Integrated CuO/Pd Nanospike Hydrogen Sensor on Silicon Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Heterostructured Nanofilm
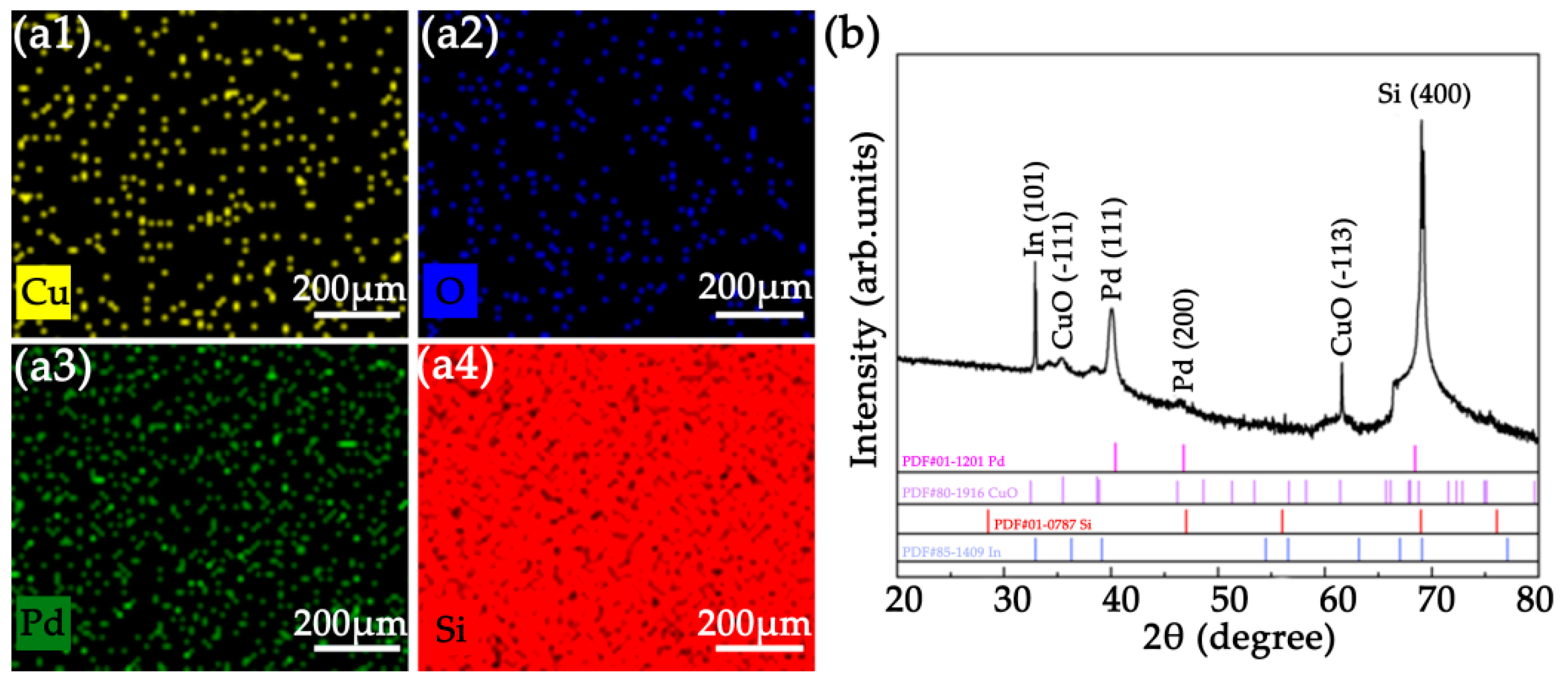
2.2. Morphological and Structural Characterization
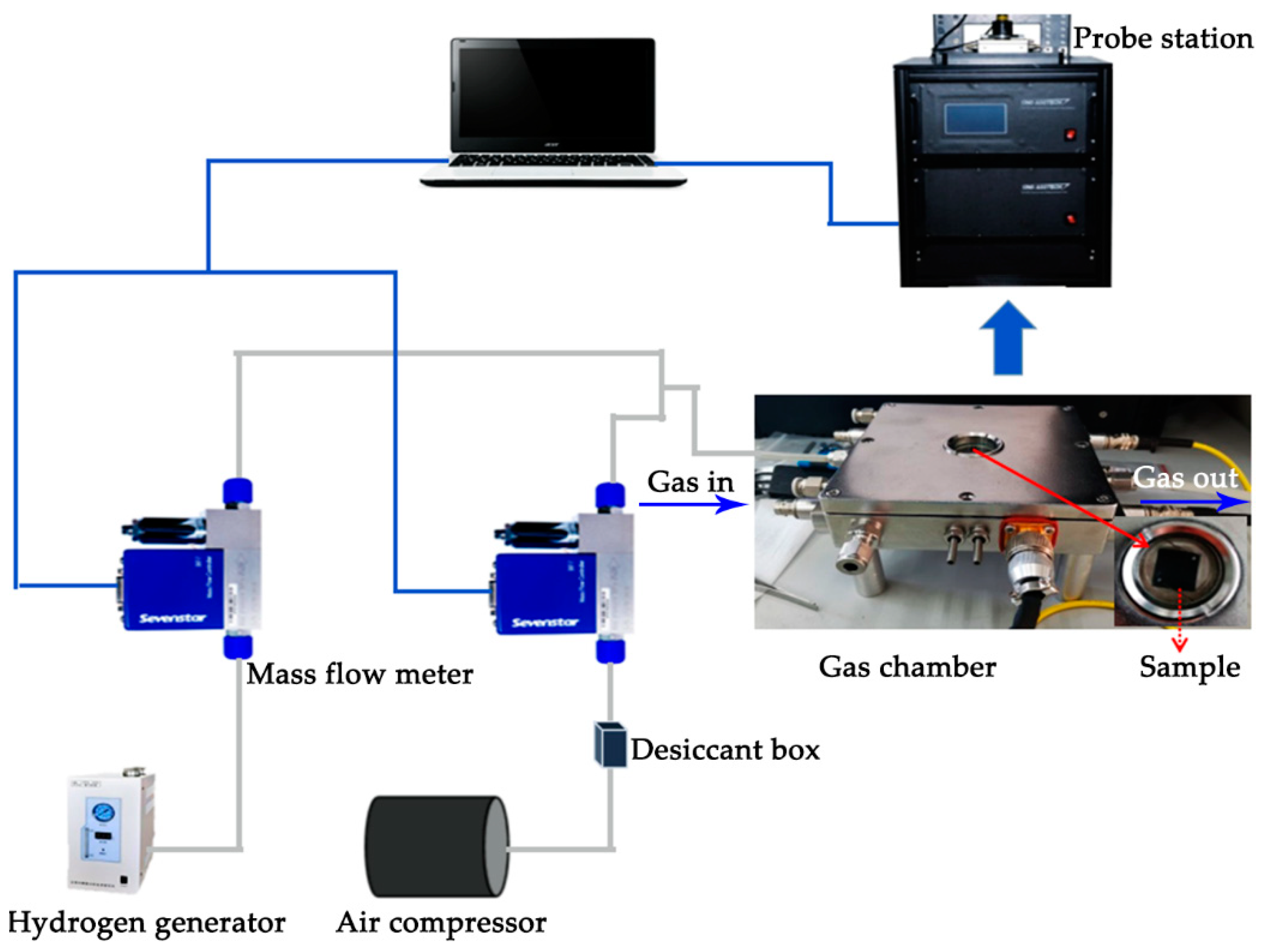
2.3. Hydrogen Response Measurement
3. Results and Discussion
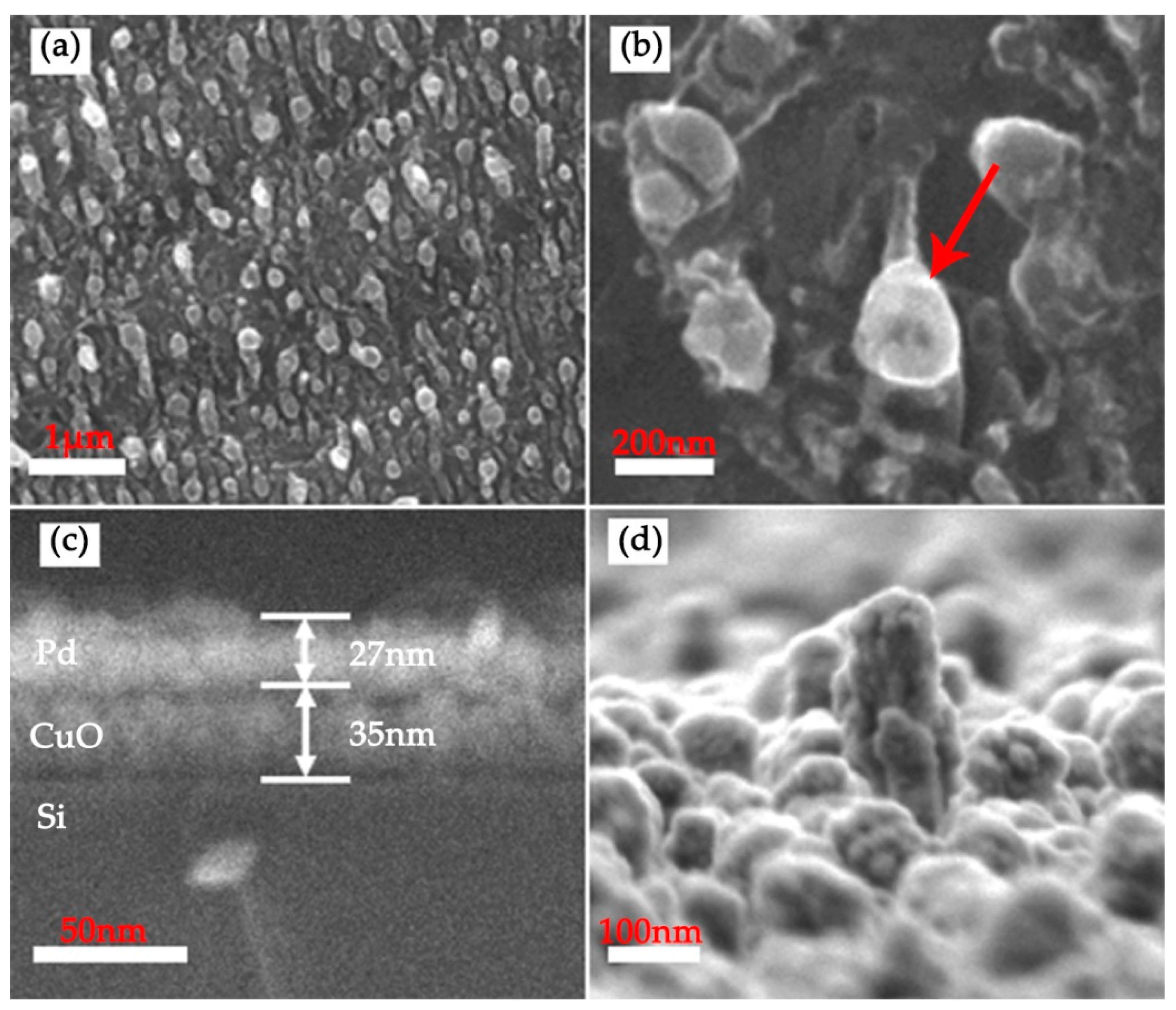
3.1. Characterization of Laser Treated Si Surface
3.2. Hydrogen Sensor Performance
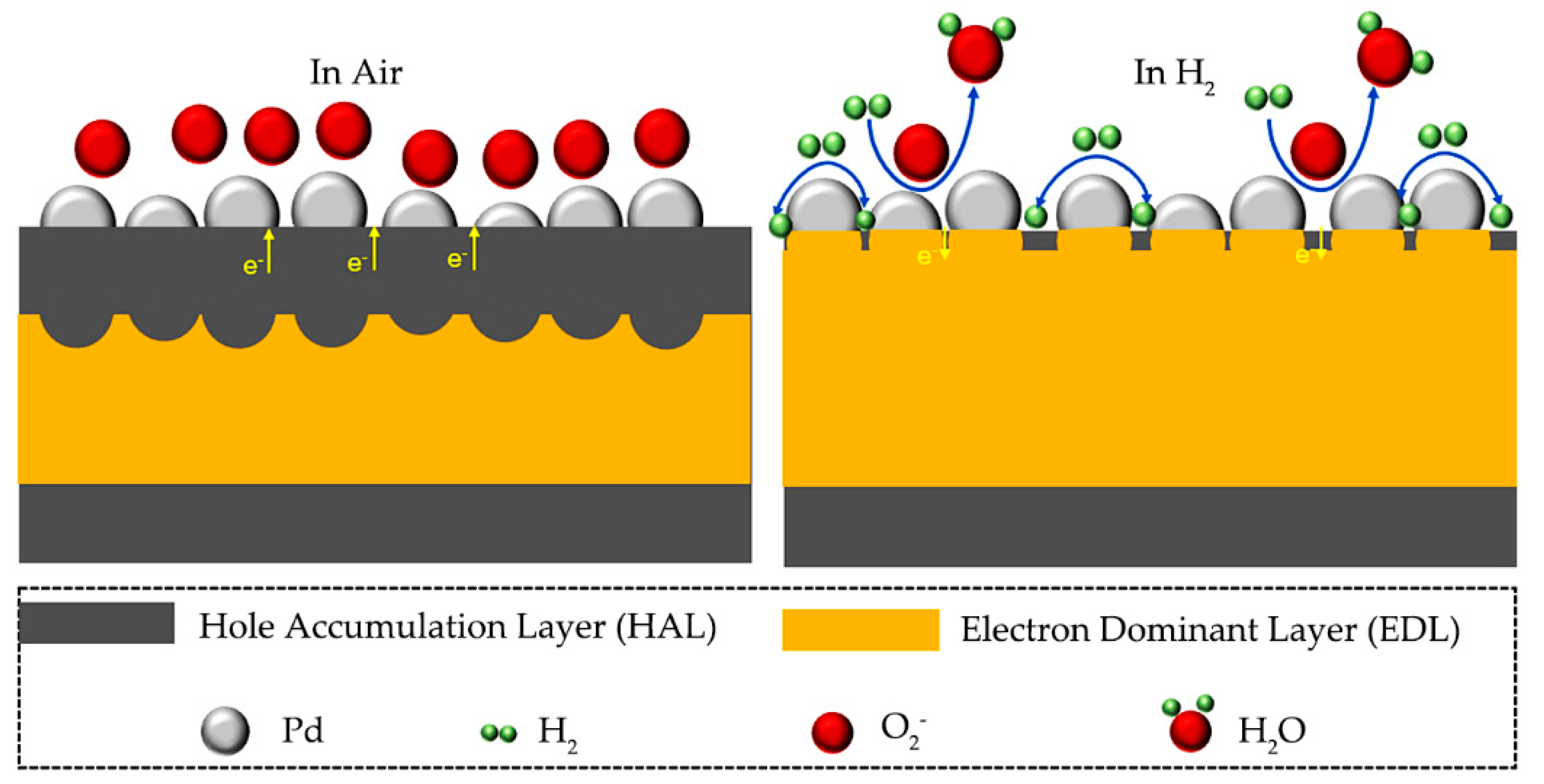
3.3. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, C.H.; Zhu, Z.; Chang, H.M.; Jiang, Z.X.; Hsieh, C.Y.; Wu, R.J. Pt@NiO core-shell nanostructure for a hydrogen gas sensor. J. Alloys Compd. 2020, 814, 151815. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Jiang, H.C.; Zhang, J.F.; Huang, Y.F.; Tian, J.W.; Deng, X.W.; Zhao, X.H.; Zhang, W.L. Flexible nanofiber sensor for low-concentration hydrogen detection. Nanotechnology 2020, 31, 015504. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Zhou, J.T.; Liao, C.R.; Zhu, M.; Wang, Y.; Liu, S.; Li, C.; Zhang, Y.F.; Zhao, Y.Y.; Gan, Z.S.; et al. Fiber-Tip Polymer Microcantilever for Fast and Highly Sensitive Hydrogen Measurement. ACS Appl. Mater. Interfaces 2020, 12, 33163–33172. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liao, N.B.; Xue, W.; Zhou, H.M. First principles investigation on MoO3 as room temperature and high temperature hydrogen gas sensor. Int. J. Hydrog. Energy 2020, 45, 9252–9259. [Google Scholar] [CrossRef]
- Tabares, G.; Redondo-Cubero, A.; Vazquez, L.; Revenga, M.; Cortijo-Campos, S.; Lorenzo, E.; de Andrés, A.; Ruiz, E.; Pau, J.L. A route to detect H2 in ambient conditions using a sensor based on reduced graphene oxide. Sens. Actuator A Phys. 2020, 304, 111884. [Google Scholar] [CrossRef]
- Koster, D.; Gutkowski, R.; Masa, J.; Schuhmann, W. H2 quantification based on selective pre-concentration and oxidative stripping at Pd modified microelectrodes. J. Electroanal. Chem. 2018, 812, 207–212. [Google Scholar] [CrossRef]
- Mai, H.D.; Jeong, S.; Nguyen, T.K.; Youn, J.S.; Ahn, S.; Park, C.M.; Jeon, K.J. Pd Nanocluster/Monolayer MoS2 Heterojunctions for Light-Induced Room-Temperature Hydrogen Sensing. ACS Appl. Mater. Interfaces 2021, 13, 14657–14665. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhang, L.B.; Han, B.; Peng, H.J.; Zhou, T.M.; Lv, R.Q. Erbium-doped fiber ring laser with SMS modal interferometer for hydrogen sensing. Opt. Laser Technol. 2018, 102, 262–267. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.P.; Hu, K.L.; Liu, H.C.; Zhao, Q.; Wang, K.Z.; Zhang, Y.X. Low temperature and fast response hydrogen gas sensor with Pd coated SnO2 nanofiber rods. Int. J. Hydrog. Energy 2020, 45, 7234–7242. [Google Scholar] [CrossRef]
- Lupan, C.; Khaledialidusti, R.; Mishra, A.K.; Postica, V.; Terasa, M.I.; Magariu, N.; Pauporte, T.; Viana, B.; Drewes, J.; Vahl, A.; et al. Pd-Functionalized ZnO:Eu Columnar Films for Room-Temperature Hydrogen Gas Sensing: A Combined Experimental and Computational Approach. ACS Appl. Mater. Interfaces 2020, 12, 24951–24964. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Abuzalat, O.; Ko, J.; Lee, J.; Kim, S.; Park, S.S. Intense Pulsed Light-Treated Near-Field Electrospun Nanofiber on a Quartz Tuning Fork for Multimodal Gas Sensors. ACS Appl. Mater. Interfaces 2020, 12, 24308–24318. [Google Scholar] [CrossRef]
- Yang, F.; Taggart, D.K.; Penner, R.M. Fast, Sensitive Hydrogen Gas Detection Using Single Palladium Nanowires That Resist Fracture. Nano Lett. 2009, 9, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Donavan, K.C.; Kung, S.C.; Penner, R.M. The Surface Scattering-Based Detection of Hydrogen in Air Using a Platinum Nanowire. Nano Lett. 2012, 12, 2924–2930. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Latimer, M.L.; Xiao, Z.L.; Panuganti, S.; Welp, U.; Kwok, W.K.; Xu, T. Hydrogen Gas Sensing with Networks of Ultrasmall Palladium Nanowires Formed on Filtration Membranes. Nano Lett. 2011, 11, 262–268. [Google Scholar] [CrossRef]
- Ambardekar, V.; Bandyopadhyay, P.P.; Majumder, S.B. Sensing Capability of Air Plasma-Sprayed SnO2 Coating in the Presence of Hydrogen and Carbon Monoxide. J. Mater. Eng. Perform. 2019, 28, 6728–6735. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Peng, H.J.; Zhou, T.M.; Zhang, L.B.; Zhang, Y.Y.; Zhao, Y. Hydrogen sensor based on high-birefringence fiber loop mirror with sol-gel Pd/WO3 coating. Sens. Actuators B Chem. 2017, 248, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Haidry, A.A.; Plecenik, T.; Vidis, M.; Grancic, B.; Roch, T.; Gregor, M.; Durina, P.; Yao, Z.J.; Plecenik, A. Influence of nanoscale TiO2 film thickness on gas sensing properties of capacitor-like Pt/TiO2/Pt sensing structure. Appl. Surf. Sci. 2020, 499, 143909. [Google Scholar] [CrossRef]
- Moumen, A.; Kaur, N.; Poli, N.; Zappa, D.; Comini, E. One Dimensional ZnO Nanostructures: Growth and Chemical Sensing Performances. Nanomaterials 2020, 10, 1940. [Google Scholar] [CrossRef]
- Sta, I.; Jlassi, M.; Kandyla, M.; Hajji, M.; Koralli, P.; Krout, F.; Kompitsas, M.; Ezzaouia, H. Surface functionalization of sol-gel grown NiO thin films with palladium nanoparticles for hydrogen sensing. Int. J. Hydrog. Energy 2016, 41, 3291–3298. [Google Scholar] [CrossRef]
- Koga, K. Electronic and Catalytic Effects of Single-Atom Pd Additives on the Hydrogen Sensing Properties of Co3O4 Nanoparticle Films. ACS Appl. Mater. Interfaces 2020, 12, 20806–20823. [Google Scholar] [CrossRef]
- Yadav, P.; Kumar, A.; Sanger, A.; Gautam, Y.K.; Singh, B.P. Sputter-Grown Pd-Capped CuO Thin Films for a Highly Sensitive and Selective Hydrogen Gas Sensor. J. Electron. Mater. 2021, 50, 192–200. [Google Scholar] [CrossRef]
- Lupan, O.; Ababii, N.; Mishra, A.K.; Gronenberg, O.; Vahl, A.; Schurmann, U.; Duppel, V.; Kruger, H.; Chow, L.; Kienle, L.; et al. Single CuO/Cu2O/Cu Microwire Covered by a Nanowire Network as a Gas Sensor for the Detection of Battery Hazards. ACS Appl. Mater. Interfaces 2020, 12, 42248–42263. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Ababii, N.; Hoppe, M.; Cretu, V.; Tiginyanu, I.; Sontea, V.; Pauporte, T.; Viana, B.; Adelung, R. Influence of CuO nanostructures morphology on hydrogen gas sensing performances. Microelectron. Eng. 2016, 164, 63–70. [Google Scholar] [CrossRef]
- Yatskiv, R.; Tiagulskyi, S.; Grym, J.; Vanis, J.; Basinova, N.; Horak, P.; Torrisi, A.; Ceccio, G.; Vacik, J.; Vrnata, M. Optical and electrical characterization of CuO/ZnO heterojunctions. Thin Solid Film. 2020, 693, 137656. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L.W. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Kheel, H.; Sun, G.J.; Lee, J.K.; Mirzaei, A.; Choi, S.; Lee, C. Hydrogen Gas Detection of Nb2O5 Nanoparticle-Decorated CuO Nanorod Sensors. Met. Mater. Int. 2017, 23, 214–219. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. An innovative gas sensor incorporating ZnO-CuO nanoflakes in planar MEMS technology. Sens. Actuators B Chem. 2016, 229, 414–424. [Google Scholar] [CrossRef]
- De, S.; Venkataramani, N.; Prasad, S.; Dusane, R.O.; Presmanes, L.; Thimont, Y.; Tailhades, P.; Baco-Carles, V.; Bonningue, C.; Thondiyanoor Pisharam, S.; et al. Ethanol and Hydrogen Gas-Sensing Properties of CuO–CuFe2O4 Nanostructured Thin Films. IEEE Sens. J. 2018, 18, 6937–6945. [Google Scholar] [CrossRef] [Green Version]
- Sihar, N.; Tiong, T.Y.; Dee, C.F.; Ooi, P.C.; Hamzah, A.A.; Mohamed, M.A.; Majlis, B.Y. Ultraviolet Light-Assisted Copper Oxide Nanowires Hydrogen Gas Sensor. Nanoscale Res. Lett. 2018, 13, 150. [Google Scholar] [CrossRef] [Green Version]
- Nakate, U.T.; Lee, G.H.; Ahmad, R.; Patil, P.; Hahn, Y.B.; Yu, Y.T.; Suh, E.K. Nano-bitter gourd like structured CuO for enhanced hydrogen gas sensor application. Int. J. Hydrog. Energy 2018, 43, 22705–22714. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Pauporte, T.; Viana, B.; Terasa, M.I.; Adelung, R. Room temperature gas nanosensors based on individual and multiple networked Au-modified ZnO nanowires. Sens. Actuators B Chem. 2019, 299, 126977. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, G.L.; Gao, W.Q.; Zhao, R.J.; Wang, Y.D. Ag decorated ZnO nanocrystallines synthesized by a low-temperature solvothermal method and their application for high response H2 gas sensor. J. Mater. Sci. Mater. Electron. 2019, 30, 18959–18969. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Ko, J.; Ahn, J.; Bok, M.; Gao, M.; Hwang, S.H.; Kang, H.J.; Jeon, S.; Park, I.; Jeong, J.H. 3D Layer-By-Layer Pd-Containing Nanocomposite Platforms for Enhancing the Performance of Hydrogen Sensors. ACS Sens. 2020, 5, 2367–2377. [Google Scholar] [CrossRef]
- Phan, D.T.; Youn, J.S.; Jeon, K.J. High-sensitivity and fast-response hydrogen sensor for safety application using Pt nanoparticle-decorated 3D graphene. Renew. Energ. 2019, 144, 167–171. [Google Scholar] [CrossRef]
- Behera, A.K.; Viswanath, R.N.; David, T.M.; Prasad, A.K.; Ajikumar, P.K.; Mathews, T. Hydrogen sensing behaviour of platinum and palladium functionalized silicon nanowalls. Appl. Phys. A 2021, 127, 40. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Pd-decorated Si nano-horns as sensitive and selective hydrogen gas sensors. Mater. Res. Bull. 2020, 132, 110985. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Wang, Y.L.; Deng, H.; Latimer, M.L.; Xiao, Z.L.; Pearson, J.; Xu, T.; Wang, H.H.; Welp, U.; Crabtree, G.W.; et al. Networks of Ultrasmall Pd/Cr Nanowires as High Performance Hydrogen Sensors. Acs Nano 2011, 5, 7443–7452. [Google Scholar] [CrossRef]
- Sarica, N.; Alev, O.; Arslan, L.C.; Ozturk, Z.Z. Characterization and gas sensing performances of noble metals decorated CuO nanorods. Thin Solid Film. 2019, 685, 321–328. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Pd functionalization on ZnO nanowires for enhanced sensitivity and selectivity to hydrogen gas. Sens. Actuators B Chem. 2019, 297, 126693. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, T.; Li, X.; Li, Q.; Zhang, X.; Cao, T.; Li, Y.; Zhang, L.; Guo, L.; Fu, Y. Pd-Decorated PdO Hollow Shells: A H2-Sensing System in Which Catalyst Nanoparticle and Semiconductor Support are Interconvertible. ACS Appl. Mater. Interfaces 2020, 12, 42971–42981. [Google Scholar] [CrossRef] [PubMed]
- Achary, L.S.K.; Maji, B.; Kumar, A.; Ghosh, S.P.; Kar, J.P.; Dash, P. Efficient room temperature detection of H2 gas by novel ZnFe2O4–Pd decorated rGO nanocomposite. Int. J. Hydrog. Energy 2020, 45, 5073–5085. [Google Scholar] [CrossRef]
- Rahamim, G.; Mirilashvili, M.; Nanikashvili, P.; Greenberg, E.; Shpaisman, H.; Grinstein, D.; Welner, S.; Zitoun, D. Hydrogen sensors with high humidity tolerance based on indium-tin oxide colloids. Sens. Actuators B Chem. 2020, 310, 127845. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Kim, J.Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. ppb-Level Selective Hydrogen Gas Detection of Pd-Functionalized In2O3-Loaded ZnO Nanofiber Gas Sensors. Sensors 2019, 19, 4276. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Chou, T.C.; Chen, W.C.; Niu, J.S.; Lin, K.W.; Cheng, S.Y.; Tsai, J.H.; Liu, W.C. Study of a WO3 thin film based hydrogen gas sensor decorated with platinum nanoparticles. Sens. Actuators B Chem. 2020, 317, 128145. [Google Scholar] [CrossRef]
- Farnood, A.; Ranjbar, M.; Salamati, H. Localized surface plasmon resonance (LSPR) detection of hydrogen gas by Pd2+/Au core/shell like colloidal nanoparticles. Int. J. Hydrog. Energy 2020, 45, 1158–1169. [Google Scholar] [CrossRef]
- Hassan, K.; Uddin, A.; Chung, G.S. Hydrogen sensing properties of Pt/Pd bimetal decorated on highly hydrophobic Si nanowires. Int. J. Hydrog. Energy 2016, 41, 10991–11001. [Google Scholar] [CrossRef]
- Xu, B.R.; Tian, Z.; Wang, J.; Han, H.; Lee, T.; Mei, Y.F. Stimuli-responsive and on-chip nanomembrane micro-rolls for enhanced macroscopic visual hydrogen detection. Sci. Adv. 2018, 4, eaap8203. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.; Dai, Y.T.; Zhou, X.; Dong, K.; Yang, M.H. Femtosecond Laser Ablated FBG with Composite Microstructure for Hydrogen Sensor Application. Sensors 2016, 16, 2040. [Google Scholar] [CrossRef] [Green Version]
- Deepti; Kumar, H.; Tripathi, A.; Dey, A.B.; Gupta, M.; Krishna, R.; Avasthi, D.K. Improved hydrogen sensing behaviour in ion-irradiated Pd-Au alloy thin films. Sens. Actuators B Chem. 2019, 301, 127006. [Google Scholar] [CrossRef]
- Gattass, R.R.; Mazur, E. Femtosecond laser micromachining in transparent materials. Nat. Photon. 2008, 2, 219–225. [Google Scholar] [CrossRef]
- Her, T.H.; Finlay, R.J.; Wu, C.; Deliwala, S.; Mazur, E. Microstructuring of silicon with femtosecond laser pulses. Appl. Phys. Lett. 1998, 73, 1673–1675. [Google Scholar] [CrossRef]
- Zappa, D.; Comini, E.; Zamani, R.; Arbiol, J.; Morante, J.R.; Sberveglieri, G. Preparation of copper oxide nanowire-based conductometric chemical sensors. Sens. Actuators B Chem. 2013, 182, 7–15. [Google Scholar] [CrossRef]
- Xu, H.Y.; Liu, Y.J.; Liu, H.; Dong, S.C.; Wu, Y.P.; Wang, Z.G.; Wang, Y.M.; Wu, M.S.; Han, Z.D.; Hao, L.Z. Pd-decorated 2D SnSe ultrathin film on SiO2/Si for room-temperature hydrogen detection with ultrahigh response. J. Alloys Compd. 2021, 851, 156844. [Google Scholar] [CrossRef]
- Al-Hazeem, N.Z.; Ahmed, N.M.; Matjafri, M.Z.; Bououdina, M. Hydrogen gas sensor based on nanofibers TiO2-PVP thin film at room temperature prepared by electrospinning. Microsyst. Technol. 2021, 27, 293–299. [Google Scholar] [CrossRef]
- Qin, Y.X.; Wang, Y.Y.; Liu, Y.; Zhang, X.J. KOH post-etching-induced rough silicon nanowire array for H2 gas sensing application. Nanotechnology 2016, 27, 465502. [Google Scholar] [CrossRef]

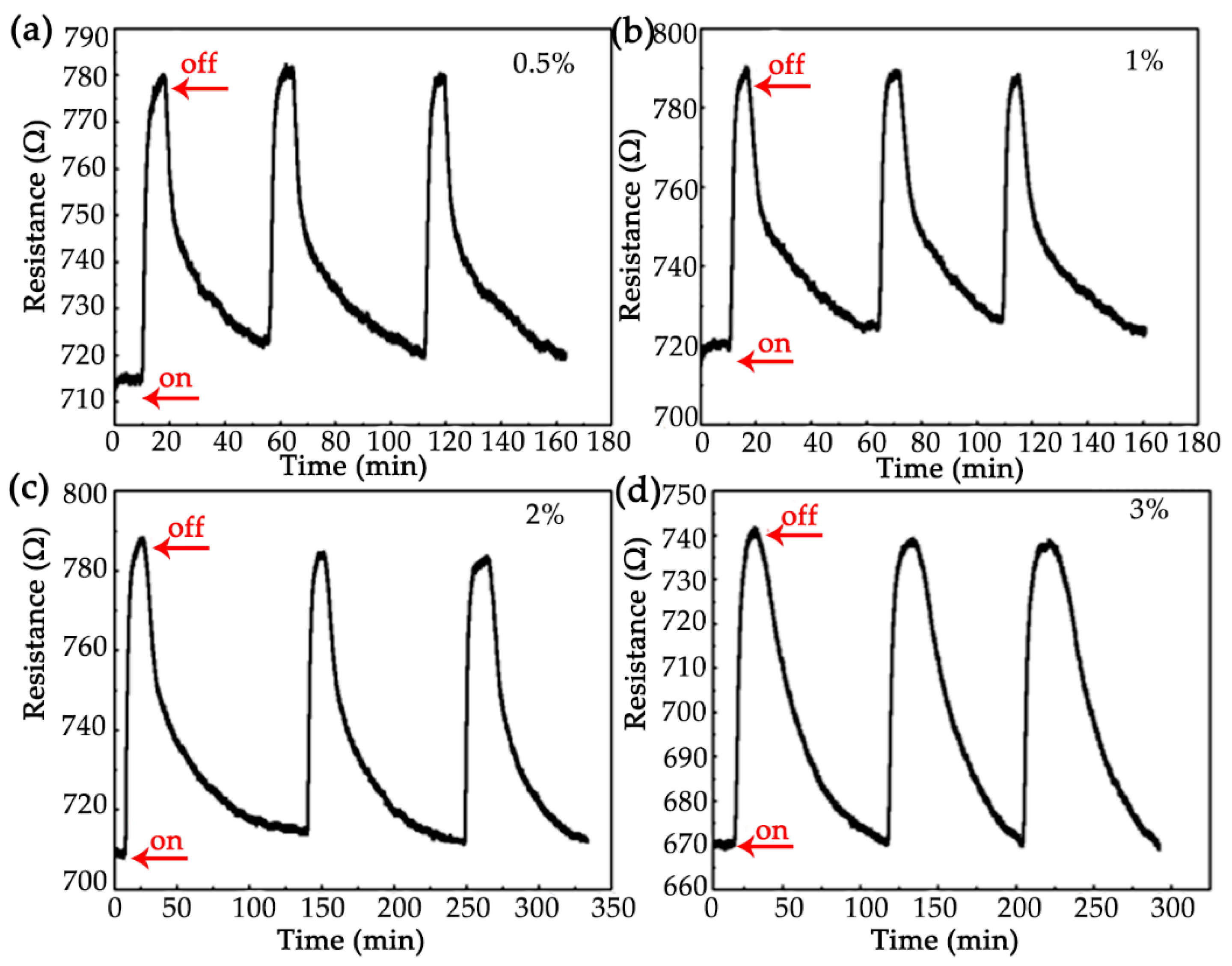

| H2 conc. | tres (s) | trec (min) | S (%) |
|---|---|---|---|
| 40 ppm | 428 | 30.15 | 0.23 |
| 50 ppm | 1250 | 20.83 | 0.28 |
| 0.01% | 1585 | 58.01 | 1.90 |
| 0.05% | 632 | 71.08 | 4.26 |
| 0.1% | 743 | 58.26 | 5.95 |
| 0.5% | 358 | 41.98 | 9.76 |
| 1% | 198 | 49.86 | 10.80 |
| 2% | 229 | 60.28 | 12.20 |
| 3% | 355 | 78.56 | 12.07 |
| Material | H2 conc. | tres | trec | S * | T. (°C) | Ref. |
|---|---|---|---|---|---|---|
| CuO NW networks | 100 ppm | 60 s | 2 s | 340 | 300 | [24] |
| Nb2O5 Nps/CuO Nanorod | 0.50% | 161 s | 163 s | 217.05% | 300 | [28] |
| Pd/CuO Nanorod | 1000 ppm | 10 min | 16 min | 4.5 | 200 | [40] |
| Nano-bitter gourd CuO | 100 ppm | 150 s | 1016 s | 175% | 200 | [32] |
| Pd/SnSe/SiO2/Si | 0.10% | 73.1 s | 23.7 s | 3225 | RT | [55] |
| TiO2 Nanofibers | 1000 ppm | 1.49 min | 0.52 min | 63 | RT | [56] |
| Pd/CuO/Si | 1% | 198 s | 49.86 min | 10.80% | RT | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, R.; Hu, Q.; Liu, Z.; Pan, S.; Chen, Z.; Zhang, W.; Liu, Z.; Zhang, S.; Zhang, C. Integrated CuO/Pd Nanospike Hydrogen Sensor on Silicon Substrate. Nanomaterials 2022, 12, 1533. https://doi.org/10.3390/nano12091533
Lin R, Hu Q, Liu Z, Pan S, Chen Z, Zhang W, Liu Z, Zhang S, Zhang C. Integrated CuO/Pd Nanospike Hydrogen Sensor on Silicon Substrate. Nanomaterials. 2022; 12(9):1533. https://doi.org/10.3390/nano12091533
Chicago/Turabian StyleLin, Ru, Qi Hu, Zuolian Liu, Shusheng Pan, Zhifeng Chen, Wei Zhang, Zhiyu Liu, Shaolin Zhang, and Chengyun Zhang. 2022. "Integrated CuO/Pd Nanospike Hydrogen Sensor on Silicon Substrate" Nanomaterials 12, no. 9: 1533. https://doi.org/10.3390/nano12091533
APA StyleLin, R., Hu, Q., Liu, Z., Pan, S., Chen, Z., Zhang, W., Liu, Z., Zhang, S., & Zhang, C. (2022). Integrated CuO/Pd Nanospike Hydrogen Sensor on Silicon Substrate. Nanomaterials, 12(9), 1533. https://doi.org/10.3390/nano12091533








