Preparation, Characterization and Application of Epitaxial Grown BiOBr (110) Film on ZnFe2O4 Surface with Enhanced Photocatalytic Fenton Oxidation Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of BiOBr/ZnFe2O4 Nanocomposites
2.2. Methods of Characterization
2.3. Electrochemical Measurements
2.4. Photocatalytic Investigation
3. Results and Discussion
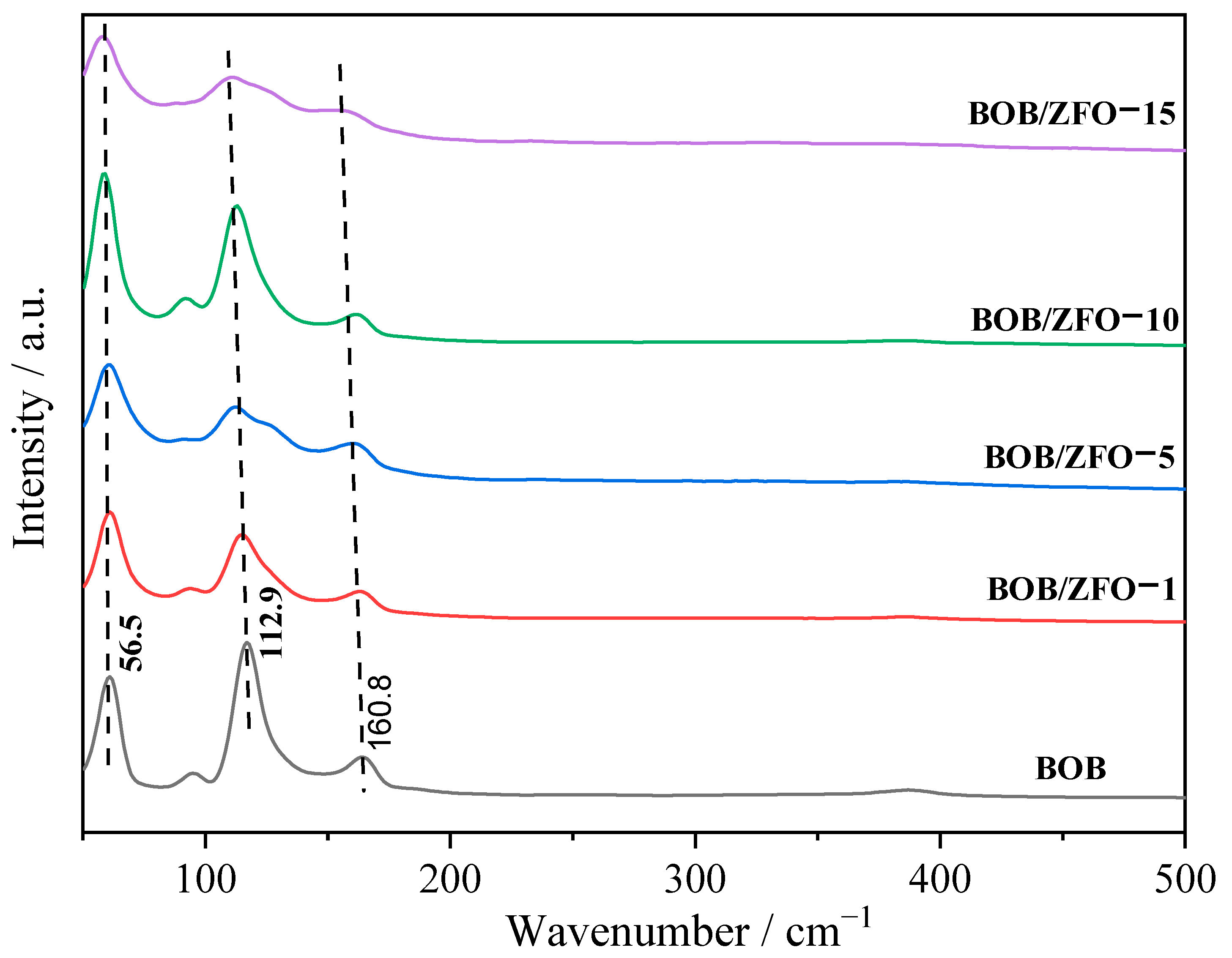
3.1. The Crystal Structure of BOB/ZFO Nanocomposite
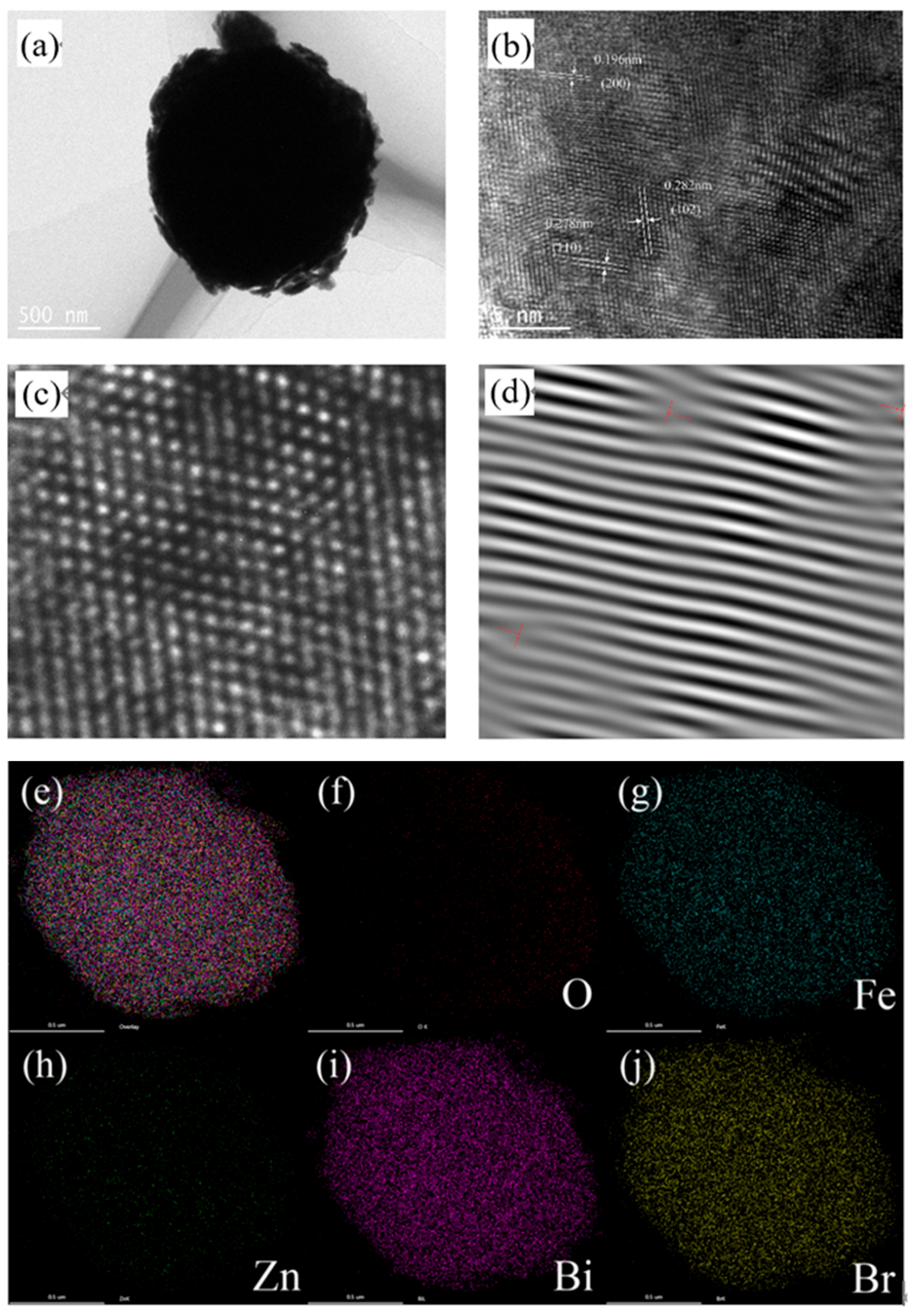
3.2. The Morphology of BOB/ZFO Nanocomposite
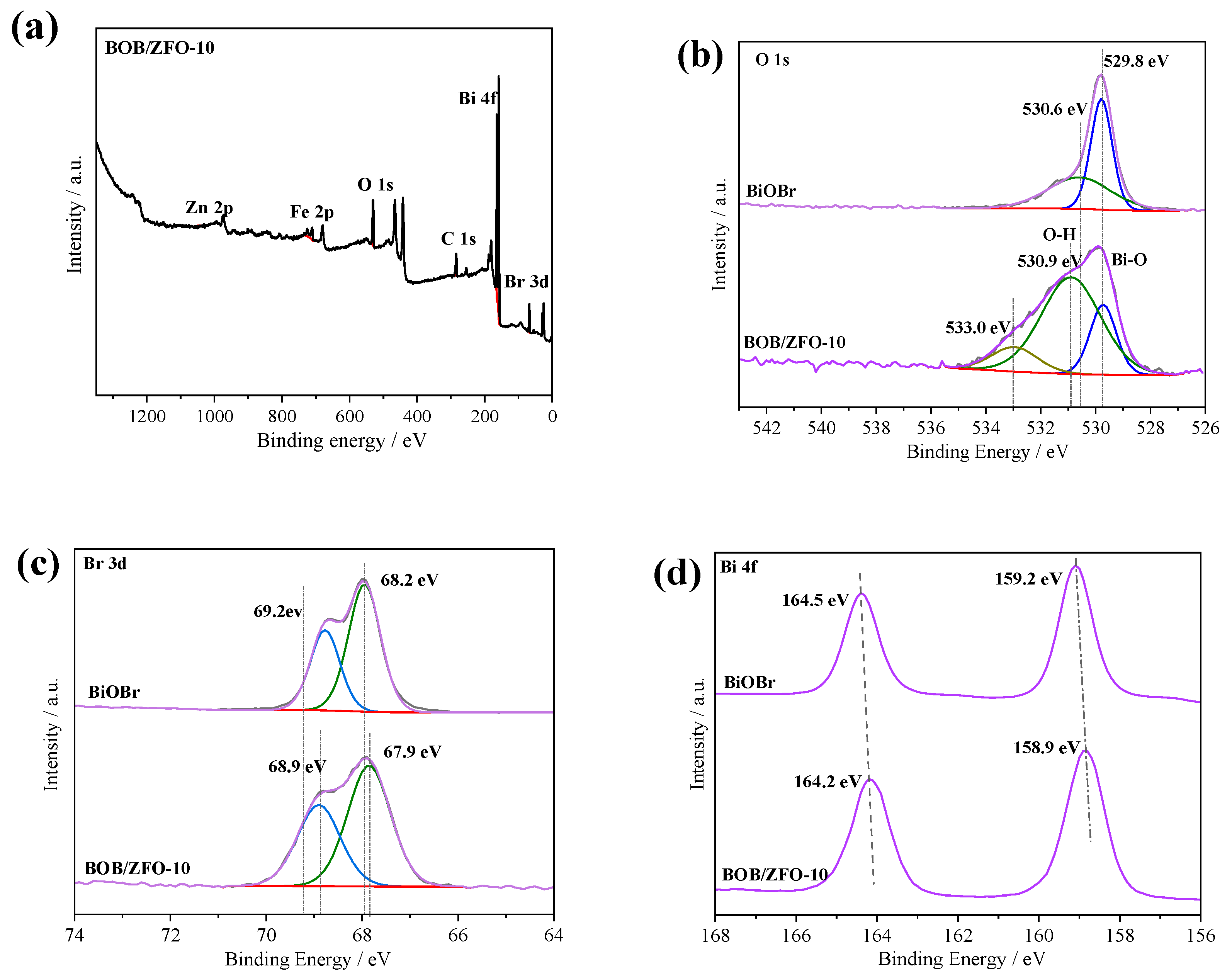
3.3. The Surface Chemical States of BOB/ZFO-x Nanocomposite
3.4. The Local Structure of BOB/ZFO Nanocomposite
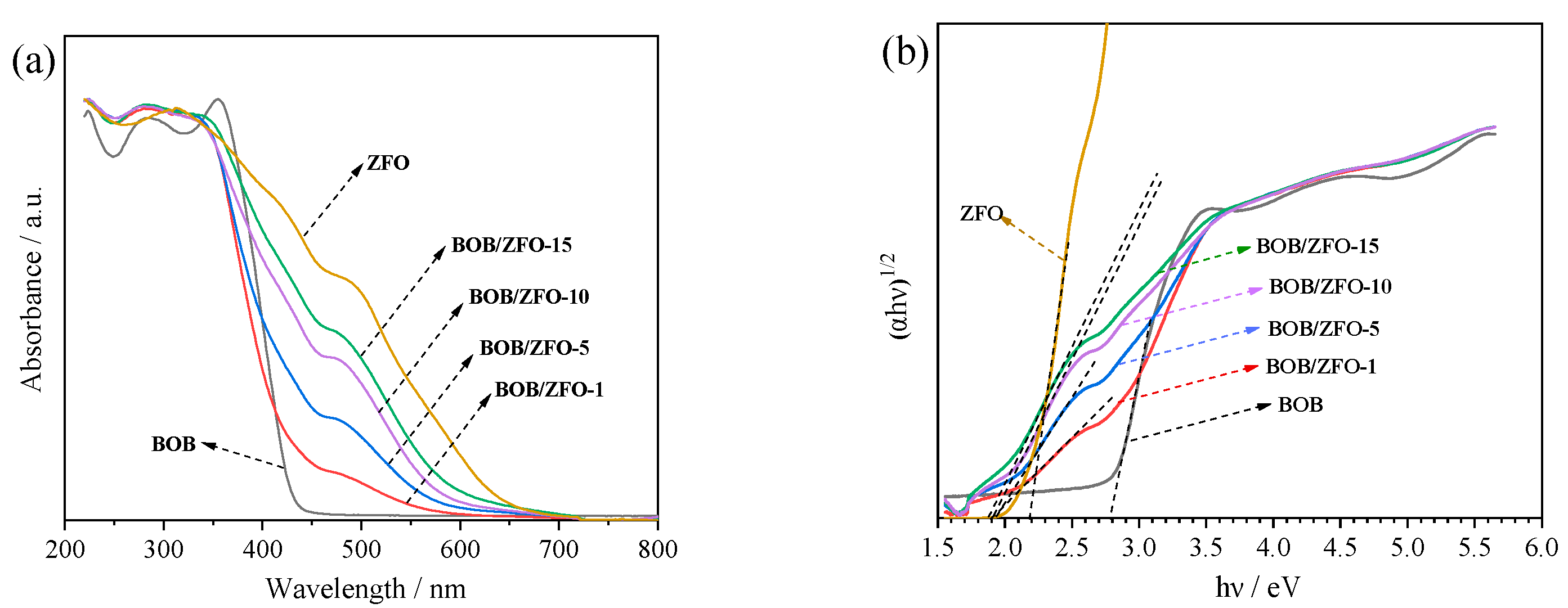
3.5. The Photophysical Property of BOB/ZFO Nanocomposite
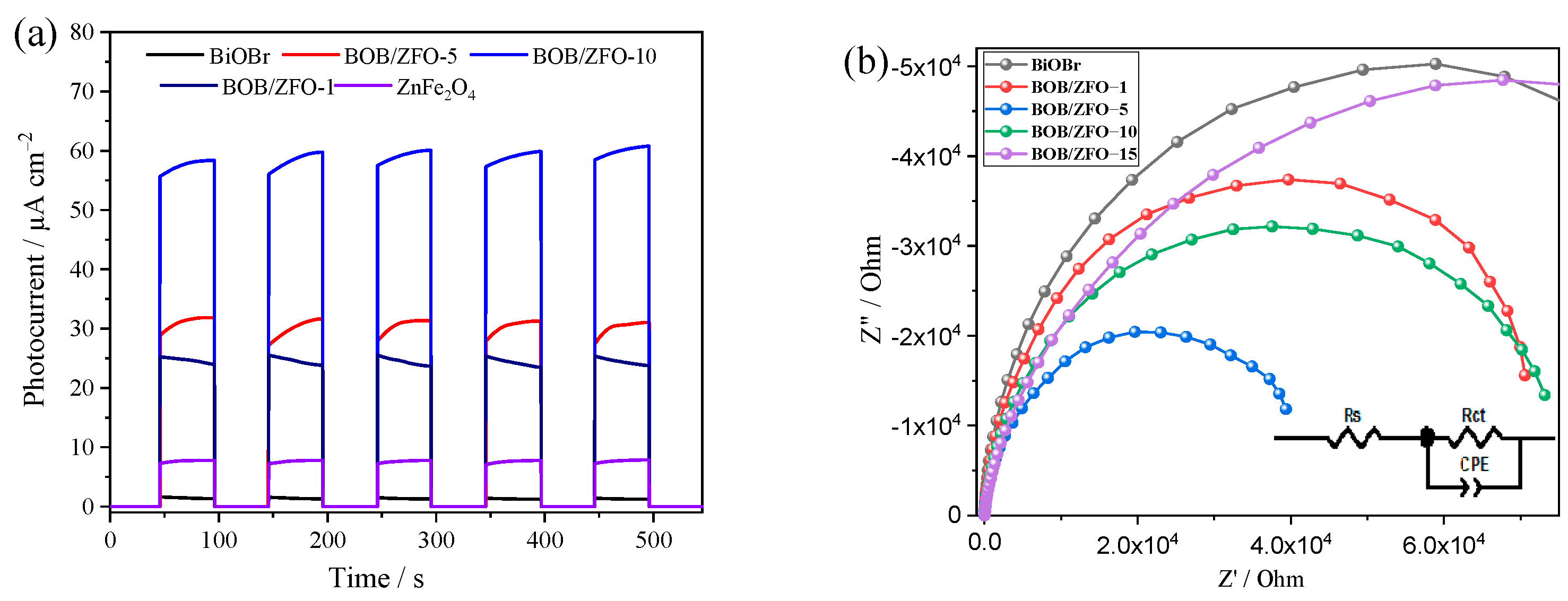
3.6. The Photoelectrochemical Properties of BOB/ZFO Nanocomposites
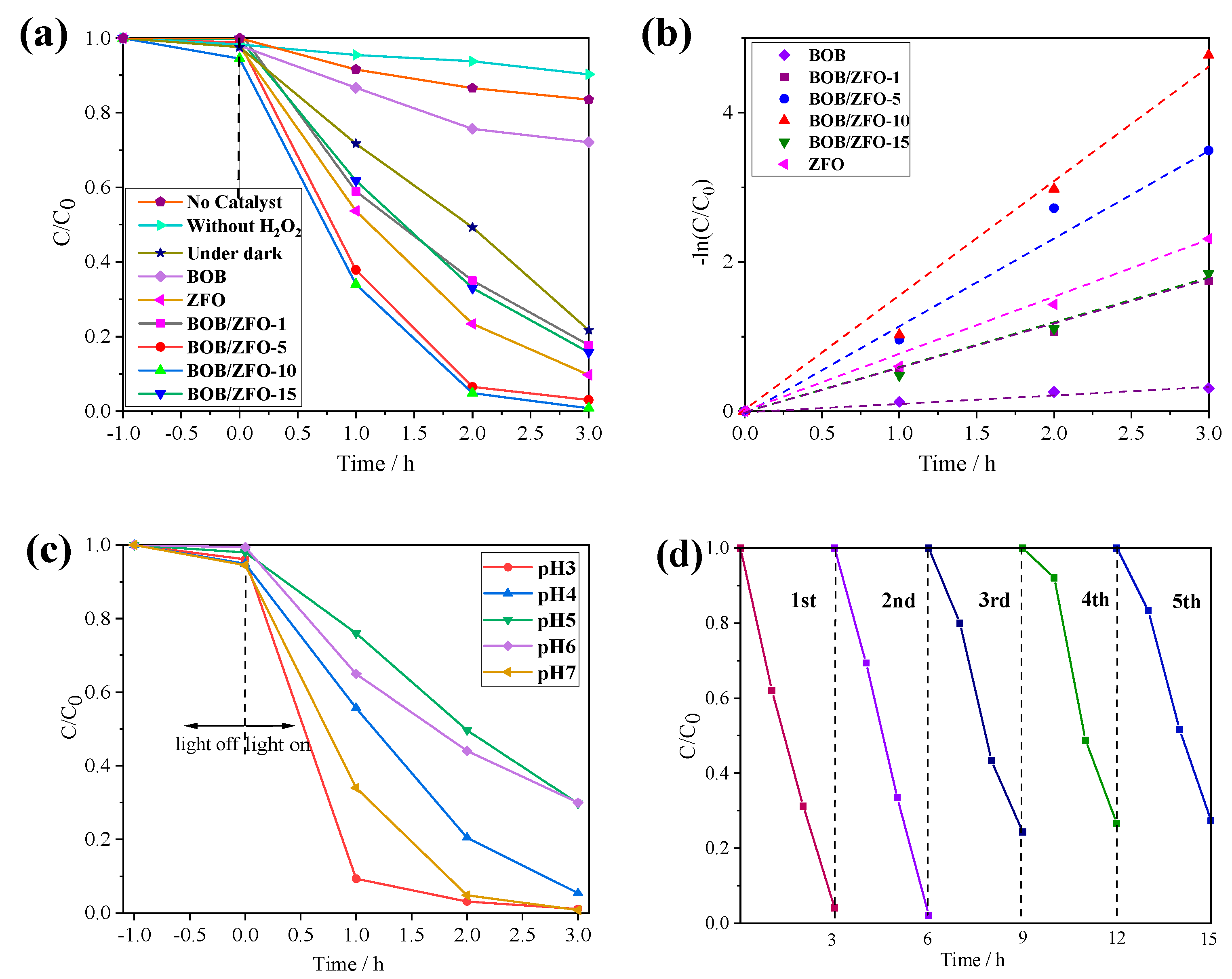
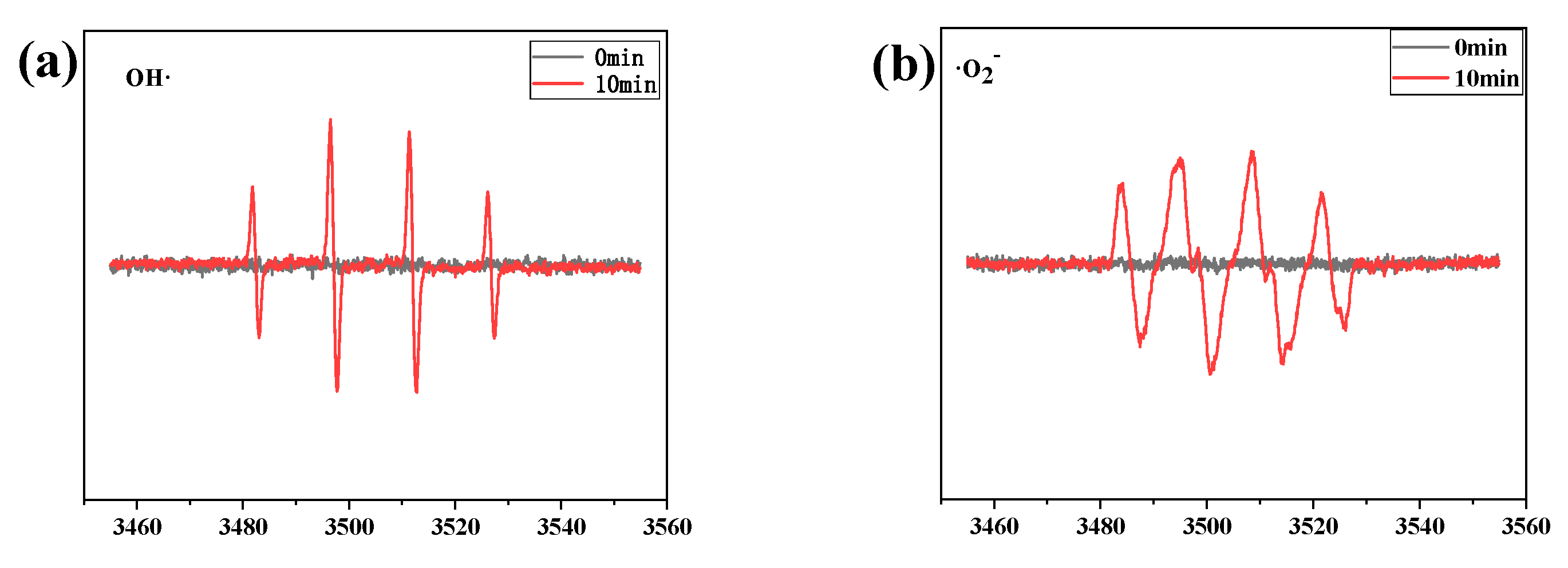
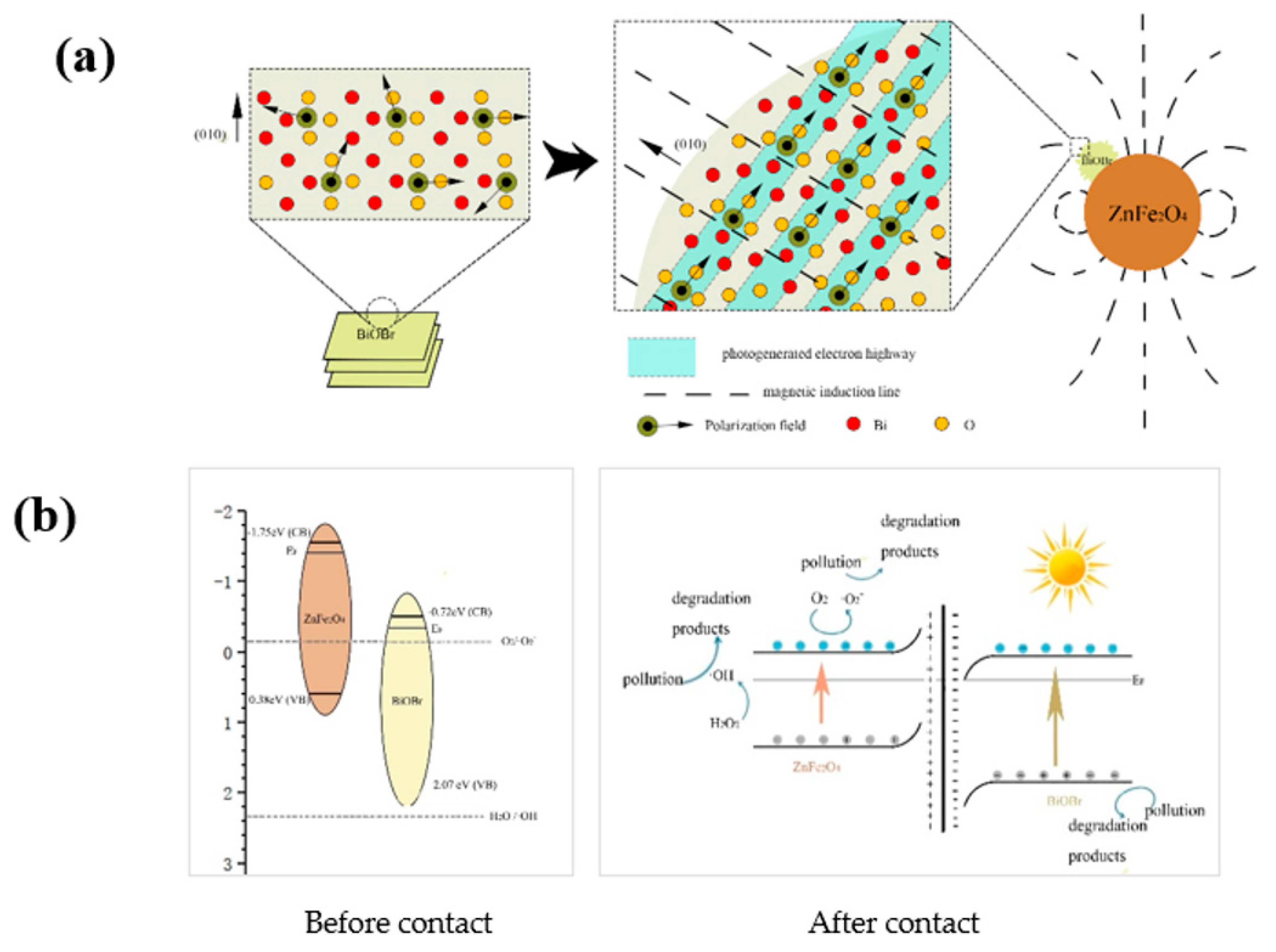
3.7. The Photo-Fenton Degradation Property of BOB/ZFO Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A Critical Review on Antibiotics and Hormones in Swine Wastewater: Water Pollution Problems and Control Approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the Biological and Chemical Treatment Technologies for Emerging Contaminant Removal from Wastewater: A Critical Review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Liu, Y.G.; Zeng, G.M.; Liu, N.; Liu, S.B. Graphene and Graphene-Based Nanocomposites Used for Antibiotics Removal in Water Treatment: A Review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, K.; El-Din, M.G.; Snyder, S. Ozonation and Advanced Oxidation Treatment of Emerging Organic Pollutants in Water and Wastewater. Ozone Sci. Eng. 2008, 30, 21–26. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Veciana, M.; Su, C.C.; Lu, M.C. Acetaminophen Degradation by Electro-Fenton and Photoelectro-Fenton Using a Double Cathode Electrochemical Cell. J. Hazard. Mater. 2012, 217, 200–207. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for Enhancing the Heterogeneous Fenton Catalytic Reactivity: A Review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Advancing Fenton and Photo-Fenton Water Treatment through the Catalyst Design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van Der Waals Heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J.; Li, M.; Xu, J.; Zhang, L.; Yao, J. Construction of Two-Dimensional Bioi on Carboxyl-Rich Mil-121 for Visible-Light Photocatalytic Degradation of Tetracycline. J. Alloys Compd. 2021, 872, 159711. [Google Scholar] [CrossRef]
- Gao, X.; Gao, K.; Li, X.; Shang, Y.; Fu, F. Hybrid Pdi/Biocl Heterojunction with Enhanced Interfacial Charge Transfer for a Full-Spectrum Photocatalytic Degradation of Pollutants. Catal. Sci. Technol. 2020, 10, 372–381. [Google Scholar] [CrossRef]
- Li, B.; Shao, L.; Wang, R.; Dong, X.; Zhao, F.; Gao, P.; Li, Z. Interfacial Synergism of Pd-Decorated Biocl Ultrathin Nanosheets for the Selective Oxidation of Aromatic Alcohols. J. Mater. Chem. A 2018, 6, 6344–6355. [Google Scholar] [CrossRef]
- Xiao, Y.; He, Z.; Wang, R.; Tao, X.; Li, B. Synthesis of Wo3 Nanofibers Decorated with Biocl Nanosheets for Photocatalytic Degradation of Organic Pollutants under Visible Light. Colloids Surf. Physicochem. Eng. Asp. 2019, 580, 123752. [Google Scholar] [CrossRef]
- Zhang, K.L.; Liu, C.M.; Huang, F.Q.; Zheng, C.; Wang, W.D. Study of the Electronic Structure and Photocatalytic Activity of the Biocl Photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Wang, H.; Yong, D.; Chen, S.; Jiang, S.; Zhang, X.; Shao, W.; Zhang, Q.; Yan, W.; Pan, B.; Xie, Y. Oxygen-Vacancy-Mediated Exciton Dissociation in Biobr for Boosting Charge-Carrier-Involved Molecular Oxygen Activation. J. Am. Chem. Soc. 2018, 140, 1760–1766. [Google Scholar] [CrossRef]
- Gu, W.; Li, X.; Zhang, W.; Wang, J.; Yin, X.; Zhu, L.; Chen, Z.; Zou, W.; Fu, Z.; Lu, Y. Self-Limited Ion-Exchange Grown Bi6Fe2Ti3O18-Biobr Ferroelectric Heterostructure and the Enhanced Photocatalytic Oxygen Evolution. Appl. Surf. Sci. 2019, 479, 137–147. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Lu, D.; Fan, Y. Optical Property and Thermal Performance of Hollow Glass Microsphere/Biobr1-Xix Composites as a Novel Colored near Infrared Reflective Pigment. Dyes Pigment. 2018, 154, 21–29. [Google Scholar] [CrossRef]
- Xiao, X.; Hao, R.; Liang, M.; Zuo, X.; Nan, J.; Li, L.; Zhang, W. One-Pot Solvothermal Synthesis of Three-Dimensional (3D) Bioi/Biocl Composites with Enhanced Visible-Light Photocatalytic Activities for the Degradation of Bisphenol-A. J. Hazard. Mater. 2012, 233, 122–130. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Wu, P.; Cui, W. Enriched Photoelectrocatalytic Degradation and Photoelectric Performance of Bioi Photoelectrode by Coupling Rgo. Appl. Catal. B Environ. 2017, 208, 22–34. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J.; Zhang, L.; Ma, H.; Yao, J. Amino-Functionalized Ti-Metal-Organic Framework Decorated Bioi Sphere for Simultaneous Elimination of Cr(Vi) and Tetracycline. J. Colloid Interface Sci. 2022, 607, 933–941. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Huang, D.; Zeng, G.; Xu, P.; Zhou, C.; Lai, C.; Wang, H.; Cheng, M.; Wang, W. Multiply Structural Optimized Strategies for Bismuth Oxyhalide Photocatalysis and Their Environmental Application. Chem. Eng. J. 2019, 374, 1025–1045. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Li, X.; Shi, W.; Ling, P.; Jiao, X.; Gao, S.; Liang, L.; Xu, J.; Yan, W.; et al. Efficient Visible-Light-Driven Co2 Reduction Mediated by Defect-Engineered Biobr Atomic Layers. Angew. Chem. 2018, 130, 8855–8859. [Google Scholar]
- Yu, Q.; Chen, J.; Li, Y.; Wen, M.; Liu, H.; Li, G.; An, T. In-Situ Decoration of Metallic Bi on Biobr with Exposed (110) Facets and Surface Oxygen Vacancy for Enhanced Solar Light Photocatalytic Degradation of Gaseous N-Hexane. Chin. J. Catal. 2020, 41, 1603–1612. [Google Scholar] [CrossRef]
- Bhachu, D.S.; Moniz, S.J.A.; Sathasivam, S.; Scanlon, D.O.; Walsh, A.; Bawaked, S.M.; Mokhtar, M.; Obaid, A.Y.; Parkin, I.P.; Tang, J.; et al. Bismuth Oxyhalides: Synthesis, Structure and Photoelectrochemical Activity. Chem. Sci. 2016, 7, 4832–4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Li, J.; Wang, Q.; Wu, Q. High {001} Facets Dominated Biobr Lamellas: Facile Hydrolysis Preparation and Selective Visible-Light Photocatalytic Activity. J. Mater. Chem. A 2013, 1, 8622–8629. [Google Scholar] [CrossRef]
- Yan, X.; Ji, Q.; Wang, C.; Xu, J.; Wang, L. In Situ Construction Bismuth Oxycarbonate/Bismuth Oxybromide Z-Scheme Heterojunction for Efficient Photocatalytic Removal of Tetracycline and Ciprofloxacin. J. Colloid Interface Sci. 2021, 587, 820–830. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel Bp/Biobr S-Scheme Nano-Heterojunction for Enhanced Visible-Light Photocatalytic Tetracycline Removal and Oxygen Evolution Activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Guin, D.; Baruwati, B.; Manorama, S.V. A Simple Chemical Synthesis of Nanocrystalline Afe(2)O(4) (a = Fe, Ni, Zn): An Efficient Catalyst for Selective Oxidation of Styrene. J. Mol. Catal. A Chem. 2005, 242, 26–31. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Adsorptive Removal of as(Iii) from Aqueous Solution Using Iron Oxide Coated Cement (Iocc): Evaluation of Kinetic, Equilibrium and Thermodynamic Models. Sep. Purif. Technol. 2006, 51, 165–172. [Google Scholar] [CrossRef]
- Mendonca, M.H.; Godinho, M.I.; Catarino, M.A.; Pereira, M.I.D.; Costa, F.M. Preparation and Characterisation of Spinel Oxide Ferrites Suitable for Oxygen Evolution Anodes. Solid State Sci. 2002, 4, 175–182. [Google Scholar] [CrossRef]
- Candeia, R.A.; Bernardi, M.I.B.; Longo, E.; Santos, I.M.G.; Souza, A.G. Synthesis and Characterization of Spinel Pigment CaFe2O4 Obtained by the Polymeric Precursor Method. Mater. Lett. 2004, 58, 569–572. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Zhou, Z.; Zhao, Y.; Liu, L. Enhanced Photocatalytic Properties in Biobr Nanosheets with Dominantly Exposed (102) Facets. J. Phys. Chem. C 2014, 118, 14662–14669. [Google Scholar] [CrossRef]
- Xiong, X.; Ding, L.; Wang, Q.; Li, Y.; Jiang, Q.; Hu, J. Synthesis and Photocatalytic Activity of Biobr Nanosheets with Tunable Exposed {0 1 0} Facets. Appl. Catal. B Environ. 2016, 188, 283–291. [Google Scholar] [CrossRef]
- Yang, X.; Yang, X.; Peng, Y.; Li, Z.; Yu, J.; Zhang, Y. Regulating the Built-in Electric Field of Biobr by a Piezoelectric Mineral Tourmaline and the Enhanced Photocatalytic Property. Ind. Eng. Chem. Res. 2022, 61, 1704–1714. [Google Scholar] [CrossRef]
- Kong, L.; Jiang, Z.; Xiao, T.; Lu, L.; Jones, M.O.; Edwards, P.P. Exceptional Visible-Light-Driven Photocatalytic Activity over Biobr-Znfe2o4 Heterojunctions. Chem. Commun. 2011, 47, 5512–5514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Geng, X.; Xiao, F.; An, G.; Wang, D. Feii/Feiii Doped Bi/BioBr Hierarchical Microspheres as a Highly Efficient Catalyst for Degradation of Organic Contaminants at Neutral Ph: The Role of Visible Light and H2O2. ChemCatChem 2017, 9, 3762–3771. [Google Scholar] [CrossRef]
- Guan, S.; Yang, H.; Sun, X.; Xian, T. Preparation and Promising Application of Novel Lafeo3/Biobr Heterojunction Photocatalysts for Photocatalytic and Photo-Fenton Removal of Dyes. Opt. Mater. 2020, 100, 109644. [Google Scholar] [CrossRef]
- Liu, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Surface Engineering of G-C3 N4 by Stacked Biobr Sheets Rich in Oxygen Vacancies for Boosting Photocatalytic Performance. Angew. Chem. Int. Ed. Engl. 2020, 59, 4519–4524. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, G.; Zhang, Y.; Wei, Y. Mediation of Band Structure for Biobrxi1-X Hierarchical Microspheres of Multiple Defects with Enhanced Visible-Light Photocatalytic Activity. CrystEngComm 2018, 20, 3647–3656. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Li, J.; Zhang, G. Boosting Interfacial Charge Separation of Ba5nb4o15/G-C3n4 Photocatalysts by 2d/2d Nanojunction Towards Efficient Visible-Light Driven H-2 Generation. Appl. Catal. B Environ. 2020, 263, 117730. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Hierarchical Core-Shell Heterostructures of Znin2s4 Nanosheets on Electrospun In2o3 Nanofibers with Highly Enhanced Photocatalytic Activity. J. Hazard. Mater. 2020, 398, 122889. [Google Scholar] [CrossRef]
- Khodaeipour, M.; Haghighi, M.; Shabani, M.; Mohseni, N. Influence of Fuel Type and Microwave Combustion on in-Situ Fabrication of Bimonbrz Mixed-Phase Nanostructured Photocatalyst: Effective Sun-Light Photo-Response Ability in Tetracycline Degradation. J. Hazard. Mater. 2020, 393, 122462. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tong, Z.; Muhammad, Y.; Huang, G.; Zhang, H.; Wang, Z.; Zhu, Y.; Tang, R. Synthesis of Sodium Dodecyl Sulfate Modified Biobr/Magnetic Bentonite Photocatalyst with Three-Dimensional Parterre Like Structure for the Enhanced Photodegradation of Tetracycline and Ciprofloxacin. Chem. Eng. J. 2020, 388, 124374. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.; Liu, X.; Li, H.; Ren, C.; Li, X.; Li, W.; Lian, Z.; Zhang, M. Engineering Design of Hierarchical G-C3n4@Bi/Biobr Ternary Heterojunction with Z-Scheme System for Efficient Visible-Light Photocatalytic Performance. J. Alloys Compd. 2019, 798, 741–749. [Google Scholar] [CrossRef]
- Huang, S.; Wang, G.; Liu, J.; Du, C.; Su, Y. A Novel Cubi2o4/Biobr Direct Z-Scheme Photocatalyst for Efficient Antibiotics Removal: Synergy of Adsorption and Photocatalysis on Degradation Kinetics and Mechanism Insight. ChemCatChem 2020, 12, 4431–4445. [Google Scholar] [CrossRef]
- Perumal, K.; Shanavas, S.; Karthigeyan, A.; Ahamad, T.; Alshehri, S.M.; Murugakoothan, P. Hydrothermal Assisted Precipitation Synthesis of Highly Stable G-C3n4/Biobr/Cds Photocatalyst with Enhanced Visible Light Photocatalytic Degradation of Tetracycline. Diam. Relat. Mater. 2020, 110, 108091. [Google Scholar] [CrossRef]
- Yan, S.; Yang, J.; Li, Y.; Jia, X.; Song, H. One-Step Synthesis of Zns/Biobr Photocatalyst to Enhance Photodegradation of Tetracycline under Full Spectral Irradiation. Mater. Lett. 2020, 276, 128232. [Google Scholar] [CrossRef]
- Li, J.; Pei, Q.; Wang, R.; Zhou, Y.; Zhang, Z.; Cao, Q.; Wang, D.; Mi, W.; Du, Y. Enhanced Photocatalytic Performance through Magnetic Field Boosting Carrier Transport. ACS Nano 2018, 12, 3351–3359. [Google Scholar] [CrossRef]
- Monzon, L.M.A.; Rode, K.; Venkatesan, M.; Coey, J.M.D. Electrosynthesis of Iron, Cobalt, and Zinc Microcrystals and Magnetic Enhancement of the Oxygen Reduction Reaction. Chem. Mater. 2012, 24, 3878–3885. [Google Scholar] [CrossRef]


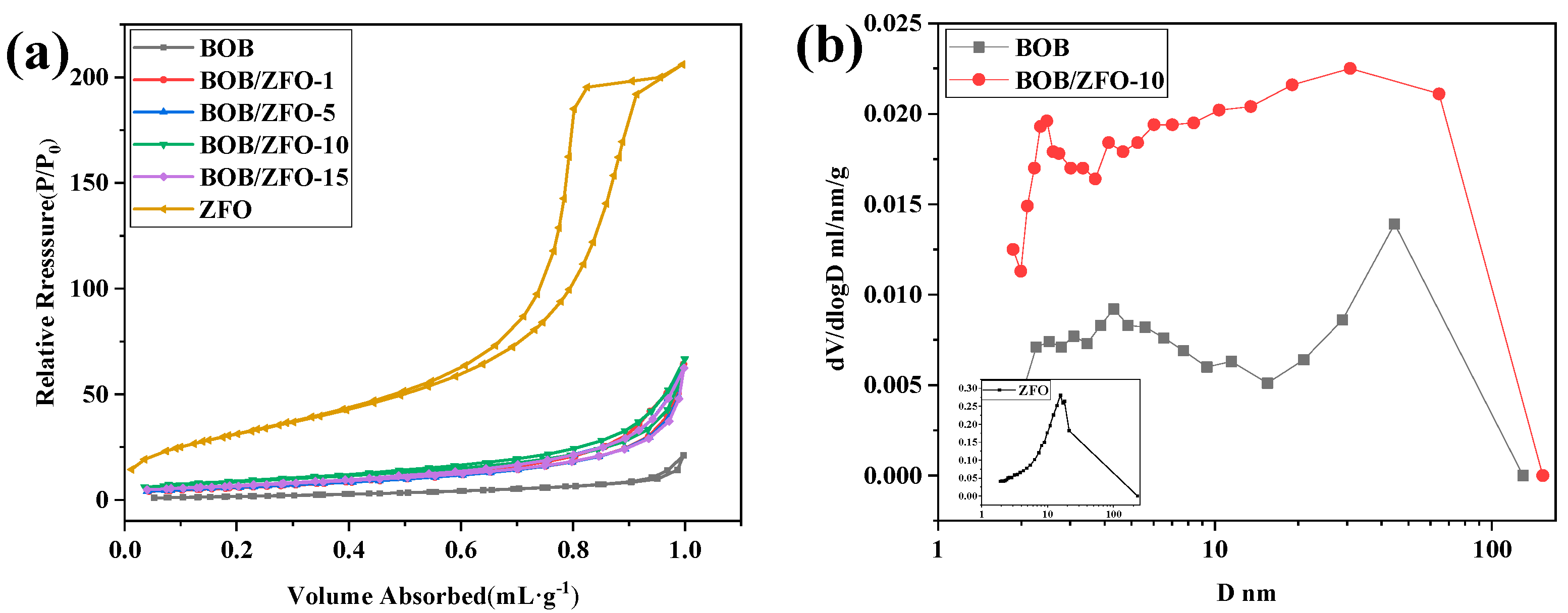
| Material | Specific Surface Area (m2/g) | Pore Volume (mL/g) | Average Pore Size (nm) |
|---|---|---|---|
| BOB | 6.98 | 0.037 | 9.9 |
| BOB/ZFO-1 | 21.9 | 0.11 | 13.2 |
| BOB/ZFO-5 | 22.4 | 0.11 | 11.3 |
| BOB/ZFO-10 | 31.2 | 0.11 | 11.4 |
| BOB/ZFO-15 | 24.2 | 0.10 | 12.9 |
| ZFO | 117.8 | 0.35 | 8.3 |
| mechanically mixture (BOB/ZFO = 100:1) | 8.09 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, Y.; Li, Z.; Yang, X.; Yang, X.; Peng, Y.; Yu, J. Preparation, Characterization and Application of Epitaxial Grown BiOBr (110) Film on ZnFe2O4 Surface with Enhanced Photocatalytic Fenton Oxidation Properties. Nanomaterials 2022, 12, 1508. https://doi.org/10.3390/nano12091508
Zhang Z, Zhang Y, Li Z, Yang X, Yang X, Peng Y, Yu J. Preparation, Characterization and Application of Epitaxial Grown BiOBr (110) Film on ZnFe2O4 Surface with Enhanced Photocatalytic Fenton Oxidation Properties. Nanomaterials. 2022; 12(9):1508. https://doi.org/10.3390/nano12091508
Chicago/Turabian StyleZhang, Zheng, Yan Zhang, Zhuo Li, Xueyuan Yang, Xiaolong Yang, Yanhua Peng, and Jianqiang Yu. 2022. "Preparation, Characterization and Application of Epitaxial Grown BiOBr (110) Film on ZnFe2O4 Surface with Enhanced Photocatalytic Fenton Oxidation Properties" Nanomaterials 12, no. 9: 1508. https://doi.org/10.3390/nano12091508





