Biocompatibility of Dextran-Coated 30 nm and 80 nm Sized SPIONs towards Monocytes, Dendritic Cells and Lymphocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis and Physicochemical Characterization
2.2. Sterility and Endotoxin Content
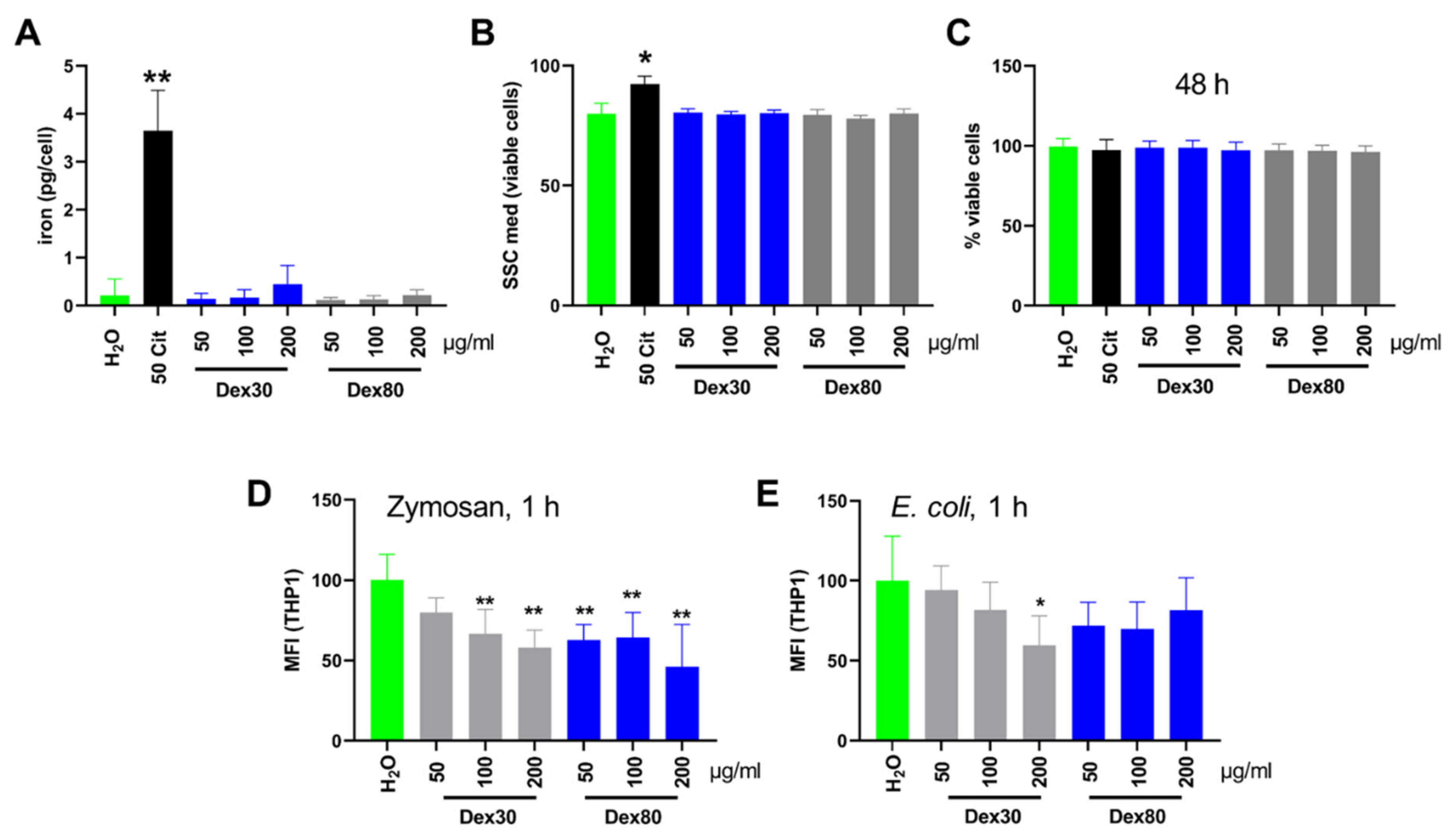
2.3. Quantification of Iron Uptake and Viability of THP-1 Cells
2.4. Phagocytosis of Zymosan A and E. coli by THP-1 Monocytes
2.5. Generation of Human Immature Dendritic Cells
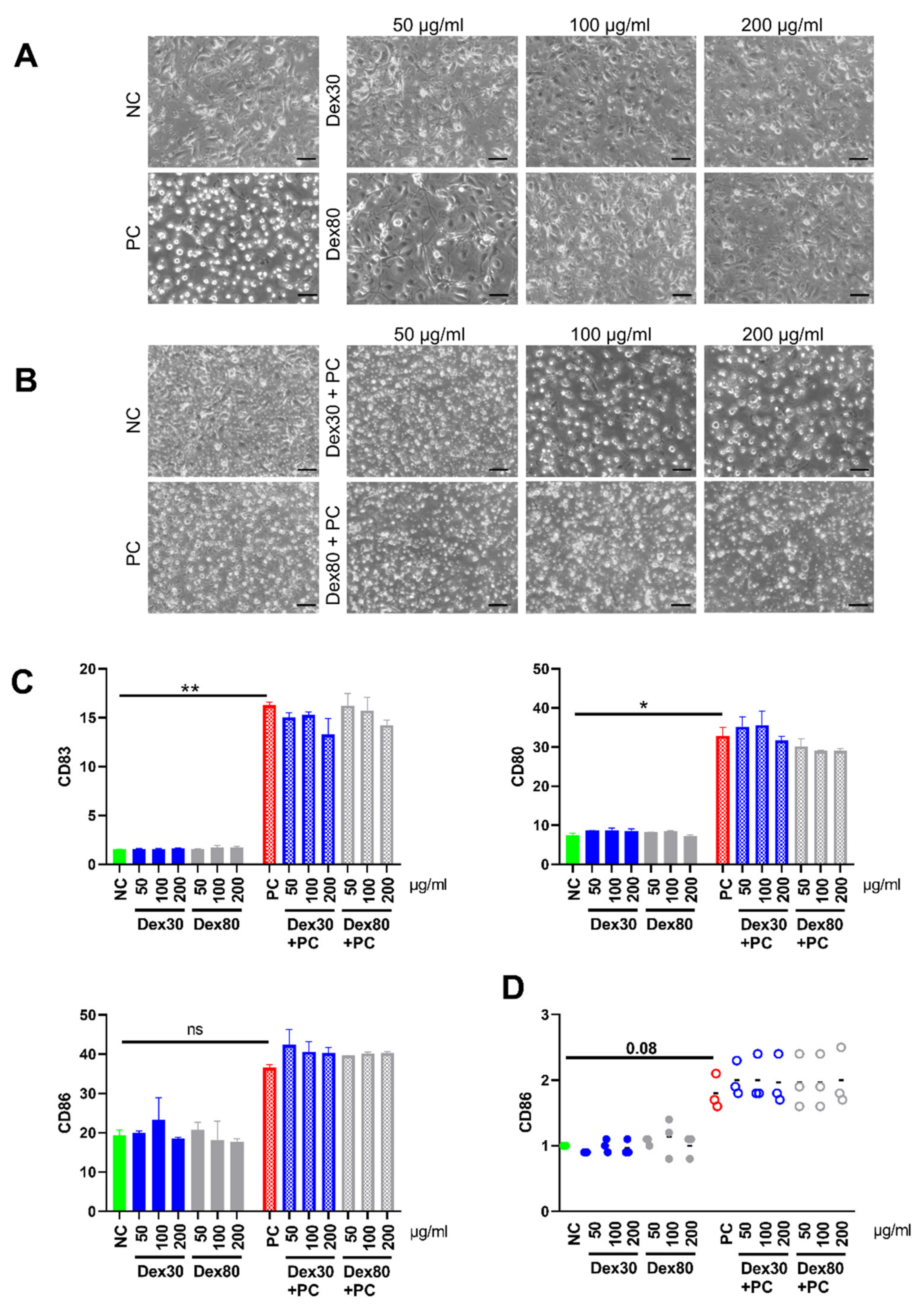
2.6. Maturation of Human Dendritic Cells and Analysis by Flow Cytometry
2.7. Lymphocyte Proliferation Assay
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
3.2. Sterility and Endotoxin Content
3.3. Uptake of SPIONDex by Monocytes and Influence on Viability and Phagocytosis
3.4. Maturation of Dendritic Cells (DCs) in the Presence of SPIONs
3.5. Lymphocyte Proliferation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shokrollahi, H. Contrast agents for MRI. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4485–4497. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khawaja, A.Z.; Cassidy, D.B.; Al Shakarchi, J.; McGrogan, D.G.; Inston, N.G.; Jones, R.G. Revisiting the risks of MRI with Gadolinium based contrast agents—Review of literature and guidelines. Insights Imaging 2015, 6, 553–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, S.; Schroeder, C.; Froehlich, J.M.; Pasch, A.; Thoeny, H.C. High signal intensity in dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in three patients with impaired renal function and vascular calcification. Contrast Media Mol. Imaging 2016, 11, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Errante, Y.; Cirimele, V.; Mallio, C.A.; Di Lazzaro, V.; Zobel, B.B.; Quattrocchi, C.C. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig. Radiol. 2014, 49, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency—Pharmacovigilance Risk Assessment Committee. PRAC Concludes Assessment of Gadolinium Agents Used in Body Scans and Recommends Regulatory Actions, Including Suspension for Some Marketing Authorisations; EMA/157486/2017; European Medicines Agency—Pharmacovigilance Risk Assessment Committee: London, UK, 2017. [Google Scholar]
- Richards, J.M.; Shaw, C.A.; Lang, N.N.; Williams, M.C.; Semple, S.I.; MacGillivray, T.J.; Gray, C.; Crawford, J.H.; Alam, S.R.; Atkinson, A.P.; et al. In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: Feasibility and safety in humans. Circ. Cardiovasc. Imaging 2012, 5, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Dahnke, H.; Rahmer, J.; Jordan, E.K.; Frank, J.A. Ultrashort T-2* Relaxometry for Quantitation of Highly Concentrated Superparamagnetic Iron Oxide (SPIO) Nanoparticle Labeled Cells. Magn. Reson. Med. 2009, 61, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.H.; Qian, X.; Mao, H.; Wang, A.Y.; Chen, Z.G.; Nie, S.; Shin, D.M. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int. J. Nanomed. 2008, 3, 311–321. [Google Scholar]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, J.; Pottler, M.; Leitinger, G.; Friedrich, R.P.; Almer, G.; Lyer, S.; Baum, E.; Tietze, R.; Heimke-Brinck, R.; Mangge, H.; et al. Pharmaceutical formulation of HSA hybrid coated iron oxide nanoparticles for magnetic drug targeting. Eur. J. Pharm. Biopharm. 2016, 101, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Kettering, M.; Hilger, I.; Muller, R.; Zeisberger, M. Magnetic multicore nanoparticles for hyperthermia—Influence of particle immobilization in tumour tissue on magnetic properties. Nanotechnology 2011, 22, 265102. [Google Scholar] [CrossRef] [PubMed]
- Brunke, O.; Odenbach, S.; Jurgons, R.; Alexiou, C.; Hilger, I.; Beckmann, F. Determination of the magnetic particle distribution in tumour tissue by means of x-ray tomography. J. Phys. Condens. Matter 2006, 18, S2903–S2917. [Google Scholar] [CrossRef] [Green Version]
- Cicha, I.; Singh, R.; Garlichs, C.D.; Alexiou, C. Nano-biomaterials for cardiovascular applications: Clinical perspective. J. Control. Release 2016, 229, 23–36. [Google Scholar] [CrossRef]
- Janko, C.; Zaloga, J.; Pöttler, M.; Dürr, S.; Eberbeck, D.; Tietze, R.; Lyer, S.; Alexiou, C. Strategies to optimize the biocompatibility of iron oxide nanoparticles—“SPIONs safe by design”. J. Magn. Magn. Mater. 2017, 431, 281–284. [Google Scholar] [CrossRef]
- Li, W.; Jan, Z.; Ding, Y.; Liu, Y.; Janko, C.; Pischetsrieder, M.; Alexiou, C.; Boccaccini, A.R. Facile preparation of multifunctional superparamagnetic PHBV microspheres containing SPIONs for biomedical applications. Sci. Rep. 2016, 6, 23140. [Google Scholar] [CrossRef] [Green Version]
- Zaloga, J.; Janko, C.; Agarwal, R.; Nowak, J.; Muller, R.; Boccaccini, A.R.; Lee, G.; Odenbach, S.; Lyer, S.; Alexiou, C. Different storage conditions influence biocompatibility and physicochemical properties of iron oxide nanoparticles. Int. J. Mol. Sci. 2015, 16, 9368–9384. [Google Scholar] [CrossRef]
- Scharlach, C.; Kratz, H.; Wiekhorst, F.; Warmuth, C.; Schnorr, J.; Genter, G.; Ebert, M.; Mueller, S.; Schellenberger, E. Synthesis of acid-stabilized iron oxide nanoparticles and comparison for targeting atherosclerotic plaques: Evaluation by MRI, quantitative MPS, and TEM alternative to ambiguous Prussian blue iron staining. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1085–1095. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Fathy, M.M.; Khalil, W.M. Preparation and characterization of magnetic gold nanoparticles to be used as doxorubicin nanocarriers. Phys. Med. 2014, 30, 843–848. [Google Scholar] [CrossRef]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef] [PubMed]
- Pardoe, H.; Chua-anusorn, W.; St. Pierre, T.G.; Dobson, J. Structural and magnetic properties of nanoscale iron oxide particles synthesized in the presence of dextran or polyvinyl alcohol. J. Magn. Magn. Mater. 2001, 225, 41–46. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.E.; Lee, S.H.; Yu, J.H.; Lee, J.H.; Park, T.G.; Hyeon, T. Designed fabrication of a multifunctional polymer nanomedical platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Adv. Mater. 2008, 20, 478–483. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Unterweger, H.; Janko, C.; Schwarz, M.; Dézsi, L.; Urbanics, R.; Matuszak, J.; Őrfi, E.; Fülöp, T.; Bäuerle, T.; Szebeni, J.; et al. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: A biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int. J. Nanomed. 2017, 12, 5223–5238. [Google Scholar] [CrossRef] [Green Version]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.; et al. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety. Int. J. Nanomed. 2018, 13, 1899–1915. [Google Scholar] [CrossRef] [Green Version]
- Schaft, N.; Dorrie, J.; Thumann, P.; Beck, V.E.; Muller, I.; Schultz, E.S.; Kampgen, E.; Dieckmann, D.; Schuler, G. Generation of an optimized polyvalent monocyte-derived dendritic cell vaccine by transfecting defined RNAs after rather than before maturation. J. Immunol. 2005, 174, 3087–3097. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.W.; Jacobs, P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: Ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging 1995, 13, 661–674. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds—Tables of Spectral Data; Springer-Verlag: Berlin/Heidelberg, Germany, 2009; Volume 4. [Google Scholar]
- Friedrich, B.; Lyer, S.; Janko, C.; Unterweger, H.; Brox, R.; Cunningham, S.; Dutz, S.; Taccardi, N.; Bikker, F.J.; Hurle, K.; et al. Scavenging of bacteria or bacterial products by magnetic particles functionalized with a broad-spectrum pathogen recognition receptor motif offers diagnostic and therapeutic applications. Acta Biomater. 2022, 141, 418–428. [Google Scholar] [CrossRef]
- Pöttler, M.; Fliedner, A.; Bergmann, J.; Bui, L.K.; Mühlberger, M.; Braun, C.; Graw, M.; Janko, C.; Friedrich, O.; Alexiou, C.; et al. Magnetic Tissue Engineering of the Vocal Fold Using Superparamagnetic Iron Oxide Nanoparticles. Tissue Eng. Part A 2019, 25, 1470–1477. [Google Scholar] [CrossRef]
- Boosz, P.; Pfister, F.; Stein, R.; Friedrich, B.; Fester, L.; Band, J.; Muhlberger, M.; Schreiber, E.; Lyer, S.; Dudziak, D.; et al. Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles Enable a Stable Non-Spilling Loading of T Cells and Their Magnetic Accumulation. Cancers 2021, 13, 4143. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.A.; Mallawarachi, C.; JM, U.K.-I.; Graves, M.J.; Horsley, J.; Goddard, M.J.; Brown, A.; Wang, L.; Kirkpatrick, P.J.; Brown, J.; et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1601–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Broeke, T.; Wubbolts, R.; Stoorvogel, W. MHC class II antigen presentation by dendritic cells regulated through endosomal sorting. Cold Spring Harb. Perspect. Biol. 2013, 5, a016873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, R.L.; Román, J.S. Biodegradable Systems in Tissue Engineering and Regenerative Medicine; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- Koike, E.; Takano, H.; Inoue, K.; Yanagisawa, R.; Kobayashi, T. Carbon black nanoparticles promote the maturation and function of mouse bone marrow-derived dendritic cells. Chemosphere 2008, 73, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.; Karp, M.; Killedar, S.; Bauer, S.M.; Guo, J.; Williams, D.; Breysse, P.; Georas, S.N.; Williams, M.A. Diesel-enriched particulate matter functionally activates human dendritic cells. Am. J. Respir. Cell Mol. Biol. 2007, 37, 706–719. [Google Scholar] [CrossRef] [Green Version]
- Tkach, A.V.; Yanamala, N.; Stanley, S.; Shurin, M.R.; Shurin, G.V.; Kisin, E.R.; Murray, A.R.; Pareso, S.; Khaliullin, T.; Kotchey, G.P.; et al. Graphene oxide, but not fullerenes, targets immunoproteasomes and suppresses antigen presentation by dendritic cells. Small 2013, 9, 1686–1690. [Google Scholar] [CrossRef] [Green Version]
- Tomic, S.; Ethokic, J.; Vasilijic, S.; Ogrinc, N.; Rudolf, R.; Pelicon, P.; Vucevic, D.; Milosavljevic, P.; Jankovic, S.; Anzel, I.; et al. Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS ONE 2014, 9, e96584. [Google Scholar] [CrossRef] [Green Version]
- Mou, Y.; Chen, B.; Zhang, Y.; Hou, Y.; Xie, H.; Xia, G.; Tang, M.; Huang, X.; Ni, Y.; Hu, Q. Influence of synthetic superparamagnetic iron oxide on dendritic cells. Int. J. Nanomed. 2011, 6, 1779–1786. [Google Scholar]
- Mou, Y.; Hou, Y.; Chen, B.; Hua, Z.; Zhang, Y.; Xie, H.; Xia, G.; Wang, Z.; Huang, X.; Han, W.; et al. In vivo migration of dendritic cells labeled with synthetic superparamagnetic iron oxide. Int. J. Nanomed. 2011, 6, 2633–2640. [Google Scholar]
- Matuszak, J.; Dorfler, P.; Zaloga, J.; Unterweger, H.; Lyer, S.; Dietel, B.; Alexiou, C.; Cicha, I. Shell matters: Magnetic targeting of SPIONs and in vitro effects on endothelial and monocytic cell function. Clin. Hemorheol. Microcirc. 2015, 61, 259–277. [Google Scholar] [CrossRef]
- Escamilla-Rivera, V.; Uribe-Ramírez, M.; González-Pozos, S.; Lozano, O.; Lucas, S.; De Vizcaya-Ruiz, A. Protein corona acts as a protective shield against Fe3O4-PEG inflammation and ROS-induced toxicity in human macrophages. Toxicol. Lett. 2016, 240, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, D.; Qu, Y.; Langer, K.; Öztürk, C.; Zhao, Y.; Chen, C.; Seebohm, G.; Düfer, M.; Fuchs, H.; Galla, H.-J.; et al. Comparison of cellular effects of starch-coated SPIONs and poly(lactic-co-glycolic acid) matrix nanoparticles on human monocytes. Int. J. Nanomed. 2016, 11, 5221–5236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strehl, C.; Gaber, T.; Maurizi, L.; Hahne, M.; Rauch, R.; Hoff, P.; Haupl, T.; Hofmann-Amtenbrink, M.; Poole, A.R.; Hofmann, H.; et al. Effects of PVA coated nanoparticles on human immune cells. Int. J. Nanomed. 2015, 10, 3429–3445. [Google Scholar] [CrossRef] [PubMed]
- Bancos, S.; Stevens, D.L.; Tyner, K.M. Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro. Int. J. Nanomed. 2015, 10, 183–206. [Google Scholar]
- Shah, A.; Dobrovolskaia, M.A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 977–990. [Google Scholar] [CrossRef]
- Anderson, S.A.; Shukaliak-Quandt, J.; Jordan, E.K.; Arbab, A.S.; Martin, R.; McFarland, H.; Frank, J.A. Magnetic resonance imaging of labeled T-cells in a mouse model of multiple sclerosis. Ann. Neurol. 2004, 55, 654–659. [Google Scholar] [CrossRef]
- Sundstrom, J.B.; Mao, H.; Santoianni, R.; Villinger, F.; Little, D.M.; Huynh, T.T.; Mayne, A.E.; Hao, E.; Ansari, A.A. Magnetic resonance imaging of activated proliferating rhesus macaque T cells labeled with superparamagnetic monocrystalline iron oxide nanoparticles. J. Acquir. Immune Defic. Syndr. 2004, 35, 9–21. [Google Scholar] [CrossRef]
- Muhlberger, M.; Janko, C.; Unterweger, H.; Friedrich, R.P.; Friedrich, B.; Band, J.; Cebulla, N.; Alexiou, C.; Dudziak, D.; Lee, G.; et al. Functionalization Of T Lymphocytes With Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles For Magnetically Controlled Immune Therapy. Int. J. Nanomed. 2019, 14, 8421–8432. [Google Scholar] [CrossRef] [Green Version]
- Mühlberger, M.; Janko, C.; Unterweger, H.; Schreiber, E.; Band, J.; Lehmann, C.; Dudziak, D.; Lee, G.; Alexiou, C.; Tietze, R. Functionalization of T lymphocytes for magnetically controlled immune therapy: Selection of suitable superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2019, 473, 61–67. [Google Scholar] [CrossRef]
- Bilyy, R.; Unterweger, H.; Weigel, B.; Dumych, T.; Paryzhak, S.; Vovk, V.; Liao, Z.; Alexiou, C.; Herrmann, M.; Janko, C. Inert Coats of Magnetic Nanoparticles Prevent Formation of Occlusive Intravascular Co-aggregates with Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 2266. [Google Scholar] [CrossRef]
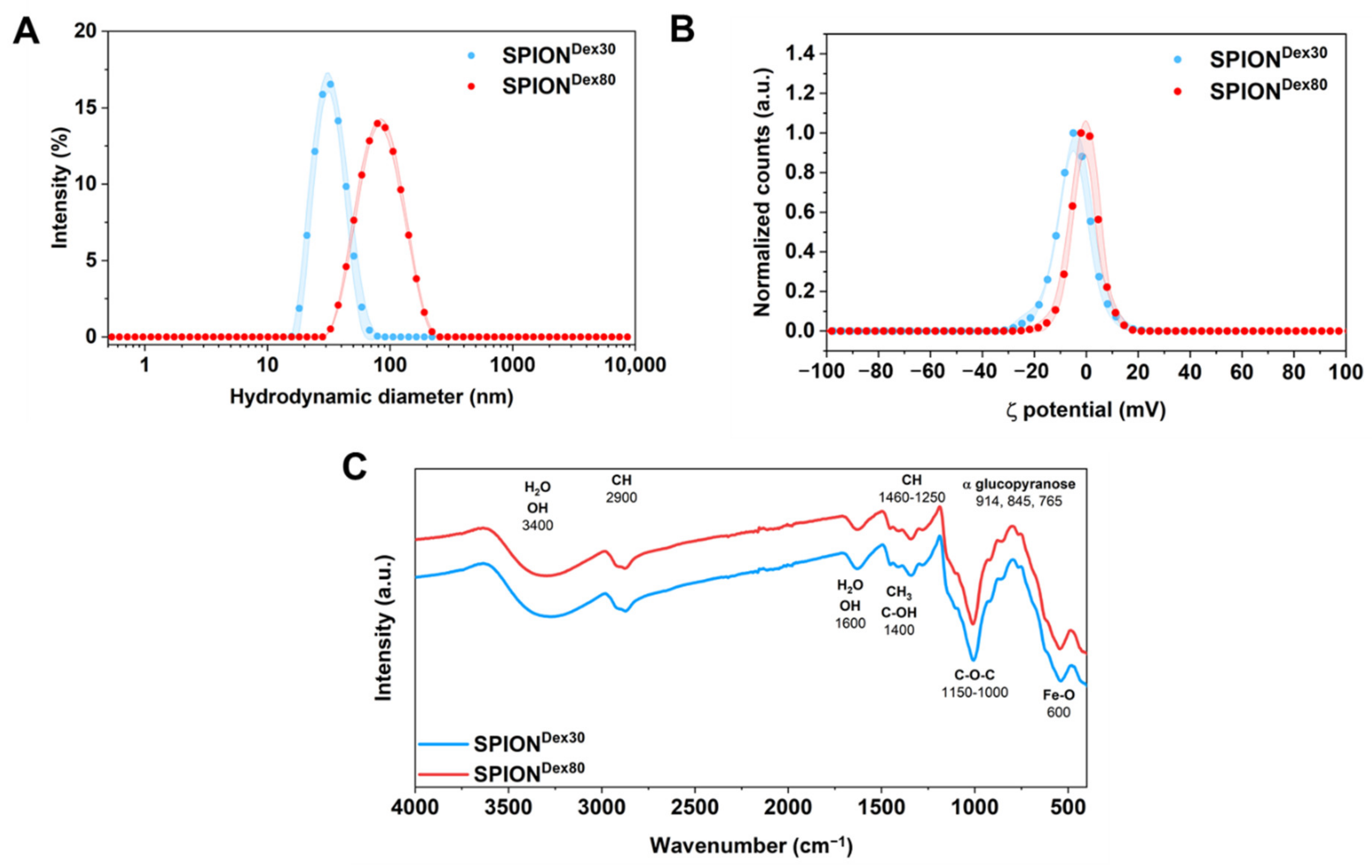


| Parameter | SPIONDex30 | SPIONDex80 |
|---|---|---|
| Hydrodynamic size (nm) | 32 ± 1 | 77 ± 2 |
| PDI (a.u.) | 0.129 ± 0.004 | 0.137 ± 0.017 |
| ζ potential (mV) at pH 7 | −4.9 ± 0.2 | −3.7 ± 0.3 |
| Magnetic volume susceptibility (SI units) | 8.0 × 10−4 ± 2.4 × 10−6 | 12.4 × 10−4 ± 1.5 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zschiesche, L.; Janko, C.; Friedrich, B.; Frey, B.; Band, J.; Lyer, S.; Alexiou, C.; Unterweger, H. Biocompatibility of Dextran-Coated 30 nm and 80 nm Sized SPIONs towards Monocytes, Dendritic Cells and Lymphocytes. Nanomaterials 2023, 13, 14. https://doi.org/10.3390/nano13010014
Zschiesche L, Janko C, Friedrich B, Frey B, Band J, Lyer S, Alexiou C, Unterweger H. Biocompatibility of Dextran-Coated 30 nm and 80 nm Sized SPIONs towards Monocytes, Dendritic Cells and Lymphocytes. Nanomaterials. 2023; 13(1):14. https://doi.org/10.3390/nano13010014
Chicago/Turabian StyleZschiesche, Lisa, Christina Janko, Bernhard Friedrich, Benjamin Frey, Julia Band, Stefan Lyer, Christoph Alexiou, and Harald Unterweger. 2023. "Biocompatibility of Dextran-Coated 30 nm and 80 nm Sized SPIONs towards Monocytes, Dendritic Cells and Lymphocytes" Nanomaterials 13, no. 1: 14. https://doi.org/10.3390/nano13010014
APA StyleZschiesche, L., Janko, C., Friedrich, B., Frey, B., Band, J., Lyer, S., Alexiou, C., & Unterweger, H. (2023). Biocompatibility of Dextran-Coated 30 nm and 80 nm Sized SPIONs towards Monocytes, Dendritic Cells and Lymphocytes. Nanomaterials, 13(1), 14. https://doi.org/10.3390/nano13010014






