic oxidation of alcohols. Acs Catal. 2015, 5, 2886–2894. [Google Scholar] [CrossRef]
- Yadavalli, G.; Lei, H.; Wei, Y.; Zhu, L.; Zhang, X.; Liu, Y.; Yan, D. Carbon dioxide capture using ammonium sulfate surface modified activated biomass carbon. Biomass Bioenergy 2017, 98, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Chen, X.; Zhang, J.; Yan, N. Chitin-derived mesoporous, nitrogen-containing carbon for heavy-metal removal and styrene epoxidation. ChemPlusChem 2015, 80, 1556. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Jiang, X.; Yao, L.; Jiang, W.; Xie, R. Preparation and evaluation of nitrogen-tailored hierarchical meso-/micro-porous activated carbon for CO2 adsorption. Environ. Technol. 2020, 41, 3544–3553. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Masteri-Farahani, M.; Ghahremani, M.; Forouzeshfar, N. New approach for sulfonation of carbonaceous materials: Highly efficient solid acid catalysts for benzaldehyde acetalization with ethylene glycol. J. Phys. Chem. Solids 2021, 150, 109846. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Hosseini, M.S.; Forouzeshfar, N. Propyl-SO3H functionalized graphene oxide as multipurpose solid acid catalyst for biodiesel synthesis and acid-catalyzed esterification and acetalization reactions. Renew. Energy 2020, 151, 1092–1101. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, X.; Yang, X.; Alghamdi, A.A.; Alharthi, F.A.; Cheng, X.; Deng, Y. Sulfonic acid-functionalized core-shell Fe3O4@carbon microspheres as magnetically recyclable solid acid catalysts. Chin. Chem. Lett. 2021, 32, 2079–2085. [Google Scholar] [CrossRef]
- Han, Z.; Yu, Y.; Zhang, Y.; Dong, B.; Kong, A.; Shan, Y. Al-coordination polymer-derived nanoporous nitrogen-doped carbon microfibers as metal-free catalysts for oxygen electroreduction and acetalization reactions. J. Mater. Chem. A 2015, 3, 23716–23724. [Google Scholar] [CrossRef]
- Miao, J.; Wan, H.; Shao, Y.; Guan, G.; Xu, B. Acetalization of carbonyl compounds catalyzed by acidic ionic liquid immobilized on silica gel. J. Mol. Catal. A Chem. 2011, 348, 77–82. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, J.; Xu, Q.; Liu, X.; Zhong, S.; Huang, H.; Zheng, M.; Kirk, S.R.; Yin, D. Imidazolyl activated carbon refluxed with ethanediamine as reusable heterogeneous catalysts for Michael addition. RSC Adv. 2019, 9, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M.A. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogen-doped carbon. ACS Catal. 2014, 4, 3749–3754. [Google Scholar] [CrossRef]









| Sample | BET/m2·g−1 | Pore Volume/cm3·g−1 | Pore Size/nm |
|---|---|---|---|
| AC-N | 418 | 0.26 | 2.5 |
| AC-N-SO4H | 384 | 0.23 | 2.5 |
| Entry | Solid Acid Catalyst | Catalyst Amount (wt.%) | Benzaldehyde: Ethylene Glycol | Time (h) | Temp. (°C) | Conv. in the First Cycle (%) | Sel. (%) | Reaction Cycle | Conv. in the Last Cycle (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AC-N-SO4H | 5 | 1:1.75 | 5 | 80 | 99 | 100 | 10 | 99 | This study |
| 2 | SulAmp-AC | 3 | 1:3 | 3 | 90 | 98 | - | 4 | 92 | [27] |
| 3 | GO-PrSO3H | 3 | 1:3 | 3 | 90 | 92 | - | 5 | 80 | [28] |
| 4 | Fe3O4@C-SO3H | 1.3 | 1:1 | 2 | 90 | 69 | 97 | 9 | 63 | [29] |
| 5 | SO3H/NCF-600 | 1.9 | 1:5 | 1 | 90 | 99 | - | 5 | 99 | [30] |
| 6 | SG-[(CH2)3SO3H-HIM]HSO4 | 8.2 | 1:1.8 | 1.5 | 110 | 95 | - | 10 | 90 | [31] |
| 7 | SulAmp-GO | 3 | 1:3 | 3 | 90 | 86 | - | - | - | [27] |
| 8 | CeFeTiO | 6.9 | 1:1.6 | 3 | 110 | 97 | - | - | - | [7] |
| 9 | [PPSH]2HPW12O40 | 5 | 1:1.8 | 3 | reflux | 85 | - | - | - | [11] |
| 10 | HMQ-STW | 7 | 1:3 | 1 | 105 | 96 | 100 | 5 | 90 | [13] |
| Raw Materials | Conv. (%) | |
|---|---|---|
| Alcohol | Aldehydes (Ketones) | |
| Glycol | 2-Pentanone | 99.20 |
| Glycol | Cyclohexanone | 98.90 |
| Glycol | Butanal | 99.12 |
| Glycol | 2-Furaldehyde | 96.00 |
| Glycol | Salicylaldehyde | 67.31 |
| 1,2-Propanediol | 2-Pentanone | 99.30 |
| 1,2-Propanediol | Cyclohexanone | 99.12 |
| 1,2-Propanediol | Butanal | 99.10 |
| 1,2-Propanediol | 2-Furaldehyde | 96.55 |
| 1,2-Propanediol | Salicylaldehyde | 66.28 |
| Butane-1,2-diol | 2-Pentanone | 97.26 |
| Butane-1,2-diol | Cyclohexanone | 98.09 |
| Butane-1,2-diol | Butanal | 98.73 |
| Butane-1,2-diol | 2-Furaldehyde | 90.50 |
| Butane-1,2-diol | Salicylaldehyde | 63.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Guo, R.; Peng, G.; Yin, D. Sulfuric Acid Immobilized on Activated Carbon Aminated with Ethylenediamine: An Efficient Reusable Catalyst for the Synthesis of Acetals (Ketals). Nanomaterials 2022, 12, 1462. https://doi.org/10.3390/nano12091462
Liu W, Guo R, Peng G, Yin D. Sulfuric Acid Immobilized on Activated Carbon Aminated with Ethylenediamine: An Efficient Reusable Catalyst for the Synthesis of Acetals (Ketals). Nanomaterials. 2022; 12(9):1462. https://doi.org/10.3390/nano12091462
Chicago/Turabian StyleLiu, Wenzhu, Ruike Guo, Guanmin Peng, and Dulin Yin. 2022. "Sulfuric Acid Immobilized on Activated Carbon Aminated with Ethylenediamine: An Efficient Reusable Catalyst for the Synthesis of Acetals (Ketals)" Nanomaterials 12, no. 9: 1462. https://doi.org/10.3390/nano12091462
APA StyleLiu, W., Guo, R., Peng, G., & Yin, D. (2022). Sulfuric Acid Immobilized on Activated Carbon Aminated with Ethylenediamine: An Efficient Reusable Catalyst for the Synthesis of Acetals (Ketals). Nanomaterials, 12(9), 1462. https://doi.org/10.3390/nano12091462