Reduced Potential Barrier of Sodium-Substituted Disordered Rocksalt Cathode for Oxygen Evolution Electrocatalysts
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Syntheses of Disordered Cathode Active Material
2.3. Materials Characterization
2.4. ICP-OES
2.5. XPS Data Collection and Analysis
2.6. OER
2.7. DFT Calculations
2.8. Genetic Algorithm
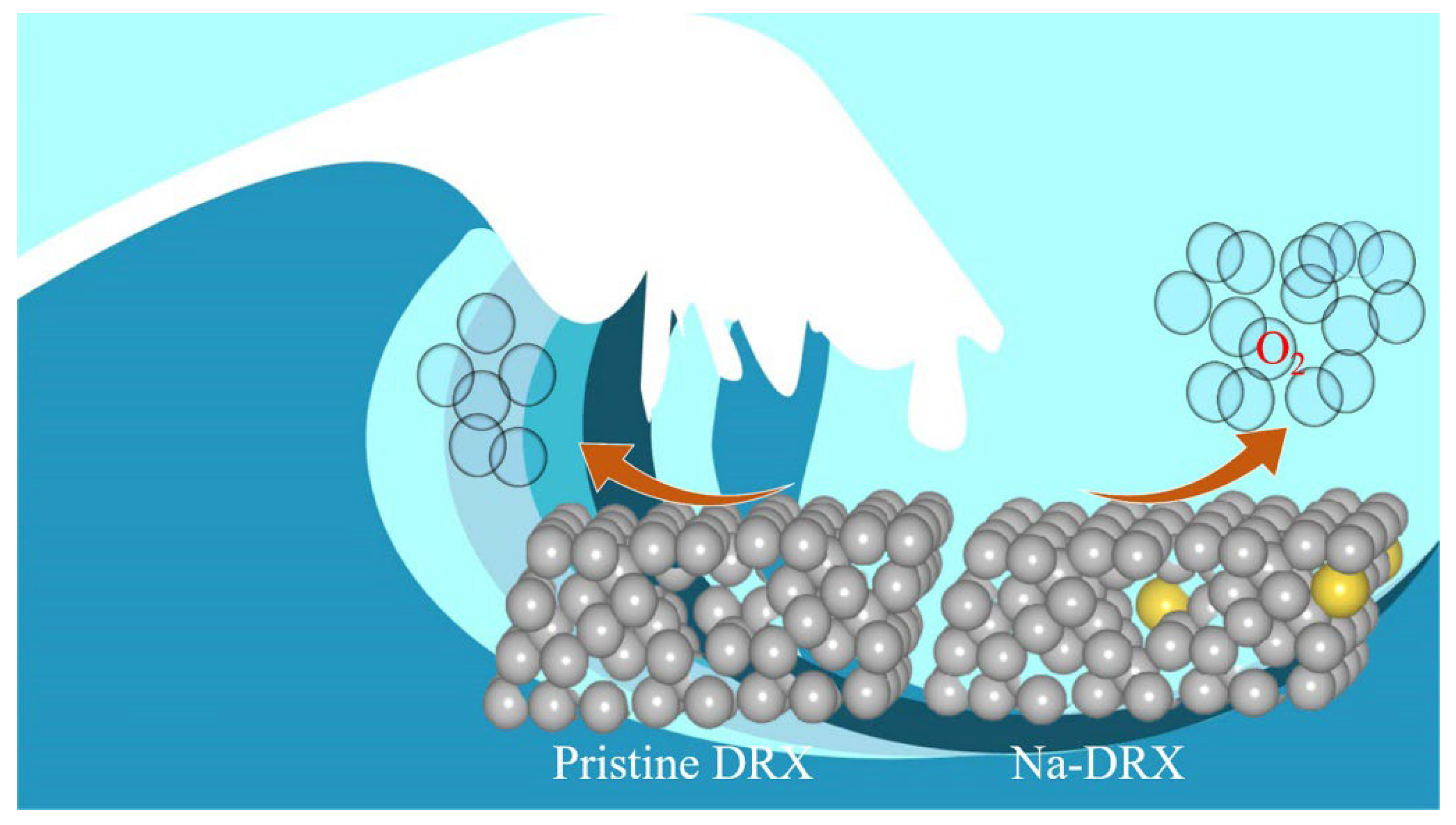
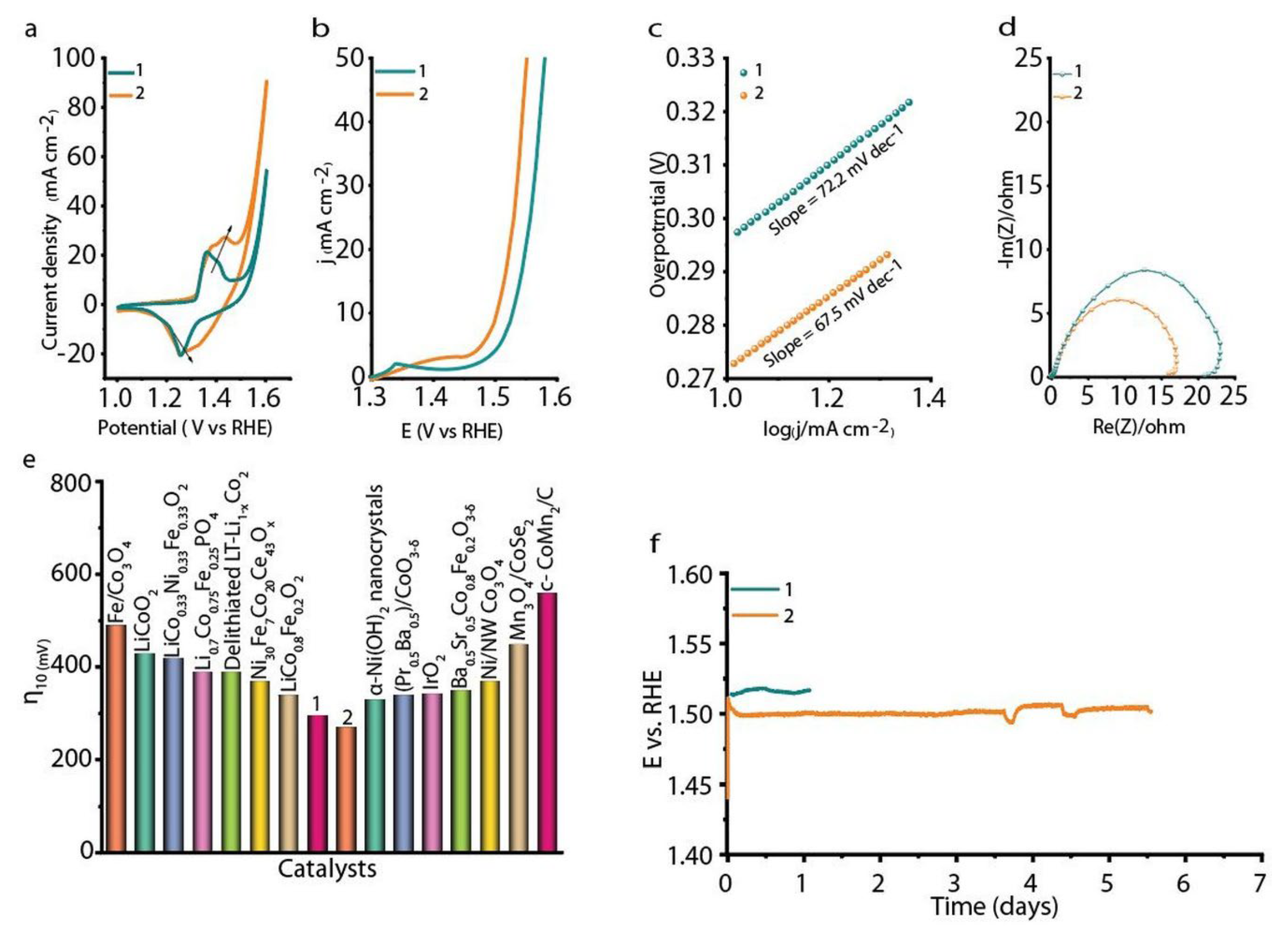
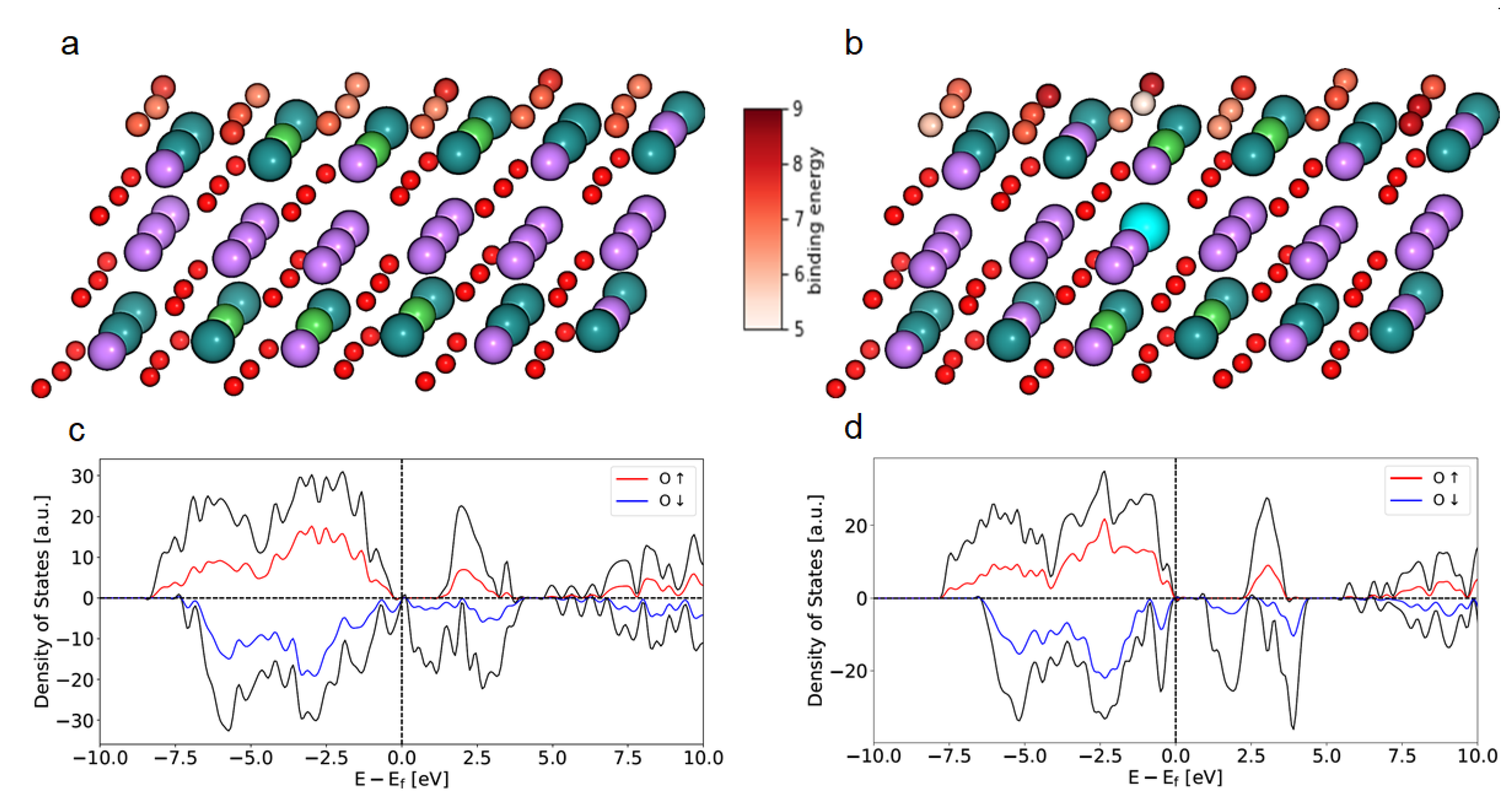
3. Results and Discussion
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.N.; Kajal, S.; Kim, J.; Jana, A.; Kim, J.Y.; Kim, K.S. Interface Engineering Driven Stabilization of Halide Perovskites against Moisture, Heat, and Light for Optoelectronic Applications. Adv. Energy Mater. 2020, 10, 2000768. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Singh, A.N.; Sultan, S.; Kim, K.S. Recent Advancement of p- and d-Block Elements, Single Atoms, and Graphene-Based Photoelectrochemical Electrodes for Water Splitting. Adv. Energy Mater. 2020, 10, 2000280. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, W.; Chen, Y.; Yu, J.; Liu, M.; Shao, Z. A High-Performance Electrocatalyst for Oxygen Evolution Reaction: LiCo0.8Fe0.2O2. Adv. Mater. 2015, 27, 7150–7155. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Kong, D.; Yan, K.; Hsu, P.C.; Zheng, G.; Yao, H.; Liang, Z.; Sun, X.; Cui, Y. Electrochemical tuning of layered lithium transition metal oxides for improvement of oxygen evolution reaction. Nat. Commun. 2014, 5, 4345. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Zhu, Y.; Li, X.; Li, M.; Lu, R.; Wang, J.; Zhu, X.; Yang, W. Layered Fe-Substituted LiNiO2 Electrocatalysts for High-Efficiency Oxygen Evolution Reaction. ACS Energy Lett. 2017, 2, 1654–1660. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Z.G.; Chen, Y.; Su, C.; Tadé, M.O.; Shao, Z. SrNb0.1Co0.7Fe0.2O3−δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem. 2015, 127, 3969–3973. [Google Scholar] [CrossRef]
- Singh, A.N.; Kim, M.; Meena, A.; Wi, T.; Lee, H.; Kim, K.S. Na/Al Codoped Layered Cathode with Defects as Bifunctional Electrocatalyst for High-Performance Li-Ion Battery and Oxygen Evolution Reaction. Small 2021, 17, e2005605. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Jiao, F. Ordered Mesoporous Cobalt Oxide as Highly Efficient Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2013, 135, 4516–4521. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Melendres, C.A.; Mini, S. X-ray Absorption Spectroscopy Studies of the Local Atomic and Electronic Structure of Iron Incorporated into Electrodeposited Hydrous Nickel Oxide Films. J. Phys. Chem. B 2000, 104, 4300–4306. [Google Scholar] [CrossRef]
- Tong, Y.; Guo, Y.; Chen, P.; Liu, H.; Zhang, M.; Zhang, L.; Yan, W.; Chu, W.; Wu, C.; Xie, Y. Spin-State Regulation of Perovskite Cobaltite to Realize Enhanced Oxygen Evolution Activity. Chem 2017, 3, 812–821. [Google Scholar] [CrossRef]
- Delmas, C.; Croguennec, L. Layered Li (Ni, M)O2 systems as the cathode material in lithium-ion batteries. MRS Bull. 2002, 27, 608–612. [Google Scholar] [CrossRef]
- Prado, G.; Fournes, L.; Delmas, C. On the LixNi0.70Fe0.15Co0.15O2 system: An X-ray diffraction and Mössbauer study. J. Solid State Chem. 2001, 159, 103–112. [Google Scholar] [CrossRef]
- Singh, A.N. A Story of Disordered Arrangements. Matter 2021, 4, 23–25. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Hajibabaei, A.; Myung, C.W.; Kim, K.S. Sparse Gaussian process potentials: Application to lithium diffusivity in superionic conducting solid electrolytes. Phys. Rev. B 2021, 103, 214102. [Google Scholar] [CrossRef]
- Hajibabaei, A.; Kim, K.S. Universal Machine Learning Interatomic Potentials: Surveying Solid Electrolytes. J. Phys. Chem. Lett. 2021, 12, 8115–8120. [Google Scholar] [CrossRef] [PubMed]
- Hajibabaei, A.; Ha, M.; Pourasad, S.; Kim, J.; Kim, K.S. Machine Learning of First-Principles Force-Fields for Alkane and Polyene Hydrocarbons. J. Phys. Chem. A 2021, 125, 9414–9420. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Yasui, Y.; Sato, M.; Igawa, N.; Kakurai, K. New-type phase transition of Li2RuO3 with honeycomb structure. J. Phys. Soc. Jpn. 2007, 76, 033705. [Google Scholar] [CrossRef]
- Xu, J.; Sun, M.; Qiao, R.; Renfrew, S.; Ma, L.; Wu, T.; Hwang, S.; Nordlund, D.; Su, D.; Amine, K.; et al. Elucidating anionic oxygen activity in lithium-rich layered oxides. Nat. Commun. 2018, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the Potential of Cation-Disordered Oxides for Rechargeable Lithium Batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef]
- Al-Tabbakh, A.A.; Karatepe, N.; Al-Zubaidi, A.B.; Benchaabane, A.; Mahmood, N.B. Crystallite size and lattice strain of lithiated spinel material for rechargeable battery by X-ray diffraction peak-broadening analysis. Int. J. Energy Res. 2019, 43, 1903–1911. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2018, 12, 322–333. [Google Scholar] [CrossRef]
- Fabbri, E.; Habereder, A.; Waltar, K.; Kötz, R.; Schmidt, T.J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2014, 4, 3800–3821. [Google Scholar] [CrossRef]
- Paoli, E.A.; Masini, F.; Frydendal, R.; Deiana, D.; Schlaup, C.; Malizia, M.; Hansen, T.W.; Horch, S.; Stephens, I.E.L.; Chorkendorff, I. Oxygen evolution on well-characterized mass-selected Ru and RuO2nanoparticles. Chem. Sci. 2014, 6, 190–196. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Zhong, Y.; Ge, L.; Chen, Y.; Veder, J.-P.M.; Guan, D.; O’Hayre, R.; Li, M.; Wang, G.; et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 2020, 11, 2002. [Google Scholar] [CrossRef]
- Liu, R.; Liang, F.; Zhou, W.; Yang, Y.; Zhu, Z. Calcium-doped lanthanum nickelate layered perovskite and nickel oxide nano-hybrid for highly efficient water oxidation. Nano Energy 2015, 12, 115–122. [Google Scholar] [CrossRef]
- Liang, F.; Yu, Y.; Zhou, W.; Xu, X.; Zhu, Z. Highly defective CeO2 as a promoter for efficient and stable water oxidation. J. Mater. Chem. A 2014, 3, 634–640. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, Y.; Wang, K.; Yu, T.; Liu, X.; Wang, G.; Xie, G.; Jiang, L. High performance of Co–P/NF electrocatalyst for oxygen evolution reaction. Mater. Chem. Phys. 2019, 235, 121772. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, J.; Liu, S.; Li, Q.; Zeng, G.; Yang, Y.; Cui, Y. Na-stabilized Ru-based lithium rich layered oxides with enhanced electrochemical performance for lithium ion batteries. Electrochim. Acta 2017, 253, 31–38. [Google Scholar] [CrossRef]
- Min, H.; Kim, M.; Lee, S.U.; Kim, H.; Kim, G.; Choi, K.; Lee, J.H.; Seok, S.I. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 2019, 366, 749–753. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect Graphene as a Trifunctional Catalyst for Electrochemical Reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef]
- Delmas, C.; Prado, G.; Rougier, A.; Suard, E.; Fournes, L. Effect of iron on the electrochemical behaviour of lithium nickelate: From LiNiO2 to 2D-LiFeO2. Solid State Ion. 2000, 135, 71–79. [Google Scholar] [CrossRef]
- Prado, G.; Rougier, A.; Fournès, L.; Delmas, C. Electrochemical Behavior of Iron-Substituted Lithium Nickelate. J. Electrochem. Soc. 2000, 147, 2880–2887. [Google Scholar] [CrossRef]
- Mansour, A.N.; Yang, X.Q.; Sun, X.; McBreen, J.; Croguennec, L.; Delmas, C. In Situ X-Ray Absorption Spectroscopy Study of Li (1− z) Ni (1+ z) O 2 (z≤ 0.02) Cathode Material. J. Electrochem. Soc. 2000, 147, 2104. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.; Markovic, V.R.S.D.S.P.P.L.N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2016, 16, 57–69. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.-W.; Zhu, H.; Kroes, G.-J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Over, H. Surface chemistry of ruthenium dioxide in heterogeneous catalysis and electrocatalysis: From fundamental to ap-plied research. Chem. Rev. 2012, 112, 3356–3426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Y.; Wu, X.; Hou, C.; Geng, Z.; Wu, J.; Huang, K.; Gao, L.; Feng, S. Charge transfer-induced O p-band center shift for an enhanced OER performance in LaCoO3 film. CrystEngComm 2019, 21, 1534–1538. [Google Scholar] [CrossRef]
- Gershinsky, Y.; Zitoun, D. Direct Chemical Synthesis of Lithium Sub-Stochiometric Olivine Li0.7Co0.75Fe0.25PO4 Coated with Reduced Graphene Oxide as Oxygen Evolution Reaction Electrocatalyst. ACS Catal. 2018, 8, 8715–8725. [Google Scholar] [CrossRef]
- Haber, J.A.; Cai, Y.; Jung, S.; Xiang, C.; Mitrovic, S.; Jin, J.; Bell, A.T.; Gregoire, J.M. Discovering Ce-rich oxygen evolution catalysts, from high throughput screening to water electrolysis. Energy Environ. Sci. 2014, 7, 682–688. [Google Scholar] [CrossRef]
- Trotochaud, L.; Ranney, J.K.; Williams, K.N.; Boettcher, S.W. Solution-Cast Metal Oxide Thin Film Electrocatalysts for Oxygen Evolution. J. Am. Chem. Soc. 2012, 134, 17253–17261. [Google Scholar] [CrossRef]
- Liang, H.; Meng, F.; Cabán-Acevedo, M.; Li, L.; Forticaux, A.; Xiu, L.; Wang, Z.; Jin, S. Hydrothermal continuous flow synthe-sis and exfoliation of NiCo layered double hydroxide nanosheets for enhanced oxygen evolution catalysis. Nano Lett. 2015, 15, 1421–1427. [Google Scholar] [CrossRef]
- Peng, X.; Wang, L.; Hu, L.; Li, Y.; Gao, B.; Song, H.; Huang, C.; Zhang, X.; Fu, J.; Huo, K.; et al. In situ segregation of cobalt nanoparticles on VN nanosheets via nitriding of Co2V2O7 nanosheets as efficient oxygen evolution reaction electrocatalysts. Nano Energy 2017, 34, 1–7. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin Spinel-Structured Nanosheets Rich in Oxygen Deficiencies for Enhanced Electrocatalytic Water Oxidation. Angew. Chem. 2015, 127, 7507–7512. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, W.; Zhu, W.; Yang, Q.; Lei, X.; Liu, J.; Li, Y.; Sun, X.; Duan, X. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 2014, 50, 6479–6482. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolu-tion catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Fan, K.; Chen, H.; Ji, Y.; Huang, H.; Claesson, H.H.P.M.; Daniel, Q.; Philippe, B.; Rensmo, B.P.H.; Li, F.; Luo, Y.; et al. Nickel–vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 2016, 7, 11981. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Luo, F.; Tan, Y.; Zeng, L.; Fang, B.; Liu, A. Rock salt type NiCo2O3 supported on ordered mesoporous carbon as a highly efficient electrocatalyst for oxygen evolution reaction. Appl. Catal. B Environ. 2019, 256, 117852. [Google Scholar] [CrossRef]
- Indra, A.; Menezes, P.W.; Sahraie, N.R.; Bergmann, A.; Das, C.; Tallarida, M.; Schmeißer, D.; Strasser, P.; Driess, M. Unifica-tion of catalytic water oxidation and oxygen reduction reactions: Amorphous beat crystalline cobalt iron oxides. J. Am. Chem. Soc. 2014, 136, 17530–17536. [Google Scholar] [CrossRef]
- Chakrapani, K.; Bendt, G.; Hajiyani, H.; Lunkenbein, T.; Greiner, M.T.; Masliuk, L.; Salamon, S.; Landers, J.; Schloegl, R.; Wende, H.; et al. The Role of Composition of Uniform and Highly Dispersed Cobalt Vanadium Iron Spinel Nanocrystals for Oxygen Electrocatalysis. ACS Catal. 2018, 8, 1259–1267. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Wang, H.; Wang, C.; Pinna, N.; Lu, X. Ni Strongly Coupled with Mo2 C Encapsulated in Nitrogen-Doped Carbon Nanofibers as Robust Bifunctional Catalyst for Overall Water Splitting. Adv. Energy Mater. 2019, 9, 1803185. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.-L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef]
- Liu, H.; He, Q.; Jiang, H.; Lin, Y.; Zhang, Y.; Habib, M.; Chen, S.; Song, L. Electronic Structure Reconfiguration toward Pyrite NiS2 via Engineered Heteroatom Defect Boosting Overall Water Splitting. ACS Nano 2017, 11, 11574–11583. [Google Scholar] [CrossRef]
- Fan, K.; Zou, H.; Lu, Y.; Chen, H.; Li, F.; Liu, J.; Sun, L.; Tong, L.; Toney, M.F.; Sui, M.; et al. Direct Observation of Structural Evolution of Metal Chalcogenide in Electrocatalytic Water Oxidation. ACS Nano 2018, 12, 12369–12379. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Dang, N.K.; Sultan, S.; Thangavel, P.; Jeong, H.Y.; Kim, K.S. Multi-heteroatom-doped carbon from waste-yeast biomass for sustained water splitting. Nat. Sustain. 2020, 3, 556–563. [Google Scholar] [CrossRef]
- Qu, M.; Ding, X.; Shen, Z.; Cui, M.; Oropeza, F.E.; Gorni, G.; O’Shea, V.A.D.L.P.; Li, W.; Qi, D.-C.; Zhang, K.H.L. Tailoring the Electronic Structures of the La2NiMnO6 Double Perovskite as Efficient Bifunctional Oxygen Electrocatalysis. Chem. Mater. 2021, 33, 2062–2071. [Google Scholar] [CrossRef]
- Baumung, M.; Schönewald, F.; Erichsen, T.; Volkert, C.A.; Risch, M. Influence of particle size on the apparent electrocatalytic activity of LiMn2O4 for oxygen evolution. Sustain. Energy Fuels 2019, 3, 2218–2226. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Z.; Tang, M.; Chen, G.; Li, Y.; Chen, W.; Lin, D.; Zhang, Z.; Zhou, G.; Li, J.; et al. Self-Selective Catalyst Synthesis for CO2 Reduction. Joule 2019, 3, 1927–1936. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.N.; Hajibabaei, A.; Ha, M.; Meena, A.; Kim, H.-S.; Bathula, C.; Nam, K.-W. Reduced Potential Barrier of Sodium-Substituted Disordered Rocksalt Cathode for Oxygen Evolution Electrocatalysts. Nanomaterials 2023, 13, 10. https://doi.org/10.3390/nano13010010
Singh AN, Hajibabaei A, Ha M, Meena A, Kim H-S, Bathula C, Nam K-W. Reduced Potential Barrier of Sodium-Substituted Disordered Rocksalt Cathode for Oxygen Evolution Electrocatalysts. Nanomaterials. 2023; 13(1):10. https://doi.org/10.3390/nano13010010
Chicago/Turabian StyleSingh, Aditya Narayan, Amir Hajibabaei, Miran Ha, Abhishek Meena, Hyun-Seok Kim, Chinna Bathula, and Kyung-Wan Nam. 2023. "Reduced Potential Barrier of Sodium-Substituted Disordered Rocksalt Cathode for Oxygen Evolution Electrocatalysts" Nanomaterials 13, no. 1: 10. https://doi.org/10.3390/nano13010010
APA StyleSingh, A. N., Hajibabaei, A., Ha, M., Meena, A., Kim, H.-S., Bathula, C., & Nam, K.-W. (2023). Reduced Potential Barrier of Sodium-Substituted Disordered Rocksalt Cathode for Oxygen Evolution Electrocatalysts. Nanomaterials, 13(1), 10. https://doi.org/10.3390/nano13010010







