Forced Convective Heat Transfer Coefficient Measurement of Low Concentration Nanorods ZnO–Ethylene Glycol Nanofluids in Laminar Flow
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of ZnO NRs
2.3. Characterization of ZnO NRs
2.4. ZnO–EG NFs Preparation
2.5. Stability Assessment of ZnO–EG NFs
2.6. Viscosity Measurements of ZnO–EG NFs
2.7. Density Measurements of ZnO–EG NFs
2.8. HTC Measurement of ZnO–EG NFs
3. Results and Discussion
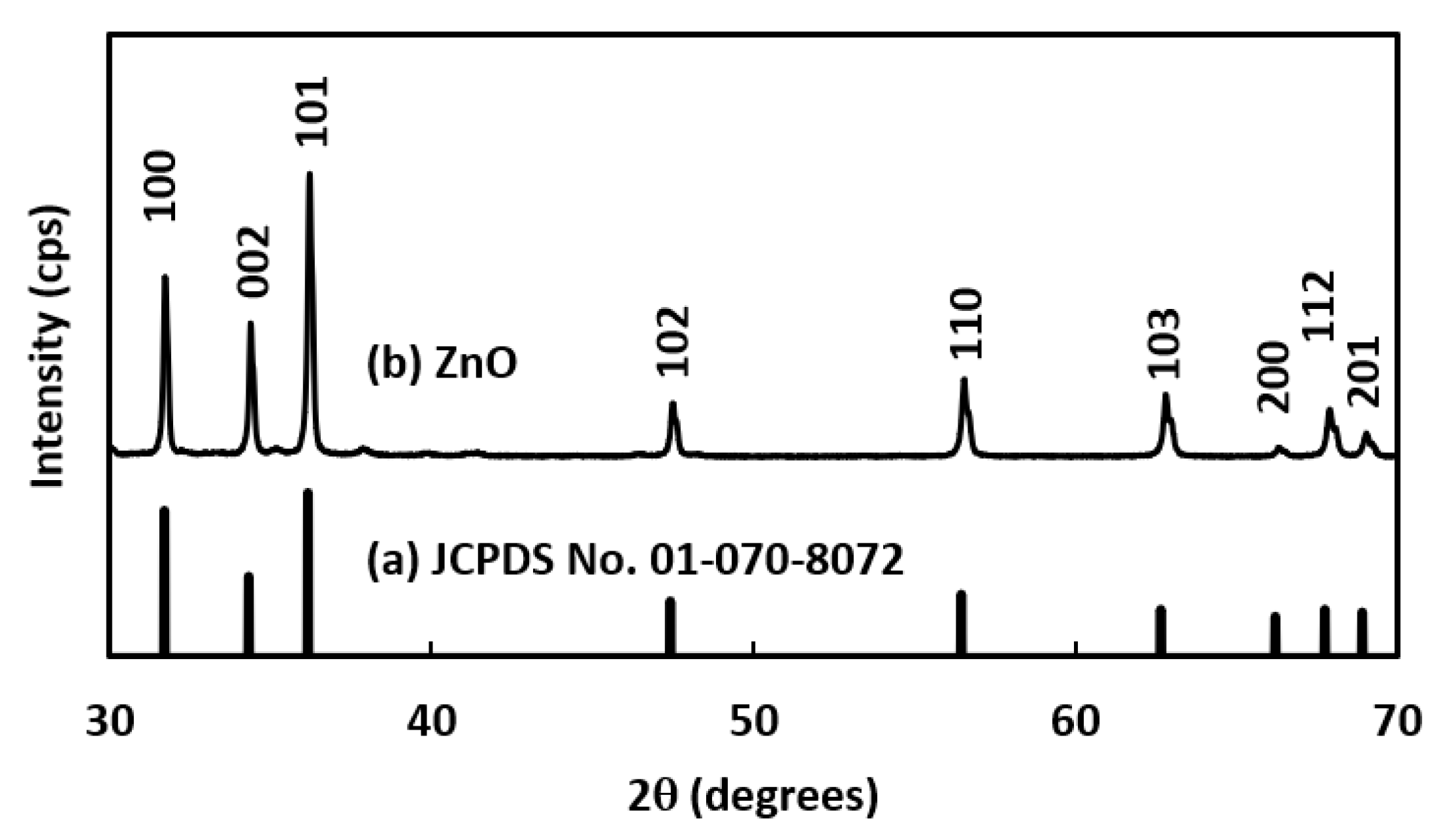
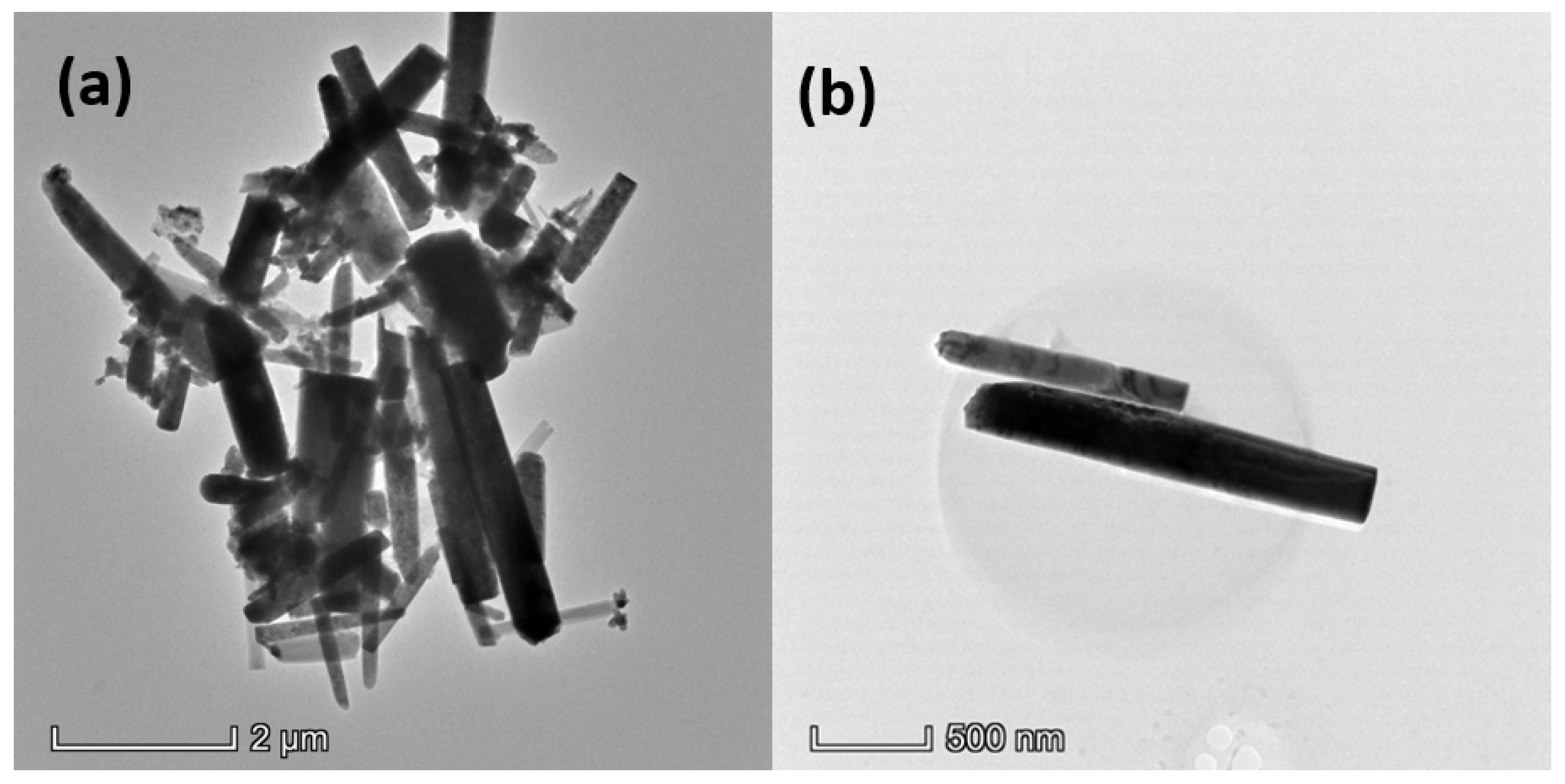
3.1. Characterization of Synthesized ZnO NRs
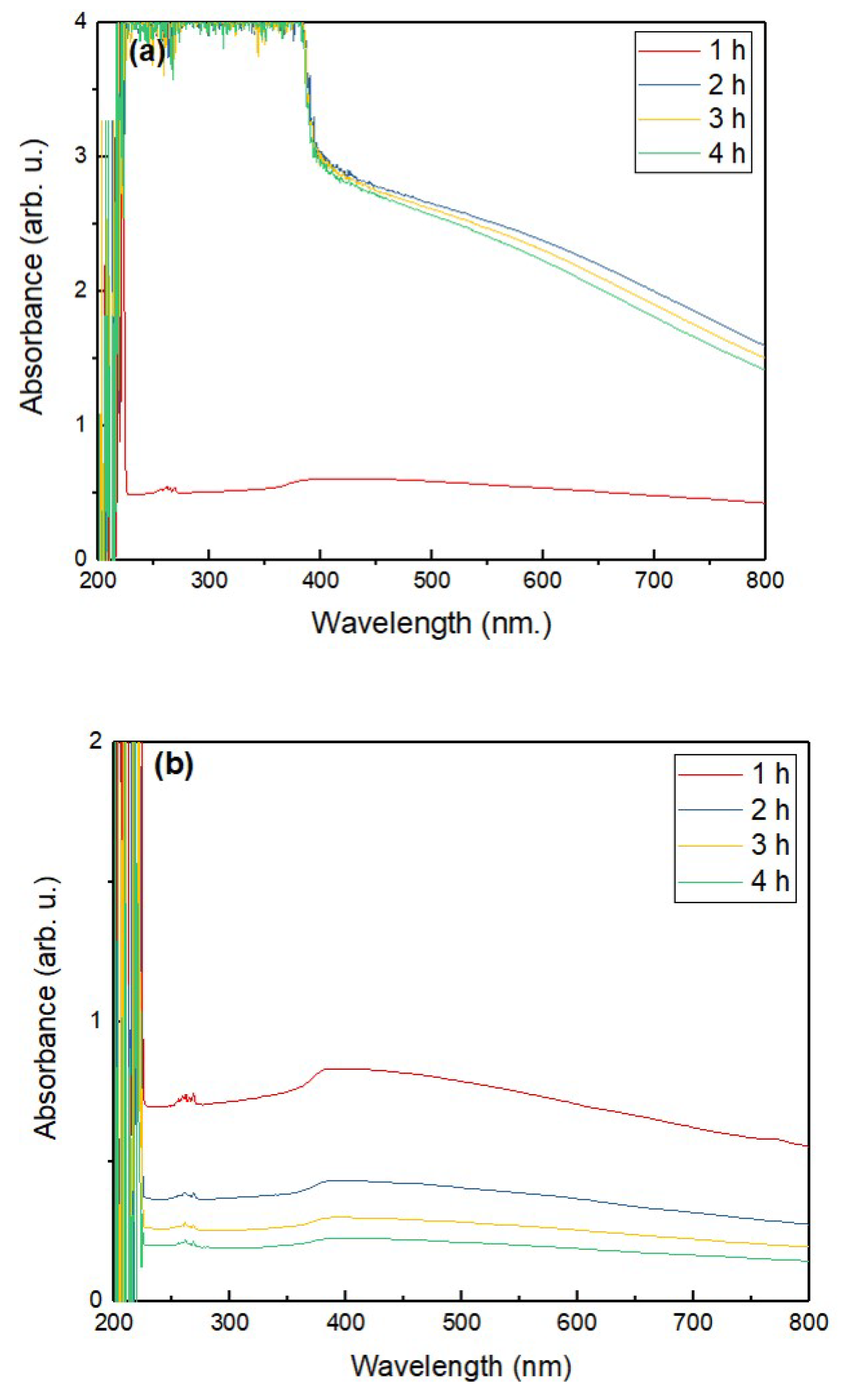
3.2. Stability Assessment of ZnO–EG NFs
3.3. Viscosity of ZnO–EG NFs
3.4. Density of ZnO–EG NFs
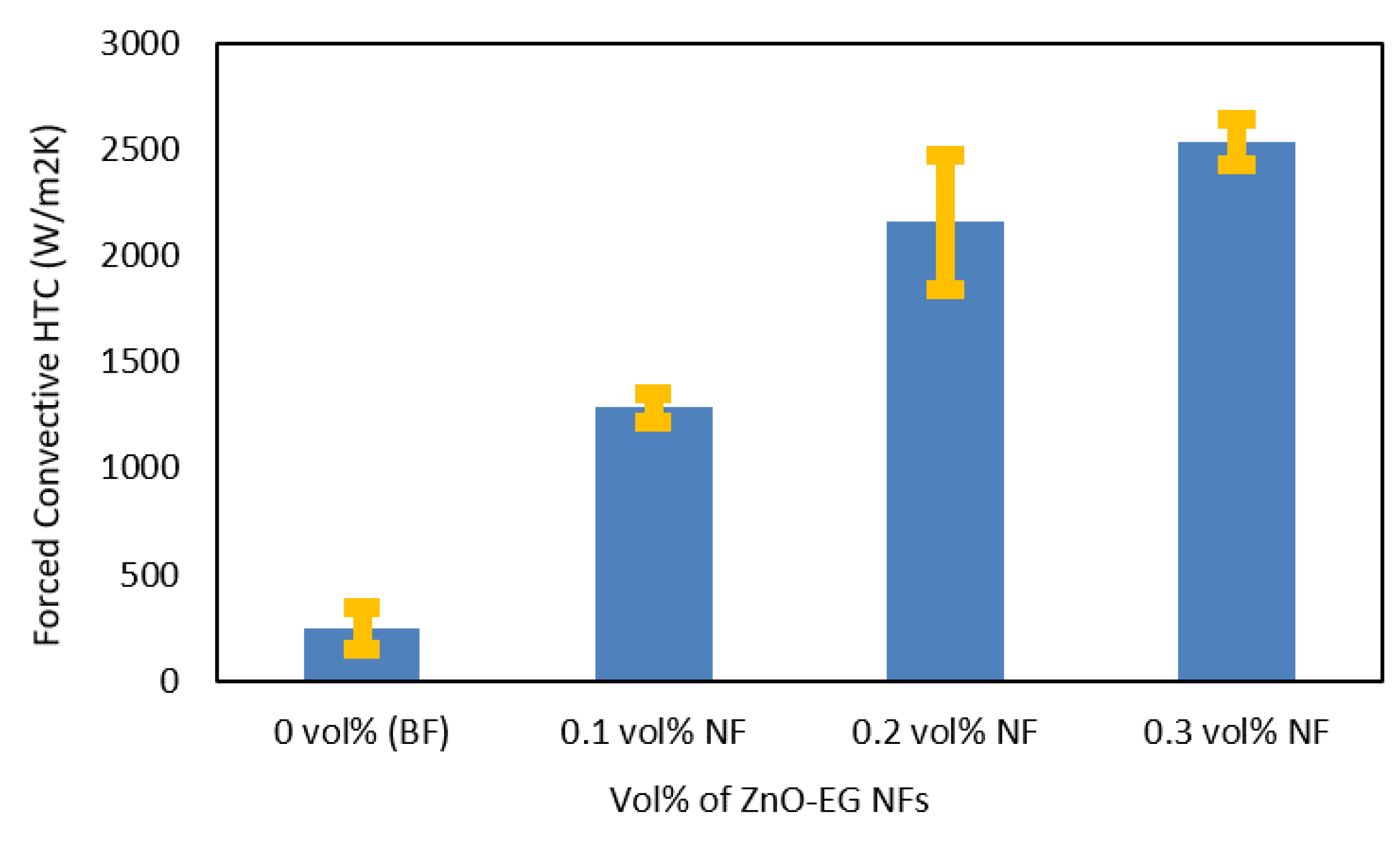
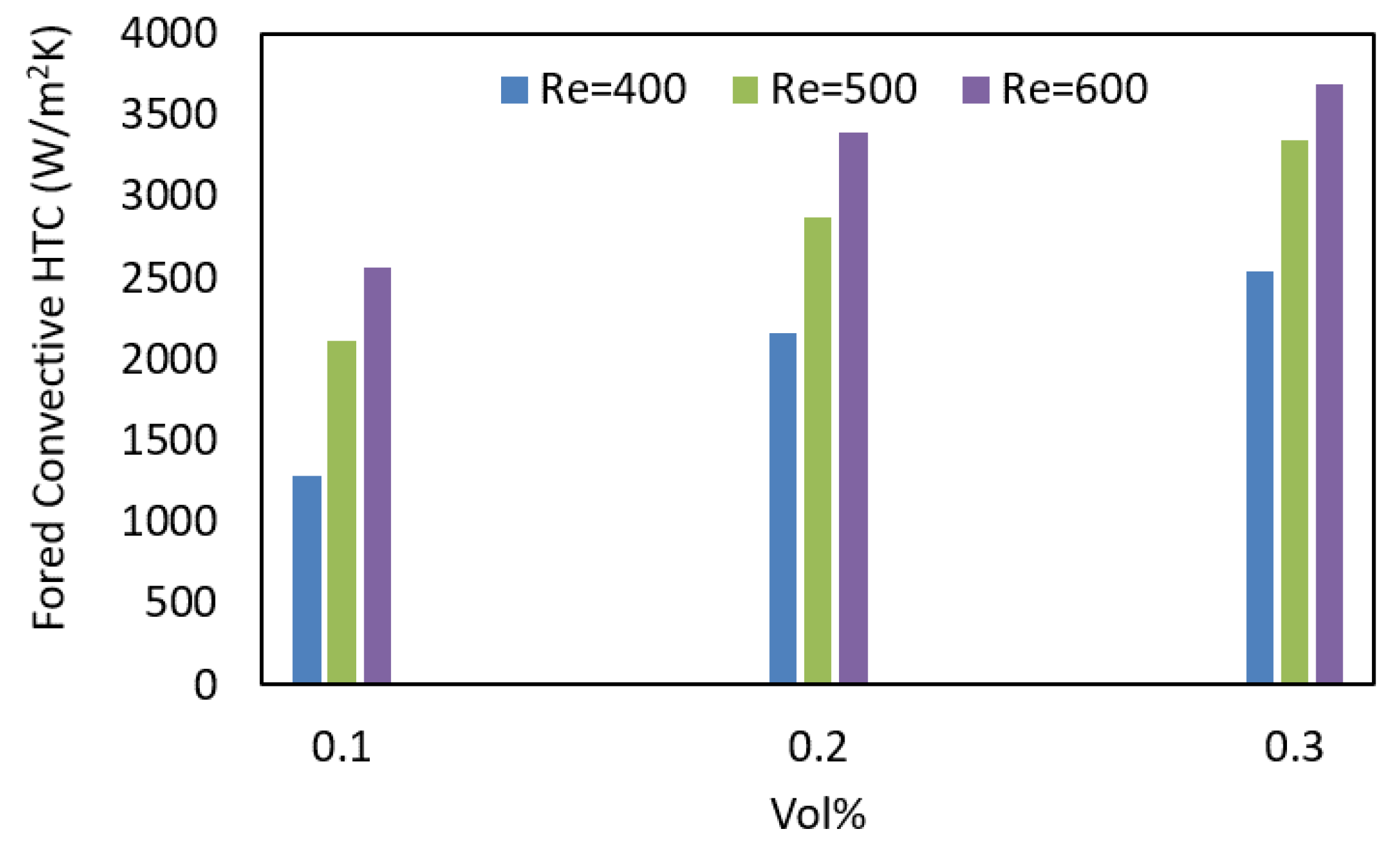
3.5. HTC of ZnO–EG NFs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| List of symbols | |
| WmK−1 | Thermal conductivity |
| m | Length of the heat exchanger, l |
| mV | Zeta potential |
| J/kg·K | Specific heat of water, S |
| m | Diameter of Cu-tube |
| m | Length |
| K | Δθ |
| K | ΔT |
| cP | Viscosity, μ |
| Re | Reynolds number |
| ζ | zeta potential |
| μ | Viscosity |
| T | Temperature |
| Q | the rate of forced convection heat transfer (W) |
| Ts | the solid surface temperature (K) |
| Tf | the fluid temperature (K) |
| A | the area of the surface that is in contact with the fluid (m2) |
| h | the convective HTC (W/m2·K) |
| m | the mass of hot water, kg, |
| S | the specific heat of water, J/kg·K |
| Δθ | the difference in water temperature before and after releasing temperature, K. |
| Abbreviations | |
| ZnO | Zinc oxide |
| NR | Nanorod |
| BF | Base fluid |
| NF | Nanofluid |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscopy |
| UV–vis | Ultraviolet-visible |
| HTC | Heat transfer coefficient |
| EG | Ethylene glycol |
| NM | Nanomaterial |
| NaOH | Sodium hydroxide |
| PVP | Polyvinylpyrrolidone |
| JCPDS | Joint committee for powder diffraction studies |
References
- Ali, A.R.I.; Salam, B. A Review on Nanofluid: Preparation, Stability, Thermophysical Properties, Heat Transfer Characteristics and Application. SN Appl. Sci. 2020, 2, 1636. [Google Scholar] [CrossRef]
- Okonkwo, E.C.; Wole-Osho, I.; Almanassra, I.W.; Abdullatif, Y.M.; Al-Ansari, T. An Updated Review of Nanofluids in Various Heat Transfer Devices. J. Therm. Anal. Calorim. 2021, 145, 2817–2872. [Google Scholar] [CrossRef]
- Choi, S.U.S. Enhancing Thermal Conductivity of Fluids with Nanoparticles. Am. Soc. Mech. Eng. Fluids Eng. Div. FED 1995, 231, 99–105. [Google Scholar]
- Das, S.K.; Choi, S.U.S.; Patel, H.E. Heat Transfer in Nanofluids—A Review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles. In Developments Applications of Non-Newtonian Flows; Signer, D.A., Wang, H.P., Eds.; Asme: New York, NY, USA, 1995; pp. 99–105. [Google Scholar]
- Hwang, Y.; Lee, J.K.; Lee, J.K.; Jeong, Y.M.; Cheong, S.I.; Ahn, Y.C.; Kim, S.H. Production and Dispersion Stability of Nanoparticles in Nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Babita; Sharma, S.K.; Gupta, S.M. Preparation and Evaluation of Stable Nanofluids for Heat Transfer Application: A Review. Exp. Therm. Fluid Sci. 2016, 79, 202–212. [Google Scholar] [CrossRef]
- Ponmani, S.; Gupta, P.; Jadhawar, P.; Nagarajan, R.; Sangwai, J. Investigations on the Thermal and Electrical Conductivity of Polyethylene Glycol-Based CuO and ZnO Nanofluids. Indian Chem. Eng. 2020, 62, 402–412. [Google Scholar] [CrossRef]
- Radkar, R.N.; Bhanvase, B.A.; Barai, D.P.; Sonawane, S.H. Intensified Convective Heat Transfer Using ZnO Nanofluids in Heat Exchanger with Helical Coiled Geometry at Constant Wall Temperature. Mater. Sci. Energy Technol. 2019, 2, 161–170. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirlohi, A.; Alhuyi Nazari, M.; Ghasempour, R. A Review of Thermal Conductivity of Various Nanofluids. J. Mol. Liq. 2018, 265, 181–188. [Google Scholar] [CrossRef]
- Rubbi, F.; Das, L.; Habib, K.; Aslfattahi, N.; Saidur, R.; Rahman, M.T. State-of-the-Art Review on Water-Based Nanofluids for Low Temperature Solar Thermal Collector Application. Sol. Energy Mater. Sol. Cells 2021, 230, 111220. [Google Scholar] [CrossRef]
- Asadi, A.; Aberoumand, S.; Moradikazerouni, A.; Pourfattah, F.; Żyła, G.; Estellé, P.; Mahian, O.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A. Recent Advances in Preparation Methods and Thermophysical Properties of Oil-Based Nanofluids: A State-of-the-Art Review. Powder Technol. 2019, 352, 209–226. [Google Scholar] [CrossRef]
- Ali, M.; Zeitoun, O.; Almotairi, S.; Al-Ansary, H. The Effect of Alumina-Water Nanofluid on Natural Convection Heat Transfer inside Vertical Circular Enclosures Heated from Above. Heat Transf. Eng. 2013, 34, 1289–1299. [Google Scholar] [CrossRef]
- Ali, H.M.; Ali, H.; Liaquat, H.; Bin Maqsood, H.T.; Nadir, M.A. Experimental Investigation of Convective Heat Transfer Augmentation for Car Radiator Using ZnO-Water Nanofluids. Energy 2015, 84, 317–324. [Google Scholar] [CrossRef]
- Xie, H.; Li, Y.; Yu, W. Intriguingly High Convective Heat Transfer Enhancement of Nanofluid Coolants in Laminar Flows. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2010, 374, 2566–2568. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Rahman, S.M.A.; Abdelkareem, M.A. Geometrical Effect Coupled with Nanofluid on Heat Transfer Enhancement in Heat Exchangers. Int. J. Thermofluids 2021, 10, 100072. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Rashidi, A.; Shariaty-Niassar, M. Convective Heat Transfer Enhancement of Graphene Nanofluids in Shell and Tube Heat Exchanger. Exp. Therm. Fluid Sci. 2014, 53, 136–141. [Google Scholar] [CrossRef]
- Haddad, Z.; Oztop, H.F.; Abu-Nada, E.; Mataoui, A. A Review on Natural Convective Heat Transfer of Nanofluids. Renew. Sustain. Energy Rev. 2012, 16, 5363–5378. [Google Scholar] [CrossRef]
- Suganthi, K.S.; Leela Vinodhan, V.; Rajan, K.S. Heat Transfer Performance and Transport Properties of ZnO-Ethylene Glycol and ZnO-Ethylene Glycol-Water Nanofluid Coolants. Appl. Energy 2014, 135, 548–559. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Sunny, V.; Philip, J. Effect of Nanoparticle Size, Morphology and Concentration on Specific Heat Capacity and Thermal Conductivity of Nanofluids. J. Nanofluids 2015, 4, 302–309. [Google Scholar] [CrossRef]
- Sadripour, S.; Chamkha, A.J. The Effect of Nanoparticle Morphology on Heat Transfer and Entropy Generation of Supported Nanofluids in a Heat Sink Solar Collector. Therm. Sci. Eng. Prog. 2019, 9, 266–280. [Google Scholar] [CrossRef]
- Ferrouillat, S.; Bontemps, A.; Poncelet, O.; Soriano, O.; Gruss, J.A. Influence of Nanoparticle Shape Factor on Convective Heat Transfer and Energetic Performance of Water-Based SiO2 and ZnO Nanofluids. Appl. Therm. Eng. 2013, 51, 839–851. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, S.R.; Kim, D. Thermal Conductivity of Metal-Oxide Nanofluids: Particle Size Dependence and Effect of Laser Irradiation. J. Heat Transfer 2007, 129, 298–307. [Google Scholar] [CrossRef]
- Ramya, M.; Nideep, T.K.; Nampoori, V.P.N.; Kailasnath, M. Shape Dependent Heat Transfer and Nonlinear Optical Limiting Characteristics of Water Stable ZnO Nanofluid. Surf. Interfaces 2021, 26, 101345. [Google Scholar] [CrossRef]
- Arora, N.; Gupta, M. Stability Evaluation and Enhancement Methods in Nanofluids: A Review. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2021; Volume 2341, p. 040022. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.H.; Lee, J.H.; Jang, S.P. Effect of Particle Shape on Suspension Stability and Thermal Conductivities of Water-Based Bohemite Alumina Nanofluids. Energy 2015, 90, 1290–1297. [Google Scholar] [CrossRef]
- Ghosh, M.M.; Ghosh, S.; Pabi, S.K. Effects of Particle Shape and Fluid Temperature on Heat-Transfer Characteristics of Nanofluids. J. Mater. Eng. Perform. 2013, 22, 1525–1529. [Google Scholar] [CrossRef]
- Jeong, J.; Li, C.; Kwon, Y.; Lee, J.; Kim, S.H.; Yun, R. Particle Shape Effect on the Viscosity and Thermal Conductivity of ZnO Nanofluids. Int. J. Refrig. 2013, 36, 2233–2241. [Google Scholar] [CrossRef]
- Wang, R.; Qian, S.; Zhang, Z. Investigation of the Aggregation Morphology of Nanoparticle on the Thermal Conductivity of Nanofluid by Molecular Dynamics Simulations. Int. J. Heat Mass Transf. 2018, 127, 1138–1146. [Google Scholar] [CrossRef]
- Lin, J.Z.; Xia, Y.; Ku, X.K. Flow and Heat Transfer Characteristics of Nanofluids Containing Rod-like Particles in a Turbulent Pipe Flow. Int. J. Heat Mass Transf. 2016, 93, 57–66. [Google Scholar] [CrossRef]
- Lin, J.; Xia, Y.; Ku, X. Friction Factor and Heat Transfer of Nanofluids Containing Cylindrical Nanoparticles in Laminar Pipe Flow. J. Appl. Phys. 2014, 116, 133513. [Google Scholar] [CrossRef]
- Milanese, M.; Iacobazzi, F.; Colangelo, G.; de Risi, A. An Investigation of Layering Phenomenon at the Liquid–Solid Interface in Cu and CuO Based Nanofluids. Int. J. Heat Mass Transf. 2016, 103, 564–571. [Google Scholar] [CrossRef]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; Lomascolo, M.; de Risi, A. An Explanation of the Al2O3 Nanofluid Thermal Conductivity Based on the Phonon Theory of Liquid. Energy 2016, 116, 786–794. [Google Scholar] [CrossRef]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; de Risi, A. A Critical Analysis of Clustering Phenomenon in Al2O3 Nanofluids. J. Therm. Anal. Calorim. 2019, 135, 371–377. [Google Scholar] [CrossRef]
- Almuzaiqer, R.; Ali, M.E.; Al-Salem, K. Effect of the Aspect Ratio and Tilt Angle on the Free Convection Heat Transfer Coefficient Inside Al2O3—Water-Filled Square Cuboid Enclosures. Nanomaterials 2022, 12, 500. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Effect of Prolonged Ultrasonication on the Thermal Conductivity of ZnO-Ethylene Glycol Nanofluids. Thermochim. Acta 2012, 535, 58–65. [Google Scholar] [CrossRef]
- Elias, M.M.; Miqdad, M.; Mahbubul, I.M.; Saidur, R.; Kamalisarvestani, M.; Sohel, M.R.; Hepbasli, A.; Rahim, N.A.; Amalina, M.A. Effect of Nanoparticle Shape on the Heat Transfer and Thermodynamic Performance of a Shell and Tube Heat Exchanger. Int. Commun. Heat Mass Transf. 2013, 44, 93–99. [Google Scholar] [CrossRef]
- Deb Majumder, S.; Das, A. A Short Review of Organic Nanofluids: Preparation, Surfactants, and Applications. Front. Mater. 2021, 8, 630182. [Google Scholar] [CrossRef]
- Ma, M.; Zhai, Y.; Yao, P.; Li, Y.; Wang, H. Effect of Surfactant on the Rheological Behavior and Thermophysical Properties of Hybrid Nanofluids. Powder Technol. 2021, 379, 373–383. [Google Scholar] [CrossRef]
- Qamar, A.; Anwar, Z.; Ali, H.; Imran, S.; Shaukat, R.; Mujtaba Abbas, M. Experimental Investigation of Dispersion Stability and Thermophysical Properties of ZnO/DIW Nanofluids for Heat Transfer Applications. Alexandria Eng. J. 2022, 61, 4011–4026. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Hojo, D.; Yoko, A.; Seong, G.; Aoki, N.; Tomai, T.; Takami, S.; Adschiri, T. Dispersion and Rheology of Nanofluids with Various Concentrations of Organic Modified Nanoparticles: Modifier and Solvent Effects. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 583, 123876. [Google Scholar] [CrossRef]
- Qi, J.; Chang, J.; Han, X.; Zhong, R.; Jiang, M.; Chen, Z.; Liu, B. Direct Synthesis of ZnO Nanorods from Solution under Electric Field. Mater. Chem. Phys. 2018, 211, 168–171. [Google Scholar] [CrossRef]
- Ghorbani, H.R.; Mehr, F.P.; Pazoki, H.; Rahmani, B.M. Synthesis of ZnO Nanoparticles by Precipitation Method. Orient. J. Chem. 2015, 31, 1219–1221. [Google Scholar] [CrossRef]
- Singh, R.P.; Shukla, V.K.; Yadav, R.S.; Sharma, P.K.; Singh, P.K.; Pandey, A.C. Biological Approach of Zinc Oxide Nanoparticles Formation and Its Characterization. Adv. Mater. Lett. 2011, 2, 313–317. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Rl, M.; Kv, U.; Naik, D. Synthesis and Characterization of ZnO Nanoparticles: A Review. J. Pharmacogn. Phytochem. 2019, 8, 1095–1101. [Google Scholar]
- Moulahi, A.; Sediri, F. ZnO Nanoswords and Nanopills: Hydrothermal Synthesis, Characterization and Optical Properties. Ceram. Int. 2014, 40, 943–950. [Google Scholar] [CrossRef]
- Mohan, S.; Vellakkat, M.; Aravind, A.; Reka, U. Hydrothermal Synthesis and Characterization of Zinc Oxide Nanoparticles of Various Shapes under Different Reaction Conditions. Nano Express 2020, 1, 030028. [Google Scholar] [CrossRef]
- Elen, K.; Van Den Rul, H.; Hardy, A.; Van Bael, M.K.; D’Haen, J.; Peeters, R.; Franco, D.; Mullens, J. Hydrothermal Synthesis of ZnO Nanorods: A Statistical Determination of the Significant Parameters in View of Reducingthe Diameter. Nanotechnology 2009, 20, 055608. [Google Scholar] [CrossRef]
- Akhoon, S.A.; Rubab, S.; Shah, M.A. A Benign Hydrothermal Synthesis of Nanopencils-like Zinc Oxide Nanoflowers. Int. Nano Lett. 2015, 5, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.; Hossain, M.Z. A Simple Hydrothermal Protocol for the Synthesis of Zinc Oxide Nanorods. Jagannath Univ. J. Sci. 2021, 7, 75–80. [Google Scholar]
- Luna, I.Z.; Chowdhury, A.M.S.; Gafur, M.A.; Khan, R.A. Measurement of Forced Convective Heat Transfer Coefficient of Low Volume Fraction CuO-PVA Nanofluids under Laminar Flow Condition. Am. J. Nanomater. 2015, 3, 64–67. [Google Scholar] [CrossRef]
- Liu, F.; Cao, P.J.; Zhang, H.R.; Shen, C.M.; Wang, Z.; Li, J.Q.; Gao, H.J. Well-Aligned Zinc Oxide Nanorods and Nanowires Prepared without Catalyst. J. Cryst. Growth 2005, 274, 126–131. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Preparation, Sedimentation, and Agglomeration of Nanofluids. Chem. Eng. Technol. 2014, 37, 2011–2021. [Google Scholar] [CrossRef]
- Sati, P.; Shende, R.C.; Ramaprabhu, S. An Experimental Study on Thermal Conductivity Enhancement of DI Water-EG Based ZnO(CuO)/Graphene Wrapped Carbon Nanotubes Nanofluids. Thermochim. Acta 2018, 666, 75–81. [Google Scholar] [CrossRef]
- Choudhary, R.; Khurana, D.; Kumar, A.; Subudhi, S. Stability Analysis of Al2O3/Water Nanofluids. J. Exp. Nanosci. 2017, 12, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Mostafizur, R.M.; Saidur, R.; Abdul Aziz, A.R.; Bhuiyan, M.H.U. Thermophysical Properties of Methanol Based Al2O3 Nanofluids. Int. J. Heat Mass Transf. 2015, 85, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Asirvatham, L.G.; Vishal, N.; Gangatharan, S.K.; Lal, D.M. Experimental Study on Forced Convective Heat Transfer with Low Volume Fraction of CuO/Water Nanofluid. Energies 2009, 2, 97–119. [Google Scholar] [CrossRef]




| Concentration (vol%) | Zeta Potential, ζ (mV) |
|---|---|
| 0.1 | −26.3 |
| 0.2 | 13.5 |
| 0.3 | 4.1 |
| Concentration (vol%) | Viscosity, μ (cP) | Relative Viscosity, μr |
|---|---|---|
| 0 (EG) | 7.40 | - |
| 0.1 | 7.60 | 1.02 |
| 0.2 | 7.85 | 1.06 |
| 0.3 | 8.46 | 1.14 |
| Sample Conc. (vol%) | Ave. Weight of BF and NFs at Different Temperatures, W2 | Ave. wt. of Blank Pycnometer (50 mL), W1 | Density, ρ (g/m3) = (W2 − W1)/50 | |||
|---|---|---|---|---|---|---|
| Ambient Temperature | 40 °C | 50 °C | 60 °C | |||
| 0 (EG) | 73.30 | 72.99 | 72.77 | 72.76 | 26.67 | 0.932 |
| 0.1 | 73.57 | 73.31 | 73.03 | 72.51 | 26.67 | 0.933 |
| 0.2 | 73.25 | 73.24 | 73.25 | 73.28 | 26.67 | 0.938 |
| 0.3 | 73.85 | 73.52 | 73.27 | 72.83 | 26.67 | 0.944 |
| Parameter | Value |
|---|---|
| Mass of water | 0.35 kg |
| Specific heat of water, S | 4178 J/kg·K |
| The outer diameter of the Cu-tube, d | 4.4 × 10−4 m |
| Heat exchange length of Cu-tube, l | 68.58 × 10−2 m |
| Δθ0 | 0.01 K |
| Δθ1 | 1.3 K |
| Δθ2 | 1.3 K |
| Δθ3 | 1.2 K |
| ΔT0 | 13.49 K |
| ΔT1 | 4.5 K |
| ΔT2 | 5.9 K |
| ΔT3 | 4.1 K |
| Concentration (vol%) | Forced Convective HTC (Wm2/K) | ||
|---|---|---|---|
| 1st Measurement | 2nd Measurement | 3rd Measurement | |
| 0 (BF) | 144 | 292 | 220 |
| 0.1 | 1341 | 1212 | 1299 |
| 0.2 | 2195 | 2449 | 1823 |
| 0.3 | 2656 | 2452 | 2500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.S.; Nahar, B.; Gafur, M.A.; Seong, G.; Hossain, M.Z. Forced Convective Heat Transfer Coefficient Measurement of Low Concentration Nanorods ZnO–Ethylene Glycol Nanofluids in Laminar Flow. Nanomaterials 2022, 12, 1568. https://doi.org/10.3390/nano12091568
Alam MS, Nahar B, Gafur MA, Seong G, Hossain MZ. Forced Convective Heat Transfer Coefficient Measurement of Low Concentration Nanorods ZnO–Ethylene Glycol Nanofluids in Laminar Flow. Nanomaterials. 2022; 12(9):1568. https://doi.org/10.3390/nano12091568
Chicago/Turabian StyleAlam, Md. Shah, Bodrun Nahar, Md. Abdul Gafur, Gimyeong Seong, and Muhammad Zamir Hossain. 2022. "Forced Convective Heat Transfer Coefficient Measurement of Low Concentration Nanorods ZnO–Ethylene Glycol Nanofluids in Laminar Flow" Nanomaterials 12, no. 9: 1568. https://doi.org/10.3390/nano12091568







