Engineering a Novel AgMn2O4@Na0.55Mn2O4 Nanosheet toward High-Performance Electrochemical Capacitors
Abstract
:1. Introduction
2. Materials and Methods
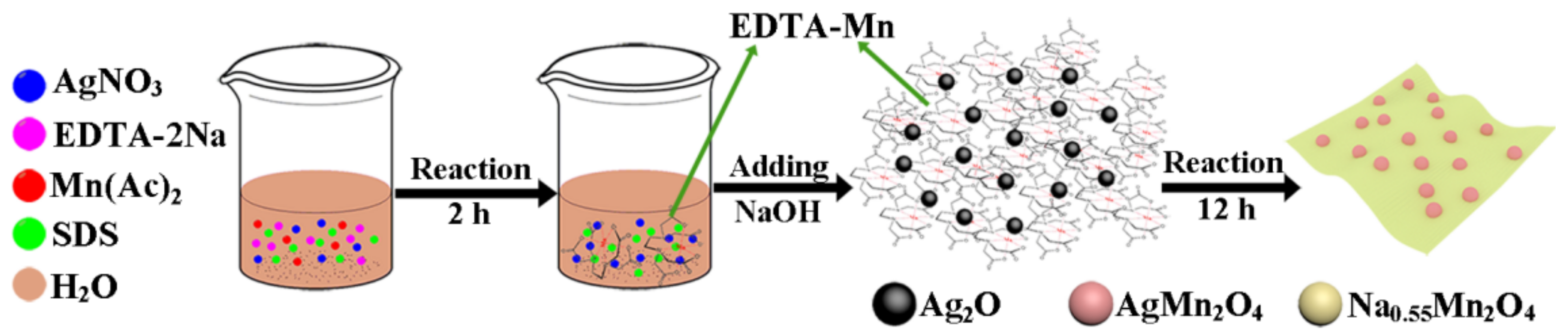
2.1. Material Synthesis
2.2. Structure Characterization
2.3. Electrochemical Properties
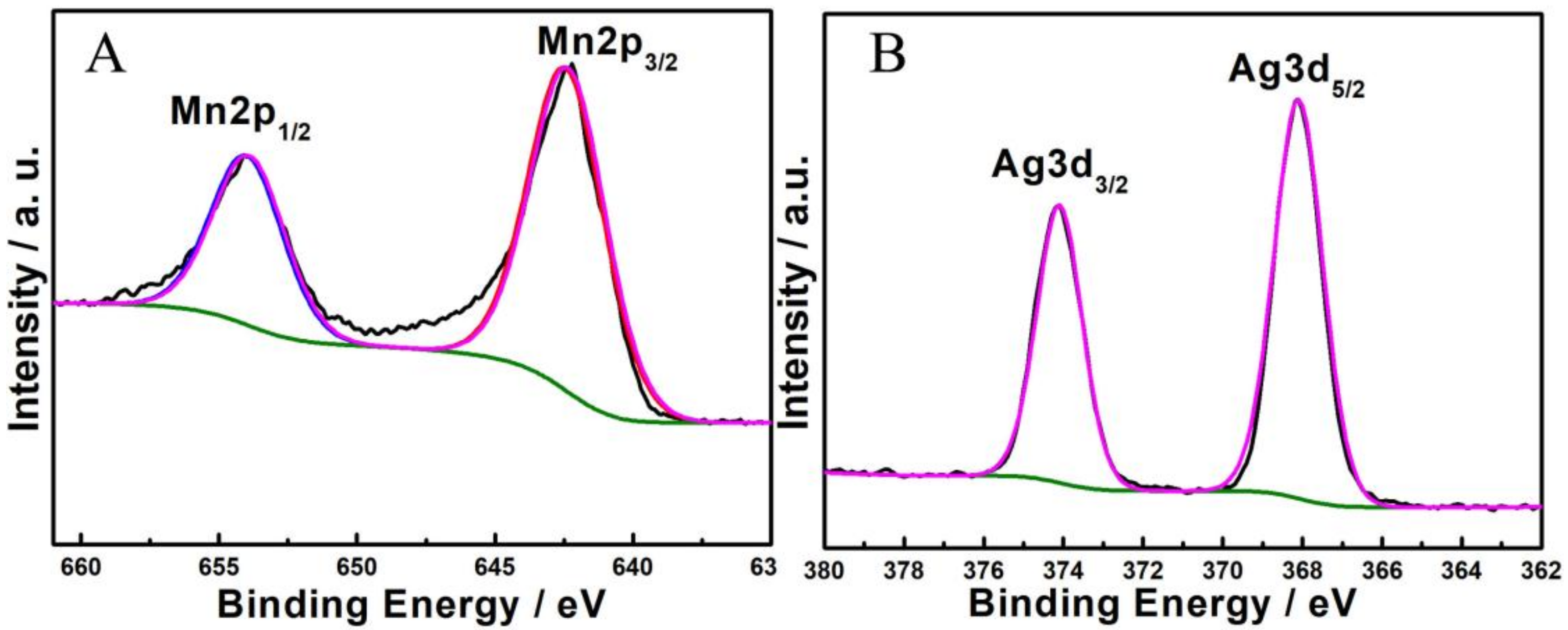
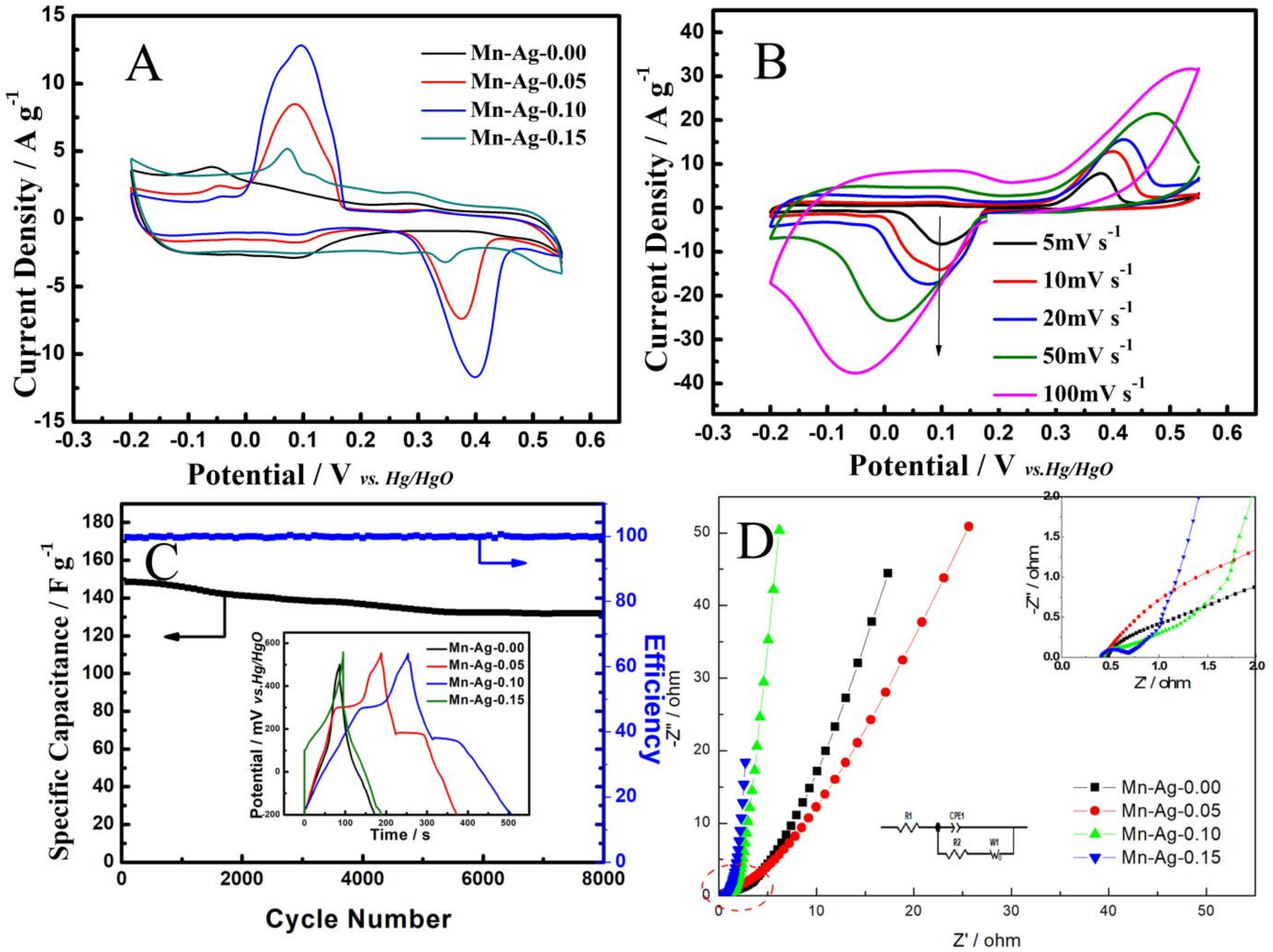
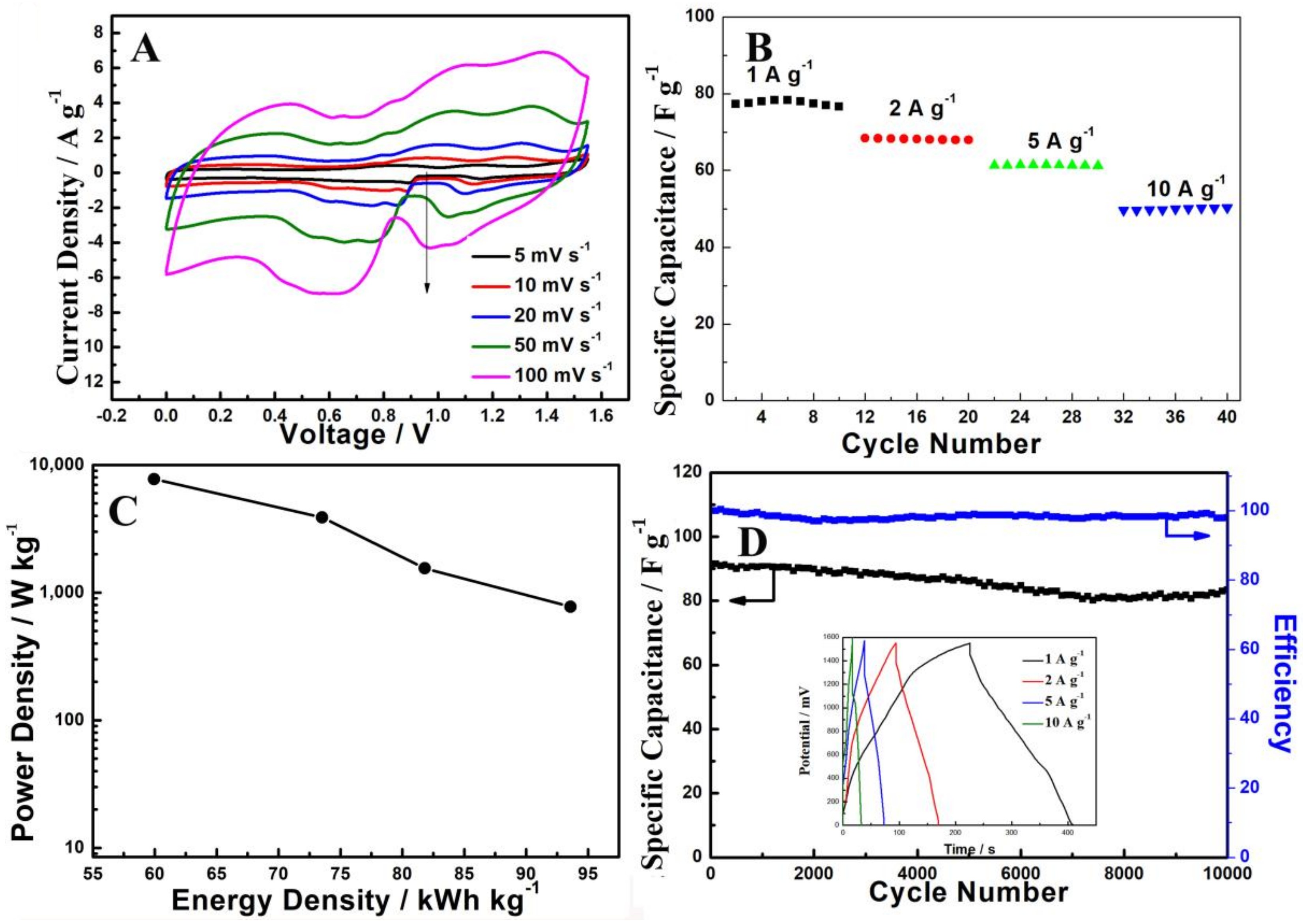
3. Results and Discussion
3.1. XRD Analysis
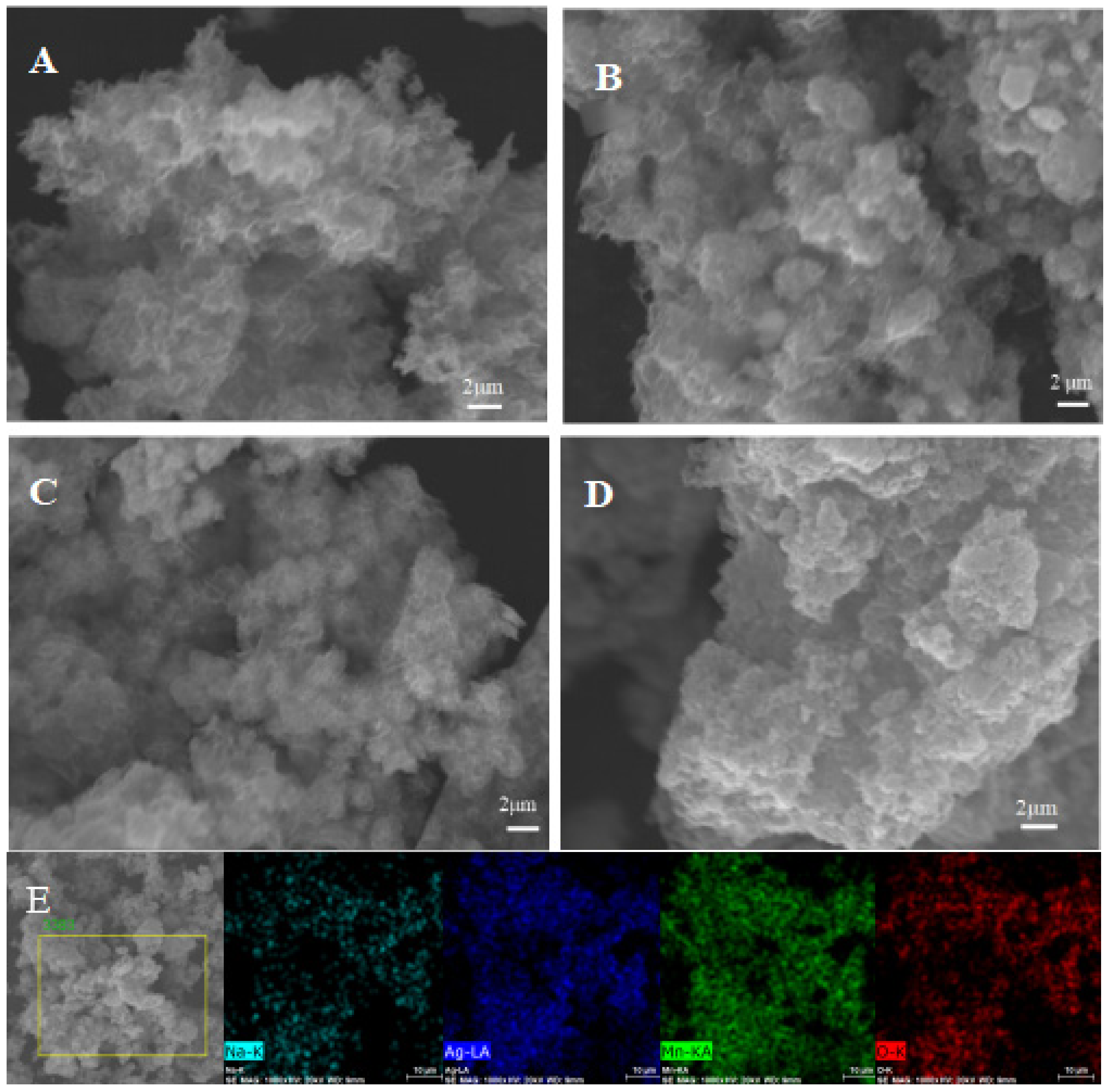
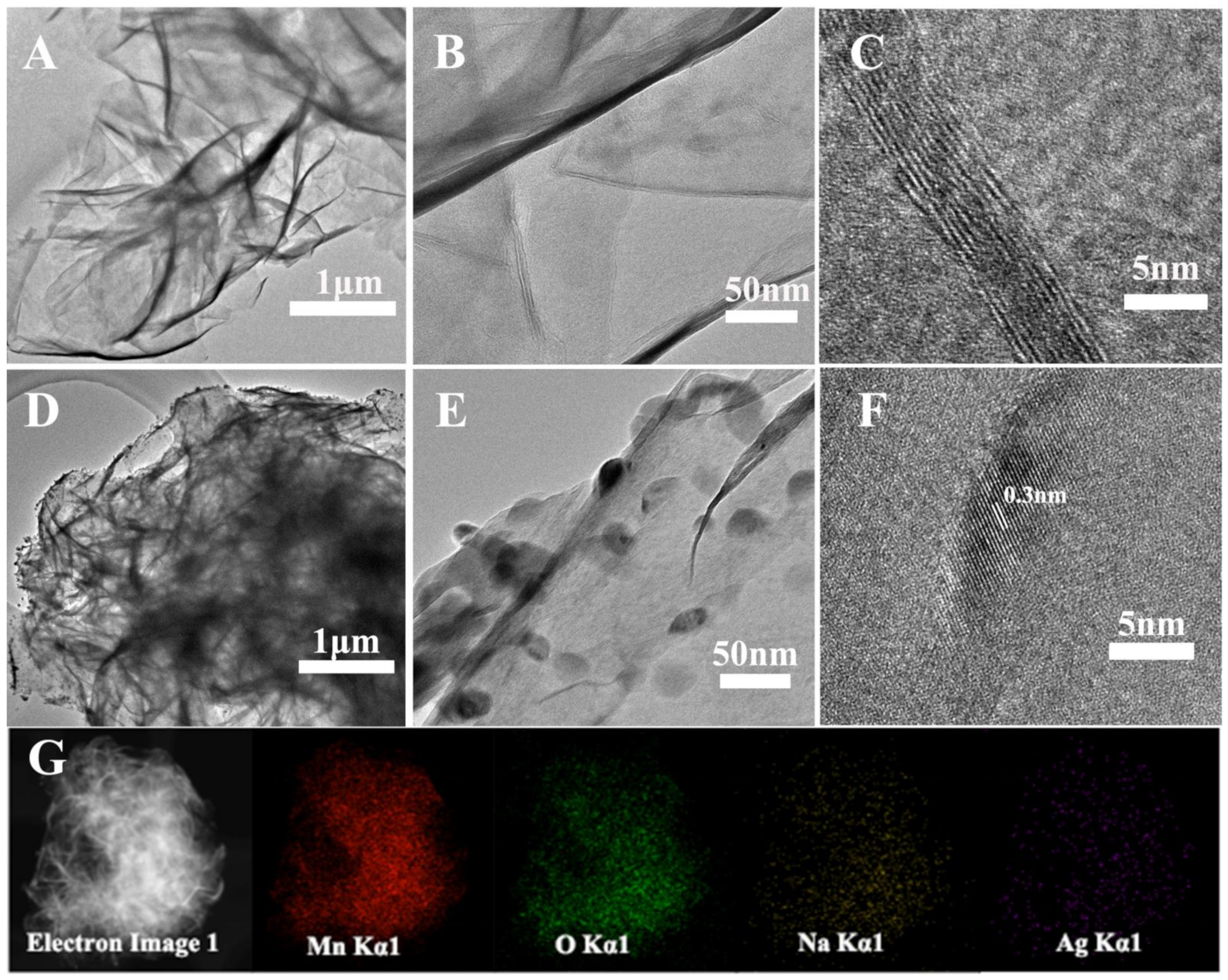
3.2. Effect of Doping on Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, W.; Zhou, N.; Wu, C.; Xie, M.; Sun, H.; Guo, Y.; Pan, L. Mini-Review on the Redox Additives in Aqueous Electrolyte for High Performance Supercapacitors. ACS Omega 2020, 5, 3801–3808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Min, L.; Ahmed, A.; Pascal, R.; Sabine, S.; Rabah, B. High Performance Flexible Hybrid Supercapacitor Based on Nickel Hydroxide Deposited on Copper Oxide Supported by Copper Foam for a Sunlight-Powered Rechargeable Energy Storage System. J. Colloid Interface Sci. 2020, 579, 520–530. [Google Scholar]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D Carbon-Based Networks Combined with Pseudocapacitive Electrode Material for High Energy Density Supercapacitor: A Review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Qi, B.; Ren, K.; Lin, Y.; Zhang, S.; Wei, T.; Fan, Z. Design of Layered-Stacking Graphene Assemblies as Advanced Electrodes for Supercapacitors. Particuology 2022, 60, 1–13. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Zhang, H.; Shen, M.; Baker, F.; Liu, Y.; Liu, L.; Yang, J.; Cao, H.; Xu, Q.; et al. Houttuynia-Derived Nitrogen-Doped Hierarchically Porous Carbon for High-Performance Supercapacitor. Carbon 2021, 161, 62–70. [Google Scholar] [CrossRef]
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Ezeigwe, E.R. Recent Development of Mixed Transition Metal Oxide and Graphene/Mixed Transition Metal Oxide Based Hybrid Nanostructures for Advanced Supercapacitors. J. Alloys Compd. 2019, 775, 1324–1356. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the Use of Transition-Metal-Oxide and Conducting Polymer-Based Fibres for High-Performance Supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Venkatkarthick, R.; Qin, J. A New 3D Composite of V2O5-Based Biodegradable Ceramic Material Prepared by an Environmentally Friendly Thermal Method for Supercapacitor Applications. Environ. Technol. Innov. 2021, 22, 101474. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Cheng, Q.; Li, J.; Liao, F.; Yang, G.; Xie, L.; Zhao, C.; Chen, L. Two-Dimensional Spinel Structured Co-Based Materials for High Performance Supercapacitors: A Critical Review. Chem. Eng. J. 2020, 387, 124081. [Google Scholar] [CrossRef]
- Sakib, M.N.; Ahmed, S.; Rahat, S.M.S.M.; Shuchi, S.B. A Review of Recent Advances in Manganese-Based Supercapacitors. J. Energy Storage 2021, 44, 103322. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Niu, H.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. NiS2/MoS2 Mixed Phases with Abundant Active Edge Sites Induced by Sulfidation and Graphene Introduction Towards High-Rate Supercapacitors. Chem. Eng. J. 2021, 406, 126713. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Khan, J. Optimization of Cobalt-Manganese Binary Sulfide for High Performance Supercapattery Devices. Electrochim. Acta 2020, 368, 137529. [Google Scholar] [CrossRef]
- Xiong, C.; Li, M.; Zhao, W.; Duan, C.; Dai, L.; Shen, M.; Xu, Y.; Ni, Y. A Smart Paper@Polyaniline Nanofibers Incorporated Vitrimer Bifunctional Device with Reshaping, Shape-Memory and Self-Healing Properties Applied in High-Performance Supercapacitors and Sensor. Chem. Eng. J. 2020, 396, 125318. [Google Scholar] [CrossRef]
- Bailmare, D.B.; Dhoble, S.J.; Deshmukh, A.D. Metal Organic Frameworks and Their Derived Materials for Capacity Enhancement of Supercapacitors: Progress and Perspective. Synth. Met. 2021, 282, 116945. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-Organic Framework-Based Materials for Hybrid Supercapacitor Application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Ojha, G.P.; Lee, M.; Kim, T.; Chae, S.-H.; Kim, H.Y. Controlled Selenium Infiltration of Cobalt Phosphide Nanostructure Arrays from a Two-Dimensional Cobalt Metal–Organic Framework: A Self-Supported Electrode for Flexible Quasi-Solid-State Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 404–415. [Google Scholar] [CrossRef]
- Hu, Z.; Xiao, X.; Jin, H.; Li, T.; Chen, M.; Liang, Z.; Guo, Z.; Li, J.; Wan, J.; Huang, L.; et al. Rapid Mass Production of Two-Dimensional Metal Oxides and Hydroxides via the Molten Salts Method. Nat. Commun. 2017, 8, 15630. [Google Scholar] [CrossRef]
- Zhao, N.; Deng, L.; Luo, D.; Zhang, P. One-Step Fabrication of Biomass-Derived Hierarchically Porous Carbon/MnO Nanosheets Composites for Symmetric Hybrid Supercapacitor. Appl. Surf. Sci. 2020, 526, 146696. [Google Scholar] [CrossRef]
- Raj, C.J.; Manikandan, R.; Sivakumar, P.; Opar, D.O.; Savariraj, A.D.; Cho, W.J.; Jung, H.; Kim, B.C. Origin of Capacitance Decay for a Flower-like δ-MnO2 Aqueous Supercapacitor Electrode: The Quantitative Surface and Electrochemical Analysis. J. Alloys Compd. 2021, 892, 162199. [Google Scholar]
- Minakshi, M.; Nallathamby, K.; Mitchell, D.R. Electrochemical Characterization of an Aqueous Lithium Rechargeable Battery: The Effect of CeO2 Additions to the MnO2 Cathode. J. Alloys Compd. 2009, 479, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated Hybrid of Graphitic Carbon-Encapsulated CuxO on Multilayered Mesoporous Carbon from Copper MOFs and Polyaniline for Asymmetric Supercapacitor and Oxygen Reduction Reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Kim, H.Y. A ZIF-8-Derived Nanoporous Carbon Nanocomposite Wrapped with Co3O4-Polyaniline as an Efficient Electrode Material for an Asymmetric Supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Wan, J.; Ji, P.; Li, B.; Xi, Y.; Gu, X.; Huang, L.; He, M.; Hu, C. Enhanced Electrochemical Performance in an Aluminium Doped δ-MnO2 Supercapacitor Cathode: Experimental and Theoretical Investigations. Chem. Commun. 2022, 58, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Gao, R.; Zhang, A.; Yang, R.; Zang, X.; Wang, S.; Yao, S.; Yang, Z.; Hao, H.; Yan, Y.-M. Cu2+ Intercalation Activates Bulk Redox Reactions of MnO2 for Enhancing Capacitive Performance. Nano Energy 2020, 74, 104891. [Google Scholar] [CrossRef]
- Zarshad, N.; Wu, J.; Rahman, A.U.; Ni, H. Fe-MnO2 Core-Shell Heterostructure for High-Performance Aqueous Asymmetrical Supercapacitor. J. Electroanal. Chem. 2020, 871, 114266. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Wang, G.; Guo, J.; Wang, X. Nickel-Doped Ultrathin K-Birnessite Manganese Oxide Nanosheet as Pseudocapacitor Electrode with Excellent Cycling Stability for High-Power Pesudocapacitors. ACS Sustain. Chem. Eng. 2017, 5, 1594–1600. [Google Scholar] [CrossRef]
- Özacar, M.; Poyraz, A.S.; Genuino, H.C.; Kuo, C.-H.; Meng, Y.; Suib, S.L. Influence of Silver on the Catalytic Properties of the Cryptomelane and Ag-Hollandite Types Manganese Oxides OMS-2 in the Low-Temperature CO Oxidation. Appl. Catal. A Gen. 2013, 462–463, 64–74. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Zhang, C.; Zhang, R.; He, H. Facile Synthesis of Ag-Modified Manganese Oxide for Effective Catalytic Ozone Decomposition. J. Environ. Sci. 2019, 80, 159–168. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Cai, H.; Qi, J. Highly Conductive Mn3O4/MnS Heterostructures Building Multi-Shelled Hollow Microspheres for High-Performance Supercapacitors. Chem. Eng. J. 2020, 392, 123890. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, F.; Zhang, X.; Wang, S.; Liu, H.; Sun, B.; Zhang, J.; Sun, Y.; Ma, R.; Bando, Y.; et al. Strain Engineering of Two-Dimensional Multilayered Heterostructures for Beyond-Lithium-Based Rechargeable Batteries. Nat. Commun. 2020, 11, 3297. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, J.; Dong, L.; Liu, W.; Mou, J.; Zhao, L.; Wang, J.; Ren, D.; Wu, J.; Xu, C.; et al. Multivalent Ion Storage Towards High-Performance Aqueous Zinc-Ion Hybrid Supercapacitors. Energy Storage Mater. 2019, 20, 335–342. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, X.; Huang, F.; Sha, Z.; Han, Z.; Chen, J.; Yang, W.; Yu, Y.; Zhang, J.; Peng, S.; et al. Hierarchically Structured Electrodes for Moldable Supercapacitors by Synergistically Hybridizing Vertical Graphene Nanosheets and MnO2. Carbon 2021, 172, 272–282. [Google Scholar] [CrossRef]
- Fang, Q.; Sun, M.; Ren, X.; Cao, B.; Shen, W.; Li, Z.; Fu, Y. Ultrafine Mn3O4 Nanowires Synthesized by Colloidal Method as Electrode Materials for Supercapacitors with a Wide Voltage Range. J. Energy Storage 2021, 44, 103260. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, M.; Li, Q.; Gu, J.; Wang, J.; Wang, D. Over-Reduction-Controlled Mixed-Valent Manganese Oxide with Tunable Mn2+/Mn3+ Ratio for High-Performance Asymmetric Supercapacitor with Enhanced Cycling Stability. Langmuir 2021, 37, 2816–2825. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined Chicken Eggshell Electrode for Battery and Supercapacitor Applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]
- Anjana, P.M.; Kumar, S.R.S.; Rakhi, R.B. Manganese Cobalt Oxide Nanoflakes for Electrochemical Energy Storage. J. Mater. Sci. Mater. Electron. 2022, 33, 8484–8492. [Google Scholar] [CrossRef]
- Shi, S.; Wan, G.; Wu, L.; He, Z.; Wang, K.; Tang, Y.; Xu, X.; Wang, G. Ultrathin Manganese Oxide Nanosheets Uniformly Coating on Carbon Nanocoils as High-Performance Asymmetric Supercapacitor Electrodes. J. Colloid Interface Sci. 2019, 537, 142–150. [Google Scholar] [CrossRef]
- Hsieh, C.E.; Chang, C.; Gupta, S.; Tai, N.H. Binder-Free CoMn2O4/Carbon Nanotubes Composite Electrodes for High-Performance Asymmetric Supercapacitor. J. Alloys Compd. 2022, 897, 163231. [Google Scholar] [CrossRef]
- Yan, L.; Niu, L.; Shen, C.; Zhang, Z.; Xu, S. Modulating the Electronic Structure and Pseudocapacitance of δ-MnO2 through Transitional Metal M (M=Fe, Co and Ni) Doping. Electrochim. Acta 2019, 306, 529–540. [Google Scholar] [CrossRef]

| Materials | Specific Capacitance | Cycle Stability | Power Density | Energy Density | Reference |
|---|---|---|---|---|---|
| C@MnO nanosheets | 162.7 F g−1(0.5 A g−1) | 93.5% (10 A g−1) 5000 cycles (ASC) | 400 W kg−1 | 57.7 Wh kg−1 | [19] |
| MnCo2O4 nanoflakes | 256 F g−1(5 mV s−1) | 85% (2 A g−1) 10,000 cycles (ASC) | 1000 W kg−1 | 25 Wh kg−1 | [37] |
| lamellarMnO2@Carbon nanocoil | 435 F g−1(1 A g−1) | 92.7% (2 A g−1) 5000 cycles (ASC) | 100 W kg−1 | 21.58 Wh kg−1 | [38] |
| CoMn2O4nanosheets /carbon nanotubes | 732 F g−1(2 mV s−1) | 77% (100 mV s−1) 5000 cycles (ASC) | 400 W kg−1 | 47.39 Wh kg−1 | [39] |
| Fe doped MnO2 nanosheets | 157 F g−1(0.5 A g−1) | 71.4% (0.5 A g−1) 5000 cycles | 1000 W kg−1 | 30.3 Wh kg−1 | [40] |
| AgMn2O4/ Na0.55Mn2O4 nanosheets | 335.94 F g−1(1 A g−1) | 90.4% (10 A g−1) 10,000 cycles (ASC) | 775 W kg−1 | 65.5 Wh kg−1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Liu, Z.; Ma, C.; Du, Z.; Liu, D.; Cheng, K.; Ye, X.; Liu, T.; Bai, L. Engineering a Novel AgMn2O4@Na0.55Mn2O4 Nanosheet toward High-Performance Electrochemical Capacitors. Nanomaterials 2022, 12, 1538. https://doi.org/10.3390/nano12091538
Wang G, Liu Z, Ma C, Du Z, Liu D, Cheng K, Ye X, Liu T, Bai L. Engineering a Novel AgMn2O4@Na0.55Mn2O4 Nanosheet toward High-Performance Electrochemical Capacitors. Nanomaterials. 2022; 12(9):1538. https://doi.org/10.3390/nano12091538
Chicago/Turabian StyleWang, Guiling, Zihao Liu, Chenchao Ma, Zhiling Du, Dongyan Liu, Kun Cheng, Xiangju Ye, Tingting Liu, and Lei Bai. 2022. "Engineering a Novel AgMn2O4@Na0.55Mn2O4 Nanosheet toward High-Performance Electrochemical Capacitors" Nanomaterials 12, no. 9: 1538. https://doi.org/10.3390/nano12091538
APA StyleWang, G., Liu, Z., Ma, C., Du, Z., Liu, D., Cheng, K., Ye, X., Liu, T., & Bai, L. (2022). Engineering a Novel AgMn2O4@Na0.55Mn2O4 Nanosheet toward High-Performance Electrochemical Capacitors. Nanomaterials, 12(9), 1538. https://doi.org/10.3390/nano12091538






