Abstract
Due to its environmental cleanliness and high energy density, hydrogen has been deemed as a promising alternative to traditional fossil fuels. Photocatalytic water-splitting using semiconductor materials is a good prospect for hydrogen production in terms of renewable solar energy utilization. In recent years, halide perovskite nanocrystals (NCs) are emerging as a new class of fascinating nanomaterial for light harvesting and photocatalytic applications. This is due to their appealing optoelectronic properties, such as optimal band gaps, high absorption coefficient, high carrier mobility, long carrier diffusion length, etc. In this review, recent progress in halide perovskite NCs for photocatalytic hydrogen evolution is summarized. Emphasis is given to the current strategies that enhance the photocatalytic hydrogen production performance of halide perovskite NCs. Some scientific challenges and perspectives for halide perovskite photocatalysts are also proposed and discussed. It is anticipated that this review will provide valuable references for the future development of halide perovskite-based photocatalysts used in highly efficient hydrogen evolution.
1. Introduction
With the rapid growth of human consumption, the world is faced with surging energy demands amidst the quick depletion of fossil fuels and severe natural environmental issues. To alleviate the threat of energy crisis and environmental deterioration, it is urgent to seek more eco-friendly and sustainable renewable energy sources. As a carbon-free and clean energy source, hydrogen has been deemed as a promising future energy source to replace traditional fossil fuels [1,2,3]. Among various approaches to hydrogen generation, photocatalytic water splitting utilizing abundant solar energy and semiconductor materials has been regarded as one of the most attractive routes. In the photocatalysis field, the rational design and fabrication of advanced photocatalysts with ideal solar-to-hydrogen (STH) energy conversion efficiency is the most critical aspect [4,5]. Since the pioneering work of photoelectrochemical water splitting on TiO2 electrodes by Fujishima and Honda [6], the field of hydrogen production from water splitting has been systematically investigated, which includes exploring the basic photocatalytic mechanism, developing novel photocatalytic materials, and designing efficient photocatalytic systems. Generally, the fundamental working principle of photocatalysis includes the absorption of light energy by semiconductors to create electron-hole pairs, which then migrate to the semiconductor’s surface to initiate redox reactions. Thus, the separated electron-hole pairs play crucial roles in the photocatalytic redox reaction. To date, various kinds of semiconductor photocatalysts have been developed for hydrogen generation, including oxides (CeO2, WO3, Ga2O3, etc.) [7,8,9,10,11,12,13,14,15,16,17], sulfides (CdS, MoS2, ZnS, etc.) [18,19,20,21,22,23,24,25,26,27], C3N4 [28,29,30,31,32,33], etc. However, conventional semiconductor photocatalysts still have a low STH energy conversion efficiency that is far from satisfactory due to their wide bandgaps and high electron-hole recombination rates.
Recently, halide perovskite materials—on account of their fascinating electronic and optical properties, including outstanding visible light harvesting ability, suitable band positions to provide sufficient driving potential, as well as their high carrier mobility and long electron-hole diffusion lengths—have emerged as a class of promising candidates for photocatalytic applications. To date, various kinds of halide perovskites, in either an organic–inorganic or all-inorganic fashion (e.g., CH3NH3PbX3, CsPbX3, X = Cl, Br, I), have shown great potential in photocatalysis fields such as CO2 reduction [34,35,36,37,38,39,40], hydrogen generation [41,42,43,44,45], pollutant degradation [46,47,48,49,50,51], phemethylol oxidation [52,53], organic reaction [54,55], etc. Since the first demonstration of using methylammonium lead iodide (CH3NH3PbI3, MAPbI3) for hydrogen generation via the solar-driven splitting of hydrogen iodide by Park et al. [44], the potential of halide perovskites for photocatalytic hydrogen production has been investigated by many researchers. In virtue of the advantages of halide perovskites mentioned above, such as the excellent light-absorption ability and suitable band positions, the applications of these materials in photocatalytic hydrogen production begin to flourish and exhibit outstanding photocatalytic performances.
In this review, we summarize the recent progress made in using halide perovskites for solar-driven photocatalytic hydrogen production. To date, numerous review articles have summarized recent advances in halide perovskites applied in solar energy conversion. In contrast to previous reviews, this review focuses exclusively on the application of halide perovskites in photocatalytic hydrogen production. Firstly, we introduced the property and photocatalytic mechanism of halide perovskites, and highlighted strategies used for enhancing the photocatalytic hydrogen production performance of these materials. Finally, we concluded by introducing a perspective on the future challenges and opportunities of this field, which could provide guidelines for further research on halide perovskite-based photocatalysis applications.
2. Properties of Halide Perovskites
2.1. The Composition of Halide Perovskites
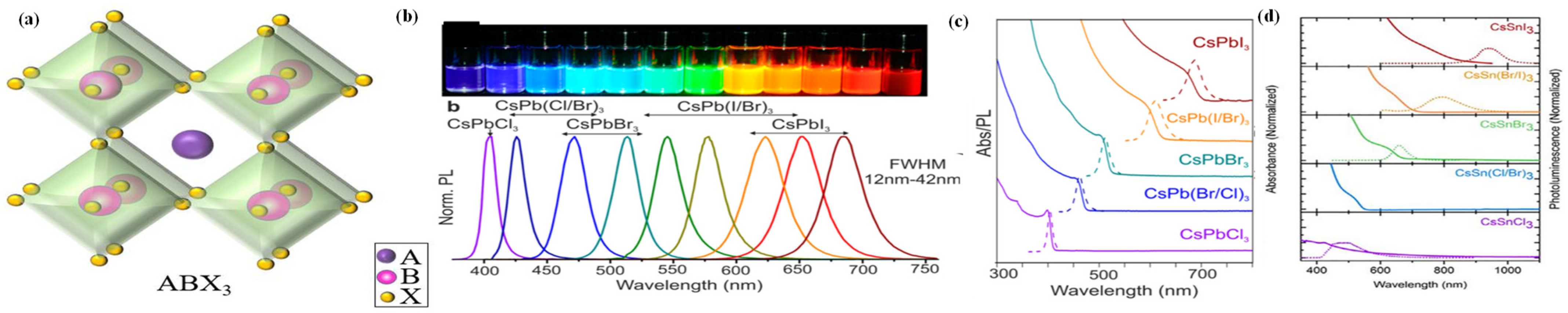
Halide perovskite materials have a general structural formula of ABX3 (Figure 1a), where A is usually a monovalent cation (Cs+, methylammonium (MA+), or formamidine (FA+)), B is a divalent metal ion (Pb2+, Sn2+, or Ge2+), and X is the halogen anion, namely I−, Br−, or Cl− [56,57]. The B cation coordinates with six halogen anions to form [BX6]4− octahedra. The large monovalent A cation behaves like a total charge neutralizer, which is bridged to a network of corner-sharing BX6 octahedrons, forming the ideal perovskite structure. The A-, B-, and C-site ions can be substituted isomorphically by other similar ions, thus altering the defect properties, electronic structure, and catalytic performance of the material [58,59,60,61]. Both the B and X ions play an important part in governing the band structure of halide perovskites that affects their catalytic performance, while the function of the A cation is ignorable. Although the A cation generally does not construct the energy level, its size plays a crucial role in judging the formability of the perovskite structure, since either a smaller or larger A cation could lead to either a contraction or expansion of the perovskite lattice. Usually, perovskites exhibit a cubic structure (space group: Pm3m), which can transform into orthorhombic (space group: Pnma) or tetragonal (space group: I4/mcm) phase when the temperature decreases [62,63,64]. As observed in the crystal structure, the B-site cation and the anion are tightly bound, while the A-site cation and the anion have a weak interaction. The BX6 octahedral structure can be distorted by the difference between the electronegativities and ionic radii of the A and B cations, leading to a weakened symmetry of the crystal structure. It has been proved that the tilt angle can affect the electronic band structure, photoluminescence, dielectric, and the charge transport properties of the perovskites [65,66,67]. Various types of perovskite crystals with desirable characteristics can be designed by adjusting these interactions via placing different types of cations at corresponding lattice sites [68,69].
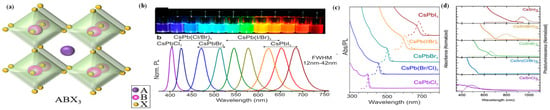
Figure 1.
(a) Schematic illustration of the crystal structure of halide perovskite. (b) Colloidal halide perovskite CsPbX3 NCs (X = Cl, Br, and I) exhibit size- and composition-tunable bandgap energies covering the entire visible spectral region with narrow and bright emission. (c) Typical optical absorption and PL spectra of CsPbX3 NCs. (d) Typical optical absorption and PL spectra of CsSnX3 NCs. (b,c) Reproduced with permission [70]. Copyright: 2015, American Chemical Society. (d) Reproduced with permission [71]. Copyright: 2016, American Chemical Society.
2.2. Optoelectronic Properties of Halide Perovskites
Understanding the composition, crystal structure, and the electronic band structure of the perovskite is of vital importance because these pivotal factors are intercorrelated in judging its potentiality for satisfactory photocatalytic performances. The energy band strucure of halide perovskites consists of an antibonding valence band maximum (VBM) and an antibonding conduction band minimum (CBM) system, rendering this crystal with high defect tolerance. Applying DFT calculations, the VBM of perovskites is composed of an antibonding hybrid state between the 6s orbital of B and the np orbital of X (n = 3, 4, and 5 for Cl, Br, and I, respectively), with the np orbital of X as the major contributor, whereas the CBM is formed from an antibonding hybrid state between 6p orbitals of B and np orbitals of X, with 6p orbitals of B as the significant contributor [72,73]. Taking cubic CsPbBr3 QDs as an example, its CB and VB positions are determined based on the 6p orbital of Pb and the 4p orbital of Br, respectively, whereas Cs has negligible effect on the energy band edge [74]. Since the A-site cations exhibit no significant effect on the VBM or CBM [75], the bandgap tuning can be easily realized by mixing or exchanging the halogen anions. For example, by altering the ratio of different halide ions, Sargent and his co-workers successfully tuned the bandgap of MAPbI3 [76]. When the iodide concentration was increased, the absorption and emission spectra of the CsPbBr3−xIx perovskite film red-shifted to longer wavelength, and its bandgap became narrower [77]. The tunable bandgap affords a good platform to modulate the energy band edge of halide perovskites toward highly efficient photocatalytic performance for various applications. For instance, Guo et al. reported that the band edge positions of CsPb(Brx/Cl1−x)3 could be tuned by regulating the ratio of Br and Cl [78], and the photocatalytic activity toward CO2 reduction is significantly enhanced.
Generally, halide perovskites are considered as direct bandgap semiconductors. For MAPbI3, the spin–orbit coupling leads to Rashba splitting of the conduction band, resulting in a weak indirect bandgap of 60 meV during the direct bandgap transition [79]. Halide perovskites exhibit photo-absorption in almost the entire visible region, indicating that charge carriers can be effectively produced upon low energy excitation, which is favourable to photocatalytic applications [80,81]. A practical strategy to realize spectral absorption diversity and bandgap tuning with perovskites is adopting mixed halides. For example, by only altering the halogen element at the X-site from Cl to Br to I, Protesescu et al. found a redshift of the emission wavelength of CsPbX3 (X = Cl, Br, or I) from 410 nm to 512 nm and 700 nm, and to any other intermediate wavelengths within the visible spectral range using mixed halide ions (Figure 1b,c) [70]. However, as the halogen composition changes, the valence band edge moves by a relatively wide margin within the energy levels, while the conduction band edge exhibits little change. For the MAPbX3 perovskite, when X was Cl to I, the emission wavelength shifted from 403 to 740 nm [82].
In addition, the B-site cation also has a significant effect on the optical properties of halide perovskites. Inevitably, the Pb element in perovskites need to be partially or completely replaced due to environmental issues. Compared to those of CsPbX3, strong red shifts of the absorption and emission spectra were observed for CsSnX3, from 443 nm (X = Cl) to 953 (X = I) (Figure 1d) [71]. By compositional modulation, the bandgaps of halide perovskites can be designed and tuned within a certain range, achieving targeted energy levels. In addition, optical properties of halide perovskites can be enhanced by a metal ion doping strategy. For example, by doping Cu2+ and Sb3+ into the three octahedral layers, the layered double perovskite Cs4CuSb2Cl12 was obtained. It has a direct band gap of 1.0 eV, and an electrical conductivity one order of magnitude higher than that of MAPbI3 [83]. In addition, the partial replacement of Pb by Mn may cause a strong Stokes shift in the emission, which can increase the utilization rate of sunlight [84]. However, the incorporation of cations (Cd2+, Al2+, and Zn2+) into the halide perovskite can cause shrinkage of the original lattice, resulting in a wider bandgap, blueshift of the absorption peak, and weaker absorption ability [85,86,87].
3. Applications of Halide Perovskites in Photocatalytic Hydrogen Evolution
3.1. Basic Principle of Photocatalysis with Halide Perovskites
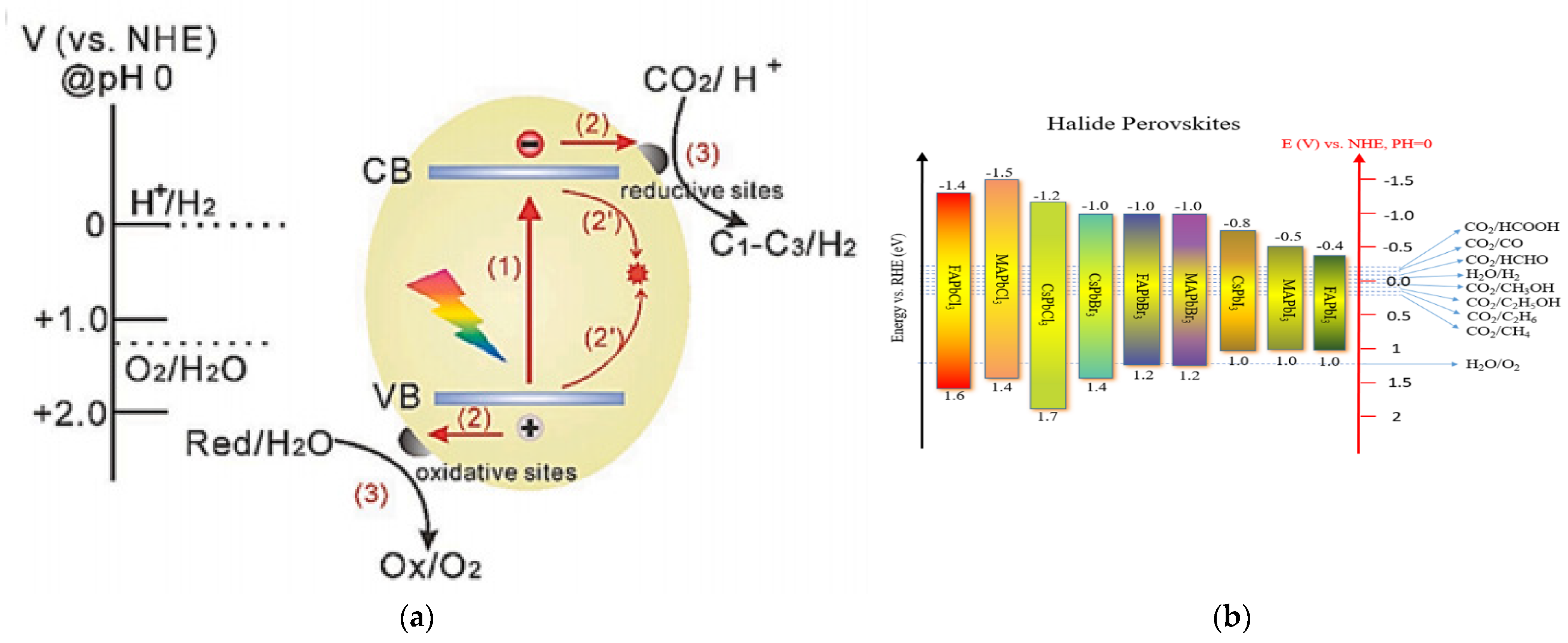
Photocatalytic redox reactions driven by semiconductor materials usually involve identically essential steps (Figure 2a) [88]: (1) generation of electron-hole pairs by the light harvesting of the photocatalyst, (2) transfer of photogenerated electrons and holes to the surface of the photocatalyst, and (3) photogenerated charge carriers participate in redox reactions. In order to drive the water-splitting redox reactions, the VB edge of the semiconductor should be more positive than the oxidation potential of H2O to O2 (1.23 V vs. normal hydrogen electrode [NHE], pH = 0), while the CB edge of the semiconductor should be more negative than the reduction potential of H+ to H2 (0 V vs. NHE, pH = 0) [89]. So, theoretically, the minimum bandgap required for water splitting is 1.23 eV. However, considering the overpotential associated with the water-splitting redox reactions, the bandgap to drive efficient overall water splitting must be further widened, usually to 1.8–2.0 eV [90,91,92]. The relative positions of CB and VB potentials for most halide perovskites are shown in Figure 2b, along with the redox potentials of photocatalytic half-reactions associated with water splitting, CO2 reduction, etc. Apparently, the CB potentials of most halide perovskites are more negative than the reduction potential of H+ to H2, meeting the thermodynamic requirements for reducing water. In other words, the relative CB positions of halide perovskites are usually sufficient for H2 production, exhibiting excellent reduction abilities. Theoretically, some members of halide perovskites (such as all-inorganic CsPbBr3) can also oxidize water to produce O2 because their VB potentials are relatively positive.
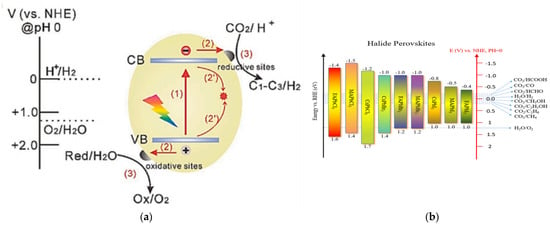
Figure 2.
(a) Schematic illustration of charge transfer reactions that may occur at the surface and in the bulk of a semiconductor photocatalyst. (b) Energy levels of halide perovskites with the relative potential in photocatalytic applications. (a) Reproduced with permission [88]. Copyright: 2020, Wiley.
In addition, factors like the molar extinction coefficient, charge carrier recombination, and defect state of a halide perovskite should also be taken into account for photocatalytic applications [93,94,95]. A high molar extinction coefficient (ε) is essential for efficient absorption of visible light and generation of excitons [96]. The ε values of colloidal perovskites, ranging from about 105 to 107 L mol−1 cm−1, are comparable to those of a-Si: H and GaAs, and an order of magnitude higher than that of c-Si, which are representative photovoltaic materials [97,98]. This is indicative of better visible light responses for halide perovskites, thus improving photon-carrier conversion efficiencies [99]. However, the ε value of a halide perovskite is also dependent on the size of the crystals. This is especially important in the nanometer size range due to the quantification effect, which needs to be taken into account for practical applications in optoelectronics [100].
Carrier diffusion length is also important for the photocatalytic applications of halide perovskites. Generally, a longer carrier diffusion length indicates a lower recombination rate. The carrier diffusion lengths of halide perovskites have been increased by various strategies. For example, Dong et al. used a solution–growth method to obtain MAPbI3 single crystals with a high carrier diffusion length exceeding 175 μm, which could be attributed to the long lifetime, high carrier mobility, and small trap densities of the single crystals [101], whereas the polycrystalline MAPbI3 has a charge carrier diffusion of only ca. 100 nm. By incorporating Cl− into MAPbI3−xClx, Zhang et al. obtained a perovskite with a carrier diffusion length of up to 380 μm [102]. This is because the incorporation of Cl− can increase the density of trap states, thus creating the medium for carrier transfer and reducing the VB, which play a dominate role in charge recombination. As a consequence, maximum values of the carrier diffusion lengths were reached for the MAPbI3−xClx single crystals with the optimum Cl content (x = 0.005). Owing to the enhanced utilization of photogenerated charge carriers, it is expected that the long carrier diffusion length of halide perovskites would significantly contribute to their catalytic activity.
In addition, the photogenerated charge transfer kinetics in halide perovskites are also vital for the design of highly efficient photocatalysts. Excitons and free carriers are produced rapidly under light excitation, with free carriers as the main light-excited species. In most cases, excitons are also rapidly decomposed into free carriers. For instance, the excitons in CsPbBr3 NCs are quickly converted into free carriers after 4–5 ps [103]. These hot carriers relax to the energy band edge via carrier–phonon and carrier–carrier interactions within fs. The larger the size of halide perovskite nanocrystals is, the faster the cooling kinetics of hot carriers is [104]. As the lifetime of a hot carrier in perovskites increases, the carrier becomes relatively easy to extract. Radiative recombination of cooled carriers takes place within ns at the band edge. For example, the capture time of non-radiative carriers in CsPbBr3 NCs is about 40–50 ps [105]. On account of the defect tolerance features of halide perovskites, the energies of carriers in the defects are similar to those of edge carriers, which implies that more high-energy carriers will make potential contributions to photocatalytic reactions. For CsPbBr3, the carriers can be extracted by electron-hole acceptors within ps, indicating that the extracted carriers can be potentially applied in photocatalysis [106]. Therefore, the ideal energy levels, along with the unique charge transfer kinetics, make halide perovskites good candidates for photocatalytic applications [107]. To sum up, halide perovskites provide distinct advantages for photocatalysis: (1) targeted electronic structures can be designed by altering the A, B, and X-site elements in the crystal structure, so that other physical properties can be tailored, such as stability, light absorption, and charge migration [108]; (2) the unique energy band structures endow halide perovskites with suitable band edge positions to drive a broad range of photocatalytic reactions [109]; (3) the long carrier diffusion lengths and high charge mobility also render halide perovskites as promising candidates for the design of high-performance photocatalysts.
3.2. Halide Perovskites for Hydrogen Evolution
3.2.1. Pristine Halide Perovskites or Solid Solutions
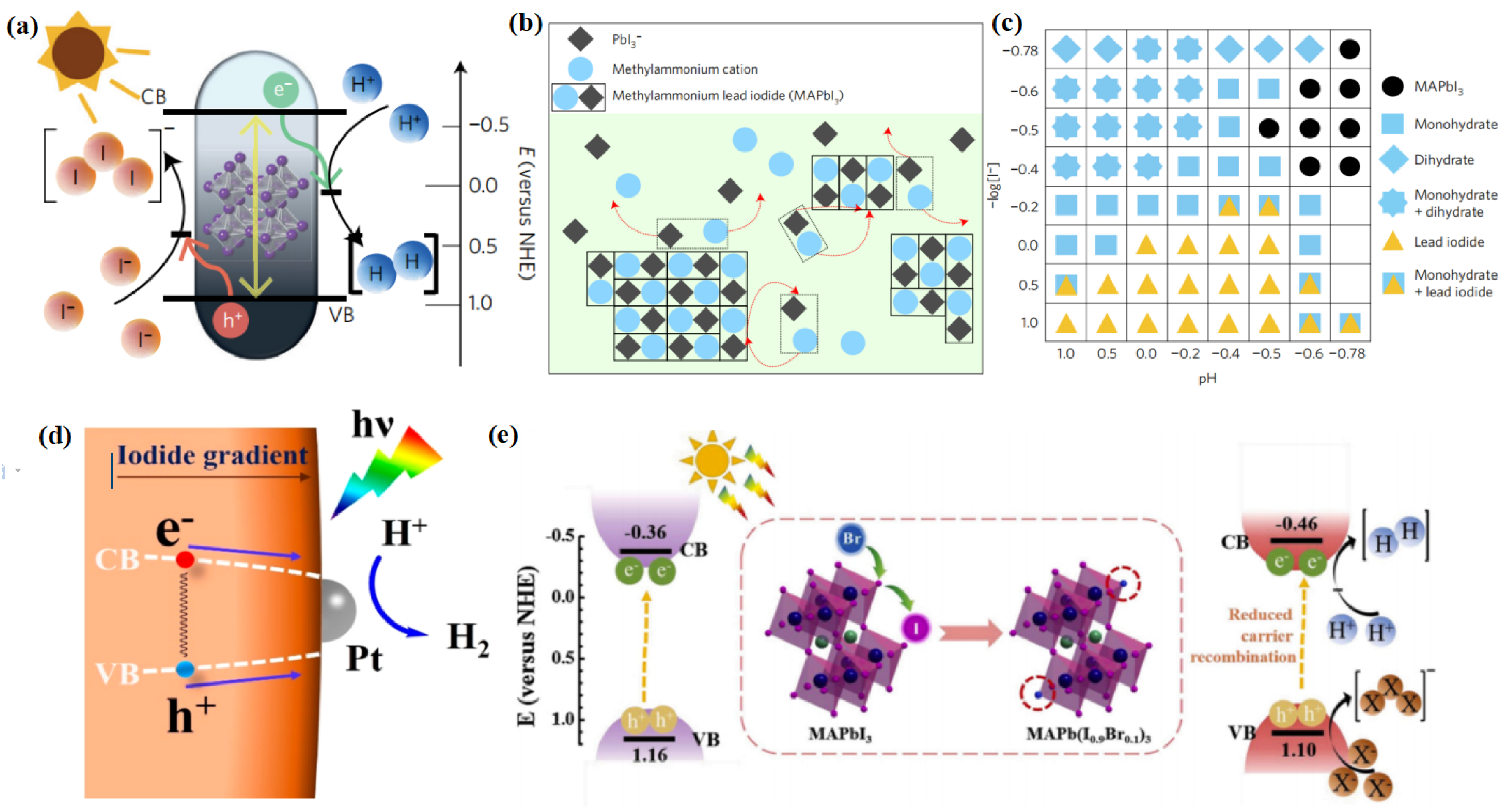
As discussed above, the unique optoelectronic properties of halide perovskites are favourable to their application in photocatalytic reactions, including hydrogen evolution. The first milestone for photocatalytic hydrogen evolution using halide perovskites was reported by Park et al. [44]. As is known, most halide perovskites are unstable in polar solvents, especially water [87]. In order to conquer this, Park and his coworkers employed the dynamically balanced HI solution as the reaction medium, which can maintain the stability of MAPbI3 (Figure 3a). MAPbI3 is regarded as an ionic crystal consisting of MA+ and PbI3−, which can be precipitated in saturated solutions. Hence, when MAPbI3 precipitates are dissolved in saturated solution, they can be decomposed into MA+ and PbI3− ions. Simultaneously, MA+ and PbI3− ions were reprecipitated into crystals at the same rate. In this way, the MAPbI3 powder could maintain stability in aqueous HI solution ((Figure 3b). They also found out that different phases of MAPbI3 can exist, depending on the concentrations of H+ and I−. However, only under the specific conditions of [I−] ≤ [H+], pH < −0.5, and −log [I−] < −0.4 can MAPbI3 remain stable (Figure 3c). This pioneering work has opened up a way for using halide perovskites in photocatalytic fields. Afterward, Wang and his group elucidated the reaction mechanism of photocatalytic hydrogen evolution using MAPbI3 [45]. In this reaction, MAPbI3 played dual roles as a visible light photoabsorber and as a catalyst reductant. Meanwhile, both the Pb atoms and surface organic molecules participated in the reaction. First, an intermediate state of Pb—H was formed by the interaction between one H atom dissociated from an MA+ ion and Pb. Subsequently, H2 was produced by the reaction of the Pb—H intermediate state with another H atom from an adjacent MA+ ion. The lost H would be replaced by protons from the solution to produce new MA+ ions through the Grotthuss mechanism.
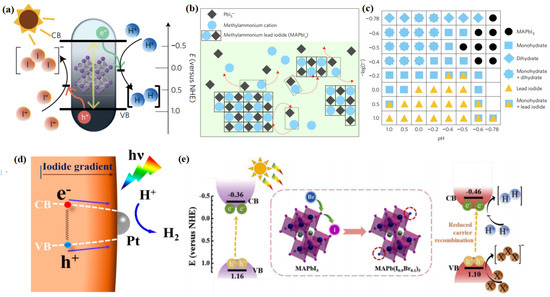
Figure 3.
(a) Schematic energy band structure of MAPbI3 powder for the photocatalytic HI splitting reaction. (b) Schematic illustration of the MAPbI3 powder in dynamic equilibrium in saturated HI solution. The red color arrows represent dissociation and reprecipitation of MAPbI3 crystal and ions. (c) Constructed phase map as a function of [I−] and [H+]. Each symbol represents the stable precipitate phases in saturated solutions at each [I−] and [H+] concentration. Main peaks of precipitated powder are not indexed under some conditions, expressed as empty boxes. (d) Promoted charge separation and enhanced photocatalytic H2 evolution by formation of a bandgap funnel structure of MAPbBr3−xIx near the surface. (e) Schematic band diagram of MAPbI3 and MAPb(I1−xBrx)3 (x = 0.10) crystal for photocatalytic HI splitting reaction. (a–c) Reproduced with permission [44]. Copyright: 2016, Nature Publishing Group. (d) Reproduced with permission [110]. Copyright: 2018, American Chemical Society. (e) Reproduced with permission [111]. Copyright: 2019, Elsevier.
As mentioned above, the CBM and VBM potentials of halide perovskites can be modified by modulating their compositions, which makes them suitable for gradient photocatalysis. By tuning the iodide concentration gradient, Huang et al. synthesized a mixed halide perovskite material (MAPbBr3−xIx) with a funnel-like bandgap structure [110]. The CBM becomes more positive with the increase of I concentration from the interior to the surface, whereas the VBM becomes more negative (Figure 3d). In this way, a smooth funnel is constructed, which promoted the charge transfer from the inside to the surface. As a consequence, this specially designed photocatalyst exhibited a H2 generation rate of 255.3 μmol h−1. When Pt was further loaded on the surface of the perovskite, the photogenerated electrons on the perovskite surface transferred rapidly to the Pt particles, which further increased the H2 generation rate to 651.2 μmol h−1. In a similar fashion, Huang’s group also constructed a bandgap funnel-structured CsPbBr3−xIx mixed halide perovskite via the graded distribution of iodide [112]. The obtained CsPbBr3−xIx/Pt photocatalysts exhibited a H2 evolution rate of 1120 μmol g−1 h−1 under visible light irradiation, along with a high stability during the 50 h of the photocatalytic experiment.
Considering the stability issues of halide perovskites, photocatalytic hydrogen generation using these materials are often conducted in HX (X = Cl, Br, or I) solution instead of direct water splitting [113]. Water splitting is a four-electron reaction, while the reduction of HI involves two electrons. When electrons drive the H2 production reaction, I3− is generated during the photocatalytic process, which will darken the reaction medium gradually. As a consequence, the light absorption of the photocatalyst will be interfered. This can be overcome by the addition of hypophosphorus acid (H3PO2) as a chemical stabilizer, which can maintain the concentration of I− and reduce the I3− ions [110]. Doping Br ions have also proved an effective way to enhance the photocatalytic HI splitting activity of MAPbI3 [111]. The resultant MAPb(I1−xBrx)3 perovskite exhibited a high H2 evolution of 1471 μmol h−1 g−1 even without a Pt cocatalyst. This is because the addition of Br ions can tune the band structure of perovskite, with a negative shift of the CB potential, thus enhancing the reduction capability of electrons for efficient H2 production (Figure 3e). In addition, the Br-incorporated perovskite has a lower H-Pb absorption energy, which makes it easier for H to transfer from MA+ to the Pb atom at the defect site, thus increasing the H2 evolution rate.
3.2.2. Halide Perovskite Composites
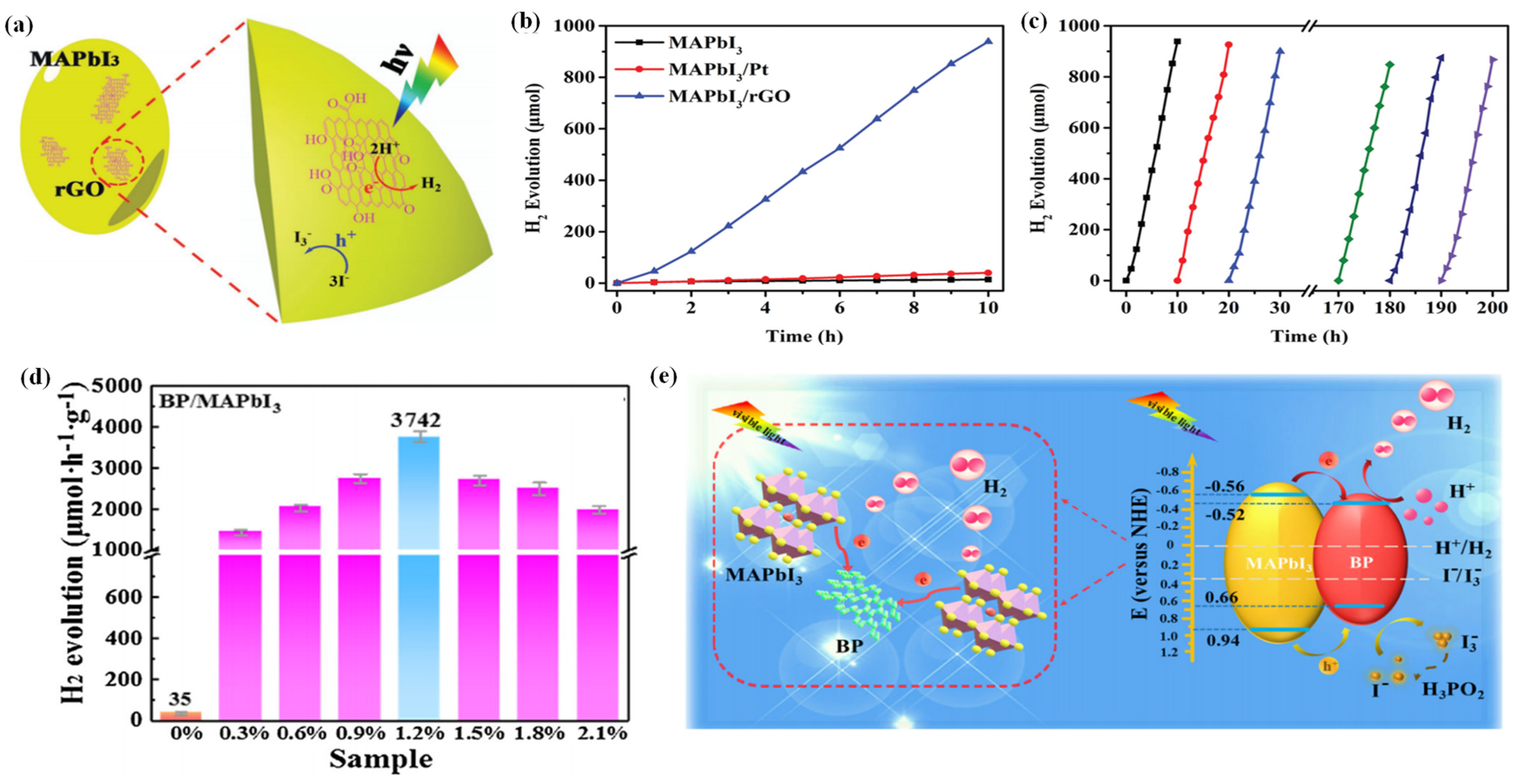
Wu et al. reported a MAPbI3/rGO composite with outstanding photocatalytic performance in aqueous HI solution (Figure 4a) [41]. It has a high H2 evolution rate of 93.9 µmol h−1 under visible light irradiation, which is 67 times and 23 times higher than that of pristine MAPbI3 and Pt-loaded MAPbI3, respectively (Figure 4b). The remarkable performance could be attributed to the introduction of rGO, which possesses good charge transport ability and facilitates charge transfer. The electrons that transfer from MAPbI3 to rGO then reduce protons to H2, resulting in excellent photocatalytic activity of the MAPbI3/rGO composite. Moreover, the composite is extremely stable, with no significant decrease of the H2 evolution activity after 200 h of cyclic experiments (Figure 4c). As confirmed by XRD, the recycled photocatalyst showed no change or failure in structure. That was because the MAPbI3 powders and the saturated HI solution were in dynamic equilibrium. When the reaction occurred, the exposed MAPbI3 surface was restored all the time, ensuring the continuous oxidation of I− to I3− on the surface in contact with HI.
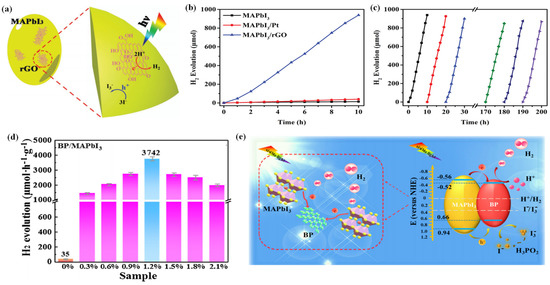
Figure 4.
(a) Schematic illustration of photocatalytic H2 evolution by MAPbI3/rGO. (b) Comparison of the H2 evolution performance of MAPbI3, MAPbI3/Pt, and MAPbI3/rGO. (c) Stability test of MAPbI3/rGO during 20 cycles of H2 evolution experiments. Lines with different colors represent different cycles. (d) Photocatalytic H2 evolution rates of BP/MAPbI3. (e) Schematic mechanism of the photogenerated charge transfer in the BP/MAPbI3 composite under visible light irradiation. (a–c) Reproduced with permission [41]. Copyright: 2018, Wiley. (d,e) Reproduced with permission [114]. Copyright: 2019, Elsevier.
Li et al. anchored a 2D few-layer black phosphorus (BP) on MAPbI3 via electrostatic coupling and fabricated a BP/MAPbI3 composite for photocatalytic hydrogen evolution [114]. The resultant BP/MAPbI3 exhibited a superb photocatalytic hydrogen evolution rate of 3742 μmol h−1 g−1 under visible light, which was far higher than that of both pure MAPbI3 and MAPbI3/Pt (Figure 4d). Moreover, the BP/MAPbI3 showed superior durability without no obvious decrease in the activity after 20 cycles. The outstanding photocatalytic activity and stability of the BP/MAPbI3 could be attributed to the broadened light harvesting, enhanced charge separation, and high chemical/optical stability of BP/MAPbI3 composite in HI solution (Figure 4e).
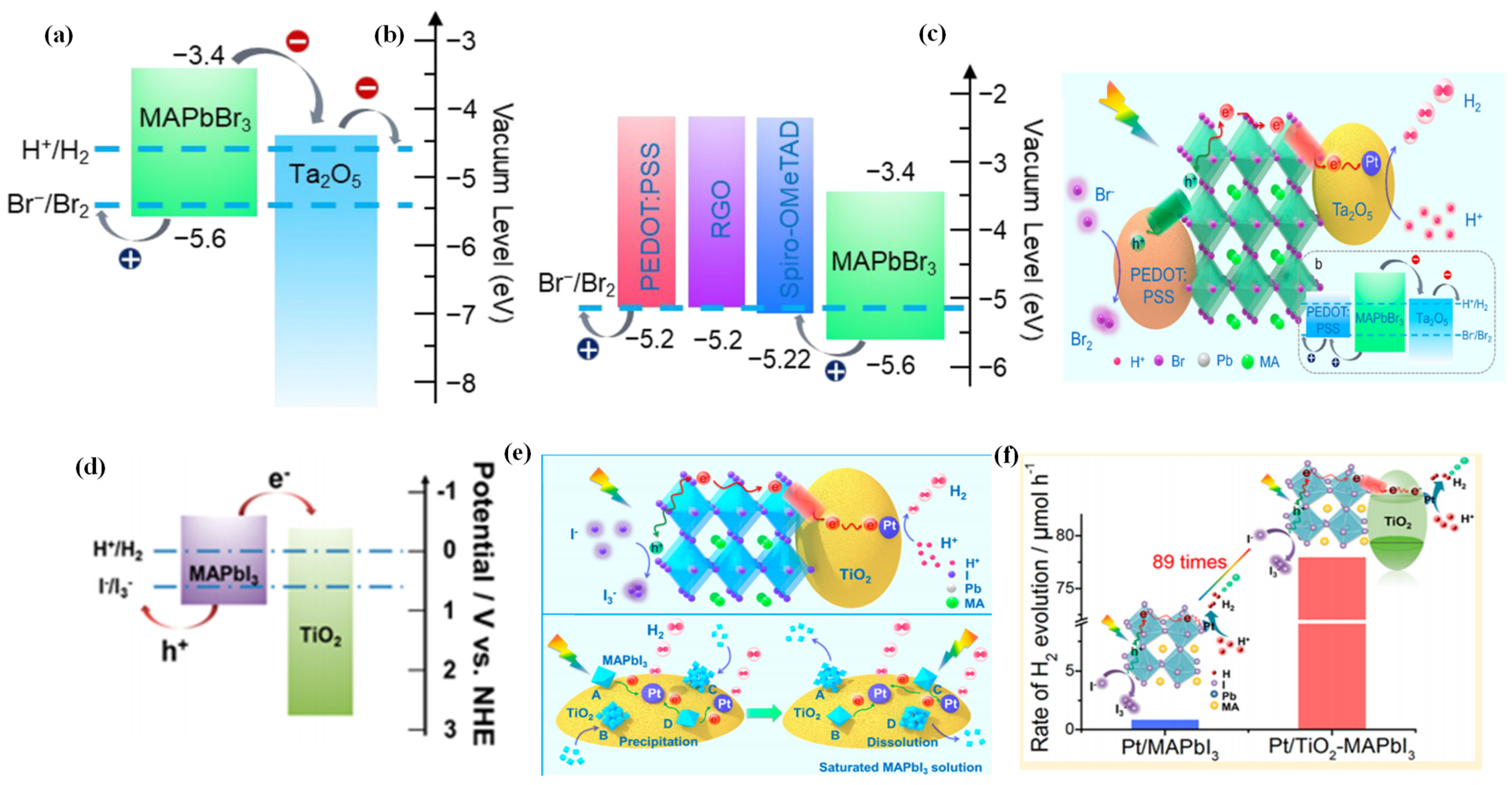
Wang et al. adopted a novel simultaneous dual-charge transportation modulation approach to improve the photocatalytic H2 evolution activity of organic–inorganic MAPbBr3 NCs [115]. They hybridized the MAPbBr3 perovskite with Pt/Ta2O5 and poly(3,-4-ethylenedioxythiophene):polystyrenesulfonate (PEDOT:PSS) nanoparticles, which acted as electron- and hole-transporting motifs, respectively. By providing new dual-charge transporting pathways, the charge separation and transportation efficiency of MAPbBr3 was significantly improved. Tantalum pentoxide (Ta2O5) was selected for its ideal conduction band edge position, which can provide an electron transport pathway to accelerate the electron transportation from MAPbBr3 (Figure 5a). Thus, Pt/Ta2O5-MAPbBr3 contributed to the increase of H2 evolution rate. PEDOT:PSS was used as an efficient hole-transporting material in the hybrid system for its more positive VBM than that of MAPbBr3, which facilitated the Br-oxidation reaction (Figure 5b). Therefore, Pt/Ta2O5-MAPbBr3-PEDOT:PSS was the most effective photocatalyst for H2 evolution (Figure 5c). The photocatalytic hydrogen evolution rate on the hybridized system was increased by ca. 52 times than that of pristine MAPbBr3, with an apparent quantum efficiency up to 16.4% at 420 nm.
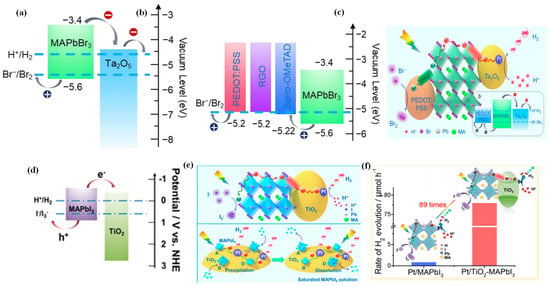
Figure 5.
(a) Schematic energy levels of MAPbBr3 and Ta2O5 and the redox potentials for HBr splitting reaction. (b) Energy level diagrams of MAPbBr3 and hole-transporting materials. (c) Schematic illustration of the reaction mechanism for MAPbBr3 with Pt/Ta2O5 and PEDOT:PSS as the electron- and hole-transporting channels, respectively. (d) Schematic diagrams of energy band of MAPbI3 and TiO2. (e) Schematic illustration of photocatalytic HI splitting for H2 evolution by Pt/TiO2-MAPbI3 hybrid system under visible light irradiation. (f) Comparison of H2 evolution activity over Pt/MAPbI3 and Pt/TiO2-MAPbI3. (a–c) Reproduced with permission [115]. Copyright: 2019, American Chemical Society. (d–f) Reproduced with permission [42]. Copyright: 2018, American Chemical Society.
Through hybridization of MAPbI3 with Pt/TiO2, Wang et al. greatly enhanced the photocatalytic hydrogen evolution rate of MAPbI3 from HI splitting [42]. Due to the suitable band alignment (Figure 5d), the TiO2 nanoparticles can act as nanoscale electron-transporting channels, which allow efficient extraction of the photogenerated electrons from MAPbI3. As illustrated in Figure 5e, the introduction of Pt/TiO2 could create dynamically existing electron-transporting channels between the MAPbI3 and Pt/TiO2, which drastically enhanced the charge transportation efficiency of MAPbI3 nanoparticles. As a consequence, the photocatalytic hydrogen evolution rate of Pt/TiO2-MAPbI3 was enhanced by ca. 89 times than that of Pt/MAPbI3 (Figure 5f).
Wang et al. employed MoS2 nanosheets as a cocatalyst to couple with MAPbI3 and fabricated a MAPbI3/MoS2 composite for photocatalytic H2 evolution [116]. Since the conduction band potential of MAPbI3 is more negative than that of MoS2, the photogenerated electrons can efficiently transfer from MAPbI3 to MoS2, which hindered the carrier recombination rates. As a result, the MAPbI3/MoS2 composite exhibited a H2 evolution rate 121 times higher than pristine MAPbI3.
3.2.3. Pb-Free Halide Perovskites
In order to overcome the toxicity of lead, Guo et al. developed an eco-friendly lead-free perovskite MA3Bi2I9 and applied it for photocatalytic H2 evolution [117]. Owing to the precipitation–solubility equilibrium reached in the system, the obtained MA3Bi2I9 exhibited excellent phase stability in HI solution. After 70 h of repeated H2 evolution, it showed no degradation or oxidization with satisfactory cycle stability. When using Pt as a cocatalyst, the H2 production rate was enhanced by 14 times compared with the pristine one, reaching 169.21 μmol g−1 h−1.
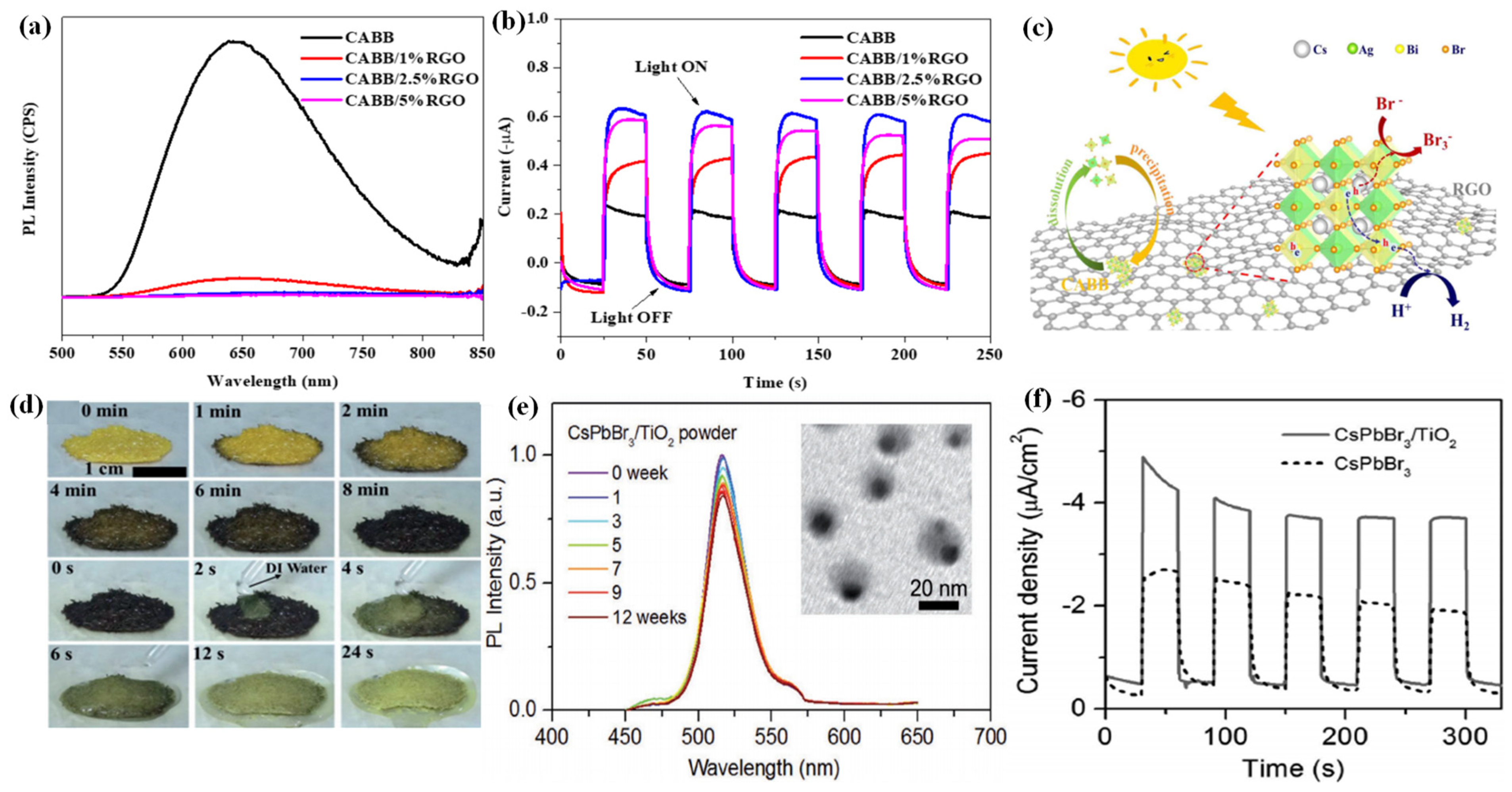
For the first time, Zhao et al. applied the Cs2AgBiBr6 (CABB) double perovskite for HBr splitting under visible light irradiation, in which RGO was introduced to extract the photogenerated electrons from CABB [118]. The resultant CABB/RGO composite exhibited a H2 evolution of 489 μmol g−1 within 10 h under visible light irradiation, which was 80 times higher than that of bare CABB. Moreover, the CABB/RGO composite with optimal RGO demonstrated ideal stability, with no significant decline in H2 evolution after 120 h continuous photocatalytic reaction. As confirmed by the photoluminescence (PL) (Figure 6a) and photoelectrochemical measurements (Figure 6b), the CABB/RGO composite exhibited suppressed charge recombination and better charge transfer ability than bare CABB. This could be attributed to the introduction of conductive RGO, which could accelerate the electron transfer from CABB through the M-O-C bonds. Subsequently, the transfered electrons reduce H+ to generate H2 at the active sites of RGO, while the holes on CABB particles oxidized Br- to produce Br3− (Figure 6c).
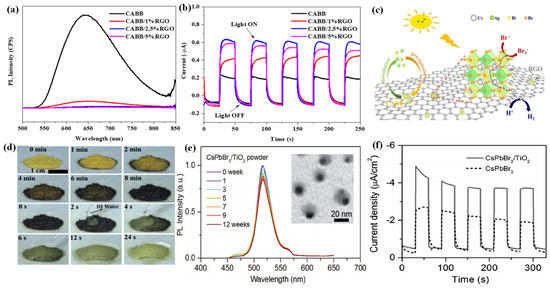
Figure 6.
(a) Steady-state PL spectra of the CABB/xRGO composites (x = 0, 1%, 2.5%, 5%). (b) Photocurrent responses of the CABB/xRGO samples (x = 0, 1%, 2.5%, 5%) recorded at 0 V vs. Ag/AgCl electrode. (c) Schematic mechanism of photocatalytic HBr splitting by CABB/RGO under visible light irradiation. (d) In situ observation of the reversible DMASnI3 transformation process at 80 °C in air. (e) The relative PL intensity of CsPbBr3/TiO2 NCs after immersing in Milli-Q water (0–12 weeks). Inset: TEM image of CsPbBr3/TiO2 NCs after immersing in Millil-Q water for 12 weeks. (f) Transient photocurrent responses of CsPbBr3 and CsPbBr3/TiO2 NCs electrodes at −0.1 V versus NHE. (a–c) Reproduced with permission [118]. Copyright: 2020, Elsevier. (d) Reproduced with permission [119]. Copyright: 2018, Wiley. (e,f) Reproduced with permission [120]. Copyright: 2018, Wiley.
3.2.4. Water Stable Halide Perovskites
In order to overcome the stability issues of most halide perovskites, Ju et al. developed a lead-free hybrid perovskite single-crystal DMASnI3 (DMA = CH3NH2CH+) with excellent water phase stability [119]. No decomposition was observed when DMASnI3 was immersed in deionized water for 16 h. Inspired by this, they applied DMASnI3 as a photocatalyst for H2 evolution in deionized water. A H2 evolution rate of 0.64 µmol h−1 was observed on the DMASnI3 crystals, accompanied by good recycling properties. Interestingly, the DMASnI3 crystals exhibited a reversible band gap narrowing behavior without phase transformation. When exposed to deionized water, the transformed samples in black can self-heal into yellow ones rapidly (Figure 6d). The narrow band gap, high stability, as well as outstanding electrical properties render DMASnI3 as a promising optoelectronic material. By the encapsulation of colloidal CsPbBr3 NCs into the TiO2 shell, Li et al. obtained nearly monodispersed CsPbBr3/TiO2 core/shell NCs with excellent water stability [120]. The size, structure, morphology, and optical properties remained identical after the CsPbBr3/TiO2 core/shell NCs were immersed in water for three months (Figure 6e), representing one of the most water-stable inorganic shell passivated perovskite NCs. Moreover, owing to the electrical conductivity of the TiO2 shell, the CsPbBr3/TiO2 core/shell NCs exhibited increased charge separation efficiency (Figure 6f), making it a potential material for optoelectronic and photocatalytic applications in aqueous phase. The photocatalytic performance of halide perovskite-based systems for hydrogen generation is summarized in Table 1.

Table 1.
Summary of photocatalytic hydrogen evolution activity of halide perovskite-based systems.
4. Conclusions and Prospects
In this review, we have introduced the recent advances made in the field of halide perovskite-based hydrogen evolution, focusing on the strategies to enhance the photocatalytic activity of these materials. Although halide perovskites have intriguing properties, their poor stabilities arising from the soft ionic crystal structures restrict their application in photocatalysis. However, via intrinsically improving the crystal stabilities of halide perovskites, several “stable” photocatalytic systems based on these kind of materials have been designed [119,120]. Moreover, by altering the external reaction conditions, such as using saturated halo acid solutions as the solvent, halide perovskites have been successfully used in photocatalytic hydrogen evolution [41,42,44,114,115,116,117,118,119,121,122,123,124,125,126,127,128]. In spite of this, there are still big challenges when applying halide perovskites in photocatalytic reactions under more common environments. On the basis of the current level of knowledge and the limitations of halide perovskites, some promising approaches to enhance the activity and stability of halide perovskite-based photocatalysis are proposed.
4.1. Improving the Long-Term Catalytic Stability of Halide Perovskites
Although conducting photocatalytic reactions in halo acid solutions have been proven effective for hydrogen evolution, such methods are not universal in nature. To limit contact between halide perovskites and the polar solvent, it is essential to explore excellent sealing technology to secure the stability of halide perovskites. There are two essential criteria to be considered when establishing this technology: one is the transparency of the sealing materials which can ensure sufficient light absorption of halide perovskites. The second criterion is the good conductivity of the sealing material that can allow the effective extraction of photo-generated carriers. At present, transparent resin (epoxy) is the most commonly used sealing material. Conductive carbon paste is also employed as the sealing agent in some studies, considering its conductive property and higher resistance towards degradation. Alternatively, some researchers encapsulate halide perovskites with electron- and hole-transport materials together to fabricate corresponding photoelectrodes for photocatalysis. In addition, halide perovskites can be separated from the polar solvent by encapsulating the perovskite layer in a solar cell structure to develop a PV–PEC reaction system. This system has the advantage of increased redox capacity of the PEC cell owing to the photovoltaic device, which allows larger voltage in series and thus supporting a wide range of applications.
4.2. Improving the H2 Generation Activity of Halide Perovskite-Based Photocatalysts
As shown in Table 1, the highest H2 evolution rate so far achieved for halide perovskite-based photocatalyst is about 13.6 mmol g−1 h−1 [126]. Although great achievements have been reached, the present H2 evolution rate of this kind of material is far from practical application. There are several strategies proposed to further improve the H2 evolution activity of halide perovskites: the first approach is the controllable synthesis of nanostructures with definite morphologies such as nanosheets, nanoplatelets to afford more exposed surfaces, and to increase the surface area and provide more active sites. Another option is the combination of single-atom catalysis with halide perovskites. Owing to the rapid increase of surface-free energy, quantum confinement effects, unsaturated coordination, and interactions between the metal with reduced size and substrate, the catalytic activity and stability of halide perovskites can be improved [43]. The construction of novel heterojunctions between halide perovskites and a suitable charge-transporting motif with desirable/well matching band alignment can enhance the charge separation [129]. For example, by coupling halide perovskites with electron- or hole-transporting materials such as GO, rGO, MXene, MOF, etc., can effectively promote charge separation and migration, thereby resulting in efficient catalytic activity. Type-II and Z-scheme are the most widely reported heterojunctions to achieve rapid charge transfer.
4.3. Enhance the Redox Ability of Halide Perovskites
The relatively narrow bandgaps of halide perovskites, which are especially associated with the VB edges, will inevitably bring about poor oxidation abilities. The weak oxidation capacity of halide perovskites will limit their application in some oxidation reactions—for instance, water oxidation (H2O/O2 at 1.23 eV vs. RHE) and organic compound mineralization (OH−/•OH at 1.67 eV vs. RHE). The combination of halide perovskites with other semiconductors with more positive VB (building a Z-scheme heterojunction) can achieve strong redox capabilities, broad light absorption, and efficient charge separation. Alternatively, by encapsulating the perovskite layer in a solar cell architecture to develop a perovskite-based PEC reaction system, the redox ability of perovskite can be enhanced by the applied voltage to achieve a broad reaction scope [43].
4.4. Exploring Mechanisms of Perovskite-Based Photocatalysis by Combining Experimental and Theoretical Research
Despite the great progress made in perovskite-based photocatalysis, there is a lack of comprehensive understanding of reaction mechanisms, such as the catalytic kinetic processes, the photophysical processes, and microscopic mechanisms of the involved surface chemical reactions. Hence, a complete theoretical model is required to interpret the roles of perovskite materials in photocatalytic redox reactions. Theoretical studies can not only help to enhance the understanding of established activities, but also can provide guidance for developing more efficient photocatalysts for redox reactions. By combining theoretical calculations and in-situ characterization techniques, mechanisms of perovskite-based photocatalysis such as reaction pathways and changes of catalysts during photocatalysis can be probed.
In this review, we introduced up-to-date progress of halide perovskites in photocatalytic hydrogen production. Up to this stage, perovskite powder (photocatalysis) and thin film (PEC and PV-PEC)-based photocatalysis systems have been proved to be effective for solar fuel production. However, from the viewpoint of commercialization, the factors of yield, reaction kinetics, stability, scalability, and cost and simplicity of production shall be reassessed. In addition, the general photocatalytic activity and stability of perovskite-based materials are currently far from optimal, and their application in photocatalysis is still in its infancy. The use of perovskites to address energy and environmental concerns still faces many challenges. These challenges also imply large opportunities for further exploration of perovskite-based photocatalysts with improved activity and more potential reactions. We hope that this review can provide some guidance toward finding optimal performance and stability for perovskite-based photocatalytic applications.
Author Contributions
Z.Z. wrote and formatted the review. R.Z., D.L. and Y.J. significantly contributed to the writing and preparation of tables and figures. X.W. and H.T. contextualized and structured the manuscript. J.X. reviewed and supervised the present manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (51972213), Natural Science Foundation of Shanghai (22ZR1460700), and Shanghai Institute of Technology (XTCX2022–28).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, Y.; Yin, W.J.; Peng, K.L.; Wang, K.; Hu, Q.; Selloni, A.; Chen, F.R.; Liu, L.M.; Sui, M.L. Self-hydrogenated shell promoting photocatalytic H2 evolution on anatase TiO2. Nat. Commun. 2018, 9, 2752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An inorganic/organic S-scheme heterojunction H2-production photocatalyst and its charge transfer mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef]
- Liu, S.; Kuang, W.; Meng, X.; Qi, W.; Adimi, S.; Guo, H.; Guo, X.; Pervaiz, E.; Zhu, Y.; Xue, D.; et al. Dual-phase metal nitrides as highly efficient co-catalysts for photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 416, 129116. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Liu, J.W.; Zhang, L.; Sun, Y.F.; Luo, Y. Bifunctional Ag-decorated CeO2 nanorods catalysts for promoted photodegradation of methyl orange and photocatalytic hydrogen evolution. Nanomaterials 2021, 11, 1104. [Google Scholar] [CrossRef]
- Rayalu, S.S.; Jose, D.; Mangrulkar, P.A.; Joshi, M.; Hippargi, G.; Shrestha, K.; Klabunde, K. Photodeposition of AuNPs on metal oxides: Study of SPR effect and photocatalytic activity. Int. J. Hydrog. Energy 2014, 309, 3617–3624. [Google Scholar] [CrossRef]
- Ma, X.H.; Liu, Y.N.; Wang, Y.P.; Jin, Z.L. Co3O4/CeO2 p-n heterojunction construction and application for efficient photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 33809–33822. [Google Scholar] [CrossRef]
- Jin, Z.L.; Li, T.; Wang, K.; Guo, X. Interface engineering: Synergism between S-scheme heterojunctions and Mo-O bonds for promote photocatalytic hydrogen evolution. Colloid. Interface Sci. 2022, 609, 212–223. [Google Scholar] [CrossRef]
- Tahir, M.B.; Sagir, M.; Abas, N. Enhanced photocatalytic performance of CdO-WO3 composite for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 24690–24697. [Google Scholar] [CrossRef]
- Jineesh, P.; Bhagya, T.C.; Remya, R.; Shibli, S.M.A. Photocatalytic hydrogen generation by WO3 in synergism with hematite-anatase heterojunction. Int. J. Hydrog. Energy 2020, 45, 18946–18960. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Zhang, L.J.; Yan, T.; Jin, Z.L. 2D CoP supported 0D WO3 constructed S-scheme for efficient photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 20560–20572. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, Y.D.; Dong, H.; Zhou, X. Theoretical investigation of loading Ni clusters on the alpha-Ga2O3 surfaces for photocatalytic hydrogen evolution. J. Energy Chem. 2019, 30, 8–18. [Google Scholar] [CrossRef]
- Yoon, H.J.; Yang, J.H.; Park, S.J.; Rhee, C.K.; Sohn, Y. Photocatalytic CO2 reduction and hydrogen production over Pt/Zn-embedded beta-Ga2O3 nanorods. Appl. Surf. Sci. 2021, 536, 147753. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, Z.Z.; Huang, H.J.; Wang, Y.; Tong, N.; Lin, J.J.; Liu, D.; Wang, X.X. Oxygen vacancy modulation of two-dimensional-Ga2O3 nanosheets as efficient catalysts for photocatalytic hydrogen evolution. Nanoscale 2018, 10, 21509–21517. [Google Scholar] [CrossRef]
- Han, C.Q.; Mao, W.T.; Bao, K.Y.; Xie, H.Q.; Jia, Z.Y.; Ye, L.Q. Preparation of Ag/Ga2O3 nanofibers via electrospinning and enhanced photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2017, 42, 19913–19919. [Google Scholar] [CrossRef]
- Li, F.H.; Yang, J.B.; Gao, J.P.; Liu, Y.; Gong, Y.L. Enhanced photocatalytic hydrogen production of CdS embedded in cationic hydrogel. Int. J. Hydrog. Energy 2020, 45, 1969–1980. [Google Scholar] [CrossRef]
- Zhang, G.X.; Guan, Z.J.; Yang, J.J.; Li, Q.Y.; Zhou, Y.; Zou, Z.G. Metal sulfides for photocatalytic hydrogen production: Current development and future challenges. Solar RRL 2022, 6, 2200587. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, C.X.; Cheng, S.; Yang, X.F. Ultrahigh photocatalytic hydrogen evolution performance of coupled 1D CdS/1T-phase dominated 2D WS2 nanoheterojunctions. Chin. J. Catal. 2022, 43, 403–409. [Google Scholar] [CrossRef]
- Yuan, M.; Zhou, W.H.; Kou, D.X.; Zhou, Z.J.; Meng, Y.N.; Wu, S.X. Cu2ZnSnS4 decorated CdS nanorods for enhanced visible-light-driven photocatalytic hydrogen production. Int. J. Hydrog. Energy 2018, 43, 20408–20416. [Google Scholar] [CrossRef]
- Yang, W.; Xu, M.; Tao, K.Y.; Zhang, J.H.; Zhong, D.C.; Lu, T.B. Building 2D/2D CdS/MOLs heterojunctions for efficient photocatalytic hydrogen evolution. Small 2022, 18, 2200332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Li, X.; Sun, X.Y.; Kong, C.; Xie, W.J.; Li, Z.; Liu, J. Surface partially oxidized MoS2 nanosheets as a higher efficient cocatalyst for photocatalytic hydrogen production. Appl. Surf. Sci. 2019, 487, 734–742. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Devarayapalli, K.C.; Shim, J.; Vattikuti, S.V.P. Highly efficient white-LED-light-driven photocatalytic hydrogen production using highly crystalline ZnFe2O4/MoS2 nanocomposites. Int. J. Hydrog. Energy 2020, 45, 32756–32769. [Google Scholar] [CrossRef]
- Wang, X.W.; Yuan, B.; Xie, Z.H.; Wang, D.X.; Zhang, R.B. ZnS-CdS/Graphene oxide heterostructures prepared by a light irradiation-assisted method for effective photocatalytic hydrogen generation. Colloid. Interface Sci. 2015, 446, 150–154. [Google Scholar] [CrossRef]
- Dong, J.; Fang, W.J.; Yuan, H.; Xia, W.W.; Zeng, X.H.; Shangguan, W.F. Few-layered MoS2/ZnCdS/ZnS heterostructures with an enhanced photocatalytic hydrogen evolution. ACS Appl. Energy Mater. 2022, 5, 4893–4902. [Google Scholar] [CrossRef]
- Chang, C.J.; Chu, K.W.; Hsu, M.H.; Chen, C.Y. Ni-doped ZnS decorated graphene composites with enhanced photocatalytic hydrogen-production performance. Int. J. Hydrog. Energy 2015, 40, 14498–14506. [Google Scholar] [CrossRef]
- Jian, L.; Zhang, H.Z.; Liu, B.; Pan, C.S.; Dong, Y.M.; Wang, G.L.; Zhong, J.; Zheng, Y.J.; Zhu, Y.F. Monodisperse Ni-clusters anchored on carbon nitride for efficient photocatalytic hydrogen evolution. Chin. J. Catal. 2022, 43, 536–545. [Google Scholar] [CrossRef]
- Du, J.; Li, S.M.; Du, Z.G.; Meng, S.M.; Li, B. Boron/oxygen-codoped graphitic carbon nitride nanomesh for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 407, 127114. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wei, M.J.; Wei, Z.W.; Pan, M.; Su, C.Y. Ultrathin graphitic carbon nitride nanosheets for photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2020, 3, 1010–1018. [Google Scholar] [CrossRef]
- Jiang, Y.; Qu, F.Q.; Tian, L.; Yang, X.F.; Zou, Z.Y.; Lin, Z.X. Self-assembled g-C3N4 nanoarchitectures with boosted photocatalytic solar-to-hydrogen efficiency. Appl. Surf. Sci. 2019, 487, 59–67. [Google Scholar] [CrossRef]
- Lu, Y.F.; Wang, W.S.; Cheng, H.R.; Qiu, H.J.; Sun, W.H.; Fang, X.; Zhu, J.F.; Zheng, Y.H. Bamboo-charcoal-loaded graphitic carbon nitride for photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2022, 47, 3733–3740. [Google Scholar] [CrossRef]
- Xu, H.T.; Xiao, R.; Huang, J.R.; Jiang, Y.; Zhao, C.X.; Yang, X.F. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yang, M.Z.; Chen, B.X.; Wang, X.D.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Jiang, Y.; Shu, M.Y.; Li, L.; Dong, Z.L.; Xu, J.Y. Artificial photosynthesis over metal halide perovskites: Achievements, challenges, and prospects. J. Phys. Chem. Lett. 2021, 12, 5864–5870. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.J.; Ding, C.; Xu, J.Y. Boosting charge separation and photocatalytic CO2 reduction of CsPbBr3 perovskite quantum dots by hybridizing with P3HT. Chem. Eng. J. 2021, 419, 129543. [Google Scholar] [CrossRef]
- Ou, M.; Tu, W.G.; Yin, S.M.; Xing, W.N.; Wu, S.Y.; Wang, H.J.; Wan, S.P.; Zhong, Q.; Xu, R. Amino-assisted anchoring of CsPbBr3 perovskite quantum dots on porous g-C3N4 for enhanced photocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2018, 57, 13570–13574. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Wang, J.; Shen, Q.; Zhang, Y.; Ding, C.; Bai, Y.; Jiang, G.; Li, Z.; Gaponik, N. Boosting photocatalytic CO2 reduction on CsPbBr3 perovskite nanocrystals by immobilizing metal complexes. Chem. Mater. 2020, 32, 1517–1525. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.T.; Zhang, Z.J.; Dong, Z.L.; Xu, J.Y. 2D/2D CsPbBr3/BiOCl heterojunction with an S-scheme charge transfer for boosting the photocatalytic conversion of CO2. Inorg. Chem. 2022, 61, 10557–10566. [Google Scholar] [CrossRef]
- Dong, Z.L.; Zhou, J.X.; Zhang, Z.J.; Jiang, Y.; Zhou, R.; Yao, C.X. Construction of a p−n type S-scheme heterojunction by incorporating CsPbBr3 nanocrystals into mesoporous Cu2O microspheres for efficient CO2 photoreduction. ACS Appl. Energy Mater. 2022, 5, 10076–10085. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, P.; Zhu, X.; Zhang, Q.; Wang, Z.; Liu, Y.; Zou, G.; Dai, Y.; Whangbo, M.; Huang, B. Composite of CH3NH3PbI3 with reduced graphene oxide as a highly efficient and stable visible-light photocatalyst for hydrogen evolution in aqueous HI solution. Adv. Mater. 2018, 30, 1704342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Zhang, H.; Yu, W.; Wang, X.; Zhao, Y.; Zong, X.; Li, C. Dynamic interaction between methylammonium lead iodide and TiO2 nanocrystals leads to enhanced photocatalytic H2 evolution from HI splitting. ACS Energy Lett. 2018, 3, 1159–1164. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.L.; Wang, S.R.; Li, X.G. How to apply metal halide perovskites to photocatalysis: Challenges and development. Nanoscale 2021, 13, 10281–10304. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chang, W.J.; Lee, C.W.; Park, S.; Ahn, H.-Y.; Nam, K.T. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. Nat. Energy 2016, 2, 16185. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, H.; Cheng, T.; Li, Y.Y.; Goddard, W.A. Pb-Activated amine-assisted photocatalytic hydrogen evolution reaction on organic-inorganic perovskites. J. Am. Chem. Soc. 2018, 140, 1994–1997. [Google Scholar] [CrossRef]
- Gao, G.; Xi, Q.Y.; Zhou, H.; Zhao, Y.X.; Wu, C.Q.; Wang, L.D.; Guo, P.R.; Xu, J.W. Novel inorganic perovskite quantum dots for photocatalysis. Nanoscale 2017, 9, 12032–12038. [Google Scholar] [CrossRef]
- Cardenas-Morcoso, D.; Gualdrón-Reyes, A.F.; Ferreira Vitoreti, A.B.; GarcíaTecedor, M.; Yoon, S.J.; Solis de la Fuente, M. Photocatalytic and photoelectrochemical degradation of organic compounds with allinorganic metal halide perovskite quantum dots. J. Phys. Chem. Lett. 2019, 10, 630–636. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, Y.; Huang, H.; Liu, X.; Li, Q.; Chen, L. Stable and highly efficient photocatalysis with lead-free double-perovskite of Cs2AgBiBr6. Angew. Chem. Int. Ed. 2019, 58, 7263–7267. [Google Scholar] [CrossRef]
- Feng, X.; Ju, H.; Song, T.; Fang, T.; Liu, W.; Huang, W. Highly efficient photocatalytic degradation performance of CsPb(Br1−xClx)3-Au nanoheterostructures. ACS Sustain. Chem. Eng. 2019, 7, 5152–5156. [Google Scholar] [CrossRef]
- Shu, M.Y.; Lu, J.L.; Zhang, Z.J.; Shen, T.; Xu, J.Y. CsPbBr3 Perovskite quantum dots/ultrathin C3N4 nanosheet 0D/2D composite: Enhanced stability and photocatalytic activity. J. Inorg. Mater. 2021, 36, 1217–1222. [Google Scholar] [CrossRef]
- Xiao, X.; Guo, S.K.; Ding, C.; Zhang, Z.J.; Huang, H.R.; Xu, J.Y. CsPbBr3@TiO2 core-shell structure nanocomposite as a water stable and efficient visible-light-driven photocatalyst. J. Inorg. Mater. 2021, 36, 507–512. [Google Scholar] [CrossRef]
- Schunemann, S.; Gastel, M.; Tuysuz, H. A CsPbBr3/TiO2 composite for visible-light-driven photocatalytic benzyl alcohol oxidation. ChemSusChem 2018, 11, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yuan, H.; Janssen, K.; Solís-Fernández, G.; Wang, Y.; Tan, C.; Jonckheere, D.; Debroye, E.; Long, J.; Hendrix, J.; et al. Efficient and selective photocatalytic oxidation of benzylic alcohols with hybrid organic-inorganic perovskite materials. ACS Energy Lett. 2018, 3, 755–759. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, Y.; Sun, Y.; Beard, M.; Yan, Y. Lead-halide perovskites for photocatalytic α-alkylation of aldehydes. J. Am. Chem. Soc. 2019, 141, 733–738. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, Y.; San Martin, J.; Sun, Y.; Zhu, D.; Yan, Y. Lead halide perovskites for photocatalytic organic synthesis. Nat. Commun. 2019, 10, 2843. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.Y.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Baikie, T.; Boix, P.P.; Yantara, N.; Mathews, N.; Mhaisalkar, S. Band-gap tuning of lead halide perovskites using a sequential deposition process. J. Mater. Chem. A 2014, 2, 9221–9225. [Google Scholar] [CrossRef]
- Nedelcu, L.G.; Protesescu, S.; Yakunin, M.I.; Bodnarchuk, M.J.; Grotevent; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing optoelectronic properties of metal halide perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef] [PubMed]
- Worhatch, R.J.; Kim, H.; Swainson, I.P.; Yonkeu, A.L.; Billinge, S.J. Study of local structure in selected organic–inorganic perovskites in the Pm3̅m Phase. Chem. Mater. 2008, 20, 1272–1277. [Google Scholar] [CrossRef]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Weller, M.T.; Weber, O.J.; Henry, P.F.; Di Pumpo, A.M.; Hansen, T.C. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem. Commun. 2015, 51, 4180–4183. [Google Scholar] [CrossRef]
- Brivio, F.; Walker, A.B.; Walsh, A. Structural and electronic properties of hybrid perovskites for high-efficiency thin-film photovoltaics from first-principles. APL Mater. 2013, 1, 042111. [Google Scholar] [CrossRef]
- Eames, C.; Frost, J.M.; Barnes, P.R.; O’Regan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015, 6, 7497. [Google Scholar] [CrossRef]
- Leppert, L.; Reyes-Lillo, S.E.; Neaton, J.B. Electric field-and strain-induced Rashba effect in hybrid halide perovskites. J. Phys. Chem. Lett. 2016, 7, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Amat, A.; Mosconi, E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M.K.; Gratzel, M.; De Angelis, F. Cation-induced band-gap tuning in organohalide perovskites: Interplay of spin–orbit coupling and octahedra tilting. Nano Lett. 2014, 14, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Manser, J.S.; Kamat, P.V. Dual nature of the excited state in organic-inorganic lead halide perovskites. Energy Environ. Sci. 2015, 8, 208–215. [Google Scholar] [CrossRef]
- Protesescu, S.; Yakunin, M.I.; Bodnarchuk, F.; Krieg, R.; Caputo, C.H.; Hendon, R.X.; Yang, A.; Walsh; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Jellicoe, T.C.; Richter, J.M.; Glass, H.F.J.; Tabachnyk, M.; Brady, R.; Dutton, S.E.; Rao, A.; Friend, R.H.; Credgington, D.; Greenham, N.C.; et al. Synthesis and optical properties of lead-free cesium tin halide perovskite nanocrystals. J. Am. Chem. Soc. 2016, 138, 2941–2944. [Google Scholar] [CrossRef]
- Yin, W.J.; Shi, T.; Yan, Y. Superior photovoltaic properties of lead halide perovskites: Insights from first-principles theory. J. Phys. Chem. C 2015, 119, 5253–5264. [Google Scholar] [CrossRef]
- Le, Q.V.; Hong, K.; Jang, H.W.; Kim, S.Y. Halide perovskite quantum dots for light-emitting diodes: Properties, synthesis, applications, and outlooks. Adv. Electron. Mater. 2018, 4, 1800335. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, P.; Wang, C.; Wang, Y.; Hu, Y.; Zhu, G.; Ma, L.; Liu, J.; Jin, Z. CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability. J. Am. Chem. Soc. 2017, 139, 14009–14012. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yang, H.; Fan, Q.; Xiong, F.; Liu, S.; Li, D.; Liu, B. Halide perovskite composites for photocatalysis: A mini review. EcoMat 2021, 3, 12079. [Google Scholar] [CrossRef]
- Ng, C.H.; Ripolles, T.S.; Hamada, K.; Teo, S.H.; Lim, H.N.; Bisquert, J.; Hayase, S. Tunable open circuit voltage by engineering inorganic cesium lead bromide/iodide perovskite solar cells. Sci. Rep. 2018, 8, 2482. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.H.; Zhou, J.; Zhao, X.; Sun, C.Y.; You, S.Q.; Wang, X.L.; Su, Z.M. Enhanced CO2 photoreduction via tuning halides in perovskites. J. Catal. 2019, 369, 201–208. [Google Scholar] [CrossRef]
- Wang, T.; Daiber, B.; Frost, J.M.; Mann, S.A.; Garnett, E.C.; Walsh, A.; Ehrler, B. Indirect to direct bandgap transition in methylammonium lead halide perovskite. Energy Environ. Sci. 2017, 10, 509–515. [Google Scholar] [CrossRef]
- Brenner, T.M.; Egger, D.A.; Kronik, L.; Hodes, G.; Cahen, D. Hybrid organic-inorganic perovskites: Low-cost semiconductors with intriguing charge-transport properties. Nat. Rev. Mater. 2016, 1, 15007. [Google Scholar] [CrossRef]
- Sutherland, B.R.; Sargent, E.H. Perovskite photonic sources. Nat. Photonics 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Xing, J.; Yan, F.; Zhao, Y.; Chen, S.; Yu, H.; Zhang, Q.; Zeng, R.; Demir, H.V.; Sun, X.; Huan, A.; et al. High-efficiency light emitting diodes of organometal halide perovskite amorphous nanoparticles. ACS Nano 2016, 10, 6623–6630. [Google Scholar] [CrossRef] [PubMed]
- Vargas, B.; Ramos, E.; Perez-Gutierrez, E.; Alonso, J.C.; Solis-Ibarra, D. A direct bandgap copper-antimony halide perovskite. J. Am. Chem. Soc. 2017, 139, 9116–9119. [Google Scholar] [CrossRef] [PubMed]
- Meinardi, F.; Akkerman, Q.A.; Bruni, F.; Park, S.; Mauri, M.; Dang, Z.; Manna, L.; Brovelli, S. Doped halide perovskite nanocrystals for reabsorption-free luminescent solar concentrators. ACS Energy Lett. 2017, 2, 2368–2377. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Yang, D.; Chen, X.; Jing, P.; Qu, S.; Zhang, L.; Zhou, D.; Zhu, J.; Xu, W.; et al. Doping lanthanide into perovskite nanocrystals: Highly improved and expanded optical properties. Nano Lett. 2017, 17, 8005–8011. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, G.; Yin, Y.; Miao, J.; Li, K.; Wang, C.; Xu, X.; Shen, C.; Meng, H. Aluminum-doped cesium lead bromide perovskite nanocrystals with stable blue photoluminescence used for display backlight. Adv. Sci. 2017, 4, 1700335. [Google Scholar] [CrossRef] [PubMed]
- Stam, W.V.D.; Geuchies, J.J.; Altantzis, T.; Bos, K.H.W.V.D.; Meeldijk, J.D.; Aert, S.V.; Bals, S.; Vanmaekelbergh, D.; Donega, C.D.M. Highly emissive divalent-ion-doped colloidal CsPb1–xMxBr3 perovskite nanocrystals through cation exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef]
- Chen, J.; Dong, C.; Idriss, H.; Mohammed, O.F.; Bakr, O.M. Metal halide perovskites for solar-to-chemical fuel conversion. Adv. Energy Mater. 2020, 10, 1902433. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Navarro, R.M.; Alvarez-Galvan, M.C.; La Villoria de Mano, J.A.; Al-Zahrani, S.M.; Fierro, J.L.G. A framework for visible-light water splitting. Energy Environ. Sci. 2010, 3, 1865. [Google Scholar] [CrossRef]
- Reza Gholipour, M.; Dinh, C.-T.; Beland, F.; Do, T.-O. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Takanabe, K. Photocatalytic water splitting: Quantitative approaches toward photocatalyst by design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Ponseca, C.S., Jr.; Savenije, T.J.; Abdellah, M.; Zheng, K.; Yartsev, A.; Pascher, T.; Harlang, T.; Chabera, P.; Pullerits, T.; Stepanov, A.; et al. Organometal halide perovskite solar cell materials rationalized: Ultrafast charge generation, high and microsecond-long balanced mobilities, and slow recombination. J. Am. Chem. Soc. 2014, 136, 5189–5192. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.M.; Petrozza, A. Defects in perovskite-halides and their effects in solar cells. Nat. Energy 2016, 1, 16149. [Google Scholar] [CrossRef]
- Walsh, A.; Stranks, S.D. Taking control of ion transport in halide perovskite solar cells. ACS Energy Lett. 2018, 3, 1983–1990. [Google Scholar] [CrossRef]
- De Wolf, S.; Holovsky, J.; Moon, S.J.; Loper, P.; Niesen, B.; Ledinsky, M.; Haug, F.J.; Yum, J.H.; Ballif, C. Organometallic halide perovskites: Sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef]
- Park, N.G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Chen, K.; Deng, X.; Dodekatos, G.; Tüysüz, H. Photocatalytic polymerization of 3, 4-ethylenedioxythiophene over cesium lead iodide perovskite quantum dots. J. Am. Chem. Soc. 2017, 139, 12267–12273. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Maes, J.; Balcaen, L.; Drijvers, E.; Zhao, Q.; De Roo, J.; Vantomme, A.; Vanhaecke, F.; Geiregat, P.; Hens, Z. Light absorption coefficient of CsPbBr3 perovskite nanocrystals. J. Phys. Chem. Lett. 2018, 9, 3093–3097. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, B.; Li, Y.; Deng, W.; He, R. Extra long electron-hole diffusion lengths in CH3NH3PbI3−xClx perovskite single crystals. J. Mater. Chem. C 2017, 5, 8431–8435. [Google Scholar] [CrossRef]
- Yettapu, G.R.; Talukdar, D.; Sarkar, S.; Swarnkar, A.; Nag, A.; Ghosh, P.; Mandal, P. Terahertz conductivity within colloidal CsPbBr3 perovskite nanocrystals: Remarkably high carrier mobilities and large diffusion lengths. Nano Lett. 2016, 16, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bhaumik, S.; Goh, T.W.; Kumar, M.S.; Yantara, N.; Gratzel, M.; Mhaisalkar, S.; Mathews, N.; Sum, T.C. Slow cooling and highly efficient extraction of hot carriers in colloidal perovskite nanocrystals. Nat. Commun. 2017, 8, 14350. [Google Scholar] [CrossRef]
- Mondal, N.; Samanta, A. Complete ultrafast charge carrier dynamics in photo-excited all-inorganic perovskite nanocrystals (CsPbX3). Nanoscale 2017, 9, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liang, G.; Shang, Q.; Ren, Y.; Kong, D.; Lian, T. Ultrafast interfacial electron and hole transfer from CsPbBr3 perovskite quantum dots. J. Am. Chem. Soc. 2015, 137, 12792–12795. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Du, Z.; Li, Z. Recent advances in metal halide perovskite photocatalysts: Properties, synthesis and applications. J. Energy Chem. 2021, 54, 770–785. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Podzorov, V. Charge carriers in hybrid organic-inorganic lead halide perovskites might be protected as large polarons. J. Phys. Chem. Lett. 2015, 6, 4758–4761. [Google Scholar] [CrossRef]
- Sun, S.; Tominaka, S.; Lee, J.H.; Xie, F.; Cheetham, A.K. Synthesis, crystal structure, and properties of a perovskite-related bismuth phase, (NH4)3Bi2I9. APL Mater. 2016, 4, 031101. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, P.; Guan, Z.; Liu, J.; Wang, Z.; Zheng, Z.; Jin, S.; Dai, Y.; Whangbo, M.H.; Huang, B. Enhancing the photocatalytic hydrogen evolution activity of mixed halide perovskite CH3NH3PbBr3−xIx achieved by bandgap funneling of charge carriers. ACS Catal. 2018, 8, 10349–10357. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, J.; Zheng, Y.Z.; Li, N.; Li, X.; Ye, Z.; Lu, S.; Tao, X.; Chen, C. Stable hybrid perovskite MAPb(I1−xBrx)3 for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 253, 41–48. [Google Scholar] [CrossRef]
- Powers, D.C.; Hwang, S.J.; Zheng, S.L.; Nocera, D.G. How to apply metal halide perovskites to photocatalysis: Challenges and development. Inorg. Chem. 2014, 53, 9122–9128. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Wu, Y.; Wang, P.; Zhang, Q.; Wang, Z.; Zheng, Z.; Liu, Y.; Dai, Y.; Whangbo, M.-H.; Huang, B. Perovskite photocatalyst CsPbBr3−xIx with a bandgap funnel structure for H2 evolution under visible light. Appl. Catal. B Environ. 2019, 245, 522–527. [Google Scholar] [CrossRef]
- Li, R.; Li, X.T.; Wu, J.J.; Lv, X.D.; Zheng, Y.Z.; Zhao, Z.J.; Ding, X.Q.; Tao, X.; Chen, J.F. Few-layer black phosphorus-on-MAPbI3 for superb visible-light photocatalytic hydrogen evolution from HI splitting. Appl. Catal. B Environ. 2019, 259, 118075. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Chen, R.; Zhang, H.; Wang, X.; Wang, J.; Zhang, J.; Mu, L.; Wu, K.; Fan, F.; et al. Promoting photocatalytic H2 evolution on organic-inorganic hybrid perovskite nanocrystals by simultaneous dual-charge transportation modulation. ACS Energy Lett. 2019, 4, 40–47. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Zhang, Z.; Min, S. A noble-metal-free MoS2 nanosheet-coupled MAPbI3 photocatalyst for efficient and stable visible-light-driven hydrogen evolution. Chem. Comm. 2020, 56, 3281–3284. [Google Scholar] [CrossRef]
- Guo, Y.M.; Liu, G.N.; Li, Z.X.; Lou, Y.B.; Chen, J.X.; Zhao, Y.X. A stable lead-free (CH3NH3)3Bi2I9 perovskite for photocatalytic hydrogen generation. ACS Sustain. Chem. Eng. 2019, 7, 15080–15085. [Google Scholar] [CrossRef]
- Wang, T.; Yue, D.; Li, X.; Zhao, Y. Lead-free double perovskite Cs2AgBiBr6/RGO composite for efficient visible light photocatalytic H2 evolution. Appl. Catal. B Environm. 2020, 268, 118399. [Google Scholar] [CrossRef]
- Ju, D.X.; Zheng, X.P.; Liu, J.L.; Chen, Y.; Zhang, J.; Cao, B.Q.; Xiao, H.; Mohammed, O.F.; Bakr, O.M.; Tao, X.T. Reversible band gap narrowing of Sn-based hybrid perovskite single crystal with excellent phase stability. Angew. Chem. Int. Ed. 2018, 57, 14868–14872. [Google Scholar] [CrossRef]
- Li, Z.J.; Hofman, E.; Li, J.; Davis, A.H.; Tung, C.-H.; Wu, L.Z.; Zheng, W.W. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2018, 28, 1704288. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, J.; Zheng, Y.Z.; Li, N.; Li, X.; Tao, X. Ni3C-decorated MAPbI3 as visible light photocatalyst for H2 evolution from HI splitting. ACS Catal. 2019, 9, 8144–8152. [Google Scholar] [CrossRef]
- Cai, C.; Teng, Y.; Wu, J.H.; Li, J.Y.; Chen, H.Y.; Chen, J.H.; Kuang, D.B. In situ photosynthesis of an MAPbI3/CoP hybrid heterojunction for efficient photocatalytic hydrogen evolution. Adv. Funct. Mater. 2020, 30, 2001478. [Google Scholar] [CrossRef]
- Zhao, H.; Chordiya, K.; Leukkunen, P.; Popov, A.; Upadhyay Kahaly, M.; Kordas, K.; Ojala, S. Dimethylammonium iodide stabilized bismuth halide perovskite photocatalyst for hydrogen evolution. Nano Res. 2021, 14, 1116–1125. [Google Scholar] [CrossRef]
- Tang, Y.; Mak, C.H.; Liu, R.; Wang, Z.; Ji, L.; Song, H.; Tan, C.; Barriere, F.; Hsu, H.Y. In situ formation of bismuth-based perovskite heterostructures for high-performance cocatalyst-free photocatalytic hydrogen evolution. Adv. Funct. Mater. 2020, 30, 2006919. [Google Scholar] [CrossRef]
- Liu, F.; Wang, M.; Liu, X.; Wang, B.; Li, C.; Liu, C.; Lin, Z.; Huang, F. A rapid and robust light-and-solution-triggered in situ crafting of organic passivating membrane over metal halide perovskites for markedly improved stability and photocatalysis. Nano Lett. 2021, 21, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, S.; Yin, H.; Jiang, S.; Zhao, K.; Kang, J.; Liu, P.F.; Jiang, L.; Zhu, Z.; Cui, D.; et al. Perovskite microcrystals with intercalated monolayer MoS2 nanosheets as advanced photocatalyst for solarpowered hydrogen generation. Matter 2020, 3, 935–949. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Ding, X.; Wu, J.; Zheng, Y.Z.; Tao, X. Stable mixed-organic-cation perovskite MA1–xFAxPbI3 integrated with MoS2 for enhanced visible-light photocatalytic H2 evolution. Ind. Eng. Chem. Res. 2020, 59, 20667–20675. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Zhang, B.; Xu, T.; Wang, C. PtIx/[(CH3)2NH2]3[BiI6] as a welldispersed photocatalyst for hydrogen production in hydroiodic acid. Nano Energy 2018, 50, 665–674. [Google Scholar] [CrossRef]
- Teo, S.; Ng, C.; Ng, Y.; Islam, A.; Hayase, S.; Taufiq-Yap, Y. Resolve deep-rooted challenges of halide perovskite for sustainable energy development and environmental remediation. Nano Energy 2022, 99, 107401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).