The Ultrahigh Adsorption Capacity and Excellent Photocatalytic Degradation Activity of Mesoporous CuO with Novel Architecture
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Mesoporous CuO
2.3. Characterization
2.4. Computational Details
2.5. Adsorption Property
2.6. Photocatalytic Activity
3. Results and Discussion
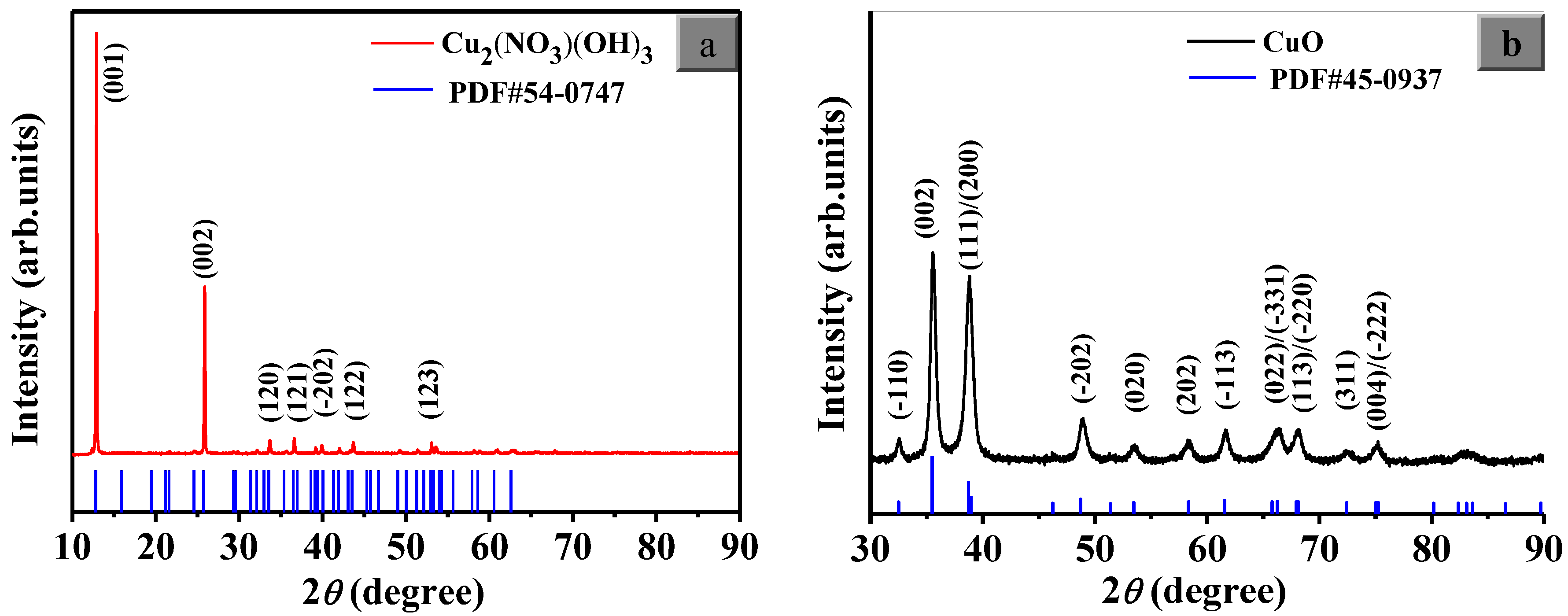
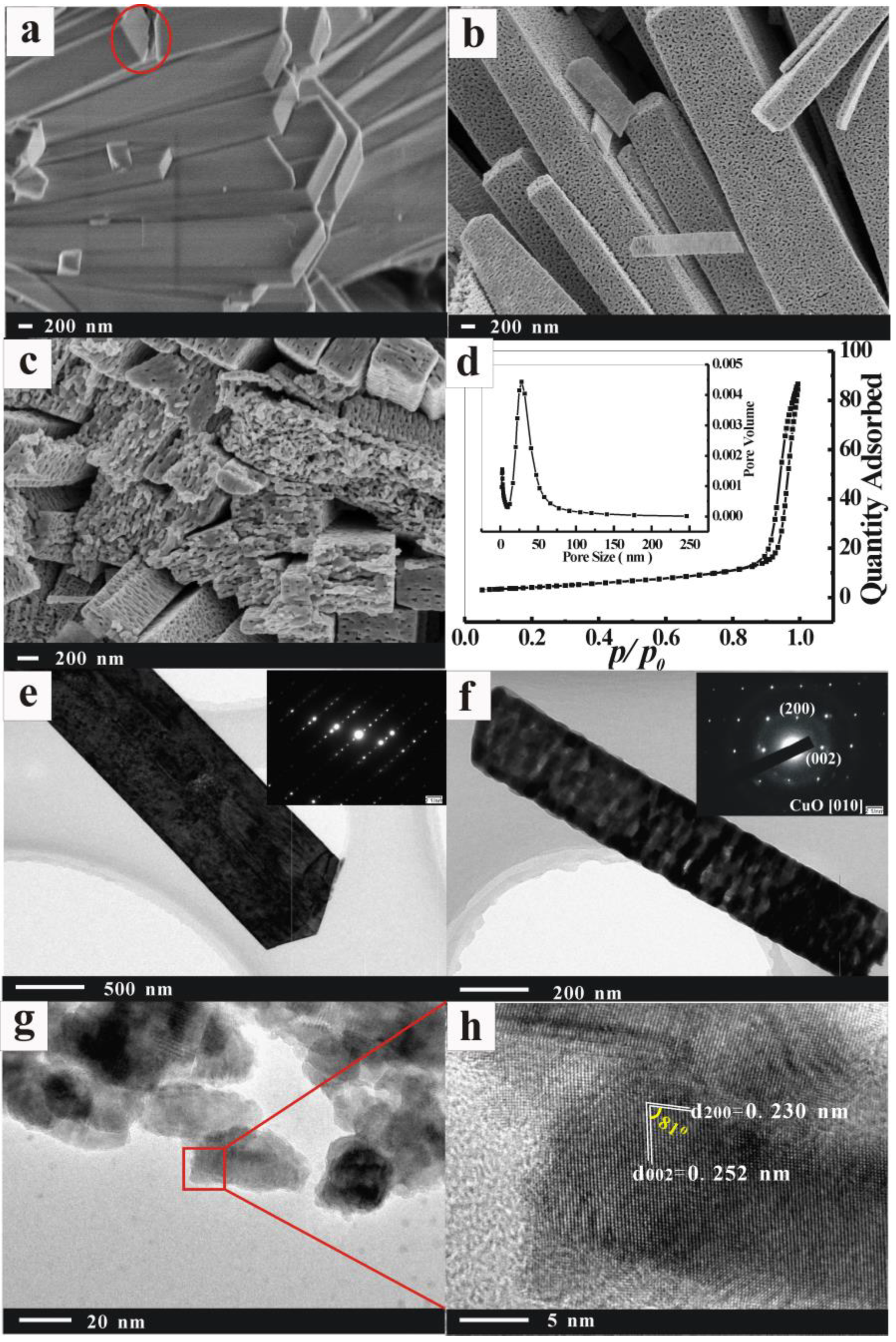
3.1. Structural Characteristics Analysis
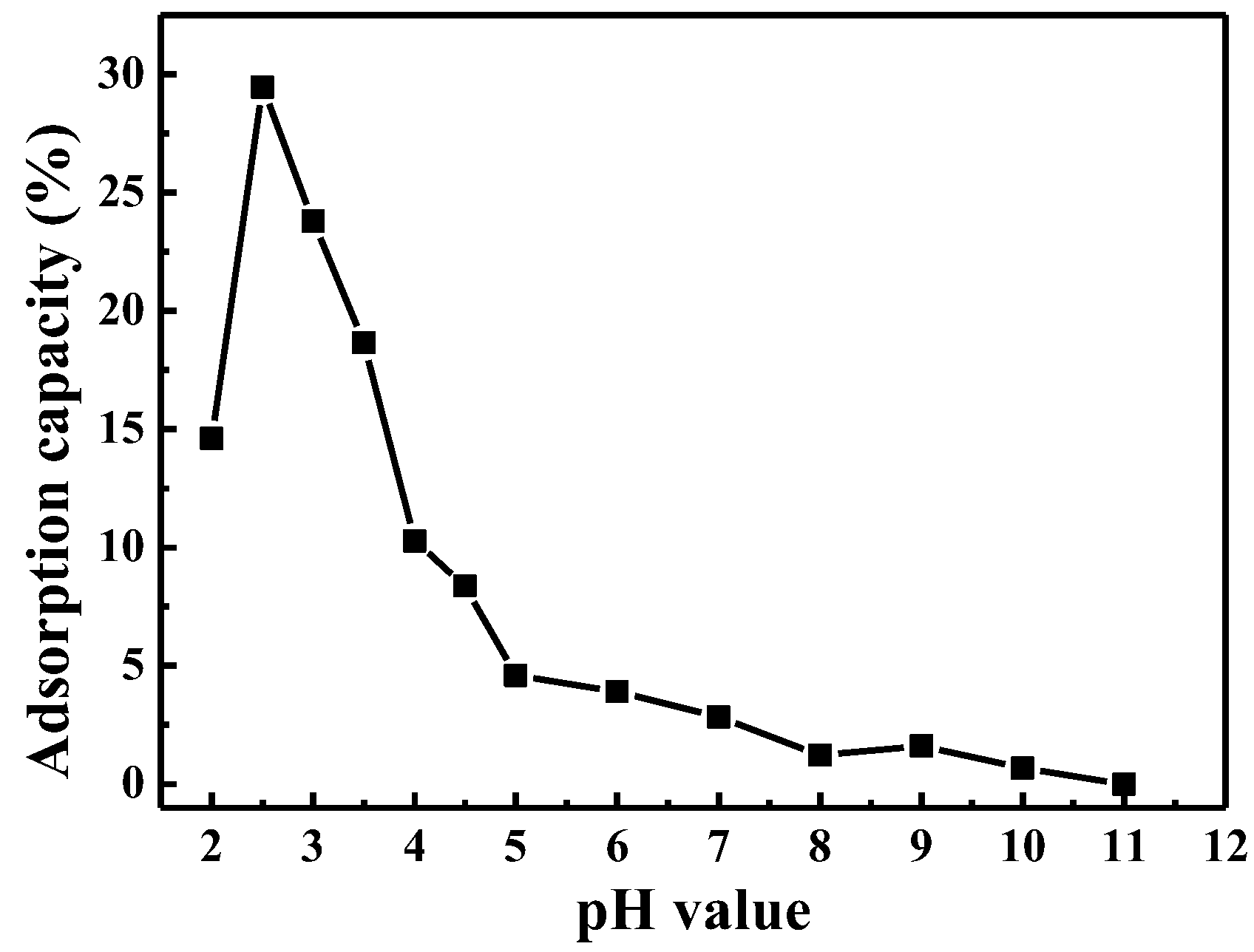
3.2. The Adsorption Capability of Mesoporous CuO
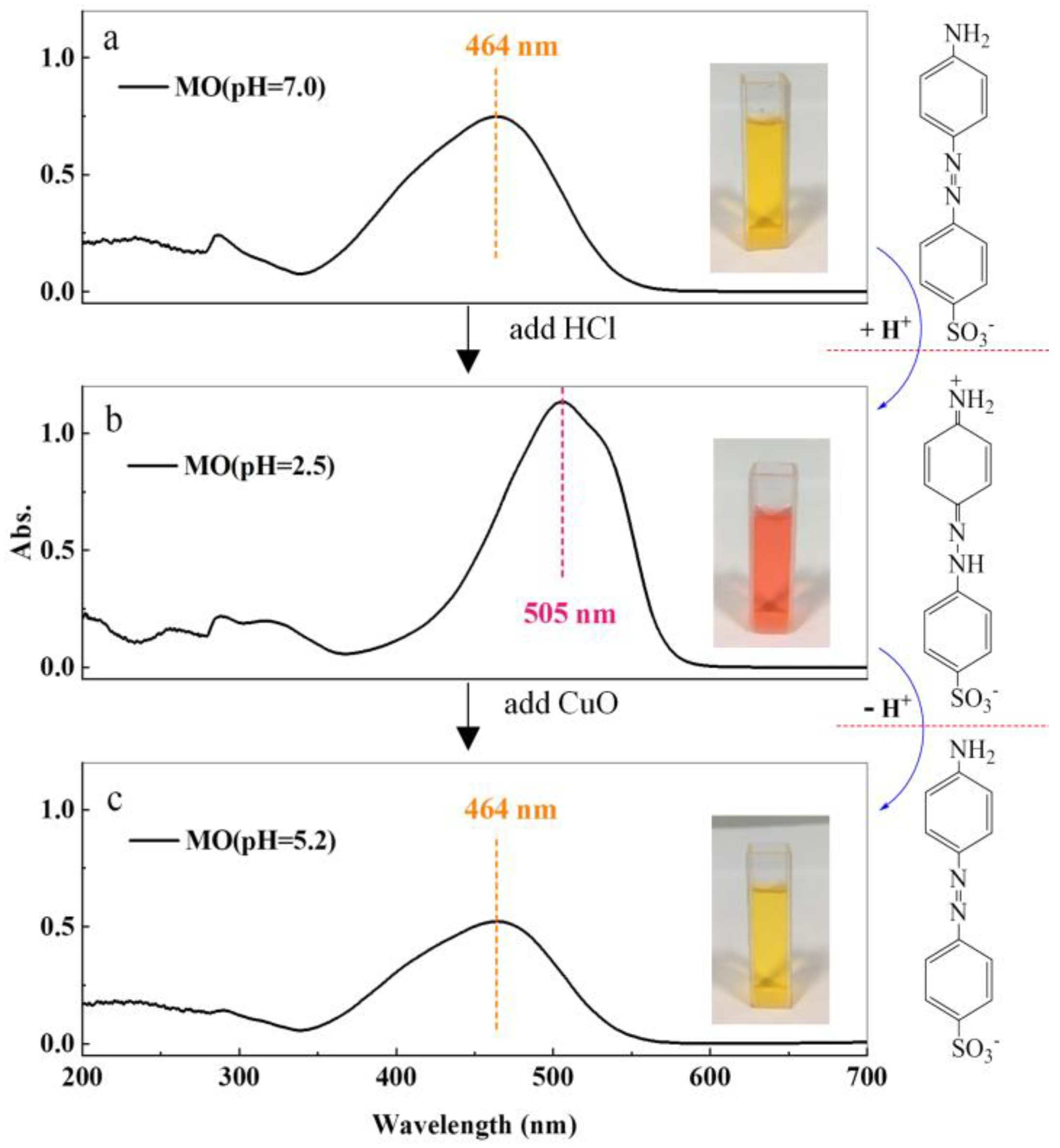
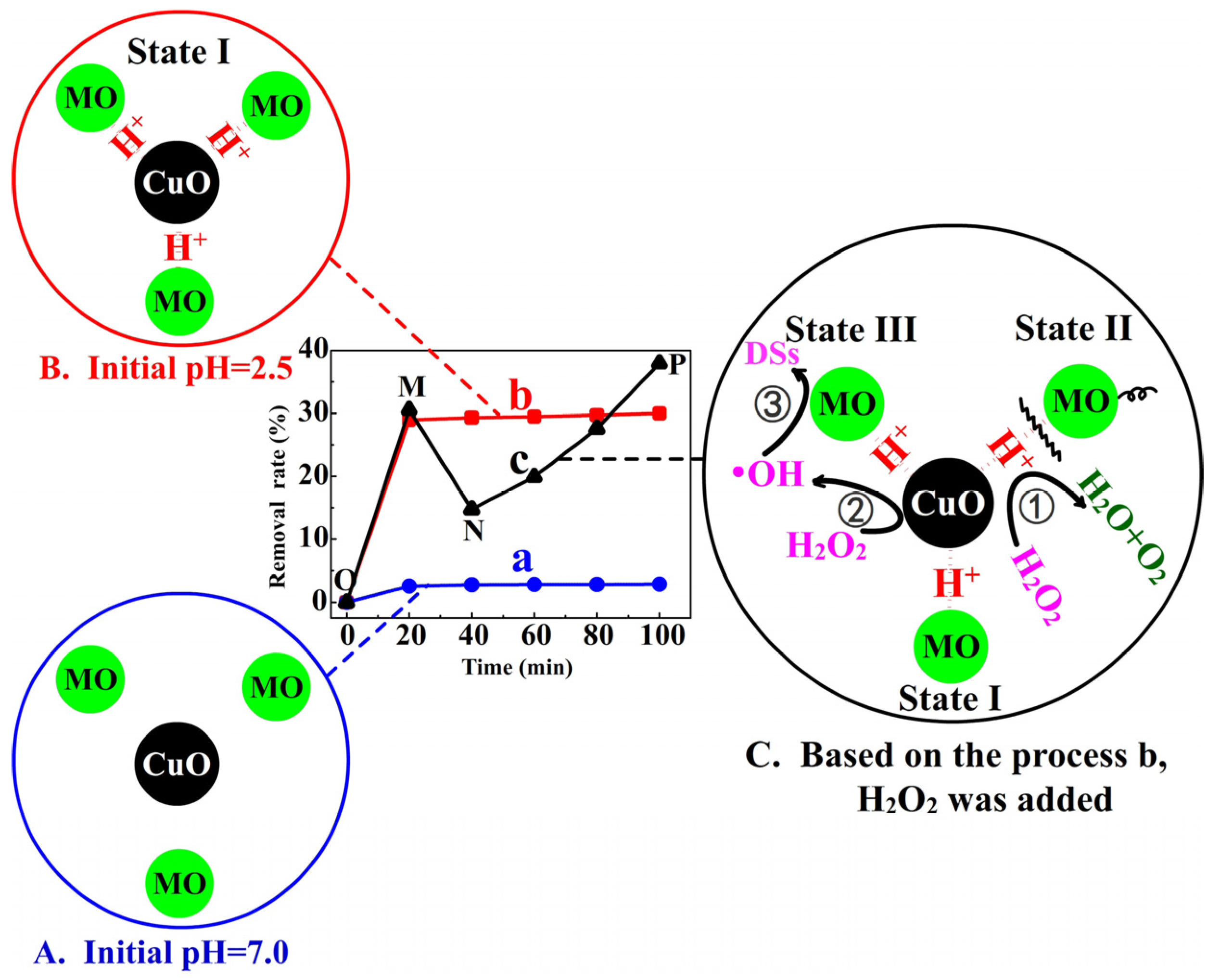
3.3. The Roles of H+ Ions and H2O2
- reaction ①:
- reaction ②
- reaction ③
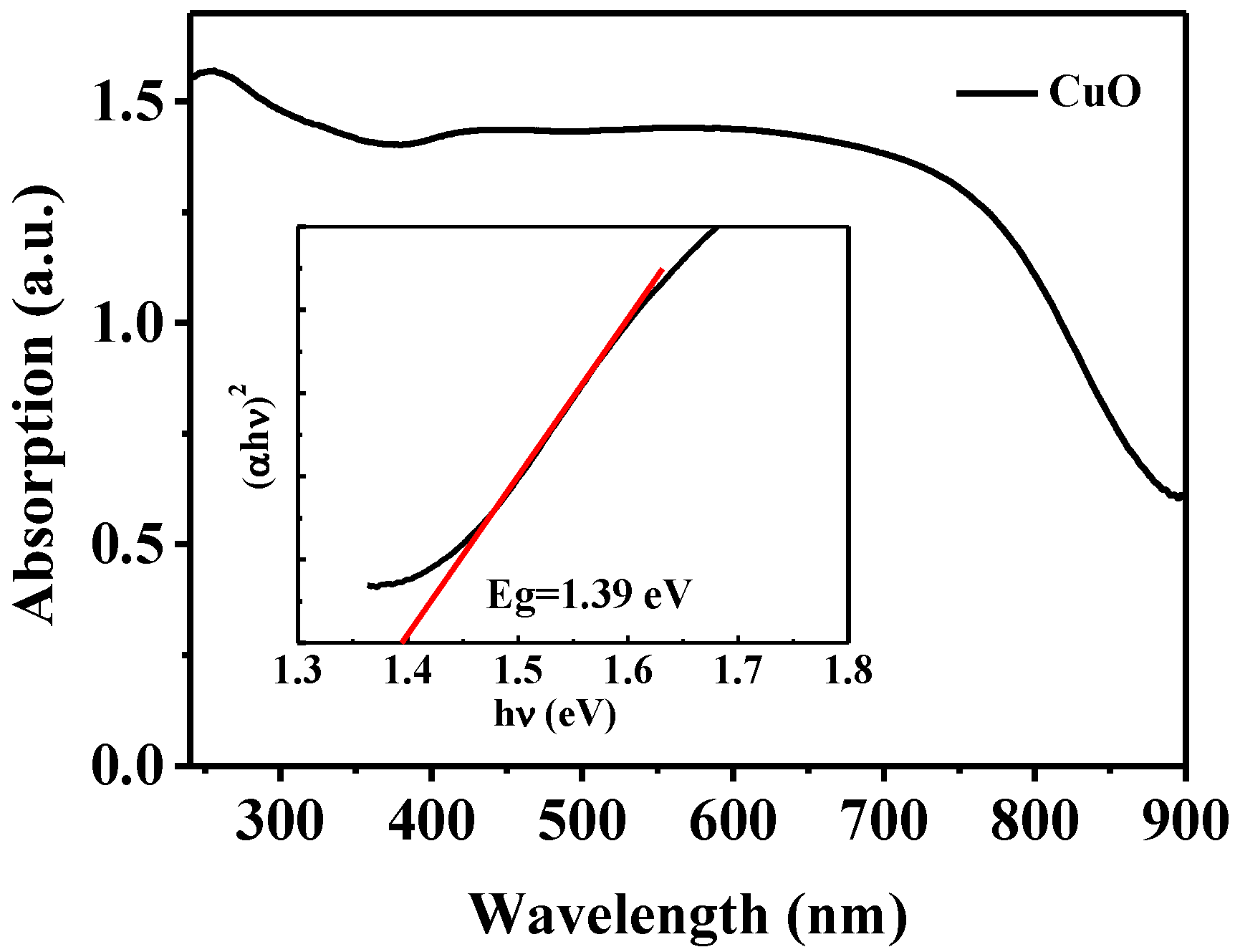
3.4. The Optical Properties of CuO
3.5. Photocatalytic Degradation of MO
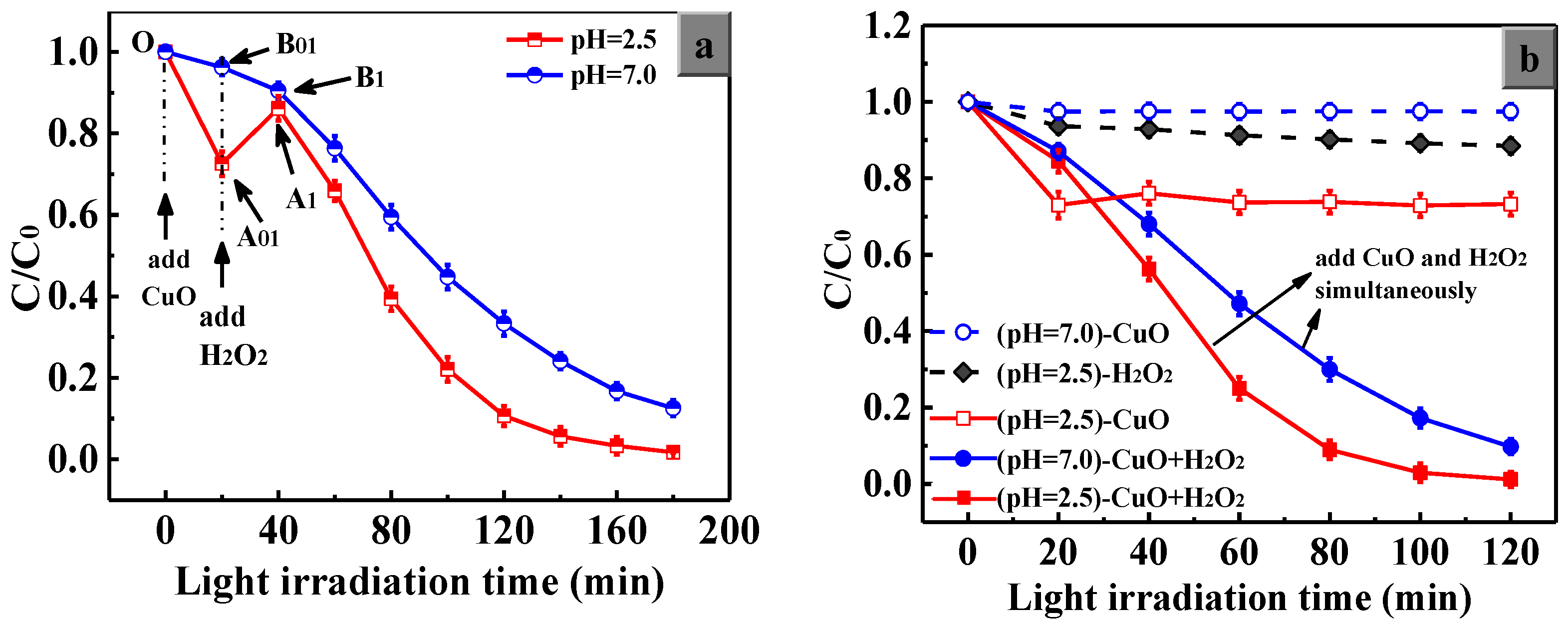
3.5.1. The Synergistic Effect of H2O2 and CuO
3.5.2. The Positive Effect of H+ Ions on the Catalytic Behavior of CuO
3.5.3. The Advantage of Simultaneous Addition of CuO and H2O2
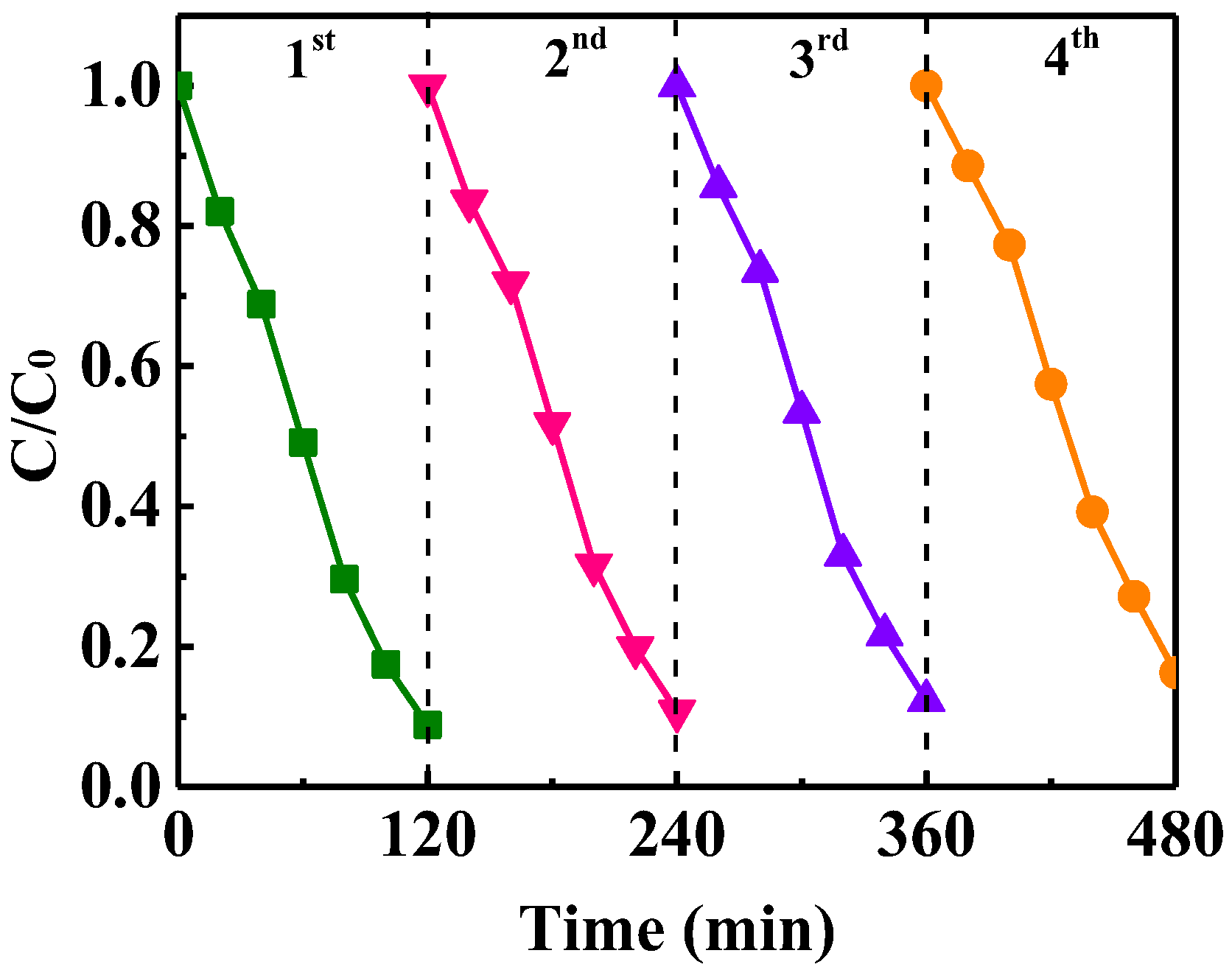
3.6. The Stability and Reusability of CuO
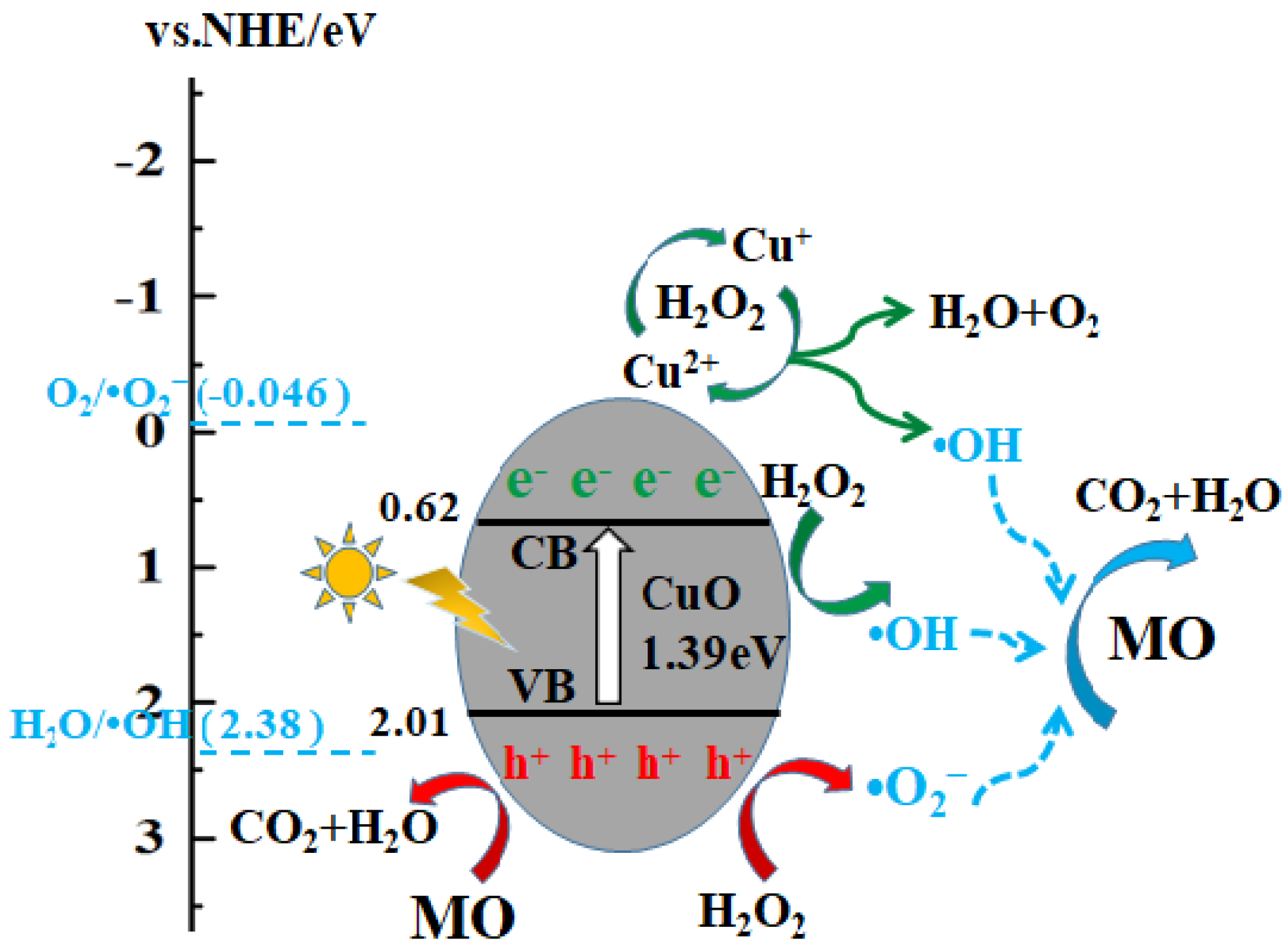
3.7. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochiai, T.; Fujishima, A. Photoelectrochemical Properties of TiO2 Photocatalyst and Its Applications for Environmental Purification. J. Photochem. Photobiol. C-Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, X.; Liu, W. The Synthesis Strategies and Photocatalytic Performances of TiO2/MOFs Composites: A State-of-the-Art Review. Chem. Eng. J. 2020, 391, 123601. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Ng, Y.H.; Ok, Y.S. Recent Advances in Photodegradation of Antibiotic Residues in Water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A Review on TiO2-Based Z-Scheme Photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for Degradation of Dyes in Industrial Effluents: Opportunities and Challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Abdel-Kader Ahmed, S.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Photocatalytic Degradation of Malachite Green Dye Using Chitosan Supported ZnO and Ce–ZnO Nano-Flowers under Visible Light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef]
- Smaali, A.; Berkani, M.; Merouane, F.; Le, V.T.; Vasseghian, Y.; Rahim, N.; Kouachi, M. Photocatalytic-Persulfate- Oxidation for Diclofenac Removal from Aqueous Solutions: Modeling, Optimization and Biotoxicity Test Assessment. Chemosphere 2021, 266, 129158. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Kumar, B.G.; Rajendran, S.; Qin, J.; Vadivel, S.; Durgalakshmi, D.; Gracia, F.; Soto-Moscoso, M.; Orooji, Y.; Karimi, F. Tuning of Metal Oxides Photocatalytic Performance Using Ag Nanoparticles Integration. J. Mol. Liq. 2020, 314, 113588. [Google Scholar] [CrossRef]
- Moussa, H.; Girot, E.; Mozet, K.; Alem, H.; Medjahdi, G.; Schneider, R. ZnO Rods/Reduced Graphene Oxide Composites Prepared via a Solvothermal Reaction for Efficient Sunlight-Driven Photocatalysis. Appl. Catal. B Environ. 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Nolan, M.; Iwaszuk, A.; Lucid, A.K.; Carey, J.J.; Fronzi, M. Design of Novel Visible Light Active Photocatalyst Materials: Surface Modified TiO2. Adv. Mater. 2016, 28, 5425–5446. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Q.; Zhang, G.; Liang, Y.; Wang, B.; Zhang, W.; Lei, B.; Wang, W. A Facile Room Temperature Solution-Phase Route to Synthesize CuO Nanowires with Enhanced Photocatalytic Performance. Mater. Lett. 2012, 74, 217–219. [Google Scholar] [CrossRef]
- Novikova, A.A.; Moiseeva, D.Y.; Karyukov, E.V.; Kalinichenko, A.A. Facile Preparation Photocatalytically Active CuO Plate-like Nanoparticles from Brochantite. Mater. Lett. 2016, 167, 165–169. [Google Scholar] [CrossRef]
- Kuz’menko, A.B.; van der Marel, D.; van Bentum, P.J.M.; Tishchenko, E.A.; Presura, C.; Bush, A.A. Infrared Spectroscopic Study of CuO: Signatures of Strong Spin-Phonon Interaction and Structural Distortion. Phys. Rev. B 2001, 63, 094303. [Google Scholar] [CrossRef] [Green Version]
- Siavash Moakhar, R.; Hosseini-Hosseinabad, S.M.; Masudy-Panah, S.; Seza, A.; Jalali, M.; Fallah-Arani, H.; Dabir, F.; Gholipour, S.; Abdi, Y.; Bagheri-Hariri, M.; et al. Photoelectrochemical Water-Splitting Using CuO-Based Electrodes for Hydrogen Production: A Review. Adv. Mater. 2021, 33, 2007285. [Google Scholar] [CrossRef]
- Sharma, K.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, P.; Kumar, R.; Thakur, V.K.; Nguyen, V.-H.; Singh, P. Fabrication of Efficient CuO/Graphitic Carbon Nitride Based Heterogeneous Photo-Fenton like Catalyst for Degradation of 2, 4 Dimethyl Phenol. Process Saf. Environ. Protect. 2020, 142, 63–75. [Google Scholar] [CrossRef]
- Zhu, G.; Jin, Y.; Ge, M. Simple Preparation of a CuO@gamma-Al2O3 Fenton-like Catalyst and Its Photocatalytic Degradation Function. Environ. Sci. Pollut. Res. 2022, 29, 68636–68651. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Takas, M.E. Synthesis and Characterization of Porous Clay Heterostructure Intercalated with CuO Nanoparticles as a Visible Light-Driven Photocatalyst. J. Iran. Chem. Soc. 2019, 16, 45–55. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Layered Double Hydroxides-Based Photocatalysts and Visible-Light Driven Photodegradation of Organic Pollutants: A Review. Chem. Eng. J. 2020, 392, 123684. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of Chloroform and Other Organic Molecules in Aqueous TiO Sub 2 Suspensions. Environ. Sci. Technol. (USA) 1991, 25, 3. [Google Scholar] [CrossRef]
- Cao, F.; Wang, T.; Ji, X. Enhanced Visible Photocatalytic Activity of Tree-like ZnO/CuO Nanostructure on Cu Foam. Appl. Surf. Sci. 2019, 471, 417–424. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, J.; Han, D.; Liu, X.; Gao, X.; Jiang, Y.; Xie, K. Highly Efficient Photocatalytic Removal of Methylene Blue by Lamellar Structured Nanocrystalline and Amorphous CuO. Mater. Lett. 2020, 276, 128217. [Google Scholar] [CrossRef]
- Rao, M.P.; Wu, J.J.; Asiri, A.M.; Anandan, S. Photocatalytic Degradation of Tartrazine Dye Using CuO Straw-Sheaf-like Nanostructures. Water Sci. Technol. 2017, 75, 1421–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, P.; Yang, Y.; Yin, Z.; Kang, F.; Fan, W.; Sheng, J.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. A Critical Review on Bismuth Oxyhalide Based Photocatalysis for Pharmaceutical Active Compounds Degradation: Modifications, Reactive Sites, and Challenges. J. Hazard. Mater. 2021, 412, 125186. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, D.; Gupta, A.; Balapure, A.; Ghugal, S.G.; Shende, A.G.; Umare, S.S. 2D/2D Wg-C3N4/g-C3N4 Composite as “Adsorb and Shuttle” Model Photocatalyst for Pollution Mitigation. J. Photochem. Photobiol. A-Chem. 2019, 370, 117–126. [Google Scholar] [CrossRef]
- Lei, J.F.; Li, L.B.; Du, K.; Ni, J.; Zhang, S.F.; Zhao, L.Z. Thermo-Catalytic Decomposition of Formaldehyde: A Novel Approach to Produce Mesoporous ZnO for Enhanced Photocatalytic Activities. Nanotechnology 2014, 25, 255701. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Dejun, W.; Wang, Y.; Liu, F. ˙OH-Initiated Heterogeneous Oxidation of Methyl Orange Using an Fe–Ce/MCM-41 Catalyst. RSC Adv. 2016, 6, 18800–18808. [Google Scholar] [CrossRef]
- Mondal, M.; Halder, M.; Pradhan, S.K. Nanoplate like Heterostructured BiOBr/BiBr/FeBr2 Nanocomposites with Enhanced Photocatalytic Activity for Wastewater Treatment by Removing Organic Dyes: Interfacial Consecutive Dual Z Scheme Electron Transfer. J. Environ. Chem. Eng. 2022, 10, 107240. [Google Scholar] [CrossRef]
- Vaez, Z.; Javanbakht, V. Synthesis, Characterization and Photocatalytic Activity of ZSM-5/ZnO Nanocomposite Modified by Ag Nanoparticles for Methyl Orange Degradation. J. Photochem. Photobiol. A-Chem. 2020, 388, 112064. [Google Scholar] [CrossRef]
- Li, L.; Sun, X.; Xian, T.; Gao, H.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Template-Free Synthesis of Bi2O2CO3 Hierarchical Nanotubes Self-Assembled from Ordered Nanoplates for Promising Photocatalytic Applications. Phys. Chem. Chem. Phys. 2022, 24, 8279–8295. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, T.; Ye, X.; Li, Q.; Guo, M.; Liu, H.; Wu, Z. Dye Adsorption by Resins: Effect of Ionic Strength on Hydrophobic and Electrostatic Interactions. Chem. Eng. J. 2013, 228, 392–397. [Google Scholar] [CrossRef]
- Segal, S.R.; Suib, S.L.; Foland, L. Decomposition of Pinacyanol Chloride Dye Using Several Manganese Oxide Catalysts. Chem. Mater. 1997, 9, 2526–2532. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Wang, C.-P.; Chen, P.-C.; Lin, K.-Y.A.; Ghosh, S.; Huang, C.-W.; Nguyen, V.-H. Perovskite Zinc Titanate Photocatalysts Synthesized by the Sol–Gel Method and Their Application in the Photocatalytic Degradation of Emerging Contaminants. Catalysts 2021, 11, 854. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Comprehensive Kinetic and Mass Transfer Modeling for Methylene Blue Dye Adsorption onto CuO Nanoparticles Loaded on Nanoporous Activated Carbon Prepared from Waste Coconut Shell. J. Mol. Liq. 2020, 307, 112949. [Google Scholar] [CrossRef]
- Rashad, M.; Al-Aoh, H.A. Promising Adsorption Studies of Bromophenol Blue Using Copper Oxide Nanoparticles. DWT 2019, 139, 360–368. [Google Scholar] [CrossRef]
- Fatima, B.; Siddiqui, S.I.; Ahmed, R.; Chaudhry, S.A. Preparation of Functionalized-CuO Nanoparticles Using Brassica Rapa Leave Extract for Water Purification. DWT 2019, 164, 192–205. [Google Scholar] [CrossRef]
- Deka, P.; Hazarika, A.; Deka, R.C.; Bharali, P. Influence of CuO Morphology on the Enhanced Catalytic Degradation of Methylene Blue and Methyl Orange. RSC Adv. 2016, 6, 95292–95305. [Google Scholar] [CrossRef]
- Bandara, J.; Kiwi, J.; Pulgarin, C.; Peringer, P.; Pajonk, G.-M.; Elaloui, A.; Albers, P. Novel Cyclic Process Mediated by Copper Oxides Active in the Degradation of Nitrophenol: Implications for the Natural Cycle. Environ. Sci. Technol. 1996, 30, 1261–1267. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P. Degradation and Mineralization of Methylene Blue Using a Heterogeneous Photo-Fenton Catalyst under Visible and Solar Light Irradiation. Catal. Sci. Technol. 2016, 6, 1222–1232. [Google Scholar] [CrossRef]
- Yin, Y.; Lv, R.; Li, X.; Lv, L.; Zhang, W. Exploring the Mechanism of ZrO2 Structure Features on H2O2 Activation in Zr-Fe Bimetallic Catalyst. Appl. Catal. B-Environ. 2021, 299, 120685. [Google Scholar] [CrossRef]
- Qian, X.; Ren, M.; Fang, M.; Kan, M.; Yue, D.; Bian, Z.; Li, H.; Jia, J.; Zhao, Y. Hydrophilic Mesoporous Carbon as Iron(III)/(II) Electron Shuttle for Visible Light Enhanced Fenton-like Degradation of Organic Pollutants. Appl. Catal. B-Environ. 2018, 231, 108–114. [Google Scholar] [CrossRef]
- Chen, F.; Guo, J.; Meng, D.; Wu, Y.; Sun, R.; Zhao, C. Strong Pyro-Electro-Chemical Coupling of Elbaite/H2O2 System for Pyrocatalysis Dye Wastewater. Catalysts 2021, 11, 1370. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bhunia, H.; Bajpai, P.K. Photocatalytic Decolorization Kinetics and Mineralization of Reactive Black 5 Aqueous Solution by UV/TiO2 Nanoparticles. CLEAN–Soil Air Water 2012, 40, 1290–1296. [Google Scholar] [CrossRef]
- Pourshirband, N.; Nezamzadeh-Ejhieh, A. An Efficient Z-Scheme CdS/g-C3N4 Nano Catalyst in Methyl Orange Photodegradation: Focus on the Scavenging Agent and Mechanism. J. Mol. Liq. 2021, 335, 116543. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Y.; Gu, S.; Mei, S.; Zhu, G.; Yu, M.; Wu, Y.; Ping, Y.; Hong, K.; Zhang, J.; et al. Degradation of Hexavalent Chromium and Methyl Orange by the Synergistic System of Graphitic Carbon Nitride and Electron Beam Irradiation. Chemosphere 2022, 287, 132228. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Cheng, F.; Dai, P. Efficient Removal of Dye from an Aqueous Phase Using Activated Carbon Supported Ferrihydrite as Heterogeneous Fenton-like Catalyst under Assistance of Microwave Irradiation. J. Taiwan Inst. Chem. Eng. 2016, 60, 376–382. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The Absolute Energy Positions of Conduction and Valence Bands of Selected Semiconducting Minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
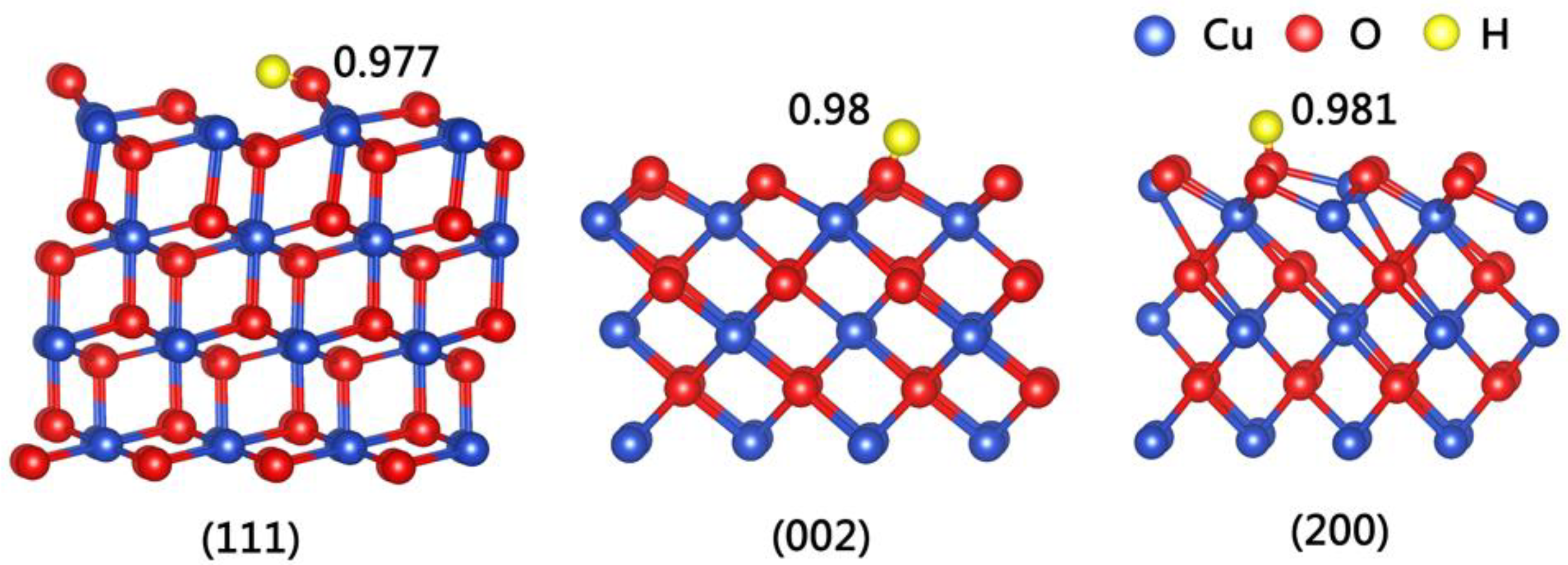

| Planes | O–H (nm) | Eads (eV) |
|---|---|---|
| (111) | 0.977 | −4.31 |
| (101) | 0.980 | −4.53 |
| (011) | 0.974 | −4.52 |
| (002) | 0.980 | −5.40 |
| (200) | 0.981 | −5.49 |
| Case | kapp (×10−2) | Intercept | R2 |
|---|---|---|---|
| (pH = 2.5)-orderly | 3.0 | −1.49 | 0.998 |
| (pH = 7.0)-orderly | 1.4 | −0.57 | 0.997 |
| (pH = 2.5)-simultanous | 4.9 | −1.47 | 0.996 |
| (pH = 7.0)-simultanous | 2.4 | −0.67 | 0.989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, J.; Lei, J.; Wang, Z.; Huang, L.; Zhu, H.; Liu, H.; Hu, F.; Qu, T.; Yang, H.; Yang, H.; et al. The Ultrahigh Adsorption Capacity and Excellent Photocatalytic Degradation Activity of Mesoporous CuO with Novel Architecture. Nanomaterials 2023, 13, 142. https://doi.org/10.3390/nano13010142
Ni J, Lei J, Wang Z, Huang L, Zhu H, Liu H, Hu F, Qu T, Yang H, Yang H, et al. The Ultrahigh Adsorption Capacity and Excellent Photocatalytic Degradation Activity of Mesoporous CuO with Novel Architecture. Nanomaterials. 2023; 13(1):142. https://doi.org/10.3390/nano13010142
Chicago/Turabian StyleNi, Jing, Jianfei Lei, Zhaowu Wang, Lanlan Huang, Hang Zhu, Hai Liu, Fuqiang Hu, Ting Qu, Huiyu Yang, Haiyang Yang, and et al. 2023. "The Ultrahigh Adsorption Capacity and Excellent Photocatalytic Degradation Activity of Mesoporous CuO with Novel Architecture" Nanomaterials 13, no. 1: 142. https://doi.org/10.3390/nano13010142
APA StyleNi, J., Lei, J., Wang, Z., Huang, L., Zhu, H., Liu, H., Hu, F., Qu, T., Yang, H., Yang, H., & Gong, C. (2023). The Ultrahigh Adsorption Capacity and Excellent Photocatalytic Degradation Activity of Mesoporous CuO with Novel Architecture. Nanomaterials, 13(1), 142. https://doi.org/10.3390/nano13010142






