Band Gap Tuning in Transition Metal and Rare-Earth-Ion-Doped TiO2, CeO2, and SnO2 Nanoparticles
Abstract
1. Introduction
2. Model and Method
3. Results and Discussion
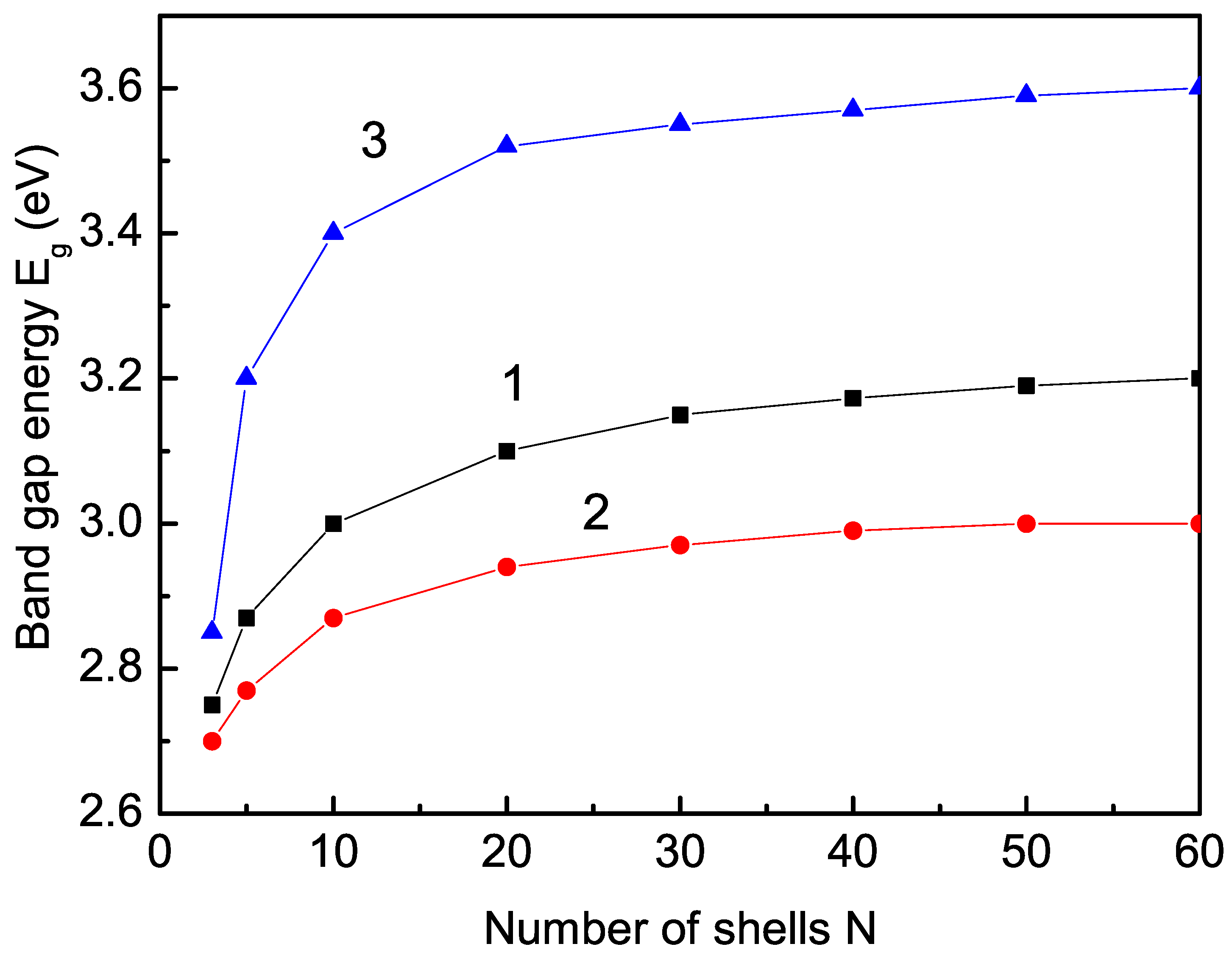
3.1. Size Dependence of the Band-Gap Energy
3.2. Ion-Doping Dependence of the Band-Gap Energy
3.2.1. Ion-Doping Dependence of the Band-Edge Energies
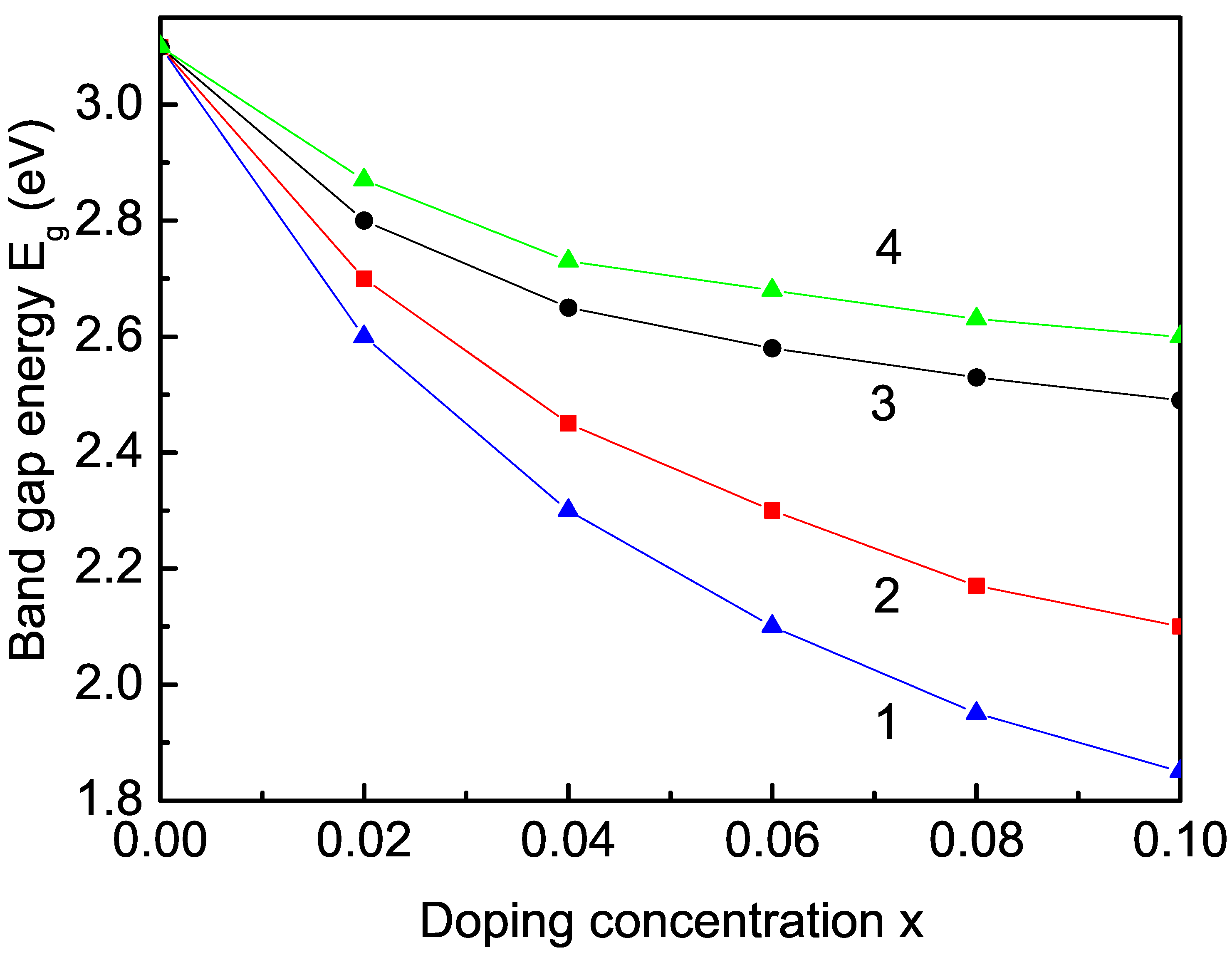
3.2.2. Transition-Metal-Ion (Co, Fe, Mn, and Cu) Doping Effect on the Band-Gap Energy
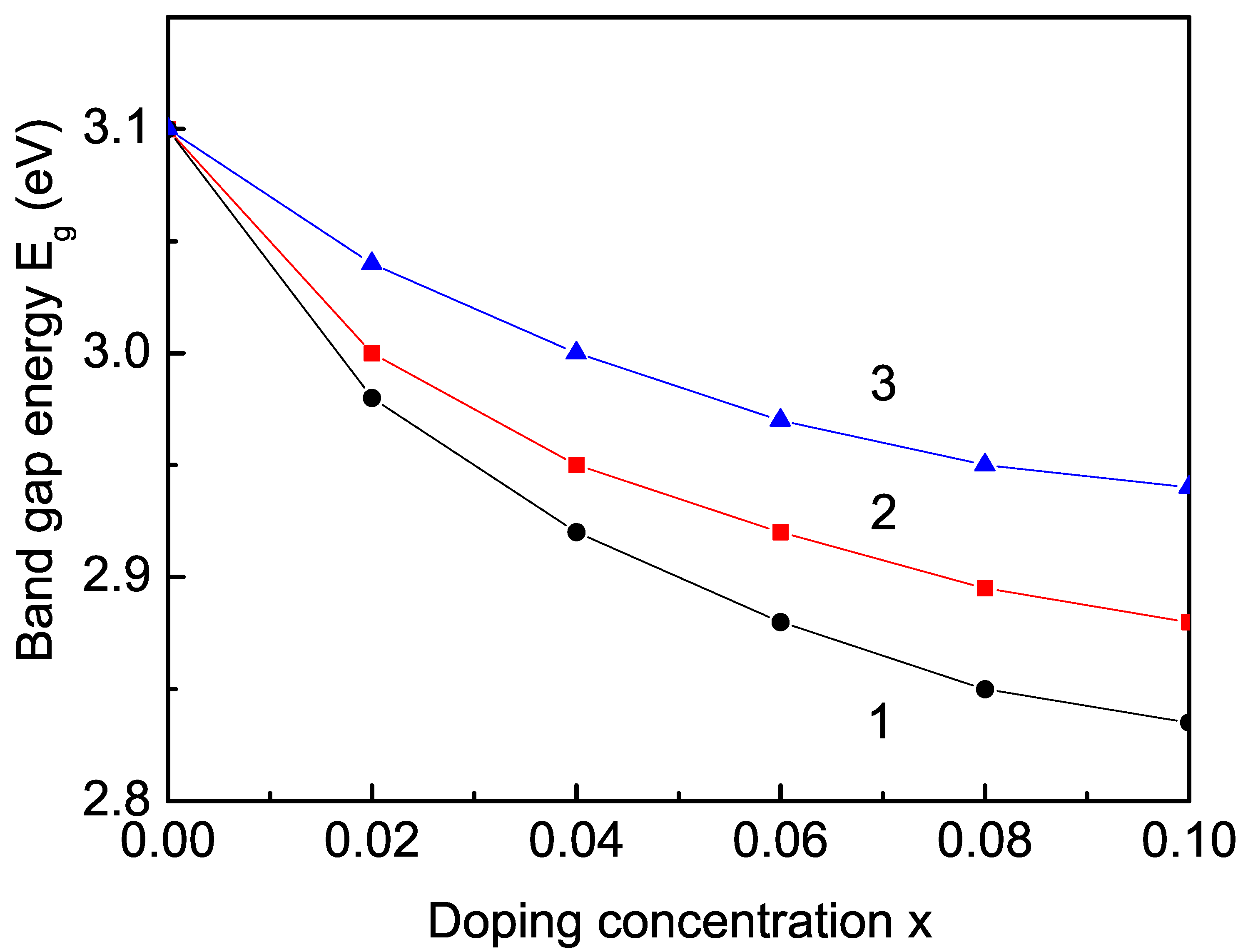
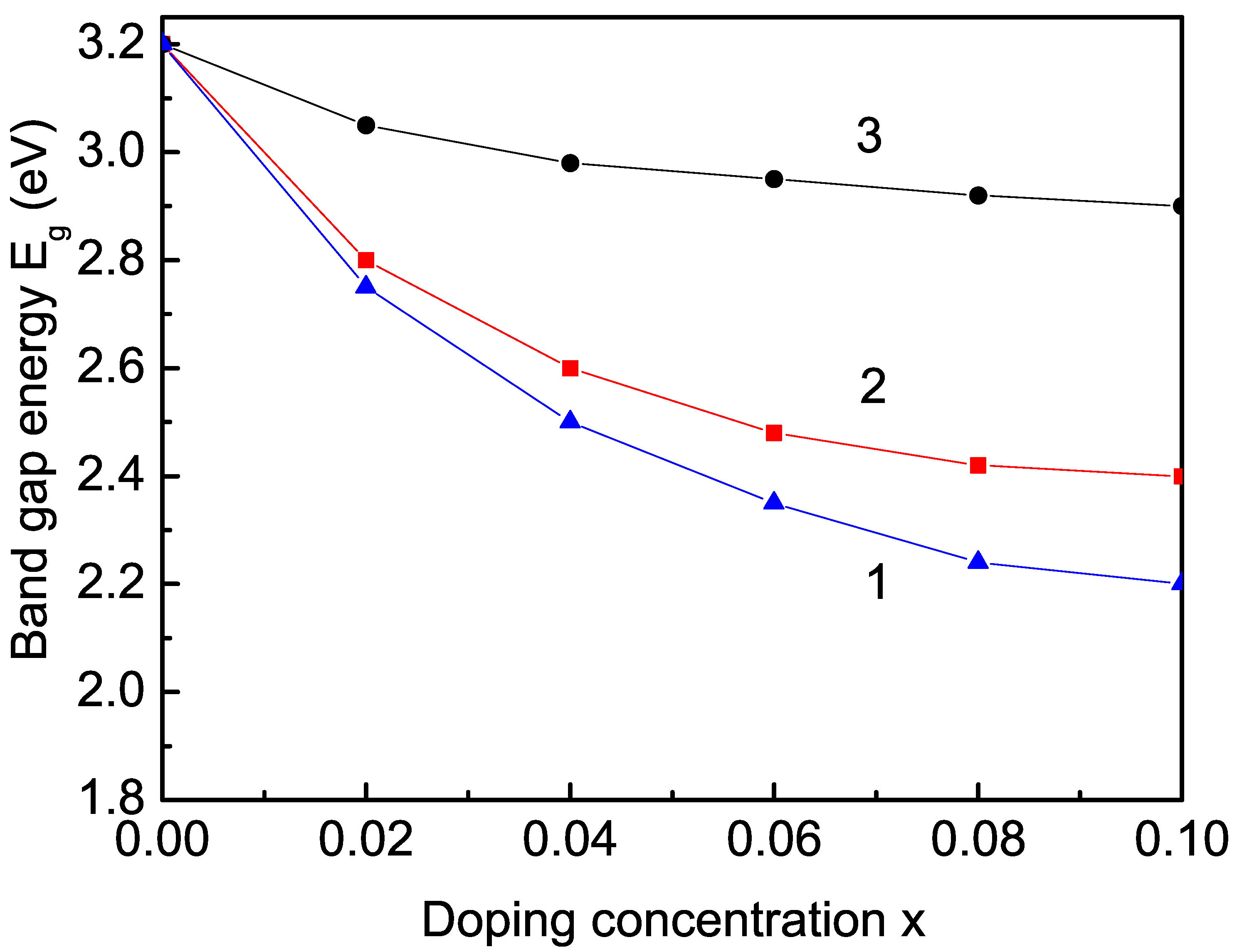
3.2.3. Rare Earth (Sm, Tb, and Er) Ion Doping Effects on the Band-Gap Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sundaresan, A.; Rao, C.N.R. Ferromagnetism as a Universal Feature of Inorganic Nanoparticles. Nano Today 2009, 4, 96. [Google Scholar] [CrossRef]
- Chang, G.S.; Forrest, J.; Kurmaev, E.Z.; Morozovska, A.N.; Glinchuk, M.D.; McLeod, J.A.; Moewes, A.; Surkova, T.P.; Hong, N.H. Oxygen-vacancy-induced ferromagnetism in undoped SnO2 thin films. Phys. Rev. B 2012, 85, 165319. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Lee, C.G. Structural and Magnetic Properties of Co Doped CeO2 Nano-Particles. J. Nanosci. Nanotechnol. 2010, 10, 7204–7207. [Google Scholar] [CrossRef] [PubMed]
- Apostolov, A.T.; Apostolova, I.N.; Wesselinowa, J.M. A comparative study of the magnetization in transition metal ion-doped CeO2, TiO2 and SnO2 nanoparticles. Phys. E 2018, 99, 202–207. [Google Scholar] [CrossRef]
- Apostolov, A.T.; Apostolova, I.N.; Wesselinowa, J.M. Magnetic Properties of Rare Earth Doped SnO2, TiO2 and CeO2 Nanoparticles. Phys. Status Solidi B 2018, 255, 1800179. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X. Machine Learning Band Gaps of Doped-TiO2 Photocatalysts from Structural and Morphological Parameters. ACS Omega 2020, 25, 15344–15352. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Le, T.T.B.; Nguyen, N.L. The preparation of SnO2 and SnO2:Sb nanopowders by a hydrothermal method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 025002. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Lee, J.; Cho, M.H. Band Gap Engineering of CeO2 Nanostructure by Electrochemically Active Biofilm for Visible Light Applications. RSC Adv. 2014, 4, 16782–16791. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Ji, Z.; Henderson, B.L.; Xia, T.; Li, L.J.; Zink, J.I.; Nel, A.E.; Maedler, L. Role of Fe Doping in Tuning the Band Gap of TiO2 for the Photo-Oxidation-Induced Cytotoxicity Paradigm. J. Am. Chem. Soc. 2011, 133, 11270–11278. [Google Scholar] [CrossRef]
- Nebi, M.; Peker, D.; Temel, S. Deposition of Co doped TiO2 films using sol gel spin coating technique and investigation of band gap. AIP Conf. Proc. 2018, 1935, 150004. [Google Scholar]
- Wu, T.S.; Chen, Y.W.; Weng, S.C.; Lin, C.N.; Chang, C.; Soo, Y.L. Dramatic band gap reduction incurred by dopant coordination rearrangement in Co-doped nanocrystals of CeO2. Sci. Rep. 2017, 7, 4715. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Varshney, M.; Kumar, S.; Verma, K.D.; Kumar, R. Magnetic properties of Fe and Ni doped SnO2 nanoparticles. Nanomater. Nanotechnol. 2011, 1, 29–33. [Google Scholar] [CrossRef]
- Kumari, K.; Aljawfi, R.N.; Vij, A.; Chae, K.H.; Hashim, M.; Alvi, P.A.; Kumar, S. Band gap engineering, electronic state and local atomic structure of Ni doped CeO2 nanoparticles. J. Mater. Sc. Mater. Electr. 2019, 30, 4562–4571. [Google Scholar] [CrossRef]
- Ungureanu, A.; Jitaru, I.; Gosnea, F. Mn-doped SnO2 prepared by a sol-gel method. UPB Sci. Bull. Series B 2013, 75, 43. [Google Scholar]
- Nakaruk, A.; Lin, C.Y.W.; Channei, D.; Koshy, P.; Sorrell, C.C. Fe-doped and Mn-doped titanium dioxide thin films. J. Sol-Gel Sci. Techn. 2012, 61, 175–178. [Google Scholar] [CrossRef]
- Deng, Q.R.; Xia, X.H.; Guo, M.L.; Gao, Y.; Shao, G. Mn-doped TiO2 nanopowders with remarkable visible light photocatalytic activity. Mater. Lett. 2011, 65, 2051–2054. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Chaudhary, R.P. Structural and photocatalytic studies of Mn-doped TiO2 nanoparticles. Spectrochim. Acta Part A 2012, 98, 256–264. [Google Scholar] [CrossRef]
- Sagadevan, S.; Chowdhury, Z.Z.; Johan, M.R.B.; Aziz, F.A.; Roselin, L.S.; Podder, J.; Lett, J.A.; Selvin, R. Cu-Doped SnO2 Nanoparticles: Synthesis and Properties. J. Nanosci. Nanotechnol. 2019, 19, 7139–7148. [Google Scholar] [CrossRef]
- Munir, S.; Shah, S.M.; Hussain, H.; Khan, R.A. Effect of carrier concentration on the optical band gap of TiO2 nanoparticles. Mater. Des. 2016, 92, 64–72. [Google Scholar] [CrossRef]
- Babu, M.H.; Dev, B.C.; Podder, J. Texture coefficient and band gap tailoring of Fe-doped SnO2 nanoparticles via thermal spray pyrolysis. Rare Metals 2022, 41, 1332–1341. [Google Scholar] [CrossRef]
- Poudel, M.B.; Ojhab, G.P.; Akim, A.; Kim, H.J. Manganese-doped tungsten disulfide microcones as binder-free electrode for high performance asymmetric supercapacitor. J. Energy Storage 2022, 47, 103674. [Google Scholar] [CrossRef]
- Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of Conducting Bifunctional Polyaniline/Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Salah, N.; Habib, S.; Azam, A.; Ansari, M.S.; Al-Shawafi, W.M. Formation of Mn-Doped SnO2 Nanoparticles Via the Microwave Technique: Structural, Optical and Electrical Properties. Nanomater. Nanotechnol. 2016, 6, 1. [Google Scholar] [CrossRef]
- Venugopal, B.; Nandan, L.; Ayyachamy, A.; Balaji, V.; Amirthapandian, S.; Panigrahi, B.K.; Paramasivam, T. Influence of manganese ions in the band gap of tin oxide nanoparticles: Structure, microstructure and optical studies. RSC Adv. 2014, 4, 6141. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Rare earth doped metal oxide nanoparticles for photocatalysis: A perspective. Nanotechn. 2022, 33, 142001. [Google Scholar] [CrossRef]
- Rehani, D.; Saxena, M.; Solanki, P.R.; Sharma, S.N. Transition Metal and Rare-Earth Metal Doping in SnO2 Nanoparticles. J. Supercond. Novel Magn. 2022, 35, 2573–2581. [Google Scholar] [CrossRef]
- Saqib, N.U.; Adnan, R.; Shah, I. A mini-review on rare earth metal-doped TiO2 for photocatalytic remediation of wastewater. Environ. Sci. Pollut. Res. Int. 2016, 23, 15941–15951. [Google Scholar] [CrossRef]
- Chandran, D.; Nair, L.S.; Balachandran, S.; Babu, K.R.; Deepa, M. Band gap narrowing and photocatalytic studies of Nd3+ ion-doped SnO2 NPs using solar energy. Bull. Mater. Sci. 2016, 39, 27–33. [Google Scholar] [CrossRef]
- Chen, X.; Luo, W. Optical spectroscopy of rare earth ion-doped TiO2 nanophosphors. J. Nanosci. Nanotechnol. 2010, 10, 1482–1494. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Tresa, S.A.; Jaseentha, O.P.; Varghese, T. Cerium doped nanotitania-extended spectral response for enhanced photocatalysis. Mater. Res. Express 2014, 1, 015003. [Google Scholar] [CrossRef]
- Song, L.; Zhao, X.; Cao, L.; Moon, J.-W.; Gu, B.; Wang, W. Synthesis Rare Earth Doped TiO2 Nanorods and Their Application in the Photocatalytic Degradation of Lignin. Nanoscale 2015, 7, 16695–16703. [Google Scholar] [CrossRef] [PubMed]
- Amritha, A.; Sundararajan, M.; Rejith, R.G.; Mohammed-Aslam, A. La-Ce doped TiO2 nanocrystals: A review on synthesis, characterization and photocatalytic activity. SN Appl. Sci. 2019, 1, 1441. [Google Scholar] [CrossRef]
- Long, R.; Dai, Y.; Huang, B. Geometric and Electronic Properties of Sn-Doped TiO2 from First-Principles Calculations. J. Phys. Chem. C 2009, 113, 650–653. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Revathy, V.R.; Rosmin, P.; Thrivedu, B.; Elsa, K.M.; Nimmymol, J.; Balakrishna, K.M.; Varghese, T. Influence of La doping on structural and optical properties of TiO2 nanocrystals. Mater. Character. 2016, 113, 144–151. [Google Scholar] [CrossRef]
- Mahanty, S.; Roy, S.; Sen, S. Effect of Sn doping on the structural and optical properties of sol-gel TiO2 thin films. J. Cryst. Growth 2004, 261, 77–81. [Google Scholar] [CrossRef]
- Lin, J.; Yu, J.C.; Lo, D.; Lam, S.K. Photocatalytic Activity of Rutile Ti1-xSnxO2 Solid Solutions. J. Catal. 1999, 183, 368–372. [Google Scholar] [CrossRef]
- Lin, C.Y.W.; Channei, D.; Koshy, P.; Nakaruk, A.; Sorrell, C.C. Multivalent Mn-doped TiO2 thin films. Phys. E 2012, 44, 1969–1972. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, J.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M.; Guo, L. A first-principles theoretical simulation on the electronic structures and optical absorption properties for O vacancy and Ni impurity in TiO2 photocatalysts. J. Phys. Chem. Solids 2010, 71, 1707–1712. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, P.; Wei, S.-H. Revisit of the band gaps of rutile SnO(2 and TiO2: A first-principles study. J. Semicond. 2019, 40, 092101. [Google Scholar] [CrossRef]
- Mendez-Galvan, M.; Celaya, C.A.; Jaramillo-Quintero, O.; Muniz, J.; Diaz, G.; Lara-Garcia, H.A. Tuning the band gap of M-doped titanate nanotubes (M = Fe, Co, Ni, and Cu): An experimental and theoretical study. Nanoscale Adv. 2021, 3, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Chetri, P.; Saikia, B.; Choudhury, A. Exploring the structural and magnetic properties of TiO2/SnO2 core/shell nanocomposite: An experimental and density functional study. J. Appl. Phys. 2013, 113, 233514. [Google Scholar] [CrossRef]
- Begna, W.B.; Gurmesa, G.S.; Zhang, Q.; Geffe, C.A. A DFT+U study of site dependent Fe-doped TiO2 diluted magnetic semiconductor material: Room-temperature ferromagnetism and improved semiconducting properties. AIP Adv. 2022, 12, 025002. [Google Scholar] [CrossRef]
- Shi, H.; Hussain, T.; Ahuja, R.; Kang, T.W.; Luo, W. Role of vacancies, light elements and rare-earth metals doping in CeO2. Sci. Rep. 2016, 6, 31345. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Song, M.; Suker, Z.; Zhao, X.; Dai, Q. Band gap calculation and photo catalytic activity of rare earths doped rutile TiO2. J. Rare Earths 2009, 27, 461–468. [Google Scholar] [CrossRef]
- Lamrani, A.F. Rare-earth-doped TiO2 rutile as a promising ferromagnetic alloy for visible light absorption in solar cells: First principle insights. RSC Adv. 2020, 10, 35505. [Google Scholar] [CrossRef]
- Xie, K.; Jia, Q.; Wang, Y.; Zhang, W.; Xu, J. The Electronic Structure and Optical Properties of Anatase TiO2 with Rare Earth Metal Dopants from First-Principles Calculations. Materials 2018, 11, 179. [Google Scholar] [CrossRef]
- Elk, K.; Gasser, W. Green’s Function Method in the Solid State Physics; Akademie: Berlin, Germany, 1979; p. 61. [Google Scholar]
- Ohtsuki, T.; Chainani, A.; Eguchi, R.; Matsunami, M.; Takata, Y.; Taguchi, M.; Nishino, Y.; Tamasaku, K.; Yabashi, M.; Ishikawa, T.; et al. Role of Ti 3 d Carriers in Mediating the Ferromagnetism of Co∶ TiO 2 Anatase Thin Films. Phys. Rev. Lett. 2011, 106, 047602. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Dalela, B.; Kumar, S.; Alvi, P.A.; Dalela, S. Role of Co doping on structural, optical and magnetic properties of TiO2. J. Alloy. Compd. 2013, 552, 274. [Google Scholar] [CrossRef]
- Sabri, N.S.; Deni, M.S.M.; Zakaria, A.; Talari, M.K. Effect of Mn Doping on Structural and Optical Properties of SnO2 Nanoparticles Prepared by Mechanochemical Processing. Phys. Procedia 2012, 25, 233–239. [Google Scholar] [CrossRef]
- Tserkovnikov, Y.A. Decoupling of chains of equations for two-time Green’s functions. Teor. Mat. Fiz. 1971, 7, 250. [Google Scholar] [CrossRef]
- Scarrozza, M.; Vindigni, A.; Barone, P.; Sessoli, R.; Picozzi, S. A combined first-principles and thermodynamic approach to M-Nitronyl Nitroxide (M=Co, Mn) spin helices. Phys. Rev. B 2015, 91, 144422. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, J.; Li, J.; Dong, P.; Wu, Z. Magnetism in Cu-Doped Rutile SnO2 Semiconductor Induced by the RKKY Interaction. J. Supercond. Nov. Mat. 2014, 27, 581. [Google Scholar] [CrossRef]
- Adhikari, R.; Das, A.K.; Karmakar, D.; Rao, T.V.C.; Ghatak, J. Structure and magnetism of Fe-doped SnO2 nanoparticles. Phys. Rev. B 2008, 78, 024404. [Google Scholar] [CrossRef]
- Peters, L.; Ghosh, S.; Sanyal, B.; van Dijk, C.; Bowlan, J.; de Heer, W.; Delin, A.; Marco, I.D.; Eriksson, O.; Katsnelson, M.I.; et al. Magnetism and exchange interaction of small rare-earth clusters; Tb as a representative. Sci. Rep. 2016, 6, 19676. [Google Scholar] [CrossRef]
- Maeda, A.; Sugimoto, H. Intramolecular exchange interaction between two rare-earth ions, RE = Sm3+-Yb3+ and Y3+. J. Chem. Soc. Faraday Trans. 1986, 82, 2019–2030. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakita, K.; Fujii, M. Microscopic origin of lattice contraction and expansion in undoped rutile TiO2 nanostructures. J. Phys. D 2014, 47, 215302. [Google Scholar] [CrossRef]
- Kalathil, S.; Khan, M.M.; Ansari, S.A.; Lee, J.; Cho, M.H. Band gap narrowing of titanium dioxide (TiO2) nanocrystals by electrochemically active biofilms and their visible light activity. Nanoscale 2013, 5, 6323–6326. [Google Scholar] [CrossRef]
- Morales-Garcia, A.; Escatllar, A.M.; Illas, F.; Bromley, S.T. Understanding the interplay between size, morphology and energy gap in photoactive TiO2 nanoparticles. Nanoscale 2019, 11, 9032–9041. [Google Scholar] [CrossRef]
- Dette, C.; Perez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Tatar, B.; Sam, E.D.; Kutlu, K.; Uergen, M. Synthesis and optical properties of CeO2 nanocrystalline films grown by pulsed electron beam deposition. J. Mater. Sci. 2008, 43, 5102–5108. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Sakthinathan, S.; Chen, S.-M.; Shanthi, K.; Sivasanker, S.; Sangeetha, P. Environmentally friendly synthesis of CeO2 nanoparticles for the catalytic oxidation of benzyl alcohol to benzaldehyde and selective detection of nitrite. Sci. Rep. 2017, 7, 46372. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Aziz, N.D.A.; Kasim, M.F.; Chayed, N.F.; Subban, R.H.Y.; Badar, N. Anomalies in wide band gap SnO2 nanostructures. J. Solid State Chem. 2019, 277, 271–280. [Google Scholar] [CrossRef]
- Asaithambi, S.; Murugan, R.; Sakthivel, P.; Karuppaiah, M.; Rajendran, S.; Ravi, G. Influence of Ni Doping in SnO2 Nanoparticles with Enhanced Visible Light Photocatalytic Activity for Degradation of Methylene Blue Dye. J. Nanosci. Nanotechn. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Fujisawa, J.; Eda, T.; Hanaya, M. Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 2017, 685, 23–26. [Google Scholar] [CrossRef]
- Dorenbos, P. The Electronic Structure of Lanthanide Impurities in TiO2, ZnO, SnO2, and Related Compounds. ECS J. Solid State Sci. Techn. 2014, 3, R19–R24. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and Photoinduced Hydrophilicity of Various Metal Oxide Thin Films. Chem. Mater. 2002, 14, 2812. [Google Scholar] [CrossRef]
- Kikoin, K.; Fleurov, V. On the nature of ferromagnetism in non-stoichiometric TiO2 doped with transition metals. J. Magn. Magn. Mater. 2007, 310, 2097–2098. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Xia, X.; Fan, R.; Su, T.; Shi, Y.; Yu, J.; Lia, L.; Jiang, Y. Band edge movement in dye sensitized Sm-doped TiO2 solar cells: A study by variable temperature spectroelectrochemistry. RSC Adv. 2015, 5, 70512–70521. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; You, W.; Lei, G.; Cao, Y.; Zhang, Y.; Jiang, L. Construction of Fe-doped TiO2-x ultrathin nanosheets with rich oxygen vacancies for highly efficient oxidation of H2S. Chem. Eng. J. 2022, 430 Pt 2, 132917. [Google Scholar] [CrossRef]
- Wu, H.-C.; Li, S.-H.; Lin, S.-W. Effect of Fe Concentration on Fe-Doped Anatase TiO2 from GGA+U Calculations. Intern. J. Photoen. 2012, 2012, 823498. [Google Scholar] [CrossRef]
- Akshay, V.R.; Arun, B.; Dash, S.; Patra, A.K.; Mandal, G.; Mutta, G.R.; Chanda, A.; Vasundhara, M. Defect mediated mechanism in undoped, Cu and Zn-doped TiO2 nanocrystals for tailoring the band gap and magnetic properties. RSC Adv. 2018, 8, 41994. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193. [Google Scholar] [CrossRef]
- Soni, S.; Vats, V.S.; Kumar, S.; Dalela, B.; Mishra, M.; Meena, R.S.; Gupta, G.; Alvi, P.A.; Dalela, S. Structural, optical and magnetic properties of Fe-doped CeO2 samples probed using X-ray photoelectron spectroscopy. J. Mater. Sci. Mater. Electr. 2018, 29, 10141–10153. [Google Scholar] [CrossRef]
- Xue, M.; Liu, X.; Wu, H.; Yu, B.; Meng, F. Construction of Cu2+-doped CeO2 nanocrystals hierarchical hollow structure and its enhanced photocatalytic performance. J. Mater. Sci. Mater. Electr. 2021, 32, 27576–27586. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Dong, C.-L.; Lu, Y.-R.; Huang, Y.-C.; Chen, C.-L.; Saravanan, P.; Asokan, K.; Kumar, R.T.R. Evolution of Visible Photocatalytic Properties of Cu-Doped CeO2 Nanoparticles: Role of Cu2+-Mediated Oxygen Vacancies and the Mixed-Valence States of Ce Ions. ACS Sust. Chem. Eng. 2018, 6, 8536–8546. [Google Scholar] [CrossRef]
- Arif, M.; Shah, M.Z.U.; Ahmad, S.A.; Shah, M.S.; Ali, Z.; Ullah, A.; Idrees, M.; Zeb, J.; Song, P.; Huang, T.; et al. High photocatalytic performance of copper-doped SnO2 nanoparticles in degradation of Rhodamine B dye. Optical Mater. A 2022, 134, 113135. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855. [Google Scholar] [CrossRef]
- Chatterjee, P.; Mukherjee, D.; Sarkar, A.; Chakraborty, A.K. Mn-doped CeO2-CNT nanohybrid for removal of water soluble organic dyes. Appl. Nanosci. 2022, 12, 3031–3043. [Google Scholar] [CrossRef]
- Mohanta, D.; Barman, K.; Jasimuddin, S.; Ahmaruzzaman, M. MnO doped SnO2 nanocatalysts: Activation of wide band gap semiconducting nanomaterials towards visible light induced photoelectrocatalytic water oxidation. J. Colloid. Interface Sci. 2017, 505, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Habib, S.S.; Naqvi, A.H. Effect of Mn doping on the structural and optical properties of SnO2 nanoparticles. J. Alloy. Compd. 2012, 523, 83–87. [Google Scholar] [CrossRef]
- Dimri, M.C.; Khanduri, H.; Kooskora, H.; Subbi, J.; Heinmaa, I.; Mere, A.; Krustok, J.; Stern, R. Ferromagnetism in rare earth doped cerium oxide bulk samples. Phys. Status Solidi A 2012, 209, 353. [Google Scholar] [CrossRef]
- Dinkar, V.A.; Shridhar, S.J.; Madhukar, E.N.; Anil, E.A.; Nitin, H.K. Sm-Doped TiO2 Nanoparticles with High Photocatalytic Activity for ARS Dye Under Visible Light Synthesized by Ultrasonic Assisted Sol-Gel Method. Orient J. Chem. 2016, 32, 933. [Google Scholar] [CrossRef]
- Mandal, B.; Mondal, A.; Ray, S.S.; Kundu, A. Sm-doped mesoporous CeO2 nanocrystals: Aqueous solution-based surfactant assisted low temperature synthesis, characterization and their improved autocatalytic activity. Dalton Trans. 2016, 45, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, J.-Q.; Chen, C.-Y.; Lan, Y.; Mao, X.; Bai, F. Optimal RE (RE = La, Y and Sm) doping conditions and the enhanced mechanism for photocatalytic application of ceria nanorods. Nanotechn. 2021, 32, 195708. [Google Scholar] [CrossRef]
- Xia, X.; Li, J.; Chen, C.; Lan, Y.-P.; Mao, X.; Chu, Z.; Ning, D.; Zhang, J.; Liu, F. Collaborative influence of morphology tuning and RE (La, Y, and Sm) doping on photocatalytic performance of nanoceria. Environ. Sci. Pollut. Res. Int. 2022, 29, 88866–88881. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, J.-T.; Park, J.-H.; Kim, Y.-H.; Lee, I.-K.; Lee, M.-H.; Kim, B.-Y. Effect of Er doping on optical band-gap energy of TiO2 thin films prepared by spin coating. Current. Appl. Phys. 2013, 13, 1301–1305. [Google Scholar] [CrossRef]
- Butt, K.M.; Farrukh, M.A.; Muneer, I. Influence of lanthanum doping via hydrothermal and reflux methods on the SnO2-TiO2 nanoparticles prepared by sol-gel method and their catalytic properties. J. Mater. Sci. Mater. Electron. 2016, 27, 8493–8498. [Google Scholar] [CrossRef]
- Nabi, G.; Ali, W.; Majid, A.; Alharbi, T.; Saeed, S.; Albedah, M.A. Controlled growth of Bi-Functional La-doped CeO2 nanorods for photocatalytic H2 production and supercapacitor applications. Int. J. Hydrogen Energy 2022, 47, 15480–15490. [Google Scholar] [CrossRef]
- Tian, A.; Ma, T.; Shi, X.; Wang, D.; Wu, W.; Liu, C.; Pei, W. Synergistic Improvement in Coating with UV Aging Resistance and Anti-Corrosion via La-Doped CeO2 Powders. Coatings 2021, 11, 1095. [Google Scholar] [CrossRef]
- Fan, Y.; Jiao, J.; Zhao, L.; Tang, J. Preparation of Lanthanide-doped (La, Nd, and Eu) polystyrene/CeO2 abrasives and investigation of slurry stability and photochemical mechanical polishing performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130508. [Google Scholar] [CrossRef]
- Joseph, J.; Mathew, V.; Abraham, K.E. Physical properties of Dy and La-doped SnO2 thin films prepared by a cost effective vapour deposition technique. Cryst. Res. Techn. 2006, 41, 1020–1026. [Google Scholar] [CrossRef]
- Babu, K.R.V.; Renuka, C.G.; Zikriya, M. Band Gap and Photo luminescence studies of Sm3+ doped TiO2 NP for wLED. Int. Adv. Res. J. Sci. Eng. Techn. 2021, 8, 24. [Google Scholar] [CrossRef]
- Bharathi, R.N.; Sankar, S. Structural, optical and magnetic properties of Pr doped CeO2 nanoparticles synthesized by citrate–nitrate auto combustion method. J. Mater. Sci. Mater. Electr. 2018, 29, 6679–6691. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolova, I.; Apostolov, A.; Wesselinowa, J. Band Gap Tuning in Transition Metal and Rare-Earth-Ion-Doped TiO2, CeO2, and SnO2 Nanoparticles. Nanomaterials 2023, 13, 145. https://doi.org/10.3390/nano13010145
Apostolova I, Apostolov A, Wesselinowa J. Band Gap Tuning in Transition Metal and Rare-Earth-Ion-Doped TiO2, CeO2, and SnO2 Nanoparticles. Nanomaterials. 2023; 13(1):145. https://doi.org/10.3390/nano13010145
Chicago/Turabian StyleApostolova, Iliana, Angel Apostolov, and Julia Wesselinowa. 2023. "Band Gap Tuning in Transition Metal and Rare-Earth-Ion-Doped TiO2, CeO2, and SnO2 Nanoparticles" Nanomaterials 13, no. 1: 145. https://doi.org/10.3390/nano13010145
APA StyleApostolova, I., Apostolov, A., & Wesselinowa, J. (2023). Band Gap Tuning in Transition Metal and Rare-Earth-Ion-Doped TiO2, CeO2, and SnO2 Nanoparticles. Nanomaterials, 13(1), 145. https://doi.org/10.3390/nano13010145







