The Controllable Ratio of the Polyaniline-Needle-Shaped Manganese Dioxide for the High-Performance Supercapacitor Application
Abstract
:1. Introduction
2. Materials
2.1. Needle-Like MnO2 Nanostructure Preparation
2.2. Synthesis of Needle-like MnO2/Polyaniline Nanocomposites
2.3. Characterization
2.4. Electrochemical Supercapacitive Performances
3. Results and discussion
Electrochemical Performance in the Half Cell
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Fan, Z.J.; Sun, W.; Ning, G.Q.; Wei, T.; Zhang, Q.; Zhang, R.F.; Zhi, L.J.; Wei, F. Advanced Asymmetric Supercapacitors Based on Ni(OH)2/Graphene and Porous Graphene Electrodes with High Energy Density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, W.; Tao, Y.; Yang, Q.H. Towards superior volumetric performance: Design and preparation of novel carbon materials for energy storage. Energy Environ. Sci. 2015, 8, 1390–1403. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, S.G.; Fouad, H.; Abd El-Salam, N.M.; Cho, M.H. Solid-state symmetrical supercapacitor based on hierarchical flower-like nickel sulfide with shape-controlled morphological evolution. Electrochim. Acta 2018, 268, 82–93. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Ansari, S.A.; Kim, S.-H. Fundamentals and recent progress of Sn-based electrode materials for supercapacitors: A comprehensive review. J. Energy Storage 2022, 53, 105187. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Alamri, H.R.; Ansari, M.O.; Khan, Z.; Cho, M.H. Facile Synthesis of SnS2 Nanostructures with Different Morphologies for High-Performance Supercapacitor Applications. ACS Omega 2018, 3, 1581–1588. [Google Scholar] [CrossRef] [Green Version]
- Parveen, N.; Ansari, M.O.; Cho, M.H. Simple route for gram synthesis of less defective few layered graphene and its electrochemical performance. RSC Adv. 2015, 5, 4920–44927. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Mu, J.; Wang, H.; Lei, Z. High-performance supercapacitor based on multi-structural CuS@polypyrrole composites prepared by in-situ oxidative polymerization. J. Mater. Chem. A 2014, 2, 3303–3307. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y. Electroactive FeS2-modified MoS2 nanosheet for high-performance supercapacitor. J. Alloy. Compd. 2020, 824, 153936. [Google Scholar] [CrossRef]
- Miao, Y.E.; Fan, W.; Chen, D.; Liu, T. High-Performance Supercapacitors Based on Hollow Polyaniline Nanofibers by Electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 4423–4428. [Google Scholar] [CrossRef]
- Zhong, S.; Sun, T.; Yuan, G.; Wen, R.; Chen, W.; Wang, Z.; Zhang, L.; Zhan, K.; Zhu, M.; Yang, J.; et al. Scalable fabrication of NiCo2O4/reduced graphene oxide composites by ultrasonic spray as binder-free electrodes for supercapacitors with ultralong lifetime. J. Mater. Sci. Technol. 2022, 99, 260–269. [Google Scholar]
- Liu, Y.; Zhou, Z.; Zhang, S.; Luo, W.; Zhang, G. Controllable synthesis of CuS hollow microflowers hierarchical structures for asymmetric supercapacitors. Appl. Surf. Sci. 2018, 442, 711–719. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.S.; Yuan, G.; Cui, C.; Lu, L. A Symmetric RuO2/RuO2 Supercapacitor Operating at 1.6 V by Using a Neutral Aqueous Electrolyte. Electrochem. Solid-State Lett. 2012, 15, A60–A63. [Google Scholar] [CrossRef] [Green Version]
- Ranganatha, S.; Munichandraiah, N. γ-MnS nanoparticles anchored reduced graphene oxide: Electrode materials for high performance supercapacitors. J. Sci. Adv. Mater. Devices 2018, 3, 359–365. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, L.; Zhang, J.; Miao, T.; Yuan, R.; Chen, W.; Wang, Z.; Yang, J.; Zhao, B. Na+ pre-intercalated Na0.11MnO2 on three-dimensional graphene as cathode for aqueous zinc ion hybrid supercapacitor with high energy density. Carbon 2022, 198, 46–56. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.X.; Zhang, L. MnO2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21380. [Google Scholar] [CrossRef]
- Roy, H.S.; Islam, M.M.; Mollah, M.Y.A.; Susan, M.A.B.H. Polyaniline-MnO2 composites prepared in-situ during oxidative polymerization of aniline for supercapacitor applications. Materials 2020, 29, 1013–1019. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Tang, J.; Jiang, X.; Bao, Y.; Chen, Y. Synthesis of γ-MnO2/PANI Composites for Supercapacitor Application in Acidic Electrolyte. J. Electrochem. Soc. 2021, 168, 030542. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Cho, M.H. Route to High Surface Area, Mesoporosity of Polyaniline–Titanium Dioxide Nanocomposites via One Pot Synthesis for Energy Storage Applications. Ind. Eng. Chem. Res. 2016, 55, 116–124. [Google Scholar] [CrossRef]
- Ansari, S.A.; Parveen, N.; Han, T.H.; Ansari, M.O.; Cho, M.H. Fibrous polyaniline@manganese oxide nanocomposites as supercapacitor electrode materials and cathode catalysts for improved power production in microbial fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 9053–9060. [Google Scholar] [CrossRef]
- Wu, J.; Huang, H.; Yu, L.; Hu, J. Controllable Hydrothermal Synthesis of MnO2 Nanostructures. Adv. Mater. Phys. Chem. 2013, 3, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Sarma, M.P.; Wary, G. Synthesis and characterization of chemically deposited nanocrystalline PbS thin film. Adv. Sci. Lett. 2016, 22, 3755–3758. [Google Scholar] [CrossRef]
- Patil, A.M.; Lokhande, A.C.; Chodankar, N.R.; Kumbhar, V.S.; Lokhande, C.D. Engineered morphologies of β-NiS thin films via anionic exchange process and their supercapacitive performance. Mater. Des. 2016, 97, 407–416. [Google Scholar] [CrossRef]
- Gospodinova, N.; Ivanov, D.A.; Anokhin, D.V.; Mihai, I.; Vidal, L.; Brun, S.; Romanova, J.; Tadjer, A. Unprecedented Route to Ordered Polyaniline: Direct Synthesis of Highly Crystalline Fibrillar Films with Strong p-p Stacking Alignment. Macromol. Rapid Commun. 2009, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Elmacı, G.; Ertürk, A.S.; Sevim, M.; Metin, Ö. MnO2 nanowires anchored on mesoporous graphitic carbon nitride (MnO2@mpg-C3N4) as a highly efficient electrocatalyst for the oxygen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 17995–18000. [Google Scholar] [CrossRef]
- Selvakumar, K.; Kumar, S.M.S.; Thangamuthu, R.; Ganesan, K.; Murugan, P.; Rajput, P.; Jha, S.N.; Bhattacharyya, D. Physiochemical Investigation of Shape-Designed MnO2 Nanostructures and Their Influence on Oxygen Reduction Reaction Activity in Alkaline Solution. J. Phys. Chem. C 2015, 119, 6604–6618. [Google Scholar] [CrossRef]
- Umek, P.; Korošec, R.C.; Gloter, A.; Pirnat, U. The control of the diameter and length of α-MnO2 nanorods by regulation of reaction parameters and their thermogravimetric properties. Mater. Res. Bull. 2011, 46, 278–284. [Google Scholar] [CrossRef]
- Rana, U.; Chakrabarti, K.; Malik, S. Benzene tetracarboxylic acid doped polyaniline nanostructures: Morphological, spectroscopic and electrical characterization. J. Mater. Chem. 2012, 22, 15665–15671. [Google Scholar] [CrossRef]
- Ansari, M.O.; Mohammad, F. Thermal Stability of HCl-Doped-Polyaniline and TiO2 Nanoparticles-Based Nanocomposites. J. Appl. Polym. Sci. 2012, 124, 4433–4442. [Google Scholar] [CrossRef]
- Ansari, M.O.; Yadav, S.K.; Cho, M.H.; Mohammad, F. Thermal stability in terms of DC electrical conductivity retention and the efficacy of mixing technique in the preparation of nanocomposites of graphene/polyaniline over the carbon nanotubes/Polyaniline. Compos. Part B 2013, 47, 155–161. [Google Scholar] [CrossRef]
- Yang, J.; Duan, X.; Guo, W.; Li, D.; Zhang, H.; Zheng, W. Electrochemical performances investigation of NiS/rGO composite as electrode material for supercapacitors. Nano Energy 2014, 5, 74–81. [Google Scholar] [CrossRef]
- Jadhav, S.K.A.; Dhas, S.K.D.; Patil, K.T.; Moholkar, A.V.; Patil, P.S. Polyaniline (PANI)-manganese dioxide (MnO2) nanocomposites as efficient electrode materials for supercapacitors. Chem. Phys. Lett. 2021, 778, 138764. [Google Scholar] [CrossRef]
- Meng, F.; Yan, X.; Zhu, Y.; Si, P. Controllable synthesis of MnO2/polyaniline nanocomposite and its electrochemical capacitive property. Nanoscale Res. Lett. 2013, 8, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; He, Y.; Pavlinek, V.; Cheng, Q.; Sahab, P.; Li, C. MnO2 nanoflake/polyaniline nanorod hybrid nanostructures on graphene paper for high performance flexible supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 17165. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Liu, Y.; Zhang, L.; Kan, E.; Zhang, S.; Tang, J.; Tang, W. MnO2 Nanorods Intercalating Graphene Oxide/Polyaniline Ternary Composites for Robust High-Performance Supercapacitors. Sci. Rep. 2014, 4, 4824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ji, L.; Zhang, S.; Yang, W. Synthesis of a novel polyaniline-intercalated layered manganese oxide nanocomposite as electrode material for electrochemical capacitor. J. Power Sources 2007, 173, 1017–1023. [Google Scholar] [CrossRef]
- Chen, L.; Song, Z.; Liu, G.; Qiu, J.; Yu, C.; Qin, J.; Ma, L.; Tian, F.; Liu, W. Synthesis and electrochemical performance of polyaniline–MnO2 nanowire composites for supercapacitors. J. Phys. Chem. Solids 2013, 74, 360–365. [Google Scholar] [CrossRef]
- Zhao, X.; He, D.; You, B. Laser engraving and punching of graphene films as flexible all-solid-state planar micro-supercapacitor electrodes. Mater. Today Sustain. 2022, 17, 100096. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, J.; Zhang, Q.; You, B.; Chen, G. Three dimensional Ni foam-supported graphene oxide for binder-free pseudocapacitor. Electrochim. Acta 2015, 152, 216–221. [Google Scholar] [CrossRef]
- You, B.; Li, N.; Zhu, H.; Zhu, X.; Yang, J. Graphene Oxide-Dispersed Pristine CNTs Support for MnO2 Nanorods as High Performance Supercapacitor Electrodes. ChemSusChem 2013, 6, 474–480. [Google Scholar] [CrossRef]






| Sample | 2θ | (hkl) | FWHM (°) | Crystalline Size (nm) |
|---|---|---|---|---|
| PMO-1 | 37.07 | (121) | 0.1597 | 1.73 |
| PMO-2 | 37.19 | (121) | 0.1382 | 2.00 |
| PMO-3 | 37.07 | (121) | 0.1603 | 1.72 |
| Electrode | Synthesis | Electrolyte | Specific Capacitance at Current Density | Retention% | Ref. |
|---|---|---|---|---|---|
| MnO2-PANI | Coating and grafting | 1 M H2SO4 | 407 F g−1 at 0.5 mA cm−2 | 96.4% after 2000 cycles | 1 |
| PAni-MnO2 composites | Chemical oxidative polymerization | 0.5 M Na2SO4 | 242 F g−1 at 0.1 A g−1 | 99% after 1000 cycles | 16 |
| γ-MnO2/PANI | In situ polymerization | 1 M H2SO4 | 232 F g−1 at 1 A g−1 | 78.6% after 3000 cycles | 17 |
| MnO2/PANI | One-step interfacial polymerization | HClO4 | 168 F g−1 at 0.2 mA cm−2 | 95% after 1000 | 4 |
| RGO/MnO2/PANI | Electrodeposition and chemical oxidative polymerization | 1.0 M Na2SO4 | 636.5 F g−1 at 1.0 A g−1 | 85% after 10,000 cycles | 5 |
| MnO2-PANI-GO | Two step coating method | 1.0 M Na2SO4 | 522 F g−1 at 0.25 A g−1 | 97% after 5100 cycles | 6 |
| fibrous Pani–MnO2 | In situ chemical oxidative method | 0.5 M H2SO4 | 525 F g−1 at a current density of 2 A g−1 | 76.9% after 1000 cycles | 19 |
| PANI-MnO2 | Exchange reaction method | 0.1 M Na2SO4 | 330 F g−1 at 1 A g−1 | 94% after 1000 cycles | 8 |
| Pani-MnO2 nanowire | In situ chemical oxidative method | 1 M KOH | 256 F g−1 at 1 A g−1 | -- | 20 |
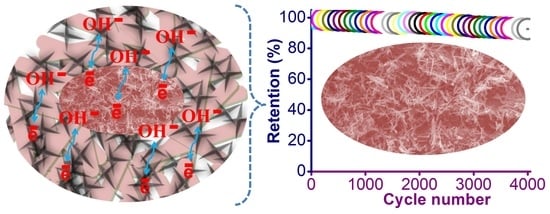
| MnO2/polyaniline nano-hybrids | Hydrothermal and chemical oxidative polymerization | 3 M KOH | 522 F g−1 at 1 A g−1 | 91% after 4000 cycles | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleithan, S.H.; Ansari, S.A.; Perdana, M.Y.; Alam, K.; Alhashem, Z.; Al-Amer, K. The Controllable Ratio of the Polyaniline-Needle-Shaped Manganese Dioxide for the High-Performance Supercapacitor Application. Nanomaterials 2023, 13, 101. https://doi.org/10.3390/nano13010101
Aleithan SH, Ansari SA, Perdana MY, Alam K, Alhashem Z, Al-Amer K. The Controllable Ratio of the Polyaniline-Needle-Shaped Manganese Dioxide for the High-Performance Supercapacitor Application. Nanomaterials. 2023; 13(1):101. https://doi.org/10.3390/nano13010101
Chicago/Turabian StyleAleithan, Shrouq H., Sajid Ali Ansari, Muhamad Yudatama Perdana, Khan Alam, Zakia Alhashem, and Kawther Al-Amer. 2023. "The Controllable Ratio of the Polyaniline-Needle-Shaped Manganese Dioxide for the High-Performance Supercapacitor Application" Nanomaterials 13, no. 1: 101. https://doi.org/10.3390/nano13010101
APA StyleAleithan, S. H., Ansari, S. A., Perdana, M. Y., Alam, K., Alhashem, Z., & Al-Amer, K. (2023). The Controllable Ratio of the Polyaniline-Needle-Shaped Manganese Dioxide for the High-Performance Supercapacitor Application. Nanomaterials, 13(1), 101. https://doi.org/10.3390/nano13010101