Influence of Laboratory Synthesized Graphene Oxide on the Morphology and Properties of Cement Mortar
Abstract
:1. Introduction
2. Methodology
2.1. Materials and Equipment
2.2. Methodology
2.2.1. Experimental Plan
2.2.2. Synthesis of GO Suspension
2.2.3. Preparation and Testing of Cement Paste
2.2.4. Preparation and Testing of Cement Mortar Specimen
3. Results
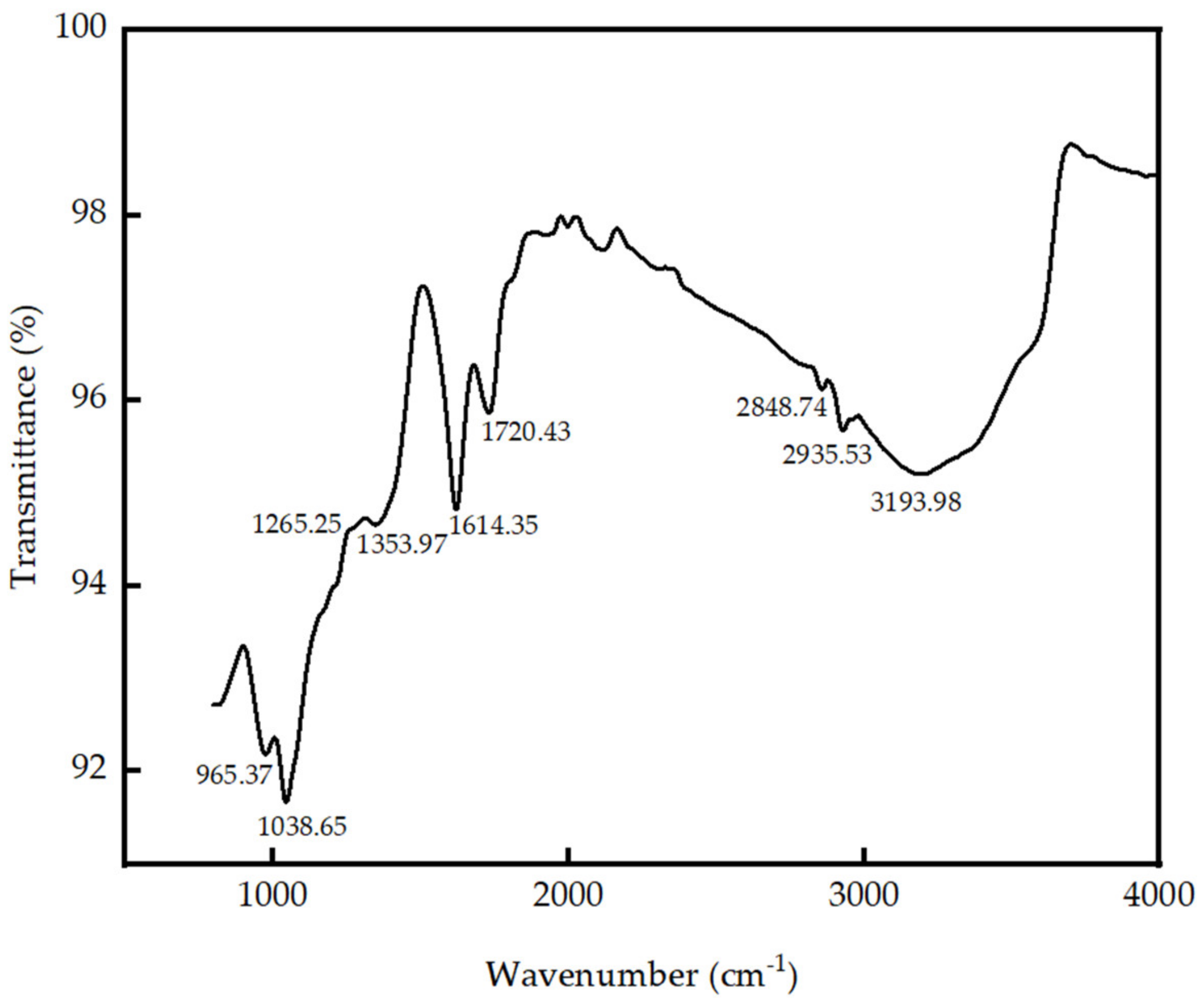
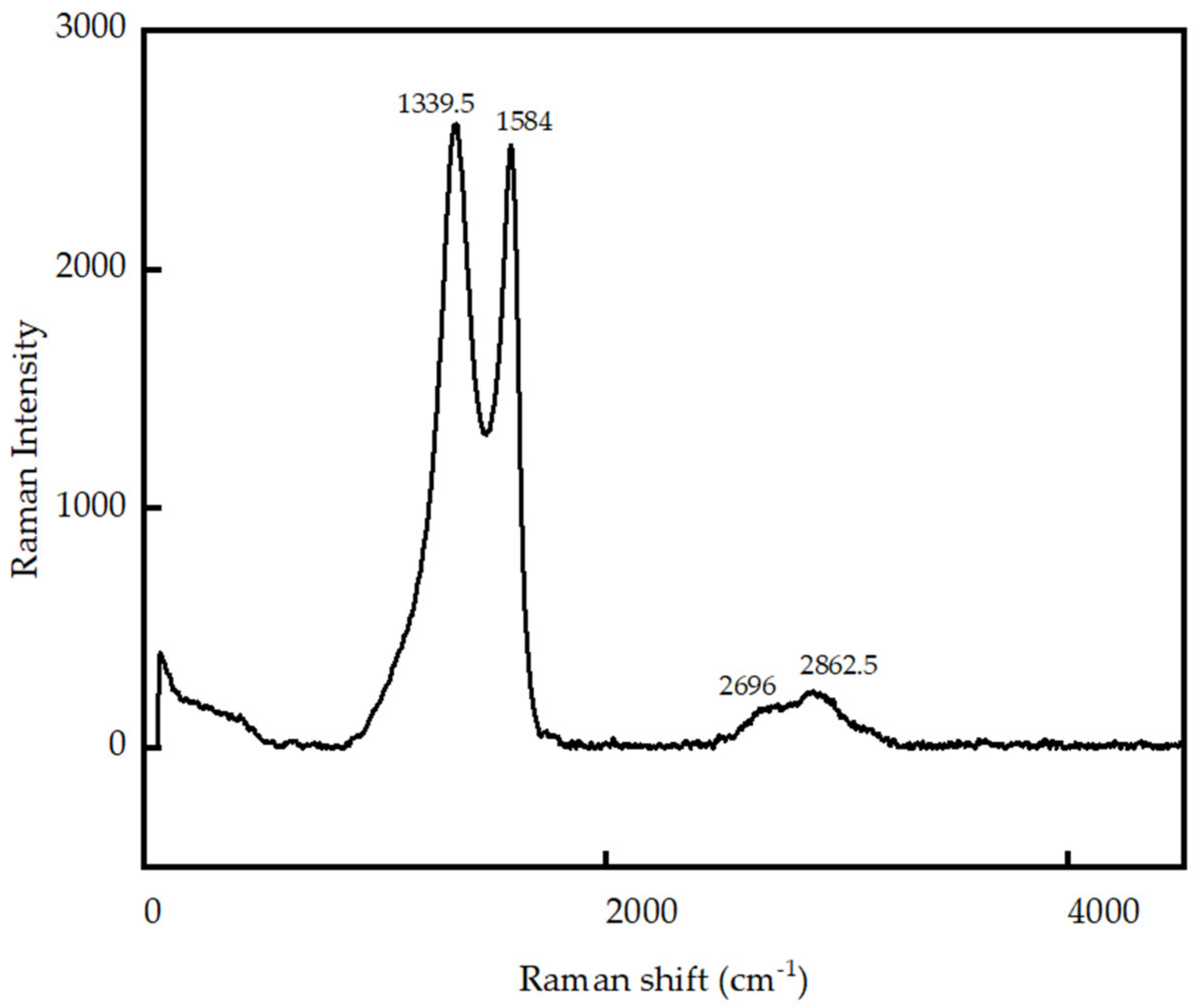
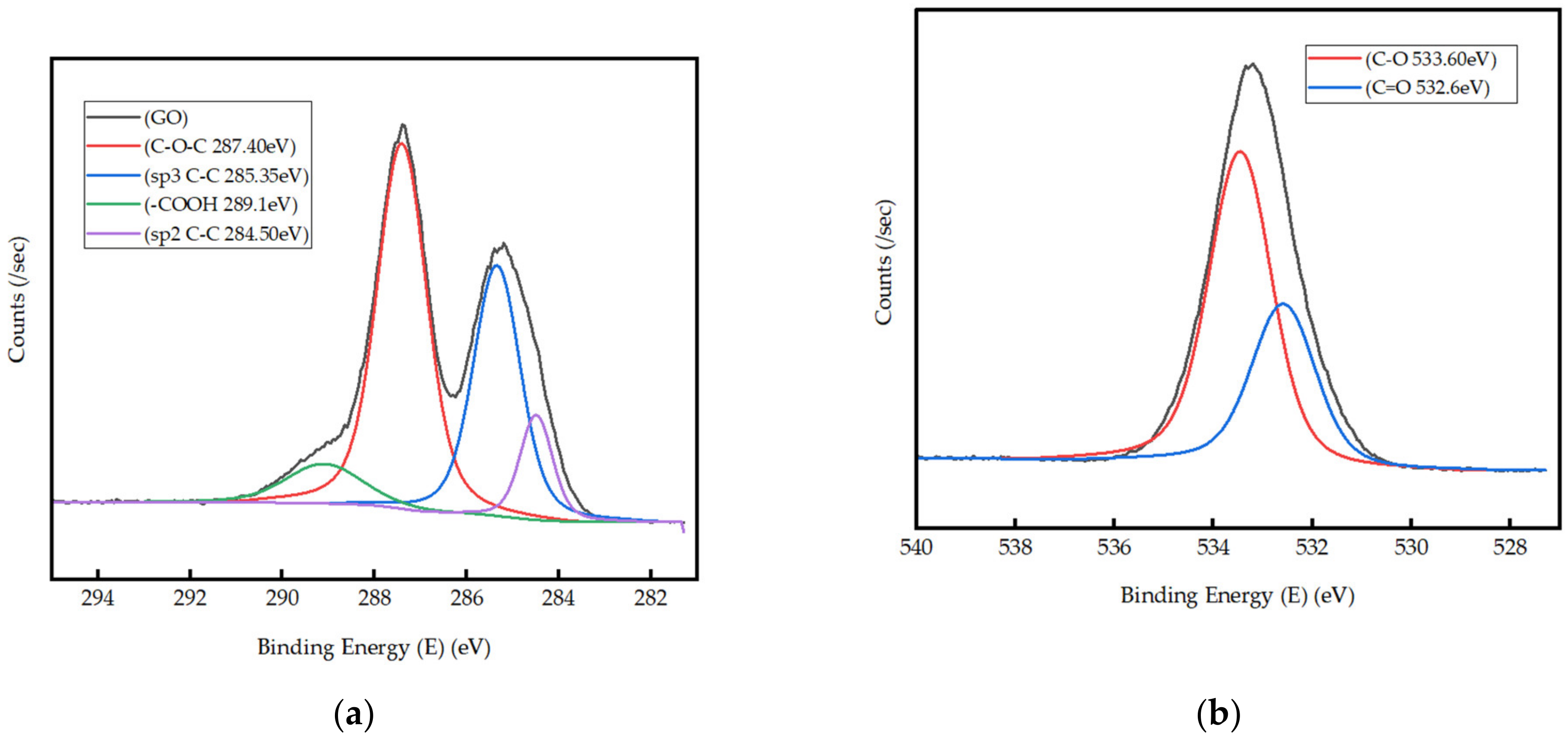
3.1. Characterization of GO Produced in Laboratory
3.2. Physical Properties of Cement Composites with GO Paste and Powder
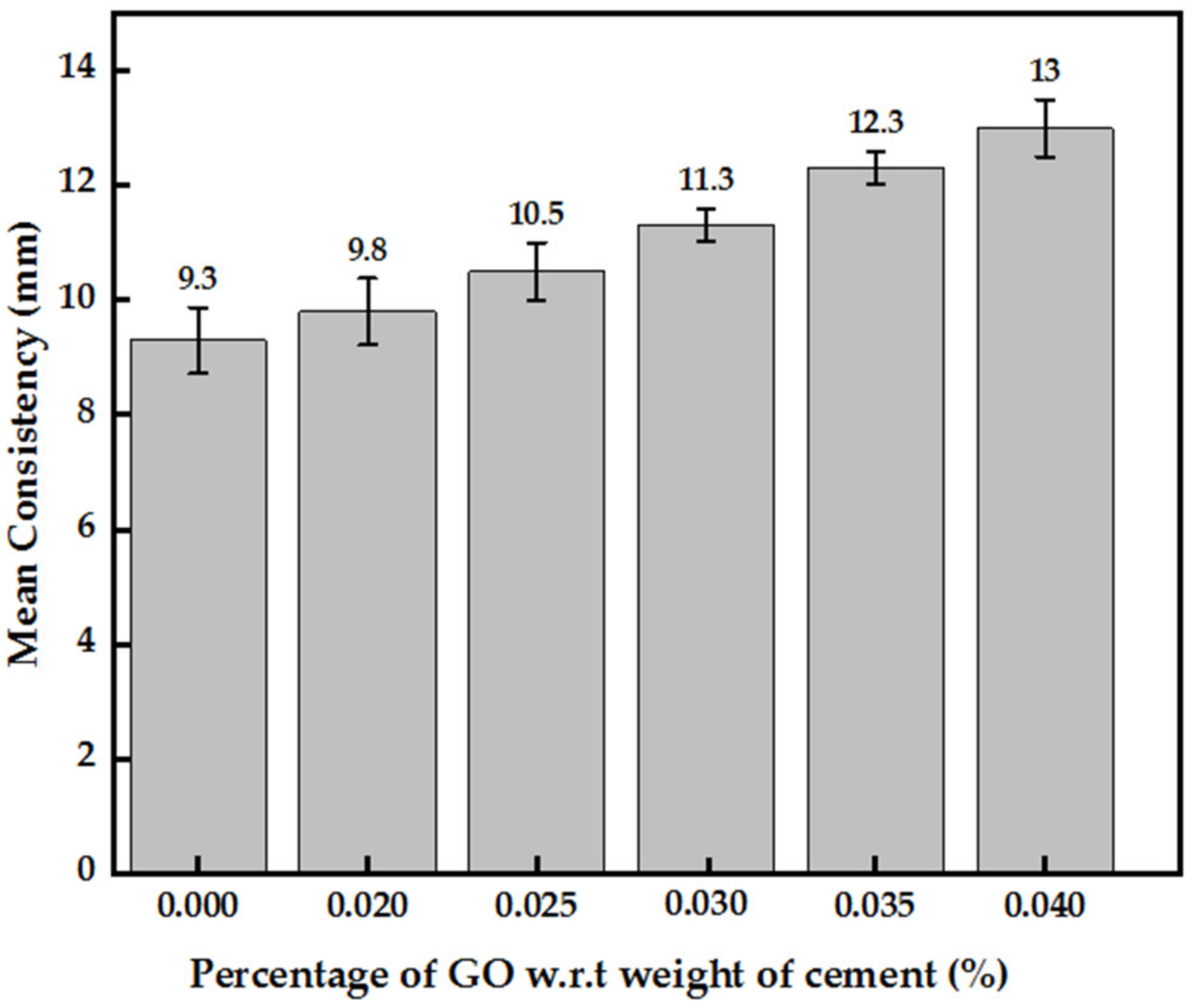
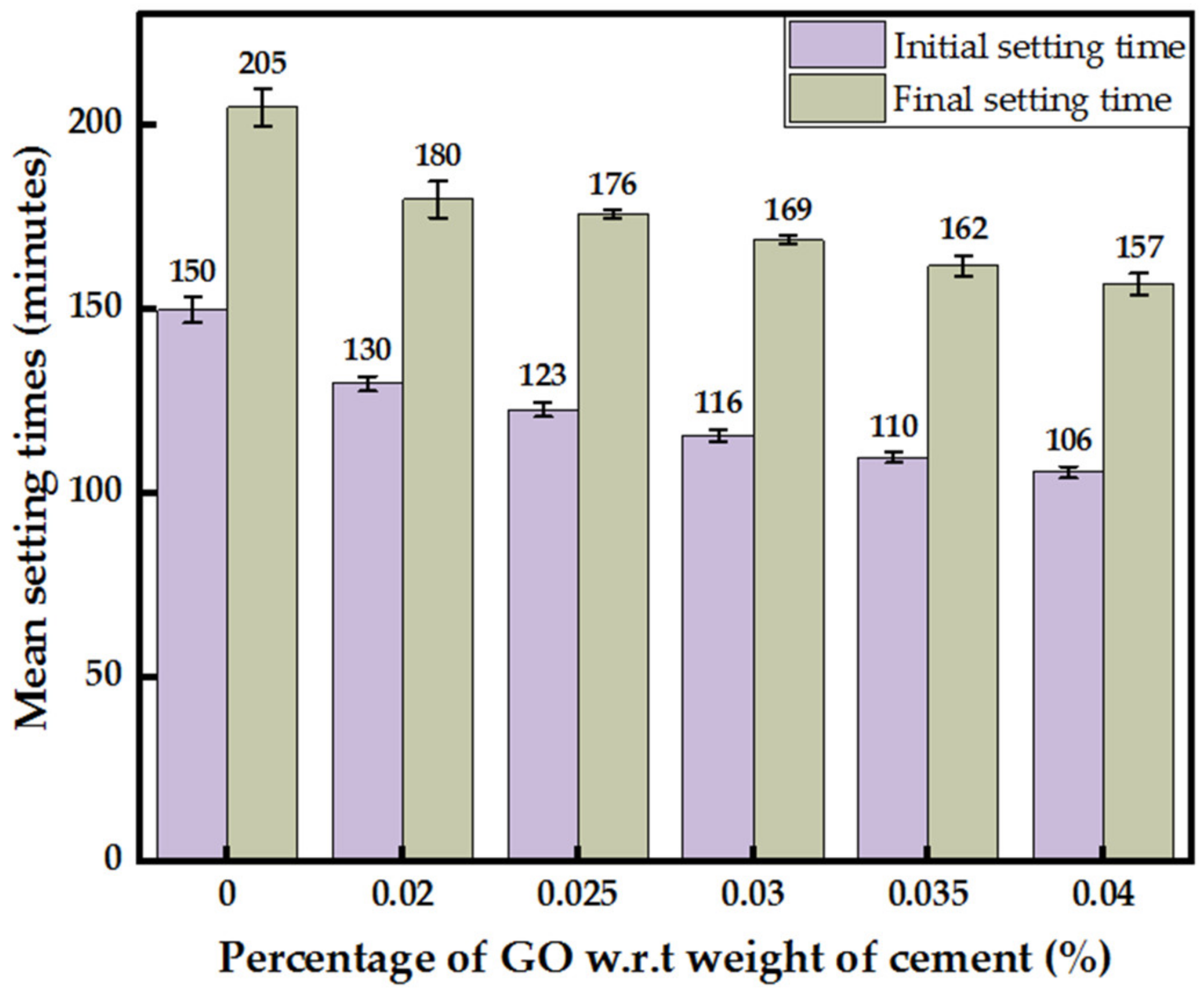
3.2.1. Consistency and Setting Times of GO Paste Incorporated Cement Paste
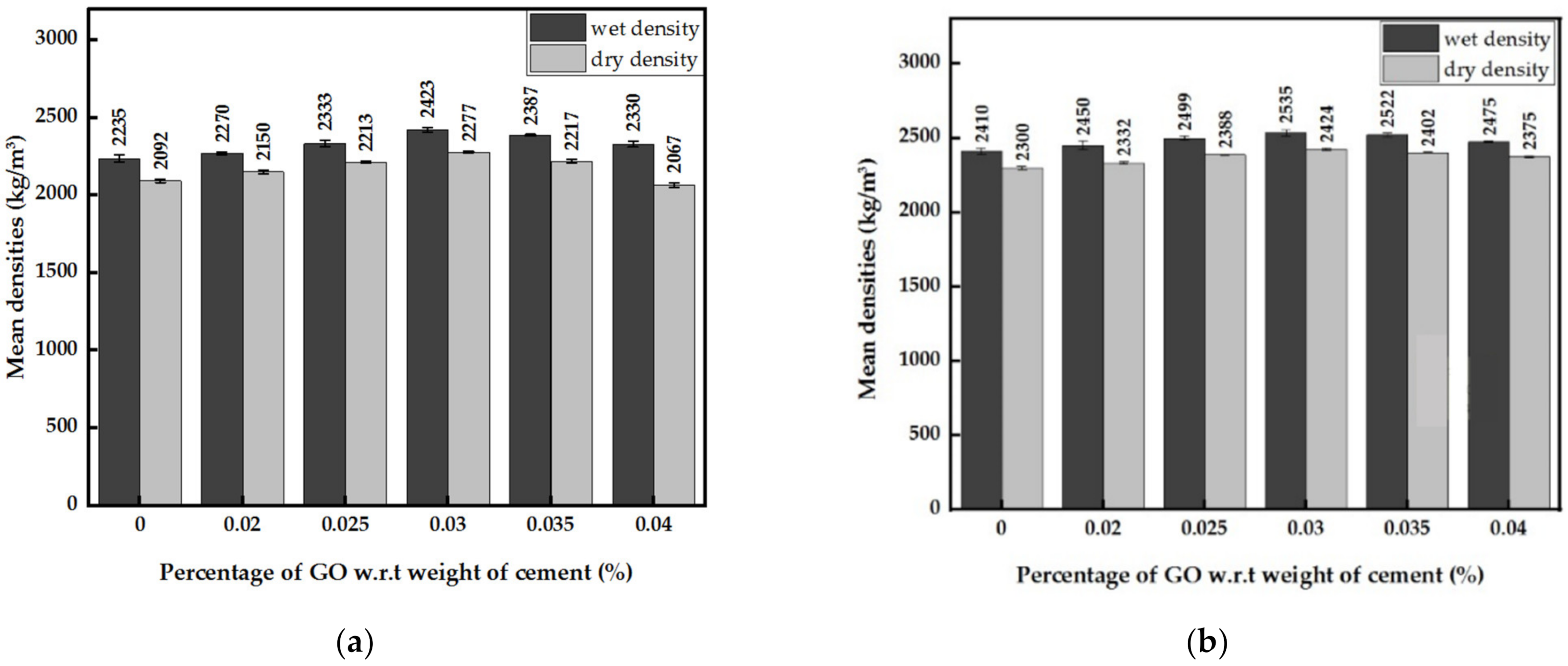
3.2.2. Densities of GO Paste- and GO Powder-Incorporated Cement Mortar
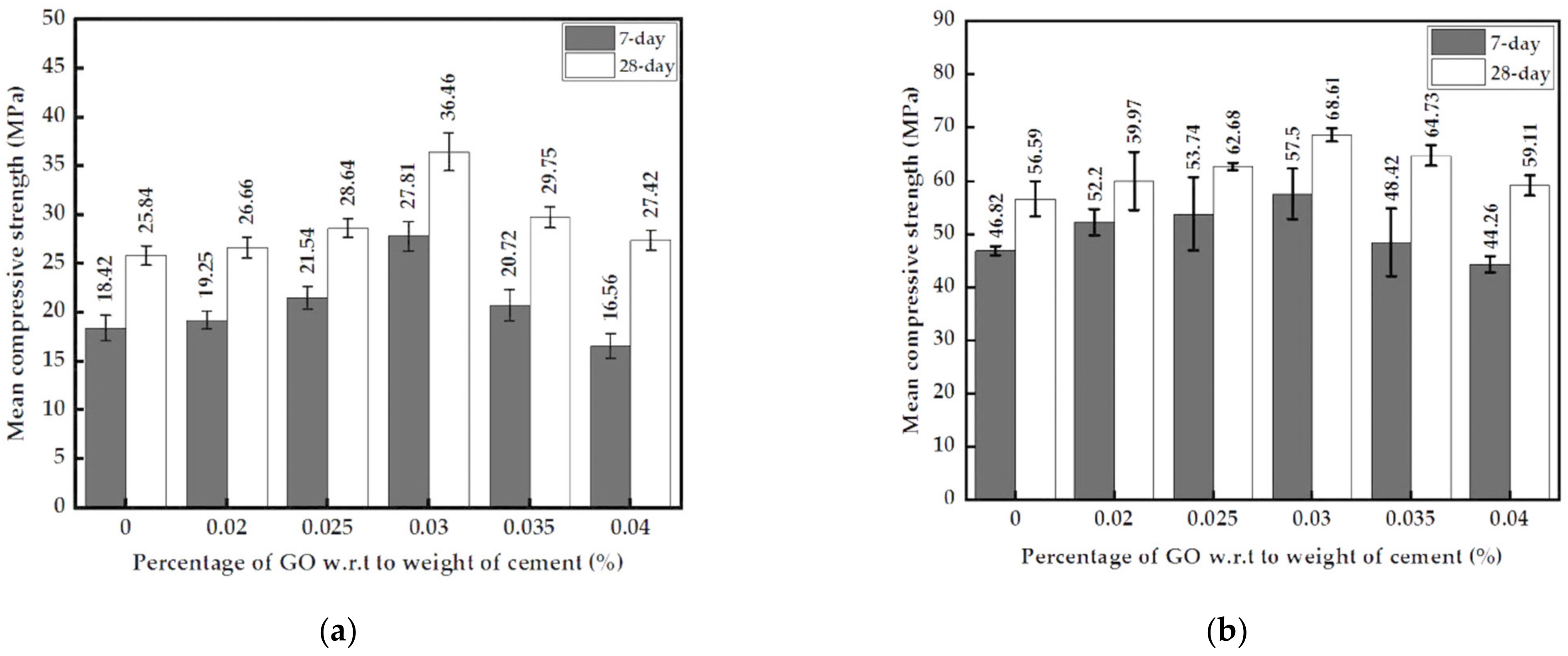
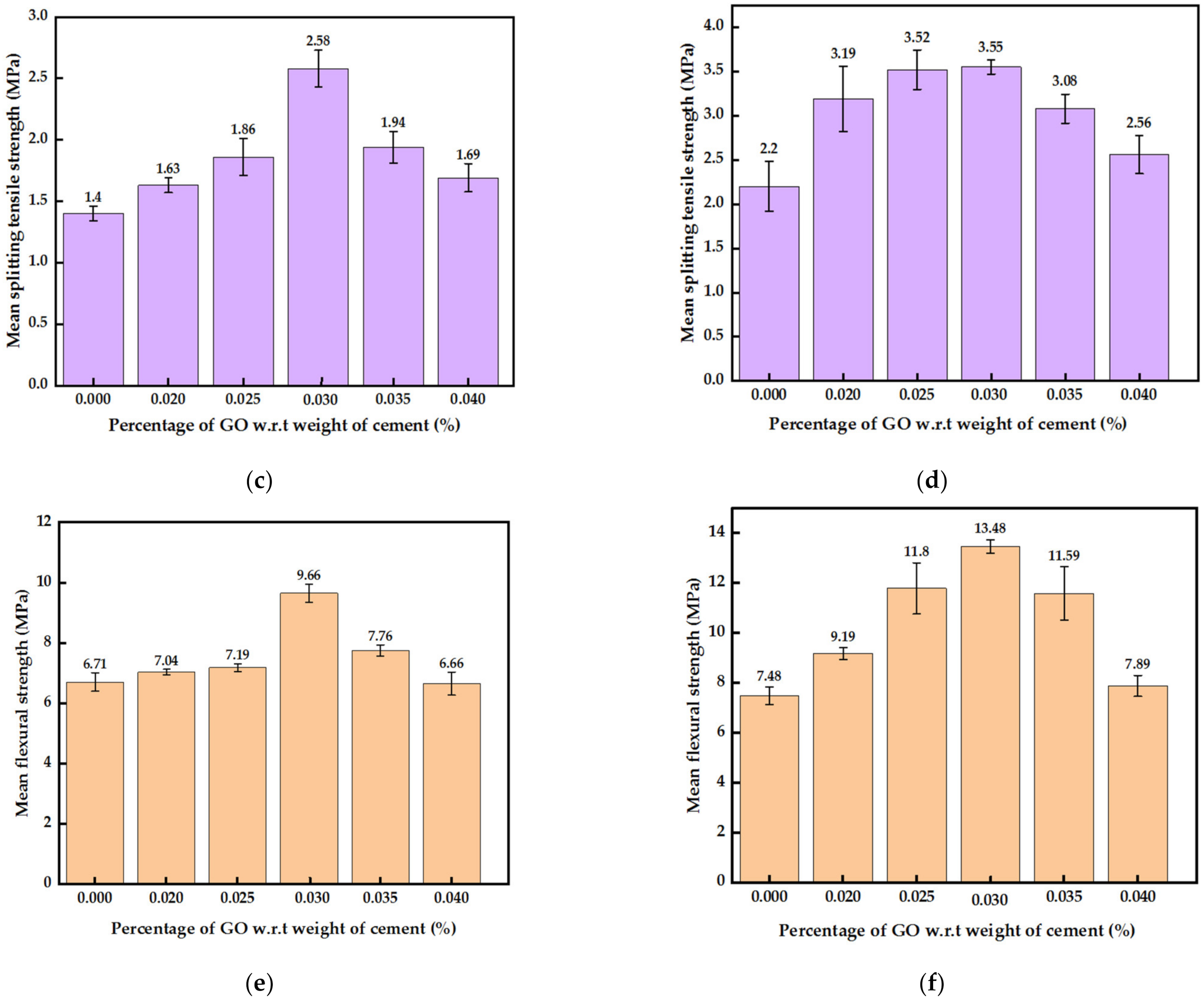
3.2.3. Mechanical Strengths of Mortar with GO Paste and GO Powder
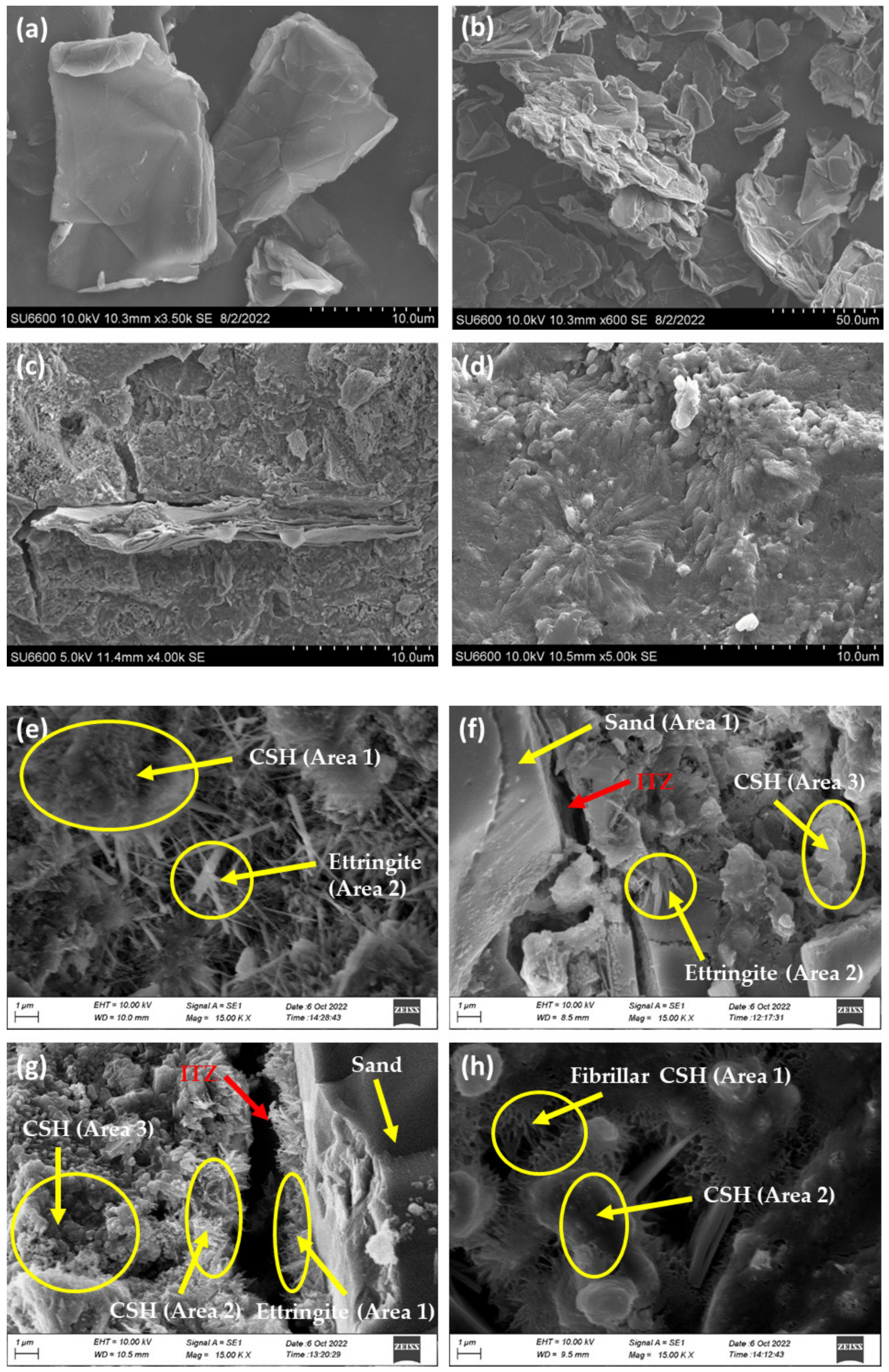
3.3. Morphology of Cement Mortar with GO Paste
3.3.1. Interpretation of SEM and EDX Results
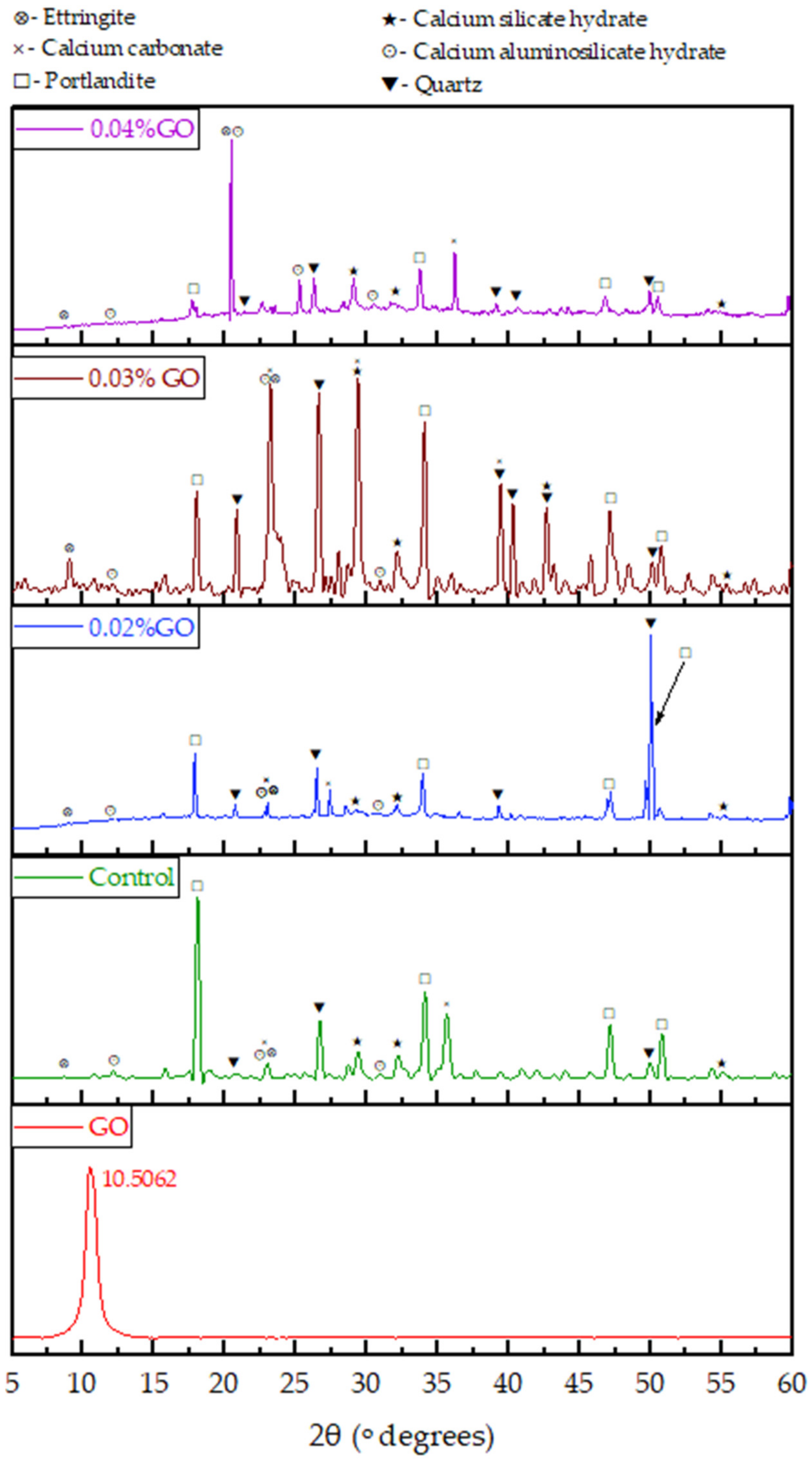
3.3.2. Interpretation of XRD Results
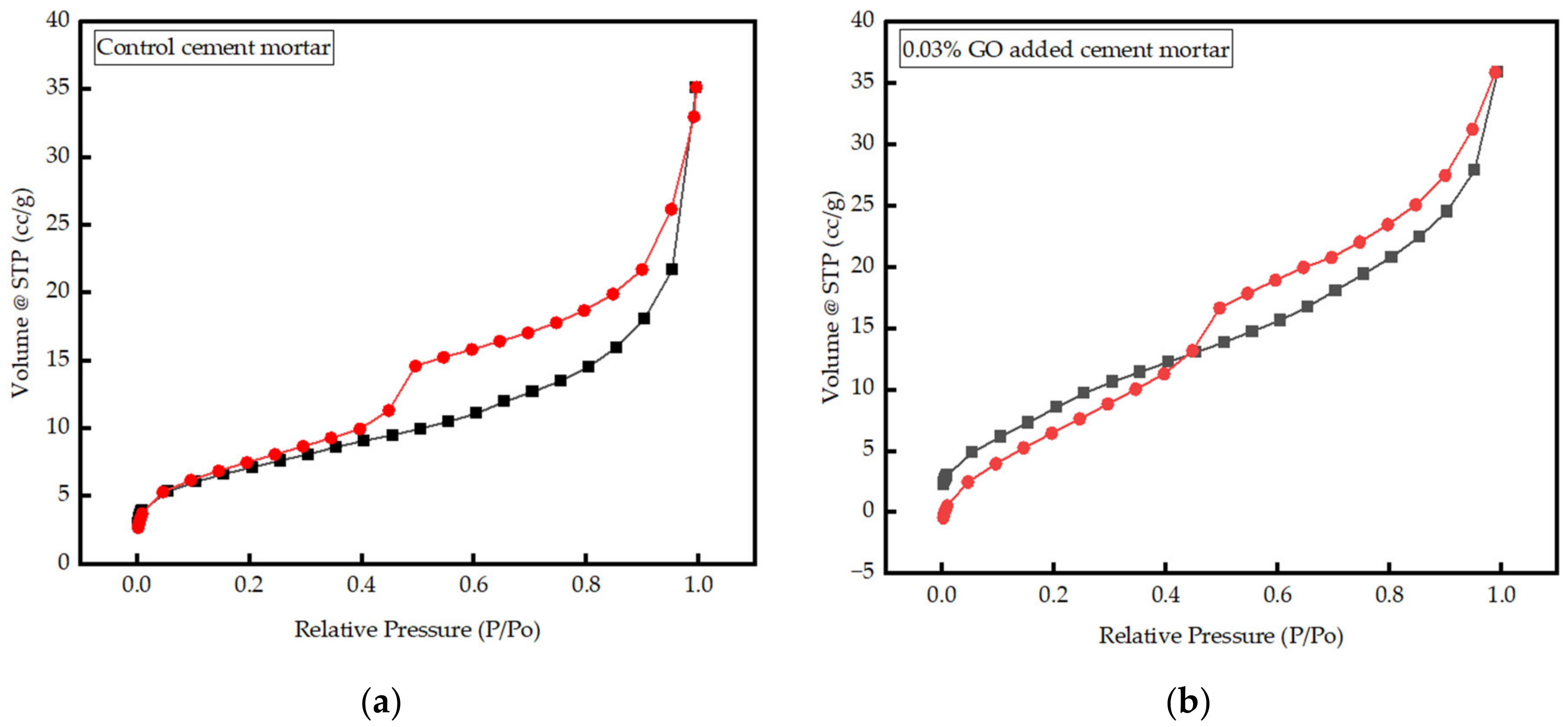
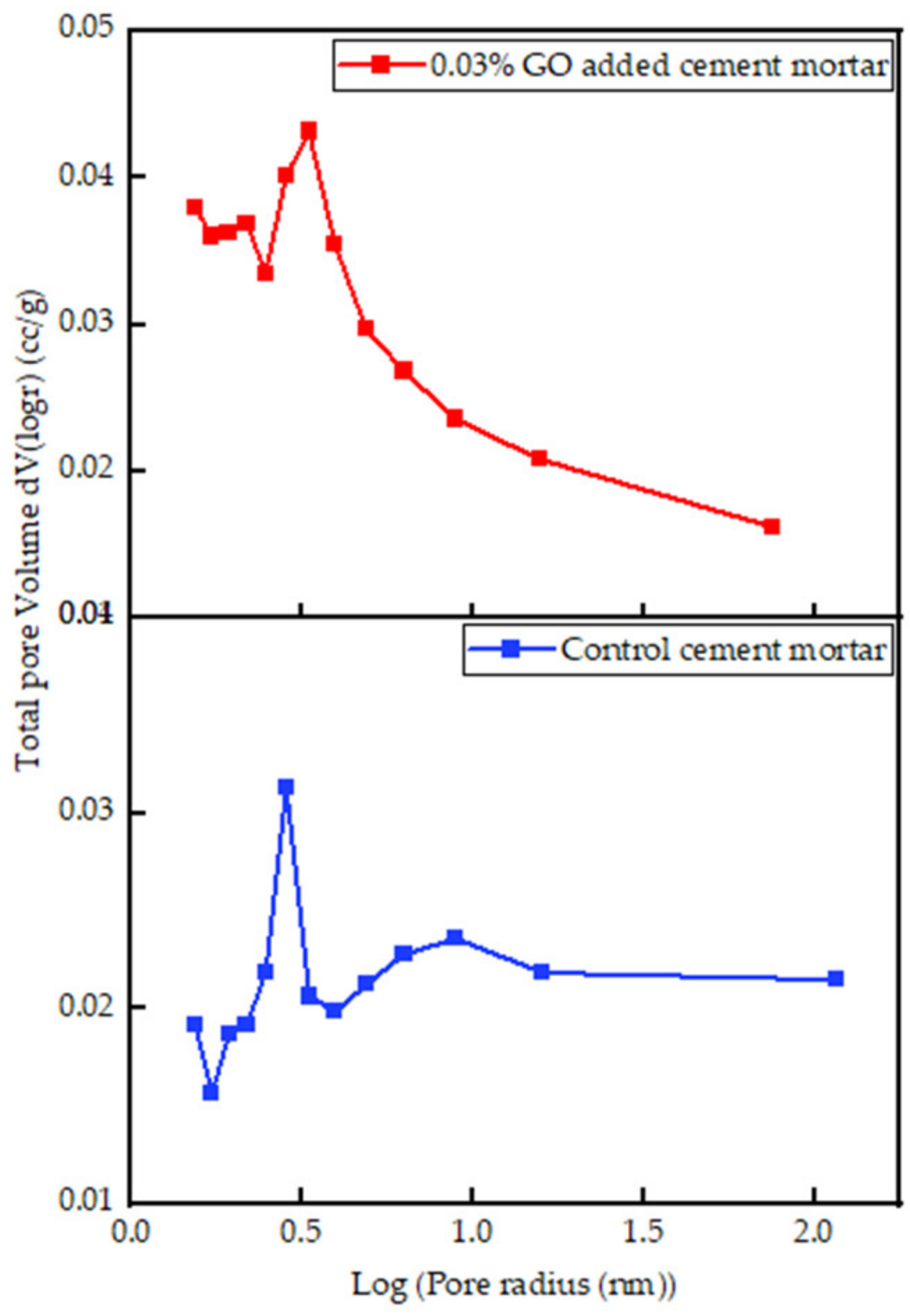
3.3.3. Interpretation of BET Results
4. Discussion
5. Conclusions
- With each 0.005% increase in GO percentage, a uniform increment in the consistency by about 7–10% was recorded, while a decrease in the initial and final setting times by an average of 6 min with the difference between the initial and final setting times constant at about 50 min was observed.
- The optimum percentage of GO paste, at 0.03%, increased the 7-day and 28-day compressive strength, splitting tensile strength and flexural strength by 51%, 41%, 84%, and 44%, respectively. The increment in compressive and splitting tensile strength with the optimum 0.03% GO powder added mortar was lesser than GO paste-containing mortar. The substantial increase in tensile strength is a major advantage as it would increase the toughness of the cementitious composite.
- Oxygen functional groups act as nucleation sites by attracting Ca2+ ions to them. Hence, CSH and Ca(OH)2 formed on the sites have a more organized structure compared to cement mortar in the absence of GO, improving the strength properties and increasing densities.
- The volume of micropores in 0.03% GO mortar increased by 10% compared to the control, keeping the overall porosity constant, leading us to the conclusion of reduced mesopores in GO-containing mortar.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neville, A.; Brooks, J. Concrete Technology, 2nd ed.; Prentice Hall: Harlow, UK, 2010; pp. 190–192. [Google Scholar]
- Ding, Y.; Yu, K.; Li, M. A Review on High-Strength Engineered Cementitious Composites (HS-ECC): Design, Mechanical Property and Structural Application. Structures 2022, 35, 903–921. [Google Scholar] [CrossRef]
- Chen, S.; Collins, F.; Macleod, A.; Pan, Z.; Duan, W.; Wang, C. Carbon Nanotube–Cement Composites: A Retrospect. IES J. Part A Civ. Struct. Eng. 2011, 4, 254–265. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Fangueiro, R. A Review on Nanomaterial Dispersion, Microstructure, And Mechanical Properties of Carbon Nanotube and Nanofiber Reinforced Cementitious Composites. J. Nanomater. 2013, 2013, 710175. [Google Scholar] [CrossRef]
- Yoo, D.; Banthia, N. Mechanical Properties of Ultra-High-Performance Fiber-Reinforced Concrete: A Review. Cem. Concr. Compos. 2016, 73, 267–280. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, J.; Chen, W.; Jin, R.; Li, B.; Pan, Z. A Holistic Review of Cement Composites Reinforced with Graphene Oxide. Constr. Build. Mater. 2018, 171, 291–302. [Google Scholar] [CrossRef]
- Rajak, M.; Majid, Z.; Ismail, M. Morphological Characteristics of Hardened Cement Pastes Incorporating Nano-Palm Oil Fuel Ash. Procedia Manuf. 2015, 2, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Marchon, D.; Flatt, R. 8—Mechanisms of cement hydration. In Science and Technology of Concrete Admixtures; Woodhead Publishing: Cambridge, UK, 2016; pp. 129–145. [Google Scholar]
- Wang, S.; Ang, P.K.; Wang, Z.; Tang, A.L.; Thong, J.T.; Loh, K.P. High Mobility, Printable, and Solution-Processed Graphene Electronics. Nano Lett. 2009, 10, 92–98. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and Graphene Oxide: Biofunctionalization and Applications in Biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-Mode Surface Plasmon Resonance Absorber Based on Dart-Type Single-Layer Graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and Application of Tetrabromobisphenol a Imprinted Electrochemical Sensor Based on Graphene/Carbon Nanotubes Three-Dimensional Nanocomposites Modified Carbon Electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Bhojaraju, C.; Mousavi, S.; Brial, V.; DiMare, M.; Ouellet-Plamondon, C. Fresh and Hardened Properties of GGBS-Contained Cementitious Composites Using Graphene and Graphene Oxide. Constr. Build. Mater. 2021, 300, 123902. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Xu, S. Reinforcing Mechanism of Graphene and Graphene Oxide Sheets on Cement-Based Materials. J. Mater. Civ. Eng. 2019, 31, 04019014, 1-9. [Google Scholar] [CrossRef]
- Sun, H.; Ling, L.; Ren, Z.; Memon, S.; Xing, F. Effect of Graphene Oxide/Graphene Hybrid on Mechanical Properties of Cement Mortar and Mechanism Investigation. Nanomaterials 2020, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Du, H. Graphene Reinforced Cement Composites: A Review. Constr. Build. Mater. 2020, 265, 120312. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Song, L.; Song, Y.; Dai, G.; Liu, J. An Intensive Review on The Role of Graphene Oxide in Cement-Based Materials. Constr. Build. Mater. 2020, 241, 117939. [Google Scholar] [CrossRef]
- Chintalapudi, K.; Pannem, R. An Intense Review on The Performance of Graphene Oxide and Reduced Graphene Oxide in An Admixed Cement System. Constr. Build. Mater. 2020, 259, 120598. [Google Scholar] [CrossRef]
- Anwar, A.; Mohammed, B.; Wahab, M.; Liew, M. Enhanced Properties of Cementitious Composite Tailored with Graphene Oxide Nanomaterial—A Review. Dev. Built Environ. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Mohammed, A.; Al-Saadi, N.; Sanjayan, J. Inclusion of Graphene Oxide in Cementitious Composites: State-Of-The-Art Review. Aust. J. Civ. Eng. 2018, 16, 81–95. [Google Scholar] [CrossRef]
- Suo, Y.; Guo, R.; Xia, H.; Yang, Y.; Zhou, B.; Zhao, Z. A Review of Graphene Oxide/Cement Composites: Performance, Functionality, Mechanisms, And Prospects. J. Build. Eng. 2022, 53, 104502. [Google Scholar] [CrossRef]
- Cote, L.; Kim, J.; Tung, V.; Luo, J.; Kim, F.; Huang, J. Graphene Oxide as Surfactant Sheets. Pure Appl. Chem. 2010, 83, 95–110. [Google Scholar] [CrossRef]
- Nebol’sin, V.; Galstyan, V.; Silina, Y. Graphene Oxide, and Its Chemical Nature: Multi-Stage Interactions Between the Oxygen and Graphene. Surf. Interfaces 2020, 21, 100763. [Google Scholar] [CrossRef]
- Tsugawa, T.; Hatakeyama, K.; Matsuda, J.; Koinuma, M.; Ida, S. Synthesis of Oxygen Functional Group-Controlled Monolayer Graphene Oxide. Bull. Chem. Soc. Jpn. 2021, 94, 2195–2201. [Google Scholar] [CrossRef]
- Usgodaarachchi, L.; Thambiliyagodage, C. Photocatalytic Activity of Go/Fe3O4 Fabricated by Sri Lankan Graphite Under Visible Light Irradiation. J. Sci. Univ. Kelaniya Sri Lanka 2021, 14, 54. [Google Scholar]
- Sun, L.; Fugetsu, B. Mass Production of Graphene Oxide from Expanded Graphite. Mater. Lett. 2013, 109, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An Improved Hummers Method for Eco-Friendly Synthesis of Graphene Oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Marcano, D.; Kosynkin, D.; Berlin, J.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.; Lu, W.; Tour, J. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Tolkou, A.K.; Efstathiou, S.; Rahdar, A.; Favvas, E.P.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials in Cementitious Composites: An Update. Molecules 2021, 26, 1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Ouyang, D. Effect of Graphene Oxide on Mechanical Properties of Cement Mortar, and Its Strengthening Mechanism. Materials 2019, 12, 3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsaei, E.; de Souza, F.B.; Yao, X.; Benhelal, E.; Akbari, A.; Duan, W. Graphene-Based Nanosheets for Stronger and More Durable Concrete: A Review. Constr. Build. Mater. 2018, 183, 642–660. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.M.; Li, W.G.; Li, C.Y.; Sanjayan, J.G.; Duan, W.H.; Li, Z. Effects of Graphene Oxide Agglomerates on Workability, Hydration, Microstructure and Compressive Strength of Cement Paste. Constr. Build. Mater. 2017, 145, 402–410. [Google Scholar] [CrossRef]
- Stephens, C.; Brown, L.; Sanchez, F. Quantification of the Re-Agglomeration of Carbon Nanofiber Aqueous Dispersion in Cement Pastes and Effect on the Early Age Flexural Response. Carbon 2016, 107, 482–500. [Google Scholar] [CrossRef] [Green Version]
- An, J.; McInnis, M.; Chung, W.; Nam, B. Feasibility of Using Graphene Oxide Nanoflake (GONF) as Additive of Cement Composite. Appl. Sci. 2018, 8, 419. [Google Scholar] [CrossRef] [Green Version]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano Reinforced Cement and Concrete Composites and New Perspective from Graphene Oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Peyvandi, A.; Soroushian, P.; Abdol, N.; Balachandran, A.M. Surface-Modified Graphite Nanomaterials for Improved Reinforcement Efficiency in Cementitious Paste. Carbon 2013, 63, 175–186. [Google Scholar] [CrossRef]
- Li, X.; Korayem, A.H.; Li, C.; Liu, Y.; He, H.; Sanjayan, J.G.; Duan, W.H. Incorporation of Graphene Oxide and Silica Fume into Cement Paste: A Study of Dispersion and Compressive Strength. Constr. Build. Mater. 2016, 123, 327–335. [Google Scholar] [CrossRef]
- Zheng, Q.; Han, B.; Cui, X.; Yu, X.; Ou, J. Graphene-Engineered Cementitious Composites. Nanomater. Nanotechnol. 2017, 7, 1847980417742304. [Google Scholar] [CrossRef]
- Long, W.-J.; Gu, Y.-C.; Xing, F.; Khayat, K.H. Microstructure Development and Mechanism of Hardened Cement Paste Incorporating Graphene Oxide during Carbonation. Cem. Concr. Compos. 2018, 94, 72–84. [Google Scholar] [CrossRef]
- Long, W.-J.; Wei, J.-J.; Xing, F.; Khayat, K.H. Enhanced Dynamic Mechanical Properties of Cement Paste Modified with Graphene Oxide Nanosheets and Its Reinforcing Mechanism. Cem. Concr. Compos. 2018, 93, 127–139. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.H.; Nazari, A. Incorporating Graphene Oxide in Cement Composites: A Study of Transport Properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Long, W.-J.; Gu, Y.-C.; Xiao, B.-X.; Zhang, Q.-M.; Xing, F. Micro-Mechanical Properties and Multi-Scaled Pore Structure of Graphene Oxide Cement Paste: Synergistic Application of Nanoindentation, X-Ray Computed Tomography, and SEM-EDS Analysis. Constr. Build. Mater. 2018, 179, 661–674. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of Graphene Oxide Nanosheets of Microstructure and Mechanical Properties of Cement Composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Qureshi, T.S.; Panesar, D.K. Impact of Graphene Oxide and Highly Reduced Graphene Oxide on Cement-Based Composites. Constr. Build. Mater. 2019, 206, 71–83. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Zhou, Q. Regulation of Go on Cement Hydration Crystals, and Its Toughening Effect. Mag. Concr. Res. 2013, 65, 1246–1254. [Google Scholar] [CrossRef]
- Dogan, U.A.; Ozkul, M.H. The Effect of Cement Type on Long-Term Transport Properties of Self-Compacting Concretes. Constr. Build. Mater. 2015, 96, 641–647. [Google Scholar] [CrossRef]
- Tong, T.; Fan, Z.; Liu, Q.; Wang, S.; Tan, S.; Yu, Q. Investigation of the Effects of Graphene and Graphene Oxide Nanoplatelets on the Micro- and Macro-Properties of Cementitious Materials. Constr. Build. Mater. 2016, 106, 102–114. [Google Scholar] [CrossRef]
- Long, W.-J.; Ye, T.H.; Gu, Y.-C.; Li, H.-D.; Xing, F. Inhibited Effect of Graphene Oxide on Calcium Leaching of Cement Pastes. Constr. Build. Mater. 2019, 202, 177–188. [Google Scholar] [CrossRef]
- Muthu, M.; Santhanam, M. Effect of Reduced Graphene Oxide, Alumina and Silica Nanoparticles on the Deterioration Characteristics of Portland Cement Paste Exposed to Acidic Environment. Cem. Concr. Compos. 2018, 91, 118–137. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.H.; Nazari, A. Graphene Oxide Impact on Hardened Cement Expressed in Enhanced Freeze-Thaw Resistance. J. Mater. Civ. Eng. 2016, 28, 04016072. [Google Scholar] [CrossRef]
- Babak, F.; Abolfazl, H.; Alimorad, R.; Parviz, G. Preparation and Mechanical Properties of Graphene Oxide: Cement Nanocomposites. Sci. World J. 2014, 2014, 276323. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, C.; Liu, Y.; Chen, S.J.; Wang, C.M.; Sanjayan, J.G.; Duan, W.H. Improvement of Mechanical Properties by Incorporating Graphene Oxide into Cement Mortar. Mech. Adv. Mater. Struct. 2017, 25, 1313–1322. [Google Scholar] [CrossRef]
- Kang, D.; Seo, K.S.; Lee, H.Y.; Chung, W. Experimental Study on Mechanical Strength of Go-Cement Composites. Constr. Build. Mater. 2017, 131, 303–308. [Google Scholar] [CrossRef]
- Basquiroto de Souza, F.; Shamsaei, E.; Sagoe-Crentsil, K.; Duan, W. Proposed Mechanism for the Enhanced Microstructure of Graphene Oxide–Portland Cement Composites. J. Build. Eng. 2022, 54, 104604. [Google Scholar] [CrossRef]
- Janjaroen, T.; Khammahong, S.; Tuichai, W.; Karaphun, A.; Phrompet, C.; Sriwong, C.; Ruttanapun, C. The Mechanical and Thermal Properties of Cement Cast Mortar/Graphene Oxide Composites Materials. Int. J. Concr. Struct. Mater. 2022, 16, 34. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Wu, Y.-Y.; Deng, X.; Zheng, Z. The Effect of Graphene Oxide on the Mechanical Properties, Impermeability and Corrosion Resistance of Cement Mortar Containing Mineral Admixtures. Constr. Build. Mater. 2021, 288, 123059. [Google Scholar] [CrossRef]
- Hewathilake, H.P.T.S.; Balasooriya, N.W.B.; Nakamura, Y.; Pitawala, H.M.T.G.A.; Wijayasinghe, H.W.M.A.; Satish–Kumar, M. Geochemical, Structural and Morphological Characterization of Vein Graphite Deposits of Sri Lanka: Witness to Carbon Rich Fluid Activity. J. Mineral. Petrol. Sci. 2018, 113, 96–105. [Google Scholar] [CrossRef] [Green Version]
- ASTM C1602/C1602M-18; Standard Specification for Mixing Water Used in the Production of Hydraulic Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM C144-18; Standard Specification for Aggregate for Masonry Mortar. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM C270-19; Standard Specification for Mortar for Unit Masonry. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM C191-08; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM C150/C150M-18; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM C187-04; Standard Test Method for Normal Consistency of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM C109/C109M-18; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM C348-21; Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C496/C496M-11; Standard Test Method for Splitting Tensile Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM C511-21; Standard Specification for Mixing Rooms, Moist Cabinets, Moist Rooms, and Water Storage Tanks Used in the Testing of Hydraulic Cements and Concretes. ASTM International: West Conshohocken, PA, USA, 2021.
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/LK/en (accessed on 5 November 2022).
- Wall, M. 52252; Tech.; Thermo Fisher Scientific: Madison, WI, USA, 2011; pp. 1–5. [Google Scholar]
- Carbon: XPS Periodic Table: Thermo Fisher Scientific—US. Available online: https://www.thermofisher.com/lk/en/home.html (accessed on 5 November 2022).
- Oxygen: XPS Periodic Table: Thermo Fisher Scientific—US. Available online: https://www.thermofisher.com/lk/en/home.html (accessed on 5 November 2022).
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. J. Carbon Res. 2021, 7, 41. [Google Scholar] [CrossRef]
| Reference | Source and Age of GO | Optimum Dosage of Dry GO %. | Percentage Change in Properties (%) | Summary on Morphological Study | |||
|---|---|---|---|---|---|---|---|
| Workability | Compression | Tension | Flexural | ||||
| [47] | Hummer’s method, age of GO unknown. | 0.03% | - | 3-day increased by 45.1%, and 28-day increased by 38.9%. | (Direct method) 3-day increased by 51.0% and 28-day increased by 78.6%. | 3-day increased by 70.7% and 28-day increased by 60.7%. | Flower-like hydration crystals formed via nucleation effect of GO. |
| [55] | Modified Hummers method, age of GO unknown. | 1.5% | - | - | (Direct method) 28-day increased by 48%. | - | More calcium silicate hydrates (CSH) formed in presence of GO. |
| [56] | GO suspension purchased, age of GO unknown. | 0.02% | Flow table test, workability reduces by 2.8%. | 3-day increased by 34.4%, and 28-day increased by 25.9%. | (Splitting method) 7-day increased by 21%, and 28-day increased by 18%. | 28-day increased by 14.8%. | Densification of ITZ and pore structure modification, in the presence of well dispersed GO. |
| [44] | GO powder purchased, and age of GO unknown | 0.2% | - | 28-day increased by 23.4%. | - | 28-day increased by 58.9%. | A refining effect on the volume and diameter of the pores is observed. The volume of loosely packed CSH reduced while the volume of highly packed CSH increased with the addition of GO. |
| [45] | GO suspension purchased, and age of GO unknown. | 0.03% | - | - | - | - | The increased quantity of gel pores enhanced the microstructure of cement paste, improving properties such as water sorptivity and chloride penetration. |
| [46] | GO suspension purchased, and age of GO unknown. | 0.10% | Mini slump test, flowability reduces by 6.0%. | 14-day increased by 18%, and 28-day increased by 20.5%. | - | 14-day increased by 15%, and 28-day increased by 28%. | Refinement of microcracks enhanced the strengths. |
| [57] | GO suspension purchased, and age of GO unknown. | 0.05% (compression) 0.01% (tension) 0.1% (flexural) | - | 28-day increased by 32%. | (Direct method) 28-day increased by 26%. | 28-day increased by 20%. | Enhancement of strengths occurred as GO sheets bridged via covalent bonding with CSH, improving its uniformity. In addition, propagation of cracks are deferred by delaying the formation of micro-cracks. |
| [58] | GO suspension purchased, and age of GO unknown. | - | - | - | - | - | GO nanosheets act as 2D platforms promoting the growth of CSH by means of the Stranski–Krastanov growth mode. Leading to a densified cement matrix. |
| [59] | Modified Hummers method, age of GO unknown. | 0.05% | - | 28-day increased by 32.71%. | (Direct method) 28-day increased by 18.93%. | - | Addition of GO in cement composites promotes hydration, reduces pore volume, accelerates crystallite formation, and improves alignment of crystallites. |
| [60] | GO suspension purchased, and age of GO unknown. | 0.03% (compression) 0.05% (flexural) | - | 28-day increased by 12.4%. | - | 28-day increased by 16.10%. | GO promotes hydration, provides a filling effect and pattern role in the matrix. Moreover, the nano-filling effect eliminates majority of the pores in the mortar and rises the density. |
| Sample Name | Percentage of GO w.r.t to Cement (%) | Dry GO Required * (g) | GO Suspension Required # (g) |
|---|---|---|---|
| 0 LGO | 0 | 0 | 0 |
| 0.02 LGO | 0.02 | 0.64 | 86.72 |
| 0.025 LGO | 0.025 | 0.8 | 108.40 |
| 0.03 LGO | 0.03 | 0.96 | 130.08 |
| 0.035 LGO | 0.035 | 1.12 | 151.76 |
| 0.04 LGO | 0.04 | 1.28 | 173.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesh, S.; Thambiliyagodage, C.; Perera, S.V.T.J.; Rajapakse, R.K.N.D. Influence of Laboratory Synthesized Graphene Oxide on the Morphology and Properties of Cement Mortar. Nanomaterials 2023, 13, 18. https://doi.org/10.3390/nano13010018
Ganesh S, Thambiliyagodage C, Perera SVTJ, Rajapakse RKND. Influence of Laboratory Synthesized Graphene Oxide on the Morphology and Properties of Cement Mortar. Nanomaterials. 2023; 13(1):18. https://doi.org/10.3390/nano13010018
Chicago/Turabian StyleGanesh, Suganthiny, Charitha Thambiliyagodage, S. V. T. Janaka Perera, and R. K. N. D. Rajapakse. 2023. "Influence of Laboratory Synthesized Graphene Oxide on the Morphology and Properties of Cement Mortar" Nanomaterials 13, no. 1: 18. https://doi.org/10.3390/nano13010018





