Single and Mixture Toxicity of Boron and Vanadium Nanoparticles in the Soil Annelid Enchytraeus crypticus: A Multi-Biomarker Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials and Characterization
2.2. Soil Invertebrates
2.3. Test Soil and Experimental Conditions
2.3.1. Enchytraeid Reproduction Test (ERT)
2.3.2. Biochemical Assays
2.4. Data Analysis
3. Results
3.1. Characterization of the Test Materials
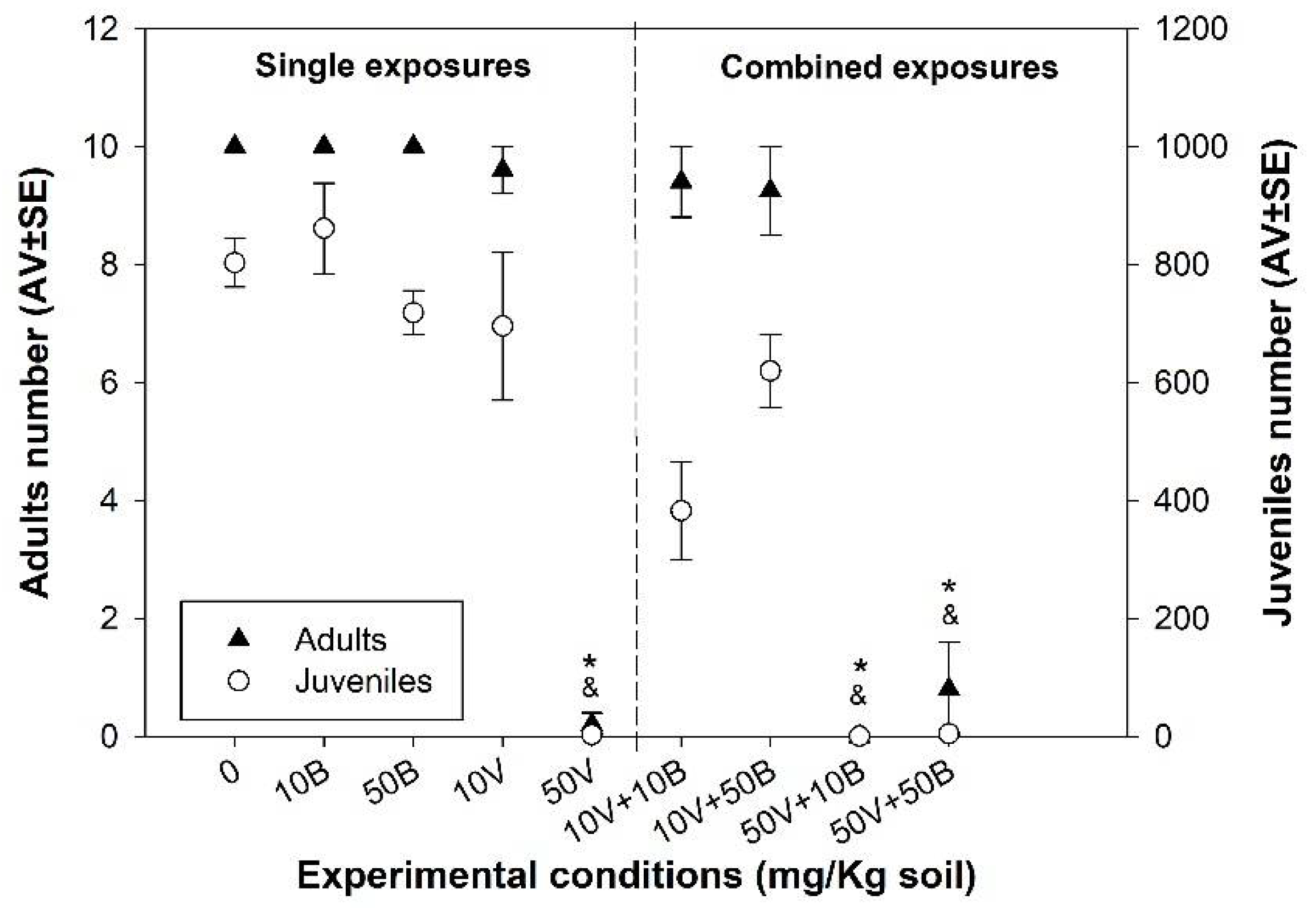
3.2. Enchytraeid Reproduction Test (ERT)
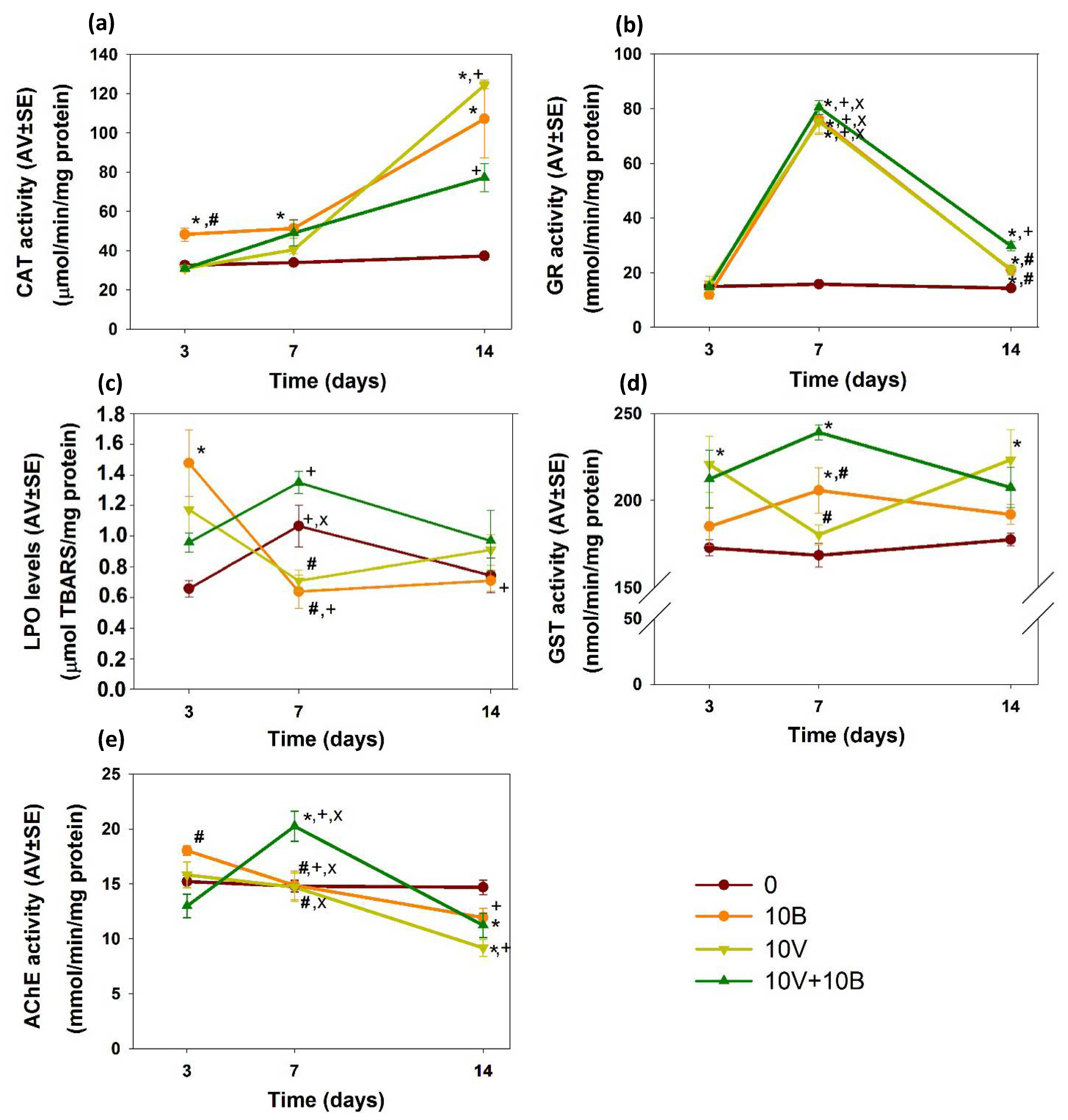
3.3. Biochemical Assays
3.3.1. Oxidative Stress/Damage and Detoxification
3.3.2. Neurotoxicity
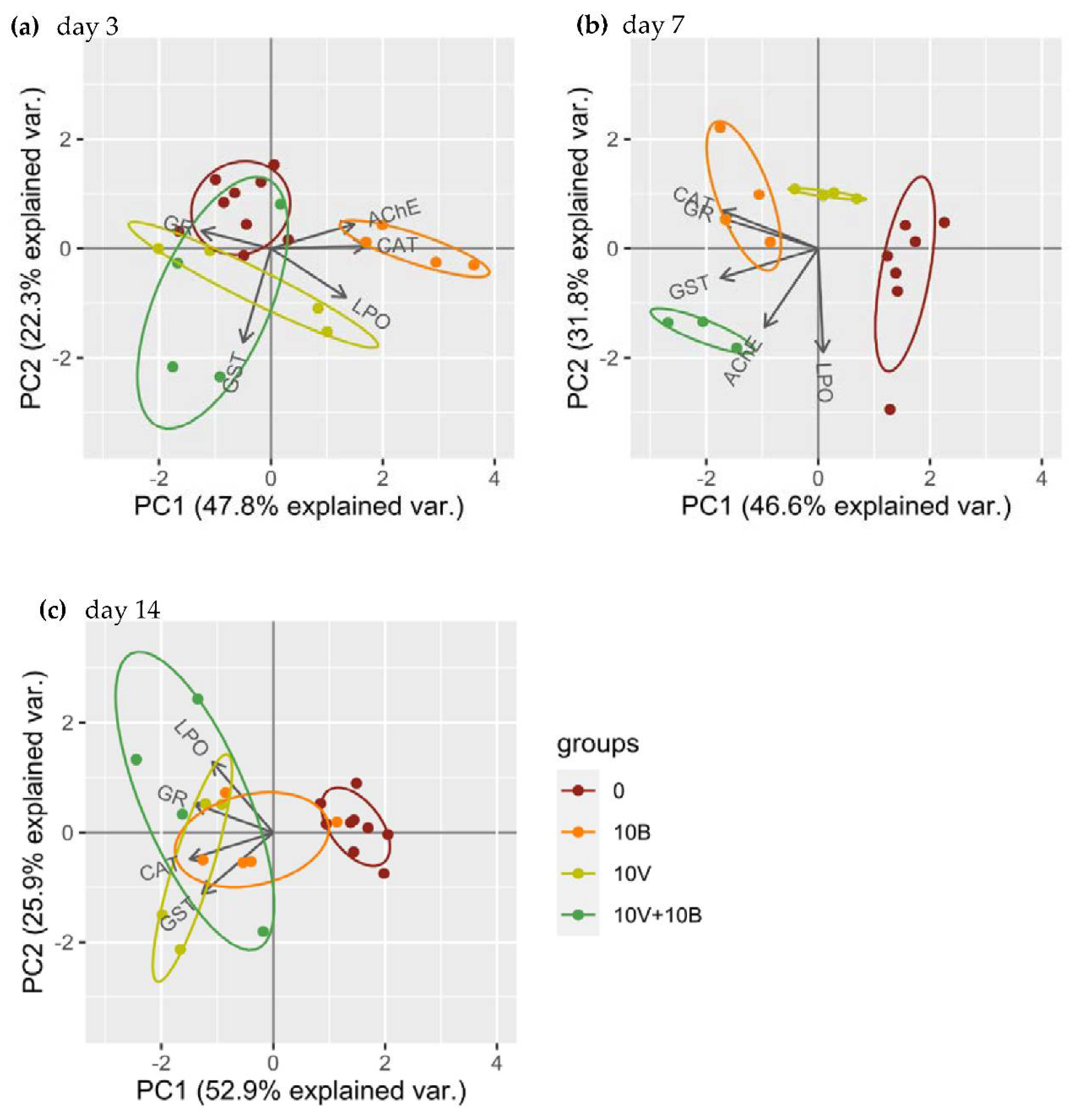
3.3.3. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F.; Baeza, A. Molecules The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- Kahru, A.; Dubourguier, H.-C. From Ecotoxicology to Nanoecotoxicology. Toxicology 2010, 269, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Bour, A.; Mouchet, F.; Verneuil, L.; Evariste, L.; Silvestre, J.; Pinelli, E.; Gauthier, L. Toxicity of CeO2 Nanoparticles at Different Trophic Levels—Effects on Diatoms, Chironomids and Amphibians. Chemosphere 2015, 120, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Chrishtop, V.V.; Prilepskii, A.Y.; Nikonorova, V.G.; Mironov, V.A. Nanosafety vs. Nanotoxicology: Adequate Animal Models for Testing In Vivo Toxicity of Nanoparticles. Toxicology 2021, 462, 152952. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.; Kanel, S.R.; Ray, C.; Snow, D.D.; Nadagouda, M.N. Nanomaterials in the Environment, Human Exposure Pathway, and Health Effects: A Review. Sci. Total Environ. 2021, 759, 143470. [Google Scholar] [CrossRef]
- Bour, A.; Mouchet, F.; Silvestre, J.; Gauthier, L.; Pinelli, E. Environmentally Relevant Approaches to Assess Nanoparticles Ecotoxicity: A Review. J. Hazard. Mater. 2015, 283, 764–777. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the Aquatic Environment: Usage, Properties, Transformation and Toxicity—A Review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Lewis, R.W.; Bertsch, P.M.; McNear, D.H. Nanotoxicity of Engineered Nanomaterials (ENMs) to Environmentally Relevant Beneficial Soil Bacteria—A Critical Review. Nanotoxicology 2019, 13, 392–428. [Google Scholar] [CrossRef]
- PJ, J.C.; Saigeetha, S.; Samrot, A.V.; Ponniah, P.; Chakravarthi, S. Overview on Toxicity of Nanoparticles, It’s Mechanism, Models Used in Toxicity Studies and Disposal Methods—A Review. Biocatal. Agric. Biotechnol. 2021, 36, 102117. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in Agriculture: Current Status, Challenges and Future Opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Garcés, M.; Cáceres, L.; Chiappetta, D.; Magnani, N.; Evelson, P. Current Understanding of Nanoparticle Toxicity Mechanisms and Interactions with Biological Systems. New J. Chem. 2021, 45, 14328–14344. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Dubey, S.P.; Sillanpää, M.; Kwon, Y.-N.; Lee, C.; Varma, R.S. Fate of Engineered Nanoparticles: Implications in the Environment. Coord. Chem. Rev. 2015, 287, 64–78. [Google Scholar] [CrossRef]
- Ma, M.; Liu, G.; Qin, Z.; Zhang, R.; Ying, Y.; Xu, L.; Liu, D. Effects of Aluminum Addition on Flash Ignition and Combustion of Boron Nanoparticles. Combust. Flame 2022, 236, 111762. [Google Scholar] [CrossRef]
- Tatiya, S.; Pandey, M.; Bhattacharya, S. Nanoparticles Containing Boron and Its Compounds—Synthesis and Applications: A Review. J. Micromanuf. 2020, 3, 159–173. [Google Scholar] [CrossRef]
- Aliyu, A.O.; Garba, S.; Bognet, S. Green Synthesis, Characterization and Antimicrobial Activity of Vanadium Nanoparticles Using Leaf Extract of Moringa oleifera. IOSR J. Appl. Chem. IOSR-JAC 2018, 11, 42–48. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization & International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland, 1998; ISBN 9241572043. [Google Scholar]
- Awan, R.S.; Liu, C.; Yang, S.; Wu, Y.; Zang, Q.; Khan, A.; Li, G. The Occurrence of Vanadium in Nature: Its Biogeochemical Cycling and Relationship with Organic Matter—A Case Study of the Early Cambrian Black Rocks of the Niutitang Formation, Western Hunan, China. Acta Geochim. 2021, 40, 973–997. [Google Scholar] [CrossRef]
- Barreto, A.; Santos, J.; Amorim, M.J.B.; Maria, V.L. Environmental Hazards of Boron and Vanadium Nanoparticles in the Terrestrial Ecosystem—A Case Study with Enchytraeus crypticus. Nanomaterials 2021, 11, 1937. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Barreto, Â.; Almeida, C.; Azevedo, C.; Domingues, I.; Amorim, M.J.B.; Maria, V.L. Toxicity of Boron and Vanadium Nanoparticles on Danio rerio Embryos—Phenotypical, Biochemical, and Behavioral Alterations. Aquat. Toxicol. 2021, 238, 105930. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.S.; Li, J.B.; Liu, Y.Y.; Wu, H.; Cao, A.; Wang, H. Cytotoxicity and Genotoxicity of Low-Dose Vanadium Dioxide Nanoparticles to Lung Cells Following Long-Term Exposure. Toxicology 2021, 459, 300–483. [Google Scholar] [CrossRef]
- Kulkarni, A.; Kumar, G.S.; Kaur, J.; Tikoo, K. A Comparative Study of the Toxicological Aspects of Vanadium Pentoxide and Vanadium Oxide Nanoparticles. Inhal. Toxicol. 2014, 26, 772–788. [Google Scholar] [CrossRef] [PubMed]
- Strigul, N.; Vaccari, L.; Galdun, C.; Wazne, M.; Liu, X.; Christodoulatos, C.; Jasinkiewicz, K. Acute Toxicity of Boron, Titanium Dioxide, and Aluminum Nanoparticles to Daphnia magna and Vibrio fischeri. Desalination 2009, 248, 771–782. [Google Scholar] [CrossRef]
- Alak, G.; Ucar, A.; Parlak, V.; Yeltekin, A.Ç.; Özgeriş, F.B.; Atamanalp, M.; Türkez, H. Antioxidant Potential of Ulexite in Zebrafish Brain: Assessment of Oxidative DNA Damage, Apoptosis, and Response of Antioxidant Defense System. Biol. Trace Elem. Res. 2021, 199, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Alak, G.; Özgeriş, F.B.; Yeltekin, A.Ç.; Parlak, V.; Ucar, A.; Caglar, O.; Turkez, H.; Atamanalp, M. Hematological and Hepatic Effects of Ulexite in Zebrafish. Environ. Toxicol. Pharmacol. 2020, 80, 103496. [Google Scholar] [CrossRef] [PubMed]
- Geyikoglu, F.; Turkez, H. Boron Compounds Reduce Vanadium Tetraoxide Genotoxicity in Human Lymphocytes. Environ. Toxicol. Pharmacol. 2008, 26, 342–347. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Lu, J.; Xiong, M.; Kaleem, M.; Huang, Y.; Bie, Z. Application of Boron Reduces Vanadium Toxicity by Altering the Subcellular Distribution of Vanadium, Enhancing Boron Uptake and Enhancing the Antioxidant Defense System of Watermelon. Ecotoxicol. Environ. Saf. 2021, 226, 112828. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ferreira, M.P.; Roelofs, D.; van Gestel, C.A.M.; Verweij, R.A.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeus crypticus as Model Species in Soil Ecotoxicology. Chemosphere 2012, 87, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, Q.-L.; O’Connor, P.; Sheng, G.D. Does Soil CuO Nanoparticles Pollution Alter the Gut Microbiota and Resistome of Enchytraeus crypticus? Environ. Pollut. 2020, 256, 113463. [Google Scholar] [CrossRef]
- Gomes, S.I.L.; Caputo, G.; Pinna, N.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effect of 10 different tio2 and zro2 (nano) materials on the soil invertebrate Enchytraeus crypticus. Environ. Toxicol. Chem. 2015, 34, 2409–2416. [Google Scholar] [CrossRef]
- Westheide, W.; Graefe, U. Two New Terrestrial Enchytraeus Species (Oligochaeta, Annelida). J. Nat. Hist. 1992, 26, 479–488. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development (OECD). Test No. 220: Enchytraeid Reproduction Test; OECD Publishing: Paris, France, 2004. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Bird, R.P.; Draper, H.H. Comparative Studies on Different Methods of Malonaldehyde Determination. Methods Enzymol. 1984, 105, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm Filho, D.; Tribess, T.; Gáspari, C.; Claudio, F.; Torres, M.; Magalhães, A.R. Seasonal Changes in Antioxidant Defenses of the Digestive Gland of the Brown Mussel (Perna perna). Aquaculture 2001, 203, 149–158. [Google Scholar] [CrossRef]
- Claiborne, A. Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Giri, U.; Iqbal, M.; Athar, M. Porphyrin-Mediated Photosensitization Has a Weak Tumor Promoting Activity in Mouse Skin: Possible Role of in Situ-Generated Reactive Oxygen Species. Carcinogenesis 1996, 17, 2023–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribb, A.E.; Leeder, J.S.; Spielberg, S.P. Use of a Microplate Reader in an Assay of Glutathione Reductase Using 5,5′-Dithiobis(2-Nitrobenzoic Acid). Anal. Biochem. 1989, 183, 195–196. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases the First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Inhibition of Acetylcholinesterase Activity as Effect Criterion in Acute Tests with Juvenile Daphnia magna. Chemosphere 1996, 32, 727–738. [Google Scholar] [CrossRef]
- Green, J.; Wheeler, J.R. The Use of Carrier Solvents in Regulatory Aquatic Toxicology Testing: Practical, Statistical and Regulatory Considerations. Aquat. Toxicol. 2013, 144–145, 242–249. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Iglewicz, B. Fine-Tuning Some Resistant Rules for Outlier Labeling. J. Am. Stat. Assoc. 1987, 82, 1147–1149. [Google Scholar] [CrossRef]
- D’Surney, S.J.; Smith, M.D. Chemicals of Environmental Concern. Encycl. Toxicol. 2005, 526–530. [Google Scholar] [CrossRef]
- Wang, H.; Adeleye, A.S.; Huang, Y.; Li, F.; Keller, A.A. Heteroaggregation of Nanoparticles with Biocolloids and Geocolloids. Adv. Colloid Interface Sci. 2015, 226, 24–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, M. Increased Lipid Peroxidation in Tissues of the Catfish Clarias batrachus Following Vanadium Treatment: In Vivo and In Vitro Evaluation. J. Inorg. Biochem. 1994, 54, 277–284. [Google Scholar] [CrossRef]
- Soares, S.S.; Martins, H.; Gutiérrez-Merino, C.; Aureliano, M. Vanadium and Cadmium in Vivo Effects in Teleost Cardiac Muscle: Metal Accumulation and Oxidative Stress Markers. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 147, 168–178. [Google Scholar] [CrossRef]
- Martinez, D.S.T.; Ellis, L.-J.A.; Da Silva, G.H.; Petry, R.; Medeiros, A.M.Z.; Davoudi, H.H.; Papadiamantis, A.G.; Fazzio, A.; Afantitis, A.; Melagraki, G.; et al. Daphnia magna and Mixture Toxicity with Nanomaterials—Current Status and Perspectives in Data-Driven Risk Prediction. Nano Today 2022, 43, 101430. [Google Scholar] [CrossRef]
- Zhao, H.-Z.; Lu, G.-H.; Xia, J.; Jin, S.-G. Toxicity of Nanoscale CuO and ZnO to Daphnia magna. Chem. Res. Chin. Univ. 2012, 28, 209–213. [Google Scholar]
- Pandey, A.; Gurbuz, Y.; Ozguz, V.; Niazi, J.H.; Qureshi, A. Probing Synergistic Toxicity Effects on Living Cells by Combination of Two Different Sized Nanoparticles by a Whole-Cell Based Biochip. Mater. Today Proc. 2017, 4, 8427–8431. [Google Scholar] [CrossRef]
- Yu, R.; Wu, J.; Liu, M.; Zhu, G.; Chen, L.; Chang, Y.; Lu, H. Toxicity of Binary Mixtures of Metal Oxide Nanoparticles to Nitrosomonas Europaea. Chemosphere 2016, 153, 187–197. [Google Scholar] [CrossRef]
- He, X.; Xue, J.; Shi, L.; Kong, Y.; Zhan, Q.; Sun, Y.; Zhang, Q.; Ramakrishna, S.; Dai, Y. Recent Antioxidative Nanomaterials toward Wound Dressing and Disease Treatment via ROS Scavenging. Mater. Today Nano 2022, 17, 100149. [Google Scholar] [CrossRef]
- Do Amaral, D.F.; Guerra, V.; Motta, A.G.C.; de Melo e Silva, D.; Rocha, T.L. Ecotoxicity of Nanomaterials in Amphibians: A Critical Review. Sci. Total Environ. 2019, 686, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, M.B.; Araújo, P.S.; Gonçalves, B.B.; Silva, L.D.; Grano-Maldonado, M.I.; Rocha, T.L. Toxicity of Engineered Nanomaterials to Aquatic and Land Snails: A Scientometric and Systematic Review. Chemosphere 2020, 260, 127654. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Takai, T.; Hisatomi, H. Cell Death via Lipid Peroxidation and Protein Aggregation Diseases. Biology 2021, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. Lipid Peroxidation 2012, 1, 3–30. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Halim, K.Y.; Osman, S.R.; Abdou, G.Y. In Vivo Evaluation of Oxidative Stress and Biochemical Alteration as Biomarkers in Glass Clover Snail, Monacha cartusiana Exposed to Zinc Oxide Nanoparticles. Environ. Pollut. 2020, 257, 113120. [Google Scholar] [CrossRef]
- Bernard, F.; Brulle, F.; Dumez, S.; Lemiere, S.; Platel, A.; Nesslany, F.; Cuny, D.; Deram, A.; Vandenbulcke, F. Antioxidant Responses of Annelids, Brassicaceae and Fabaceae to Pollutants: A Review. Ecotoxicol. Environ. Saf. 2014, 114, 273–303. [Google Scholar] [CrossRef]
- Schuijt, L.M.; Peng, F.-J.; van den Berg, S.J.P.; Dingemans, M.M.L.; Van den Brink, P.J. (Eco)Toxicological Tests for Assessing Impacts of Chemical Stress to Aquatic Ecosystems: Facts, Challenges, and Future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally Induced Oxidative Stress in Aquatic Animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Scott-Fordsmand, J.J.; Weeks, J.M. Biomarkers in Earthworms. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2000; Volume 165, pp. 117–159. [Google Scholar]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.N.; Prasad, M. Metal/Metal Oxide Nanoparticles: Toxicity Concerns Associated with Their Physical State and Remediation for Biomedical Applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef]
- Ma, X.; Deng, D.; Chen, W. Inhibitors and Activators of SOD, GSH-Px, and CAT. In Enzyme Inhibitors and Activators; InTech: London, UK, 2017; ISBN 978-953-51-3058-1. [Google Scholar]
- Ribeiro, M.; Maria, V.; Scott-Fordsmand, J.; Amorim, M. Oxidative Stress Mechanisms Caused by Ag Nanoparticles (NM300K) Are Different from Those of AgNO3: Effects in the Soil Invertebrate Enchytraeus crypticus. Int. J. Environ. Res. Public Health 2015, 12, 9589–9602. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.I.L.; Novais, S.C.; Gravato, C.; Guilhermino, L.; Scott-Fordsmand, J.J.; Soares, A.M.V.M.; Amorim, M.J.B. Effect of Cu-Nanoparticles versus One Cu-Salt: Analysis of Stress Biomarkers Response in Enchytraeus albidus (Oligochaeta). Nanotoxicology 2012, 6, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.M.S.; Novais, S.C.; Soares, A.M.V.M.; Amorim, M.J.B. Dimethoate Affects Cholinesterases in Folsomia candida and Their Locomotion-False Negative Results of an Avoidance Behaviour Test. Sci. Total Environ. 2012, 443, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lu, G.; Hou, K.; Qin, D.; Yan, Z.; Chen, W. Bioconcentration, Metabolism and Effects of Diphenhydramine on Behavioral and Biochemical Markers in Crucian Carp (Carassius auratus). Sci. Total Environ. 2016, 544, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Bicho, R.C.; Gomes, S.I.L.; Soares, A.M.V.M.; Amorim, M.J.B. Non-Avoidance Behaviour in Enchytraeids to Boric Acid Is Related to the GABAergic Mechanism. Environ. Sci. Pollut. Res. 2015, 22, 6898–6903. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Series on the Safety of Manufactured Nanomaterials, No. 36: Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials; OECD: Paris, France, 2012. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capitão, A.; Santos, J.; Barreto, A.; Amorim, M.J.B.; Maria, V.L. Single and Mixture Toxicity of Boron and Vanadium Nanoparticles in the Soil Annelid Enchytraeus crypticus: A Multi-Biomarker Approach. Nanomaterials 2022, 12, 1478. https://doi.org/10.3390/nano12091478
Capitão A, Santos J, Barreto A, Amorim MJB, Maria VL. Single and Mixture Toxicity of Boron and Vanadium Nanoparticles in the Soil Annelid Enchytraeus crypticus: A Multi-Biomarker Approach. Nanomaterials. 2022; 12(9):1478. https://doi.org/10.3390/nano12091478
Chicago/Turabian StyleCapitão, Ana, Joana Santos, Angela Barreto, Mónica J. B. Amorim, and Vera L. Maria. 2022. "Single and Mixture Toxicity of Boron and Vanadium Nanoparticles in the Soil Annelid Enchytraeus crypticus: A Multi-Biomarker Approach" Nanomaterials 12, no. 9: 1478. https://doi.org/10.3390/nano12091478
APA StyleCapitão, A., Santos, J., Barreto, A., Amorim, M. J. B., & Maria, V. L. (2022). Single and Mixture Toxicity of Boron and Vanadium Nanoparticles in the Soil Annelid Enchytraeus crypticus: A Multi-Biomarker Approach. Nanomaterials, 12(9), 1478. https://doi.org/10.3390/nano12091478








