Sulfur Vacancy-Rich CuS for Improved Surface-Enhanced Raman Spectroscopy and Full-Spectrum Photocatalysis
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of CuS Nanoplates
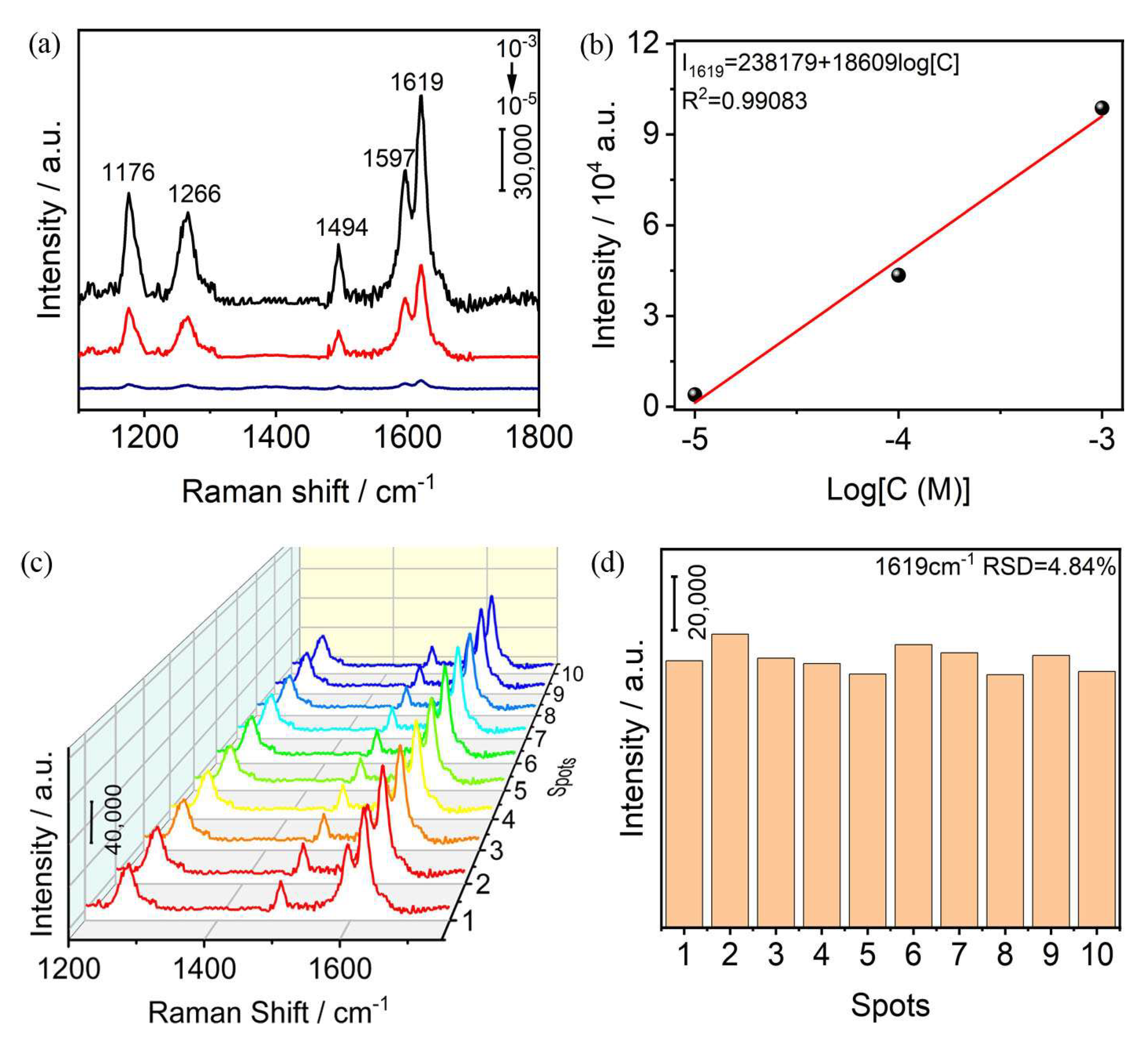
2.2. SERS Performance
2.3. Monitoring MO Photodegradation
2.4. Photoreduction of Cr(VI)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perumal, J.; Wang, Y.; Attia, A.B.E.; Dinish, U.S.; Olivo, M. Towards a point-of-care SERS sensor for biomedical and agri-food analysis applications: A review of recent advancements. Nanoscale 2021, 13, 553–580. [Google Scholar] [CrossRef] [PubMed]
- Dan, L.; Li, Z.; Gu, Y.; Ge, S.; Mao, Y.; Gu, Y.; Cao, X. A novel SERS biosensor for ultrasensitive detection of HPV-E7 and OPN based on a cascade signal amplification strategy of catalytic hairpin assembly and hybridization chain reaction. Mater. Chem. Front. 2022, 6, 1331–1343. [Google Scholar] [CrossRef]
- Dong, J.-C.; Zhang, X.-G.; Briega-Martos, V.; Jin, X.; Yang, J.; Chen, S.; Yang, Z.-L.; Wu, D.-Y.; Feliu, J.; Williams, C.; et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces. Nat. Energy 2019, 4, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Choi, H.-K.; Park, S.-M.; Jeong, J.; Lee, H.; Yeon, G.J.; Kim, D.-S.; Kim, Z. Spatially controlled fabrication of surface-enhanced Raman scattering hot spots through photoinduced dewetting of silver thin films. J. Phys. Chem. Lett. 2022, 13, 2969–2975. [Google Scholar] [CrossRef]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman scattering with dielectrics. Chem. Rev. 2016, 116, 14921–14981. [Google Scholar] [CrossRef] [PubMed]
- Samriti Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2022, 10, 73–95. [Google Scholar] [CrossRef]
- Liu, D.; Yi, W.; Fu, Y.; Kong, Q.; Xi, G. In situ surface restraint-induced synthesis of transition-metal nitride ultrathin nanocrystals as ultrasensitive SERS substrate with ultrahigh durability. ACS Nano 2022, 16, 13123–13133. [Google Scholar] [CrossRef]
- Li, Y.; Bai, H.; Zhai, J.; Yi, W.; Li, J.; Yang, H.; Xi, G. Alternative to noble metal substrates: Metallic and plasmonic Ti3O5 hierarchical microspheres for surface enhanced Raman spectroscopy. Anal. Chem. 2019, 91, 4496–4503. [Google Scholar] [CrossRef]
- Muehlethaler, C.; Considine, C.R.; Menon, V.; Lin, W.-C.; Lee, Y.-H.; Lombardi, J.R. Ultrahigh Raman enhancement on monolayer MoS2. ACS Photonics 2016, 3, 1164–1169. [Google Scholar] [CrossRef]
- Sarycheva, A.; Makaryan, T.; Maleski, K.; Satheeshkumar, E.; Melikyan, A.; Minassian, H.; Yoshimura, M.; Gogotsi, Y. Two-dimensional titanium carbide (MXene) as Surface-enhanced Raman scattering substrate. J. Phys. Chem. C 2017, 121, 19983–19988. [Google Scholar] [CrossRef]
- Sun, H.; Cong, S.; Zheng, Z.; Wang, Z.; Chen, Z.; Zhao, Z. Metal-organic frameworks as surface enhanced Raman scattering substrates with high tailorability. J. Am. Chem. Soc. 2019, 141, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.; Gong, W.; Cong, S.; Zhao, Z. Ultrathin two-dimensional nanostructures: Surface defects for morphology-driven enhanced semiconductor SERS. Angew. Chem. Int. Edit. 2021, 60, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.; Quesada-Cabrera, R.; Bardey, S.; Promdet, P.; Sapienza, R.; Keller, V.; Maier, S.A.; Caps, V.; Parkin, I.P.; Cortes, E. Probing the role of atomic defects in photocatalytic systems through photoinduced enhanced Raman scattering. ACS Energy Lett. 2021, 6, 4273–4281. [Google Scholar] [CrossRef]
- Jiang, Y.; Cong, S.; Song, G.; Sun, H.; Zhang, W.; Yao, W.; Zhao, Z. Defective cuprous oxide as a selective surface-enhanced Raman scattering sensor of dye adulteration in Chinese herbal medicines. J. Raman Spectrosc. 2021, 52, 1265–1274. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, X.; Ma, Y.; Cho, H.S.; Ding, D.; Wang, C.; Wu, J.; Oleynikov, P.; Jia, M.; Cheng, J.; et al. Filling metal–organic framework mesopores with TiO2 for CO2 photoreduction. Nature 2020, 586, 549–554. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Wolff, C.M.; Frischmann, P.D.; Schulze, M.; Bohn, B.J.; Wein, R.; Livadas, P.; Jackel, F.; Feldmann, J.; Wurthner, F.; Stolarczyk, J.K. All-in-one visible-light-driven water splitting by combining nanoparticulate and molecular co-catalysts on CdS nanorods. Nat. Energy 2018, 3, 862–869. [Google Scholar] [CrossRef]
- Xiong, J.; Di, J.; Xia, J.; Zhu, W.; Li, H. Surface defect engineering in 2D nanomaterials for photocatalysis. Adv. Funct. Mater. 2018, 28, 1801983. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Yao, S.; Liu, F.; Li, Y.; He, J.; Lin, Z.; Huang, F.; Liu, C.; Wang, M. Vacancy engineering in nanostructured semiconductors for enhancing photocatalysis. J. Mater. Chem. A 2021, 9, 17143–17172. [Google Scholar] [CrossRef]
- Kumar, A.; Krishnan, V. Vacancy engineering in semiconductor photocatalysts: Implications in hydrogen evolution and nitrogen fixation applications. Adv. Funct. Mater. 2021, 31, 2009807. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Shi, R.; Wang, B.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Tuning oxygen vacancies in ultrathin TiO2 nanosheets to boost photocatalytic nitrogen fixation up to 700 nm. Adv. Mater. 2019, 31, 1806482. [Google Scholar] [CrossRef]
- Singh, M.; Jampaiah, D.; Kandjani, A.E.; Sabri, Y.M.; Della Gaspera, E.; Reineck, P.; Judd, M.; Langley, J.; Cox, N.; van Embden, J.; et al. Oxygen-deficient photostable Cu2O for enhanced visible light photocatalytic activity. Nanoscale 2018, 10, 6039–6050. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Wang, Y.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. Zinc vacancy-promoted photocatalytic activity and photostability of ZnS for efficient visible-light-driven hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 302–311. [Google Scholar] [CrossRef]
- Xiao, B.; Lv, T.; Zhao, J.; Rong, Q.; Zhang, H.; Wei, H.; He, J.; Zhang, J.; Zhang, Y.; Peng, Y.; et al. Synergistic effect of the surface vacancy defects for promoting photocatalytic stability and activity of ZnS nanoparticles. ACS Catal. 2021, 11, 13255–13265. [Google Scholar] [CrossRef]
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519–3564. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X.; Yang, Z.; Shen, Y.; Xie, A. A novel bi-functional SiO2@TiO2/CDs nanocomposite with yolk-shell structure as both efficient SERS substrate and photocatalyst. Appl. Surf. Sci. 2019, 475, 135–142. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, R.; Sarangi, R. Quantitative X-ray absorption and emission spectroscopies: Electronic structure elucidation of Cu2S and CuS. J. Mater. Chem. C 2013, 1, 2448–2454. [Google Scholar] [CrossRef]
- Ishii, M.; Shibata, K.; Nozaki, H. Anion distributions and phase transitions in CuS1-xSex (x = 0–1) studied by Raman spectroscopy. J. Solid State Chem. 1993, 105, 504–511. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Tan, D.; Xian, Y.; Zhang, D.; Zhang, Z.; Liu, Y.; Liu, X.; Qiu, J. Photochemically derived plasmonic semiconductor nanocrystals as an optical switch for ultrafast photonics. Chem. Mater. 2020, 32, 3180–3187. [Google Scholar] [CrossRef]
- Cai, R.; Xiang, H.; Yang, D.; Lin, K.-T.; Wu, Y.; Zhou, R.; Gu, Z.; Yan, L.; Zhao, Y.; Tan, W. Plasmonic AuPt@CuS heterostructure with enhanced synergistic efficacy for radiophotothermal therapy. J. Am. Chem. Soc. 2021, 143, 16113–16127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, M.; Kang, Z.; Wang, B.; Wang, B.; Jiang, F.; Wang, X.; Yang, D.-P.; Luque, R. NIR-triggered photocatalytic/photothermal/photodynamic water remediation using eggshell-derived CaCO3/CuS nanocomposites. Chem. Eng. J. 2020, 388, 124304. [Google Scholar] [CrossRef]
- Khanchandani, S.; Kumar, S.; Ganguli, A.K. Comparative Study of TiO2/CuS core/shell and composite nanostructures for efficient visible light photocatalysis. ACS Sustain. Chem. Eng. 2016, 4, 1487–1499. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, H.Q.; Zhang, W.M.; Yin, J.; Cao, F.H.; Pan, Y.X. Noble-metal-free CuS/CdS photocatalyst for efficient visible-light-driven photocatalytic H2 production from water. Catal. Today 2019, 330, 203–208. [Google Scholar] [CrossRef]
- Hurma, T.; Kose, S. XRD Raman analysis and optical properties of CuS nanostructured film. Optik. 2016, 127, 6000–6006. [Google Scholar] [CrossRef]
- Balaji, S.; Djaoued, Y.; Robichaud, J. Phonon confinement studies in nanocrystalline anatase-TiO2 thin films by micro Raman spectroscopy. J. Raman Spectrosc. 2006, 37, 1416–1422. [Google Scholar] [CrossRef]
- Georgescu, D.; Baia, L.; Ersen, O.; Baia, M.; Simon, S. Experimental assessment of the phonon confinement in TiO2 anatase nanocrystallites by Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 876–883. [Google Scholar] [CrossRef]
- An, L.; Li, Y.; Luo, M.; Yin, J.; Zhao, Y.-Q.; Xu, C.; Cheng, F.; Yang, Y.; Xi, P.; Guo, S. Atomic-level coupled interfaces and lattice distortion on CuS/NiS2 nanocrystals boost oxygen catalysis for flexible Zn-Air batteries. Adv. Funct. Mater. 2017, 27, 1703779. [Google Scholar] [CrossRef]
- Peng, C.; Luo, G.; Zhang, J.; Chen, M.; Wang, Z.; Sham, T.-K.; Zhang, L.; Li, Y.; Zheng, G. Double sulfur vacancies by lithium tuning enhance CO2 electroreduction to n-propanol. Nat. Commun. 2021, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, W.; Xi, P.; Xi, S.; Du, Y.; Gao, D.; Ding, J. Activating and optimizing activity of CoS2 for hydrogen evolution reaction through the synergic effect of N dopants and S vacancies. ACS Energy Lett. 2017, 2, 1022–1028. [Google Scholar] [CrossRef]
- An, L.; Huang, L.; Zhou, P.; Yin, J.; Liu, H.; Xi, P. A Self-standing high-performance hydrogen evolution electrode with nanostructured NiCo2O4/CuS heterostructures. Adv. Funct. Mater. 2015, 25, 6814–6822. [Google Scholar] [CrossRef]
- Liang, H.; Shuang, W.; Zhang, Y.; Chao, S.; Han, H.; Wang, X.; Zhang, H.; Yang, L. Graphene-like multilayered CuS nanosheets assembled into flower-like microspheres and their electrocatalytic oxygen evolution properties. ChemElectroChem. 2018, 5, 494–500. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, W.; Liu, Q.; Cui, C.; Zhou, W.; Wang, X.; Zhang, Z.L.; Zhao, L.; Sang, Y.; Liu, H. Enhanced photo-induced carrier separation of CdS/MoS2 via micro-potential of Mo microsheet derived from electromagnetic induction. Chem. Eng. J. 2021, 404, 126972. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, B.; Dong, T.; Yuan, H.; Li, Y.; Tang, Z.; Liu, Z.; Liu, H.; Zhang, X.; Zhou, W. Laser synthesis of amorphous CoSx nanospheres for efficient hydrogen evolution and nitrogen reduction reactions. J. Mater. Chem. A 2022, 10, 20071–20079. [Google Scholar] [CrossRef]
- Wu, J.; Liu, B.; Ren, Z.; Ni, M.; Li, C.; Gong, Y.; Qin, W.; Huang, Y.L.; Sun, C.Q.; Liu, X.J. CuS/RGO hybrid photocatalyst for full solar spectrum photoreduction from UV/Vis to near-infrared light. J. Colloid Interf. Sci. 2018, 517, 80–85. [Google Scholar] [CrossRef]
- Thomas, M.; Mühlig, S.; Deckert-Gaudig, T.; Rockstuhl, C.; Deckert, V.; Marquetand, P. Distinguishing chemical and electromagnetic enhancement in surface-enhanced Raman spectra: The case of para-nitrothiophenol. J. Raman Spectrosc. 2013, 44, 1497–1505. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Y.; Lin, M.; Wang, Q.; Zhao, L.; Yang, Y.; Yao, K.X.; Han, Y. Site-specific growth of Au–Pd alloy horns on Au nanorods: A platform for highly sensitive monitoring of catalytic reactions by surface enhancement Raman spectroscopy. J. Am. Chem. Soc. 2013, 135, 8552–8561. [Google Scholar] [CrossRef]
- Prakash, O.; Kumar, S.; Singh, P.; Deckert, V.; Chatterjee, S.; Ghosh, A.K.; Singh, R.K. Surface-enhanced Raman scattering characteristics of CuO : Mn/Ag heterojunction probed by methyl orange: Effect of Mn2+ doping. J. Raman Spectrosc. 2016, 47, 813–818. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, Y. Adsorption orientations and interactions of methyl orange on negatively and positively charged colloidal silver particles. J. Colloid Interf. Sci. 2007, 305, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jin, B.; Park, C.; Cho, Y.; Song, X.; Shi, X.; Zhang, S.; Kim, W.; Zeng, H.; Park, J.H. Black phosphorene as a hole extraction layer boosting solar water splitting of oxygen evolution catalysts. Nat. Commun. 2019, 10, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Tian, F.; Gao, M.; Yang, W.; Yu, Y. L-Cysteine capped Mo2C/Zn0.67Cd0.33S heterojunction with intimate covalent bonds enables efficient and stable H2-Releasing photocatalysis. Chem. Eng. J. 2022, 428, 132628. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, J.; Zhu, X.; Yuan, J.; Zhou, M.; She, X.; Yu, Q.; Song, Y.; She, Y.; Hua, Y.; et al. Exploring deep effects of atomic vacancies on activating CO2 photoreduction via rationally designing indium oxide photocatalysts. Chem. Eng. J. 2021, 422, 129888. [Google Scholar] [CrossRef]
- Guan, R.; Wang, D.; Zhang, Y.; Liu, C.; Xu, W.; Wang, J.; Zhao, Z.; Feng, M.; Shang, Q.; Sun, Z. Enhanced photocatalytic N2 fixation via defective and fluoride modified TiO2 surface. Appl. Catal. B Environ. 2021, 282, 119580. [Google Scholar] [CrossRef]
- Li, A.; Lin, J.; Huang, Z.; Wang, X.; Guo, L. Surface-enhanced Raman spectroscopy on amorphous semiconducting rhodium sulfide microbowl substrates. iScience 2018, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Mirzaei, A.; Wang, Y.; Song, G.; Wang, C.; Besteiro, L.V.; Govorov, A.O.; Chaker, M.; Ma, D. Extracting hot holes from plasmonic semiconductors for photocatalysis. Appl. Catal. B Environ. 2022, 317, 121792. [Google Scholar] [CrossRef]










| Samples | Lattice Parameters (Å) | Cell Volume (Å3) | |
|---|---|---|---|
| A | C | ||
| CS-1 | 3.778 | 9.502 | 135.6 |
| CS-2 | 3.791 | 9.501 | 136.5 |
| CS-3 | 3.791 | 9.483 | 136.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Gong, Y.; Niu, L.; Li, C.; Liu, X. Sulfur Vacancy-Rich CuS for Improved Surface-Enhanced Raman Spectroscopy and Full-Spectrum Photocatalysis. Nanomaterials 2023, 13, 128. https://doi.org/10.3390/nano13010128
Hu J, Gong Y, Niu L, Li C, Liu X. Sulfur Vacancy-Rich CuS for Improved Surface-Enhanced Raman Spectroscopy and Full-Spectrum Photocatalysis. Nanomaterials. 2023; 13(1):128. https://doi.org/10.3390/nano13010128
Chicago/Turabian StyleHu, Jiapei, Yinyan Gong, Lengyuan Niu, Can Li, and Xinjuan Liu. 2023. "Sulfur Vacancy-Rich CuS for Improved Surface-Enhanced Raman Spectroscopy and Full-Spectrum Photocatalysis" Nanomaterials 13, no. 1: 128. https://doi.org/10.3390/nano13010128
APA StyleHu, J., Gong, Y., Niu, L., Li, C., & Liu, X. (2023). Sulfur Vacancy-Rich CuS for Improved Surface-Enhanced Raman Spectroscopy and Full-Spectrum Photocatalysis. Nanomaterials, 13(1), 128. https://doi.org/10.3390/nano13010128