Graphene Oxide Deposited with Transition Metal Chalcogenide for Selective Extraction and Determination of Hg(II): Experimental and Computational Analysis
Abstract
1. Introduction
2. Experiment
2.1. Materials and Chemicals
2.2. Instruments
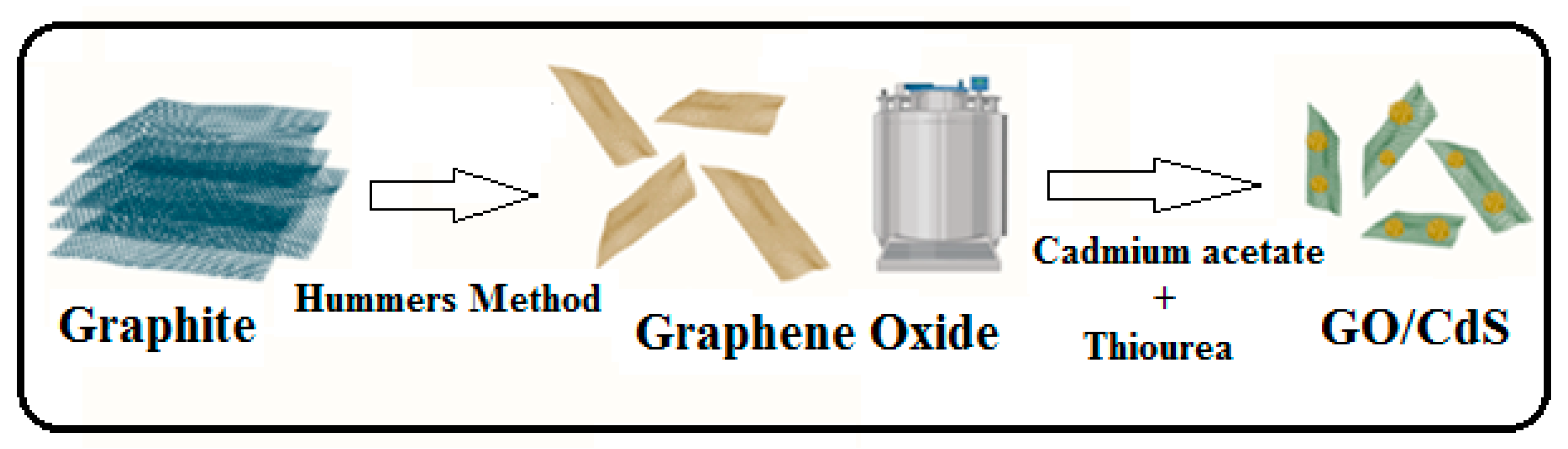
2.3. Synthesis
2.4. Preconcentration Procedure of Hg(II) Ions
3. Results and Discussion
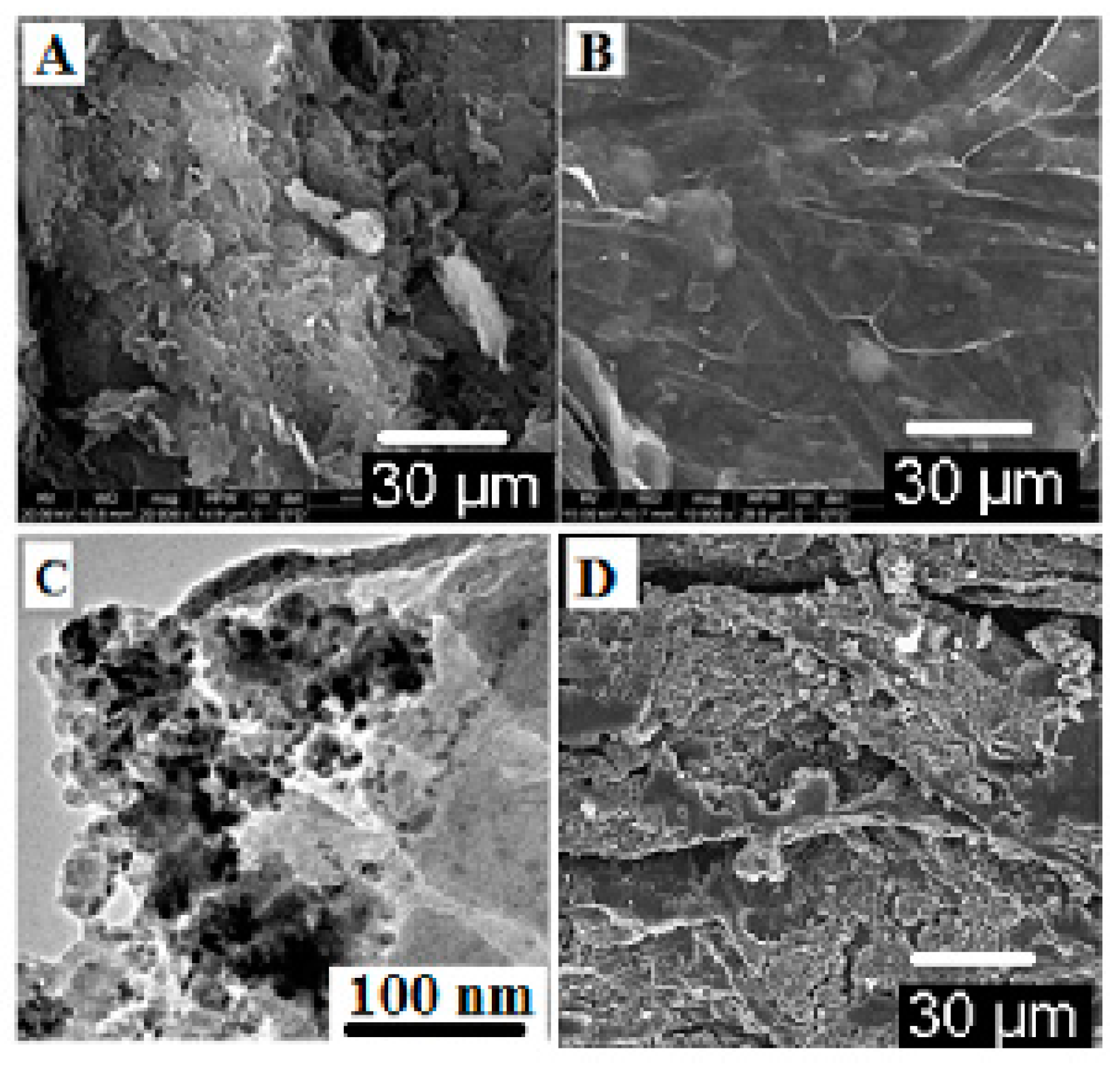
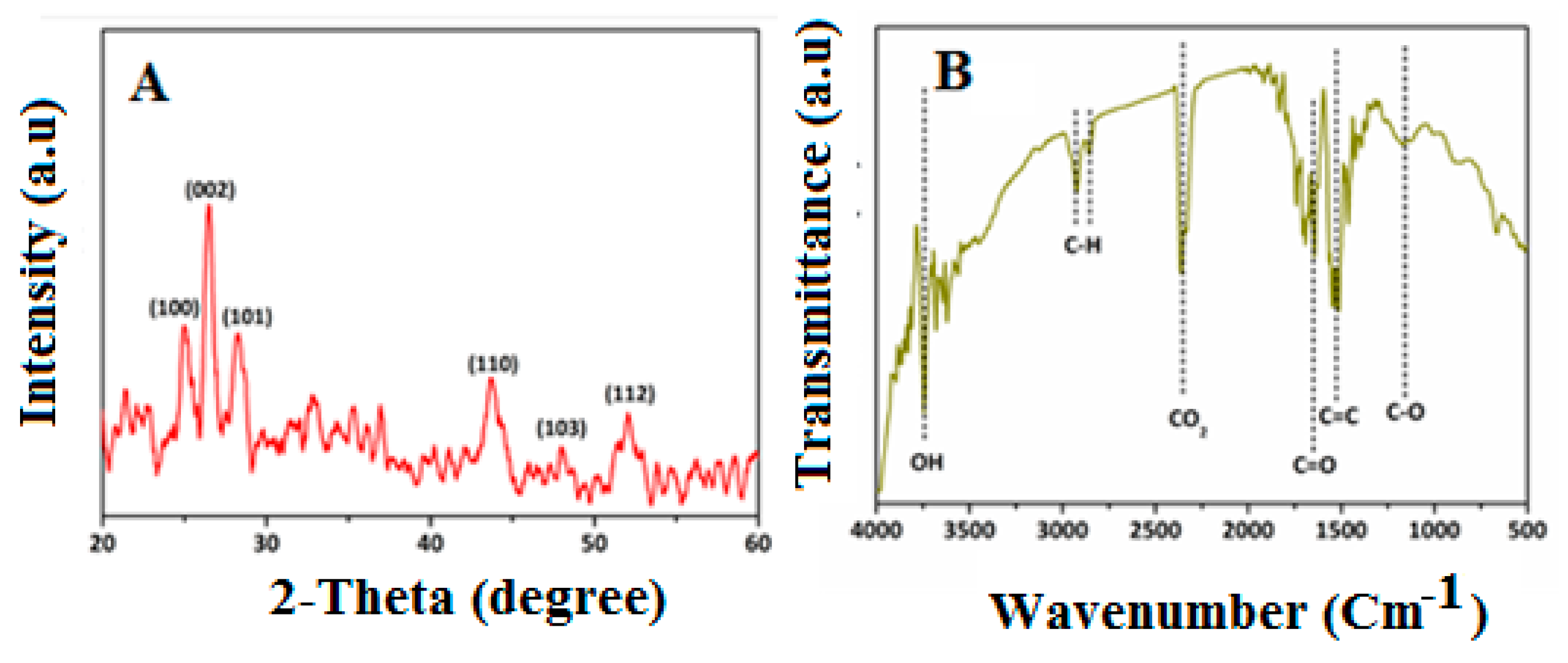
3.1. Characterization
3.2. Effect of Solution pH
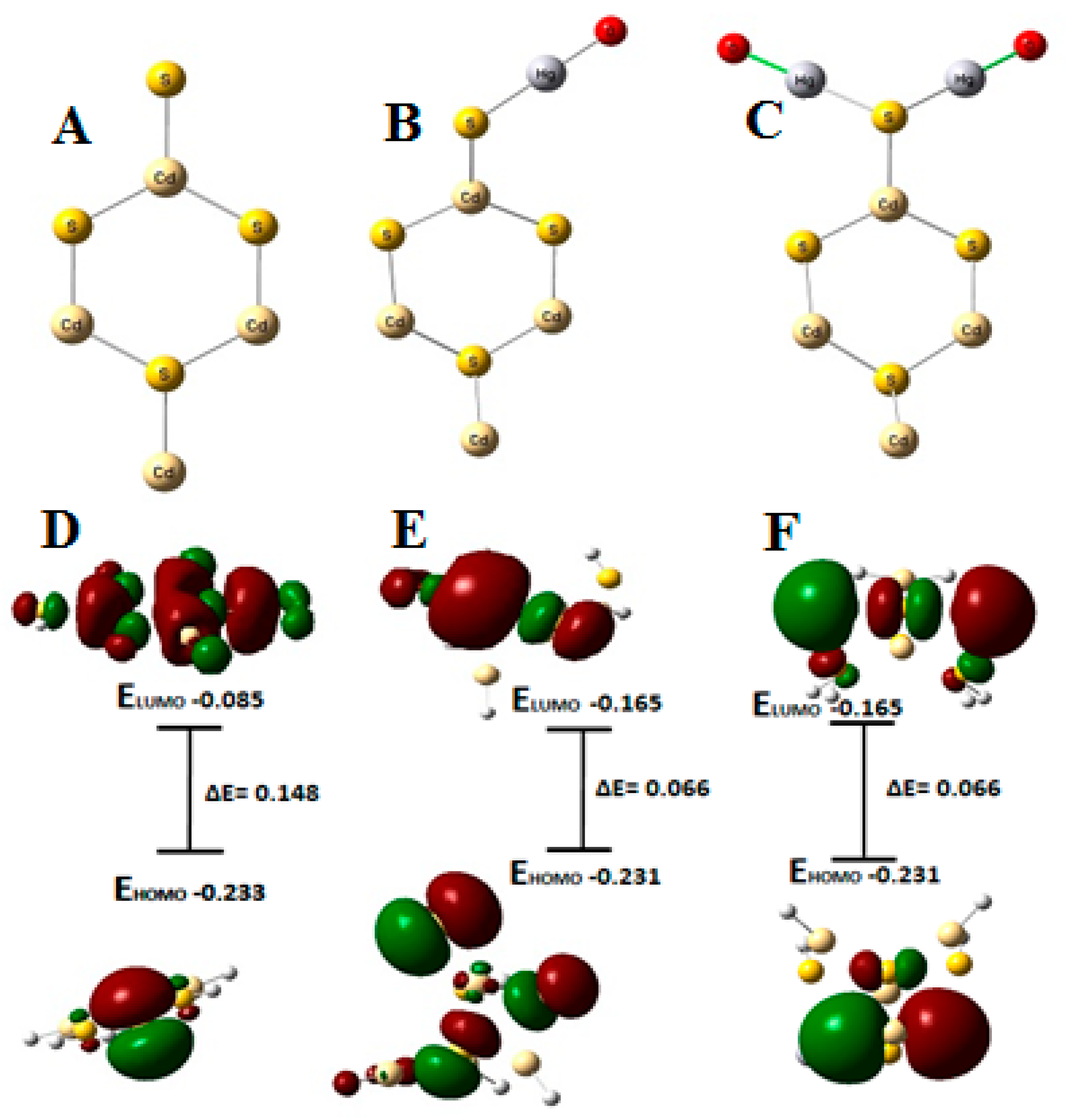
3.3. Theoretical Calculations
3.4. Effect of Sample Volume and Contact Time
3.5. Eluent Effects and Reusability
3.6. Study of Coexisting Ions including Humic Acid and Other Organic Acids
3.7. Analytical Figures of Merit
3.8. Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haseen, U.; Ahmad, H. Preconcentration and Determination of Trace Hg(II) Using a Cellulose Nanofiber Mat Functionalized with MoS2 Nanosheets, Ind. Eng. Chem. Res. 2020, 59, 3198–3204. [Google Scholar] [CrossRef]
- Wen, G.X.; Han, M.L.; Wu, X.Q.; Wu, Y.P.; Dong, W.W.; Zhao, J.; Li, D.S.; Ma, L.F. A multi-responsive luminescent sensor based on a super-stable sandwich-type terbium(iii)–organic framework. Dalton Trans. 2016, 45, 15492–15499. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yin, R.; Li, C.; Chen, D.; Grasby, S.E.; Li, T.; Ji, S.; Tian, H.; Peng, P.A. Global Hg cycle over Ediacaran–Cambrian transition and its implications for environmental and biological evolution. Earth Planet. Sci. Lett. 2022, 587, 117551. [Google Scholar] [CrossRef]
- Peirce, J.J.; Weiner, R.F.; Vesilind, P.A. Chapter 3—Water Pollution. In Environmental Pollution and Control, 4th ed.; Peirce, J.J., Weiner, R.F., Vesilind, P.A., Eds.; Butterworth-Heinemann: Woburn, UK, 1998; pp. 31–55. [Google Scholar]
- Souza-Araujo, J.D.; Hussey, N.E.; Hauser-Davis, R.A.; Rosa, A.H.; Lima, M.d.O.; Giarrizzo, T. Human risk assessment of toxic elements (As, Cd, Hg, Pb) in marine fish from the Amazon. Chemosphere 2022, 301, 134575. [Google Scholar] [CrossRef] [PubMed]
- Metal Ion Imbalance in the Body, Metals in Medicine. S.J Lippard. pp. 329–356. Available online: https://authors.library.caltech.edu/25052/10/BioinCh_chapter9.pdf (accessed on 24 November 2022).
- Abalaka, S.E.; Enem, S.I.; Idoko, I.S.; Sani, N.A.; Tenuche, O.Z.; Ejeh, S.A.; Sambo, W.K. Heavy Metals Bioaccumulation and Health Risks with Associated Histopathological Changes in Clarias gariepinus from the Kado Fish Market, Abuja, Nigeria. J. Health Pollut. 2020, 10, 200602. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Horvat, M.; David, C.E.; Zastenskaya, I.; Weihe, P.; Tempowski, J. A State-of-the-Science Review of Mercury Biomarkers in Human Populations Worldwide between 2000 and 2018. Environ. Health Perspect. 2018, 126, 106001. [Google Scholar] [CrossRef] [PubMed]
- Sioen, I.; de Henauw, S.; van Camp, J.; Volatier, J.-L.; Leblanc, J.-C. Comparison of the nutritional–toxicological conflict related to seafood consumption in different regions worldwide. Regul. Toxicol. Pharmacol. 2009, 55, 219–228. [Google Scholar] [CrossRef]
- Fu, H.R.; Zhao, Y.; Zhou, Z.; Yang, X.G.; Ma, L.F. Neutral ligand TIPA-based two 2D metal–organic frameworks: Ultrahigh selectivity of C2H2/CH4 and efficient sensing and sorption of Cr(vi). Dalton Trans. 2018, 47, 3725–3732. [Google Scholar] [CrossRef]
- Environmental Protection Agency. 2019.
- World Health Organisation Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Abdolmohammad-Zadeh, H.; Mohammad-Rezaei, R.; Salimi, A. Preconcentration of mercury(II) using a magnetite@carbon/dithizone nanocomposite, and its quantification by anodic stripping voltammetry. Mikrochim. Acta 2019, 187, 2. [Google Scholar] [CrossRef]
- Abdollahi, N.; Razavi, S.A.A.; Morsali, A.; Hu, M.L. High capacity Hg(II) and Pb(II) removal using MOF-based nanocomposite: Cooperative effects of pore functionalization and surface-charge modulation. J. Hazard. Mater. 2020, 387, 121667. [Google Scholar] [CrossRef]
- Proch, J.; Niedzielski, P. Recent applications of continuous flow chemical vapor and hydride generation (CVG, HG) coupled to plasma–based optical emission spectrometry (ICP OES, MIP OES). Talanta 2022, 243, 123372. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, J.; Olchowski, R.; Zięba, E.; Dobrowolski, R. A hybrid Zr/amine-modified mesoporous silica for adsorption and preconcentration of as before its FI HG AAS determination in water. Microporous Mesoporous Mater. 2021, 328, 111484. [Google Scholar] [CrossRef]
- Souza, J.P.; Cerveira, C.; Miceli, T.M.; Moraes, D.P.; Mesko, M.F.; Pereira, J.S.F. Evaluation of sample preparation methods for cereal digestion for subsequent As, Cd, Hg and Pb determination by AAS-based techniques. Food Chem. 2020, 321, 126715. [Google Scholar] [CrossRef] [PubMed]
- Oreste, E.Q.; de Jesus, A.; de Oliveira, R.M.; da Silva, M.M.; Vieira, M.A.; Ribeiro, A.S. New design of cold finger for sample preparation in open system: Determination of Hg in biological samples by CV-AAS. Microchem. J. 2013, 109, 5–9. [Google Scholar] [CrossRef]
- Li, C.; Duan, L.; Cheng, X. Facile method to synthesize fluorescent chitosan hydrogels for selective detection and adsorption of Hg2+/Hg+. Carbohydr. Polym. 2022, 288, 119417. [Google Scholar] [CrossRef]
- Qian, X.; Wang, R.; Zhang, Q.; Sun, Y.; Li, W.; Zhang, L.; Qu, B. A delicate method for the synthesis of high-efficiency Hg (II) The adsorbents based on biochar from corn straw biogas residue. J. Clean. Prod. 2022, 355, 131819. [Google Scholar] [CrossRef]
- Alam, M.F.; Begum, Z.A.; Furusho, Y.; Hasegawa, H.; Rahman, I.M.M. Selective separation of radionuclides from environmental matrices using proprietary solid-phase extraction systems: A review. Microchem. J. 2022, 181, 107637. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, H.; Yang, L.; Fei, P.; Liu, H. Development trend and prospect of solid phase extraction technology. Chin. J. Chem. Eng. 2022, 42, 245–255. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wen, T.; Liu, X.; Wang, Y.; Yang, H.; Sun, J.; Feng, J.; Dong, S.; Sun, J. Highly effective remediation of Pb(II) and Hg(II) contaminated wastewater and soil by flower-like magnetic MoS2 nanohybrid. Sci. Total Environ. 2020, 699, 134341. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jang, S.B.; Wong, K.T.; Kim, H.; Kim, M.J.; Choong, C.E.; Yang, J.-K.; Chang, Y.-Y.; Oh, S.-E.; Yoon, Y.; et al. Sulfur-anchored palm shell waste-based activated carbon for ultrahigh sorption of Hg(II) for in-situ groundwater treatment. J. Hazard. Mater. 2021, 417, 125995. [Google Scholar] [CrossRef]
- Kim, D.-W.; Wee, J.-H.; Yang, C.-M.; Yang, K.S. Efficient removals of Hg and Cd in aqueous solution through NaOH-modified activated carbon fiber. Chem. Eng. J. 2020, 392, 123768. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Yang, F. Removal of aqueous Hg(II) and Cr(VI) using phytic acid doped polyaniline/cellulose acetate composite membrane. J. Hazard. Mater. 2014, 280, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, J.; Luo, Z.; Wang, J.; Li, Y.; Han, Y.; Liu, J. Fluorescence detection of Mn2+, Cr2O72− and nitroexplosives and photocatalytic degradation of methyl violet and rhodamine B based on two stable metal–organic frameworks. RSC Adv. 2017, 7, 10415–10423. [Google Scholar] [CrossRef]
- Herce-Sesa, B.; López-López, J.A.; Moreno, C. Advances in ionic liquids and deep eutectic solvents-based liquid phase microextraction of metals for sample preparation in Environmental Analytical Chemistry. TrAC Trends Anal. Chem. 2021, 143, 116398. [Google Scholar] [CrossRef]
- Wang, K.; Chen, K.; Xiang, L.; Zeng, M.; Liu, Y.; Liu, Y. Relationship between Hg(II) adsorption property and functional group of different thioamide chelating resins. Sep. Purif. Technol. 2022, 292, 121044. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, X.; Guan, H.-K.; Liu, Q.; Zhao, S.-S.; Cheng, X.-L.; Xu, Q.-Q.; Li, S.-S. Hypersensitized electrochemical detection of Hg(II) based on tunable sulfur-doped porous Co3O4 nanosheets: Promotion Co2+/Co3+ valence change cycle and adsorption via introducing S. Chem. Eng. J. 2022, 435, 134950. [Google Scholar] [CrossRef]
- Ahmad, H.; Husain, F.M.; Khan, R.A. Graphene oxide lamellar membrane with enlarged inter-layer spacing for fast preconcentration and determination of trace metal ions. RSC Adv. 2021, 11, 11889–11899. [Google Scholar] [CrossRef]
- Haseen, U.; Ali, S.G.; Umar, K.; Ali, A.; Ahmad, H.; Khan, H.M. Dimercaptosuccinic Acid Functionalized Polystyrene Column for Trace Concentration Determination of Heavy Metal Ions: Experimental and Theoretical Calculation Studies. Water 2021, 13, 3056. [Google Scholar] [CrossRef]
- Ahmad, H.; Cai, C.; Liu, C. Separation and preconcentration of Pb(II) and Cd(II) from aqueous samples using hyperbranched polyethyleneimine-functionalized graphene oxide-immobilized polystyrene spherical adsorbents. Microchem. J. 2019, 145, 833–842. [Google Scholar] [CrossRef]
- Martell, A.E. Chelation: Stability and selectivity. Ann. N. Y. Acad. Sci. 1960, 88, 284–292. [Google Scholar] [CrossRef]
- Galvan, M.; Vela, A.; Gazquez, J.L. Chemical reactivity in spin-polarized density functional theory. J. Phys. Chem. 1988, 92, 6470–6474. [Google Scholar] [CrossRef]
- Ahmad, H.; Khan, R.A.; Koo, B.H.; Alsalme, A. Cellulose Nanofibers@ZrO2 membrane for the separation of Hg(II) from aqueous media. J. Phys. Chem. Solids 2022, 168, 110812. [Google Scholar] [CrossRef]

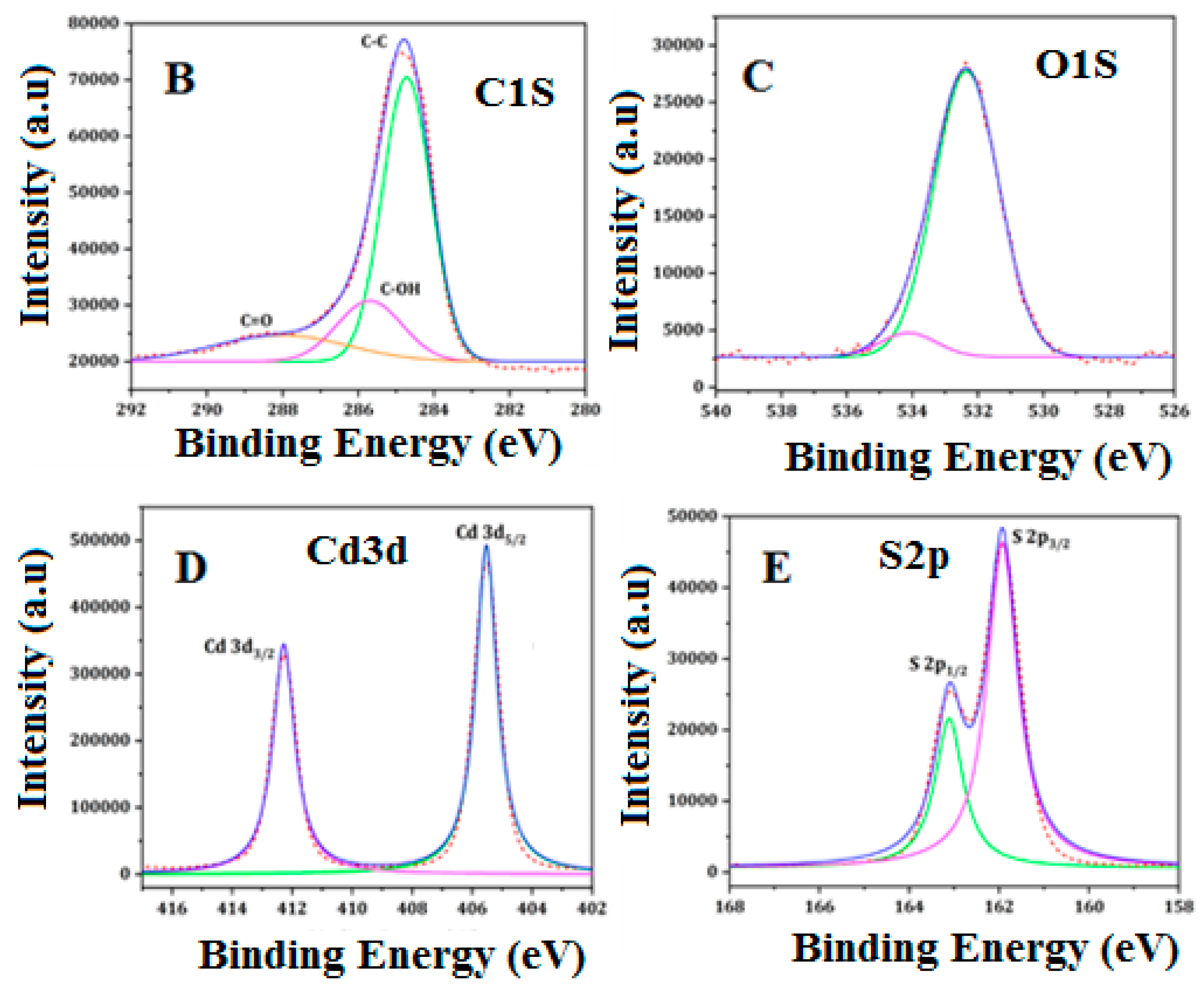

| Element | Peak Position (eV) | FWHM | Area (P) cps | Atomic % |
|---|---|---|---|---|
| C 1s | 284.8 | 1.77 | 128,088 | 55.82 |
| O 1s | 532.38 | 2.43 | 76,245 | 13.75 |
| S 2p | 161.92 | 1.03 | 72,277 | 15.53 |
| Cd 3d | 405.52 | 1.00 | 914,344 | 14.90 |
| Foreign Ions | Added as | Amount Added (µg) | % Recovery | RSD (N = 3) |
|---|---|---|---|---|
| Cl− | NaCl | 8.5 × 103 | 98.5 | 1.69 |
| Br− | NaBr | 9.0 × 103 | 100.0 | 2.48 |
| PO42− | Na2HPO4 | 5.0 × 103 | 95.0 | 3.65 |
| NO3− | NaNO3 | 2.5 × 103 | 100.0 | 3.30 |
| CO32− | Na2CO3 | 1.8 × 102 | 100.0 | 2.97 |
| SO42− | Na2SO4 | 5.0 × 102 | 95.5 | 1.95 |
| Na+ | NaCl | 6.5 × 103 | 99.0 | 2.50 |
| K+ | KCl | 5.0 × 103 | 100.6 | 1.56 |
| Ca2+ | CaCl2 | 2.0 × 103 | 97.5 | 3.45 |
| Mg2+ | MgCl2 | 2.0 × 103 | 98.0 | 1.68 |
| Zn2+ | ZnCl2 | 2.5 × 102 | 98.4 | 1.15 |
| Cd2+ | CdCl2 | 1.0 × 102 | 98.5 | 4.3 |
| Ni2+ | NiNO3 | 3.0 × 102 | 99.8 | 4.0 |
| Cu2+ | CuNO3 | 2.5 × 102 | 98.0 | 3.68 |
| Co2+ | CoNO3 | 2.5 × 102 | 96.8 | 4.26 |
| Humic acid | C6H9NO6 | 50 | 94.5 | 3.87 |
| Fulvic acid | C12H12O8 | 35 | 94.0 | 3.65 |
| Samples | Certified Value (µg g−1) | Value Found by Proposed Method (µg g−1) a ± Standard Deviation | Value of t-Test b |
|---|---|---|---|
| NISTSRM 1641d | 1.56 ± 0.02 | 1.52 ± 0.04 | 1.36 |
| Samples | Amount Added (µg) | Hg(II) Found (µg L−1) ± Standard Deviation a | Recovery Percent of Added Amount (RSD)c | Value of t-Test d |
| Tap water | 0 | ND b | - | - |
| 5 | 5.05 ± 0.24 | 101 (0.18) | 0.78 | |
| 10 | 9.92 ± 0.46 | 99.2 (0.22) | 1.18 | |
| River water | 0 | 5.5 | - | - |
| 5 | 10.60 ± 0.35 | 102. (0.35) | 1.28 | |
| 10 | 15.56 ± 0.82 | 100.6 (0.32) | 1.45 | |
| Groundwater | 0 | 1.30 ± 0.45 | - | 1.41 |
| 5 | 6.28 ± 0.15 | 99.6 (0.28) | 1.94 | |
| 10 | 11.32 ± 0.52 | 100.2 (0.36) | 2.41 |
| Adsorbent | Sorption Capacity (mg g−1) | Method | Detection Limit (µg L−1) | Ref. |
|---|---|---|---|---|
| MoS2 nanocomposite | 160.4 | SPE-icpoes | 0.09 | [1] |
| Magnetite carbon/dithizone nanocomposite | - | SPE-voltammetry | 2.7 | [13] |
| Magnetic MoS2 nano-hybrid | 428.0 | SPE | 3.2 | [23] |
| Metal–organic framework | 1600 | Dispersive SPE-icpoes | - | [14] |
| Cellulose/ZrO2 | 180 | SPE-icpoes | 0.05 | [36] |
| GO/CdS | 186.0 | SPE-icpoes | 0.07 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaeedi, H.; Ahmad, H.; Altowairqi, M.F.; Almuryyi, N.A.; Alsalme, A. Graphene Oxide Deposited with Transition Metal Chalcogenide for Selective Extraction and Determination of Hg(II): Experimental and Computational Analysis. Nanomaterials 2023, 13, 137. https://doi.org/10.3390/nano13010137
Alsaeedi H, Ahmad H, Altowairqi MF, Almuryyi NA, Alsalme A. Graphene Oxide Deposited with Transition Metal Chalcogenide for Selective Extraction and Determination of Hg(II): Experimental and Computational Analysis. Nanomaterials. 2023; 13(1):137. https://doi.org/10.3390/nano13010137
Chicago/Turabian StyleAlsaeedi, Huda, Hilal Ahmad, Malak Faisal Altowairqi, Nouf AbdulRahman Almuryyi, and Ali Alsalme. 2023. "Graphene Oxide Deposited with Transition Metal Chalcogenide for Selective Extraction and Determination of Hg(II): Experimental and Computational Analysis" Nanomaterials 13, no. 1: 137. https://doi.org/10.3390/nano13010137
APA StyleAlsaeedi, H., Ahmad, H., Altowairqi, M. F., Almuryyi, N. A., & Alsalme, A. (2023). Graphene Oxide Deposited with Transition Metal Chalcogenide for Selective Extraction and Determination of Hg(II): Experimental and Computational Analysis. Nanomaterials, 13(1), 137. https://doi.org/10.3390/nano13010137






