Development of New Health Risk Assessment of Nanoparticles: EPA Health Risk Assessment Revised
Abstract
:1. Introduction
1.1. Nanoparticles: Specific Toxicity and Size
1.2. Adverse Effects of Nanoparticles
1.3. Quantitative Assessment of the Health Cancer Risks of Nanoparticles
2. Methods
2.1. Case Study Sample Collection and Analysis
2.2. Quantitative Assessment of the Health Cancer Risks
3. Results
3.1. Development of a New Method for the NP Health Risk Assessment
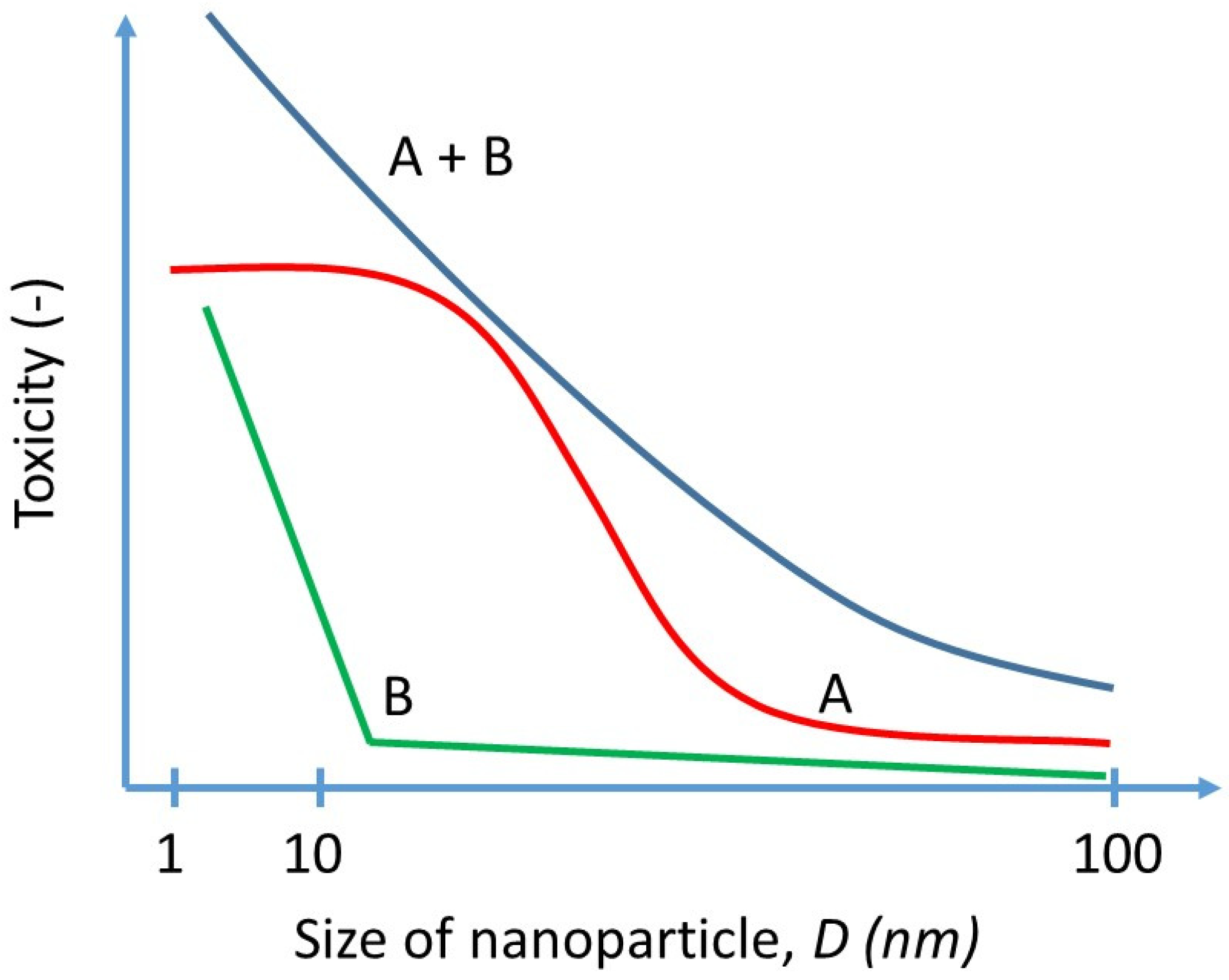
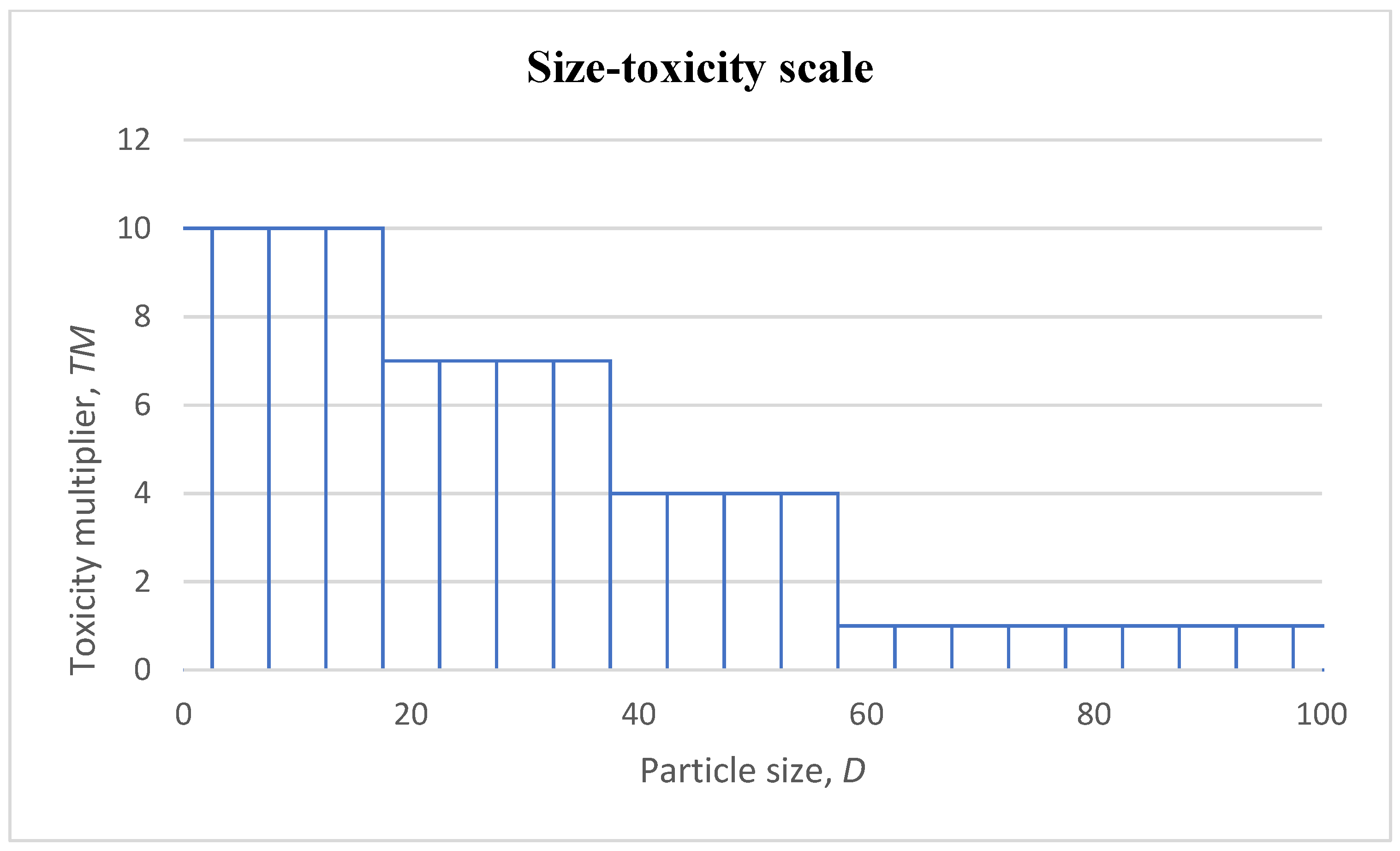
3.1.1. Draft of Size–Toxicity Scale Relationship
3.1.2. Draft of Size–Distribution Toxicity Relationship
- 1
- Point effects occur when a single particle causes a localized effect or a series of effects or triggers a cascade of effects. All direct effects, such as the following:
- NPs may damage cell membranes by perforating them;
- NPs damage components of the cytoskeleton, disturbing intracellular transport and cell division;
- NPs disturb transcription and damage DNA, thus accelerating mutagenesis;
- NPs damage mitochondria and disturb their metabolism, which leads to cell energy imbalance;
- NPs interfere with the formation of lysosomes, thereby hampering autophagy and degradation of macromolecules and triggering apoptosis;
- NPs cause structural changes in membrane proteins and disturb the transport of substances into and out of cells, including intercellular transport;
- NPs that activate the synthesis of inflammatory mediators by disturbing the normal mechanisms of cell metabolism, as well as tissue and organ metabolism, belong to this group.
- These effects scale with the total number of particles in the organism.
- 2
- Surface effects. The effect depends on the surface area of the particle either due to a specific surface catalytic mechanism of toxic agent production or another specific interaction between the nanoparticle surface and the biological surfaces, e.g., adherence of nanoparticles to the membranes.
- NPs may cause oxidation via the formation of ROS and other free radicals;
- NPs may damage cell membranes by perforating them;
- NPs interfere with the formation of lysosomes, thereby hampering autophagy and degradation of macromolecules and triggering apoptosis;
- Short-term NP effects related to the leakage of free ions of metals contained in their cores, such as cadmium, lead, and arsenic, upon oxidation by environmental agents. The larger the surface of the NPs, the faster the ion release is.
- These effects scale with the total surface of NPs present in the organism.
- 3
- Volume effects.
- Long-term NP effects related to the leakage of free ions of metals or other toxic agents contained in their cores, such as cadmium, lead, and arsenic, upon oxidation by environmental agents or simple dissolution. If the dissolution time is long enough, the final dose of the ions depends on the total volume of nanoparticles only. These effects also include oxidative stress caused by some ions.
- This effect scales with the total volume of NPs present in the organism.
3.1.3. Draft of a New Quantitative Assessment of the Health Risks of Nanoparticles
3.1.4. Implementation of Toxicity and Size Multipliers
3.2. Case Study: Demonstration of Traffic Generated Cadmium Nanoparticle Health Risk Assessment
3.2.1. Health Risk Assessment Procedure
3.2.2. Assessment of the Distribution of Cadmium Pollutant in the Air Depending on the Diameter of Solid Particles
3.2.3. Quantification of Health Risks of Cadmium Particles and Evaluation of the New Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pope, C.A. Epidemiology of Fine Particulate Air Pollution and Human Health: Biologic Mechanisms and Who’s at Risk? Environ. Health Perspect. 2000, 108, 713. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Zeger, S.L.; Dominici, F.; Curriero, F.; Coursac, I.; Dockery, D.W.; Schwartz, J.; Zanobetti, A. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and Mortality from Air Pollution in the United States. Res. Rep. Health. Eff. Inst. 2000, 94, 5–79. [Google Scholar] [PubMed]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased Particulate Air Pollution and the Triggering of Myocardial Infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groneberg-Kloft, B.; Kraus, T.; Van Mark, A.; Wagner, U.; Fischer, A. Analysing the Causes of Chronic Cough: Relation to Diesel Exhaust, Ozone, Nitrogen Oxides, Sulphur Oxides and Other Environmental Factors. J. Occup. Med. Toxicol. 2006, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfman, L.; Riihimäki, M.; Pringle, J.; Wlinder, R. Influence of Horse Stable Environment on Human Airways. J. Occup. Med. Toxicol. 2009, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Groneberg, D.A.; Fischer, A. Occupational Medicine and Toxicology. J. Occup. Med. Toxicol. 2006, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Vargas, M.; Guzman-Grenfell, A.; Blanco-Jimenez, S.; Sepulveda-Sanchez, J.; Bernabe-Cabanillas, R.; Cardenas-Gonzalez, B.; Ceballos, G.; Hicks, J. Airborne Particulate Matter PM2.5 from Mexico City Affects the Generation of Reactive Oxygen Species by Blood Neutrophils from Asthmatics: An in Vitro Approach. J. Occup. Med. Toxicol. 2009, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Uibel, S.; Scutaru, C.; Mueller, D.; Klingelhoefer, D.; Hoang, D.M.L.; Takemura, M.; Fischer, A.; Spallek, M.F.; Unger, V.; Quarcoo, D.; et al. Mobile Air Quality Studies (MAQS) in Inner Cities: Particulate Matter PM10 Levels Related to Different Vehicle Driving Modes and Integration of Data into a Geographical Information Program. J. Occup. Med. Toxicol. 2012, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Groneberg, D.A.; Nowak, D.; Wussow, A.; Fischer, A. Chronic Cough Due to Occupational Factors. J. Occup. Med. Toxicol. 2006, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Orach, J.; Rider, C.F.; Carlsten, C. Concentration-Dependent Health Effects of Air Pollution in Controlled Human Exposures. Environ. Int. 2021, 150, 106424. [Google Scholar] [CrossRef]
- Liu, N.M.; Miyashita, L.; Maher, B.A.; McPhail, G.; Jones, C.J.P.; Barratt, B.; Thangaratinam, S.; Karloukovski, V.; Ahmed, I.A.; Aslam, Z.; et al. Evidence for the Presence of Air Pollution Nanoparticles in Placental Tissue Cells. Sci. Total Environ. 2021, 751, 142235. [Google Scholar] [CrossRef] [PubMed]
- Pryor, J.T.; Cowley, L.O.; Simonds, S.E. The Physiological Effects of Air Pollution: Particulate Matter, Physiology and Disease. Front. Public Health 2022, 10, 882569. [Google Scholar] [CrossRef] [PubMed]
- Sevalnev, A.I.; Sharavara, L.P.; Kutsak, A.V.; Nefodov, O.O.; Zemliynyi, O.A.; Pisarevskyi, K.I.; Shevchenko, O.S. Nanoparticles in the air of the working zone as a risk factor for the health of workers of various industries. Med. Perspect. Perspekt. 2020, 25, 169–176. [Google Scholar] [CrossRef]
- Maher, B.A.; Gonzalez-Maciel, A.; Reynoso-Robles, R.; Torres-Jardon, R.; Calderon-Garciduenas, L. Iron-Rich Air Pollution Nanoparticles: An Unrecognised Environmental Risk Factor for Myocardial Mitochondrial Dysfunction and Cardiac Oxidative Stress. Environ. Res. 2020, 188, 109816. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Ayala, A. Air Pollution, Ultrafine Particles, and Your Brain: Are CombustionNanoparticle Emissions and Engineered Nanoparticles CausingPreventable Fatal Neurodegenerative Diseases and CommonNeuropsychiatric Outcomes? Environ. Sci. Technol. 2022, 56, 6847–6856. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.D.; Teixeira, E.C.; Hower, J.C.; Civeira, M.S.; Ramirez, O.; Yang, C.-X.; Oliveira, M.L.S.; Silva, L.F.O. Metal-Enriched Nanoparticles and Black Carbon: A Perspective from the Brazil Railway System Air Pollution. Geosci. Front. 2021, 12, 101129. [Google Scholar] [CrossRef]
- Schraufnagel, D.E. The Health Effects of Ultrafine Particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Ris, C.U.S. EPA Health Assessment for Diesel Engine Exhaust: A Review. Inhal. Toxicol. 2007, 19, 229–239. [Google Scholar] [CrossRef]
- Uibel, S.; Takemura, M.; Mueller, D.; Quarcoo, D.; Klingelhoefer, D.; Groneberg, D.A. Nanoparticles and Cars—Analysis of Potential Sources. J. Occup. Med. Toxicol. 2012, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Westerdahl, D.; Fruin, S.; Sax, T.; Fine, P.M.; Sioutas, C. Mobile Platform Measurements of Ultrafine Particles and Associated Pollutant Concentrations on Freeways and Residential Streets in Los Angeles. Atmos. Environ. 2005, 39, 3597–3610. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, Incidental, and Engineered Nanomaterials and Their Impacts on the Earth System. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigalke, M.; Keller, A.A.; Lespes, G. Natural Nanoparticles, Anthropogenic Nanoparticles, Where Is the Frontier? Front. Environ. Sci. 2020, 8, 71. [Google Scholar] [CrossRef]
- Polichetti, G.; Cocco, S.; Spinali, A.; Trimarco, V.; Nunziata, A. Effects of Particulate Matter (PM10, PM2.5 and PM1) on the Cardiovascular System. Toxicology 2009, 261, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Li, G.; Zhang, Y.; Li, J.; Chen, H. Fluorescent Reconstitution on Deposition of PM2.5 in Lung and Extrapulmonary Organs. Proc. Natl. Acad. Sci. USA 2019, 116, 2488–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, E.; Salar-Behzadi, S. Toxicological Assessment of Inhaled Nanoparticles: Role of in Vivo, Ex Vivo, in Vitro, and in Silico Studies. Int. J. Mol. Sci. 2014, 15, 4795–4822. [Google Scholar] [CrossRef] [PubMed]
- Nho, R. Pathological Effects of Nano-Sized Particles on the Respiratory System. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102242. [Google Scholar] [CrossRef]
- Williams, D.; (SCENIHR). The Appropriateness of Existing Methodologies to Assess the Potential Risks Associated with Engineered and Adventitious Products of Nanotechnologies. Available online: https://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_003b.pdf (accessed on 18 December 2022).
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [Green Version]
- Guidi, P.; Nigro, M.; Bernardeschi, M.; Lucchesi, P.; Scarcelli, V.; Frenzilli, G. Does the Crystal Habit Modulate the Genotoxic Potential of Silica Particles? A Cytogenetic Evaluation in Human and Murine Cell Lines. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 792, 46–52. [Google Scholar] [CrossRef]
- Guidi, P.; Nigro, M.; Bernardeschi, M.; Scarcelli, V.; Lucchesi, P.; Onida, B.; Mortera, R.; Frenzilli, G. Genotoxicity of Amorphous Silica Particles with Different Structure and Dimension in Human and Murine Cell Lines. Mutagenesis 2013, 28, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Board of Editors. Biology Dictionary. Available online: https://biologydictionary.net/toxicity/ (accessed on 12 June 2022).
- Viswanath, B.; Kim, S. Influence of Nanotoxicity on Human Health and Environment: The Alternative Strategies. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 242, pp. 61–104. [Google Scholar] [CrossRef]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.J. Ultrasmall Gold Nanoparticles as Carriers for Nucleus-Based Gene Therapy Due to Size-Dependent Nuclear Entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular Toxicity of Inorganic Nanoparticles: Common Aspects and Guidelines for Improved Nanotoxicity Evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Schmid, G. The Relevance of Shape and Size of Au55 Clusters. Chem. Soc. Rev. 2008, 37, 1909–1930. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and in Vitro/In Vivo Toxicity Evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of Zinc Oxide Nanostructures with Mammalian Cells: Cytotoxicity and Photocatalytic Toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Zhang, Y.; Ma, L.; Song, E.; Song, Y. “Iron Free” Zinc Oxide Nanoparticles with Ion-Leaking Properties Disrupt Intracellular ROS and Iron Homeostasis to Induce Ferroptosis. Cell Death Dis. 2020, 11, 183. [Google Scholar] [CrossRef] [Green Version]
- Paunovic, J.; Vucevic, D.; Radosavljevic, T.; Mandić-Rajčević, S.; Pantic, I. Iron-Based Nanoparticles and Their Potential Toxicity: Focus on Oxidative Stress and Apoptosis. Chem. Biol. Interact. 2020, 316, 108935. [Google Scholar] [CrossRef]
- Landsiedel, R.; Ma-Hock, L.; Kroll, A.; Hahn, D.; Schnekenburger, J.; Wiench, K.; Wohlleben, W. Testing Metal-Oxide Nanomaterials for Human Safety. Adv. Mater. 2010, 22, 2601–2627. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Nuseibeh, S.; Dybowska, A.; Berhanu, D.; Tetley, T.D.; Valsami-Jones, E. Comparative Study Using Spheres, Rods and Spindle-Shaped Nanoplatelets on Dispersion Stability, Dissolution and Toxicity of CuO Nanomaterials. Nanotoxicology 2014, 8, 422–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbamaki, N.; Rasulev, B.; Cassano, A.; Marchese Robinson, R.L.; Benfenati, E.; Leszczynski, J.; Cronin, M.T.D. Genotoxicity of Metal Oxide Nanomaterials: Review of Recent Data and Discussion of Possible Mechanisms. Nanoscale 2015, 7, 2154–2198. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where Does the Toxicity of Metal Oxide Nanoparticles Come from: The Nanoparticles, the Ions, or a Combination of Both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Eslami, H.; Esmaeili, A.; Razaeian, M.; Salari, M.; Hosseini, A.N.; Mobini, M.; Barani, A. Potentially Toxic Metal Concentration, Spatial Distribution, and Health Risk Assessment in Drinking Groundwater Resources of Southeast Iran. Geosci. Front. 2022, 13, 101276. [Google Scholar] [CrossRef]
- Mielcarek, K.; Nowakowski, P.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Markiewicz-Żukowska, R.; Naliwajko, S.K.; Grabia, M.; Bielecka, J.; Żmudzińska, A.; et al. Arsenic, Cadmium, Lead and Mercury Content and Health Risk Assessment of Consuming Freshwater Fish with Elements of Chemometric Analysis. Food Chem. 2022, 379, 132167. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Sun, J.; Li, X.; Geng, X.; Zhao, M.; Sun, T.; Fan, Z. Health Risk Assessment of Heavy Metal (Loid)s in Park Soils of the Largest Megacity in China by Using Monte Carlo Simulation Coupled with Positive Matrix Factorization Model. J. Hazard. Mater. 2021, 415, 125629. [Google Scholar] [CrossRef]
- Dahmardeh Behrooz, R.; Kaskaoutis, D.G.; Grivas, G.; Mihalopoulos, N. Human Health Risk Assessment for Toxic Elements in the Extreme Ambient Dust Conditions Observed in Sistan, Iran. Chemosphere 2021, 262, 127835. [Google Scholar] [CrossRef]
- Kang, D.; Lee, S.H.; Kim, Y.J.; Kim, T.K.; Kim, J.Y.; Kim, Y. Development of Nationwide Excess Lifetime Cancer Risk Evaluation Methods with Comprehensive Past Asbestos Exposure Reconstruction. Int. J. Environ. Res. Public Health 2021, 18, 2819. [Google Scholar] [CrossRef]
- EPA. Cadmium Compounds Hazard Summary. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/cadmium-compounds.pdf (accessed on 18 December 2022).
- EN14902:2005; Ambient Air Quality—Standard Method for Measurement of Pb, Cd, As and Ni in PM10 Fraction of Suspended Particulate Matter. European Committee for Standardization: Brussels, Belgium, 2005.
- Ministry of the Environment of the Czech Republic. Methodological instruction of the Department of Environmental Damage of the Ministry of the Environment—Risk Analysis of Contaminated Territory. Bulletin of Ministry of the Environment. Available online: https://www.mzp.cz/web/edice.nsf/46861B2409735127C125784E0043418B/$file/OVV-Vestnik_3_2011-09032011.pdf (accessed on 18 December 2022).
- Su, R.; Fan, W.; Yu, Q.; Dong, X.; Qi, J.; Zhu, Q.; Zhao, W.; Wu, W.; Chen, Z.; Li, Y.; et al. Size-Dependent Penetration of Nanoemulsions into Epidermis and Hair Follicles: Implications for Transdermal Delivery and Immunization. Oncotarget 2017, 8, 38214–38226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The European Commission. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reco/2011/696/oj (accessed on 18 December 2022).
- U.S. Department of Health and Human Services. Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considering-whether-fda-regulated-product-involves-application-nanotechnology (accessed on 18 December 2022).
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The Effect of Particle Size on the Cytotoxicity, Inflammation, Developmental Toxicity and Genotoxicity of Silver Nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Kim, M.; Park, H.-S.; Shin, U.S.; Gong, M.-S.; Kim, H.-W. Size-Dependent Cellular Toxicity of Silver Nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100A, 1033–1043. [Google Scholar] [CrossRef]
- Passagne, I.; Morille, M.; Rousset, M.; Pujalté, I.; L’Azou, B. Implication of Oxidative Stress in Size-Dependent Toxicity of Silica Nanoparticles in Kidney Cells. Toxicology 2012, 299, 112–124. [Google Scholar] [CrossRef]
- Park, Y.-H.; Bae, H.C.; Jang, Y.; Jeong, S.H.; Lee, H.N.; Ryu, W.-I.; Yoo, M.G.; Kim, Y.-R.; Kim, M.-K.; Lee, J.K.; et al. Effect of the Size and Surface Charge of Silica Nanoparticles on Cutaneous Toxicity. Mol. Cell. Toxicol. 2013, 9, 67–74. [Google Scholar] [CrossRef]
- Seiffert, J.; Hussain, F.; Wiegman, C.; Li, F.; Bey, L.; Baker, W.; Porter, A.; Ryan, M.P.; Chang, Y.; Gow, A.; et al. Pulmonary Toxicity of Instilled Silver Nanoparticles: Influence of Size, Coating and Rat Strain. PLoS ONE 2015, 10, e0119726. [Google Scholar] [CrossRef] [Green Version]
- Lee, U.; Yoo, C.-J.; Kim, Y.-J.; Yoo, Y.-M. Cytotoxicity of Gold Nanoparticles in Human Neural Precursor Cells and Rat Cerebral Cortex. J. Biosci. Bioeng. 2016, 121, 341–344. [Google Scholar] [CrossRef]
- Cho, Y.-M.; Mizuta, Y.; Akagi, J.; Toyoda, T.; Sone, M.; Ogawa, K. Size-Dependent Acute Toxicity of Silver Nanoparticles in Mice. J. Toxicol. Pathol. 2018, 31, 73–80. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, B.; Engstrom, A.M.; Harper, B.J.; Harper, S.L.; Mackiewicz, M.R. Silver Nanoparticles Stable to Oxidation and Silver Ion Release Show Size-Dependent Toxicity In Vivo. Nanomaterials 2021, 11, 1516. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tian, L.; Wang, S.; Wang, D. Size-Dependent Transgenerational Toxicity Induced by Nanoplastics in Nematode Caenorhabditis Elegans. Sci. Total Environ. 2021, 790, 148217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, L.; Ma, Y.; Liu, J.; Huang, Y.; Fu, X.; Peng, S.; Wang, X.; Yang, Y.; Zhang, X.; et al. Mechanistic Study of Silica Nanoparticles on the Size-Dependent Retinal Toxicity in Vitro and in Vivo. J. Nanobiotechnol. 2022, 20, 146. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lowry, G.V. Progress towards Standardized and Validated Characterizations for Measuring Physicochemical Properties of Manufactured Nanomaterials Relevant to Nano Health and Safety Risks. NanoImpact 2018, 9, 14–30. [Google Scholar] [CrossRef]
- Garcés, M.; Cáceres, L.; Chiappetta, D.; Magnani, N.; Evelson, P. Current Understanding of Nanoparticle Toxicity Mechanisms and Interactions with Biological Systems. New J. Chem. 2021, 45, 14328–14344. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USEPA. Basic Information about the Integrated Risk Information System|Integrated Risk Information System|US EPA. Integrated Risk Information System. Available online: https://www.epa.gov/iris/basic-information-about-integrated-risk-information-system (accessed on 18 December 2022).
- The World Bank. Life Expectancy at Birth. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=CZ (accessed on 18 December 2022).
- Noël, A.; L’Espérance, G.; Cloutier, Y.; Plamondon, P.; Boucher, J.; Philippe, S.; Dion, C.; Truchon, G.; Zayed, J. Assessment of the Contribution of Electron Microscopy to Nanoparticle Characterization Sampled with Two Cascade Impactors. J. Occup. Environ. Hyg. 2013, 10, 155–172. [Google Scholar] [CrossRef]
- Dodge, D.G.; Engel, A.M.; Prueitt, R.L.; Peterson, M.K.; Goodman, J.E. US EPA’s TSCA Risk Assessment Approach: A Case Study of Asbestos in Automotive Brakes. Inhal. Toxicol. 2021, 33, 295–307. [Google Scholar] [CrossRef]
- Paustenbach, D.; Brew, D.; Ligas, S.; Heywood, J. A Critical Review of the 2020 EPA Risk Assessment for Chrysotile and Its Many Shortcomings. Crit. Rev. Toxicol. 2021, 51, 509–539. [Google Scholar] [CrossRef]
- USEPA. Human Health Risk Assessment—Asbestos Air Sampling Clear Creek Management Area, California. 2004. Available online: https://archive.epa.gov/region9/toxic/web/pdf/tech-memo-for-9-15-04-ccma-sampling-event.pdf (accessed on 18 December 2022).
- Rodricks Joseph, V. Calculated Risks: The Toxicity and Human Health Risks of Chemicals in Our Environment, 2nd ed.; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Macphail, R.C.; Grulke, E.A.; Yokel, R.A. Assessing Nanoparticle Risk Poses Prodigious Challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 374–387. [Google Scholar] [CrossRef]
| Article | NP Size (nm) | Toxicity | Source | ||
|---|---|---|---|---|---|
| Pan et al., 2007 | 1.4 | highest | [34] | ||
| 15 | lowest | ||||
| Carlson et al., 2008 | 15 | highest | |||
| 30 | lower | [60] | |||
| 55 | lowest | ||||
| M.V.D.Z. Park et al., 2011 | 20 | highest | [61] | ||
| 80 | lower | ||||
| 110 | lowest | ||||
| Kim et al., 2012 | 10 | highest | [62] | ||
| 50 | lower | ||||
| 100 | lowest | ||||
| Passagne et al., 2012 | 20 | highest | [63] | ||
| 100 | lowest | ||||
| Y.-H. Park et al., 2013 | 20 | highest | [64] | ||
| 100 | lowest | ||||
| Huo et al., 2014 | 2 | highest | [33] | ||
| 6 | highest | ||||
| 10 | lower | ||||
| 16 | lower | ||||
| Seiffert et al., 2015 | 20 | highest | [65] | ||
| 110 | lowest | ||||
| Lee et al., 2016 | 5 | highest | [66] | ||
| 100 | lowest | ||||
| Cho et al., 2018 | 10 | highest | [67] | ||
| 60 | lower | ||||
| 100 | lowest | ||||
| Carnovale et al., 2019 | 25–50 | highest | [68] | ||
| 50+ | lower | ||||
| Cunningham et al., 2021 | 20 | highest | [69] | ||
| 40 | lower | ||||
| 60 | lower | ||||
| 80 | lower | ||||
| 100 | Lowest | ||||
| H. Liu et al., 2021 | 20 | highest | [70] | ||
| 100 | lowest | ||||
| Z. Zhang et al., 2022 | 15 | highest | [71] | ||
| 50 | lowest | ||||
| Particle Size (nm) | Concentration (pg·m−3) | Particle Size (nm) | Concentration (pg·m−3) |
|---|---|---|---|
| 15.1 | 0.45 | 599 | 65 |
| 29.4 | 1.0 | 942 | 35 |
| 53.9 | 4.3 | 1620 | 11 |
| 95.2 | 9.5 | 2460 | 4.3 |
| 154 | 16 | 3640 | 2.0 |
| 254 | 39 | 5340 | 1.1 |
| 379 | 69 | 9830 | 0.73 |
| Size Group (nm) | SM | |||||
|---|---|---|---|---|---|---|
| 15.1 | 4.5 × 10−7 | 4.5 × 10−7 | 8.1 × 10−10 | 10 | 942 | 7.63 × 10−6 |
| 29.4 | 1.0 × 10−6 | 1.0 × 10−6 | 1.8 × 10−9 | 7 | 112 | 1.41 × 10−6 |
| 53.9 | 4.3 × 10−6 | 4.3 × 10−6 | 7.7 × 10−9 | 4 | 21 | 6.38 × 10−7 |
| 95.2 | 9.5 × 10−6 | 9.5 × 10−6 | 1.7 × 10−8 | 1 | 5 | 8.91 × 10−8 |
| 95.2–9830 | 2.4 × 10−4 | 2.4 × 10−4 | 4.4 × 10−7 | 1 | 3 | 1.32 × 10−6 |
| Method | Health Risk of Measured Cadmium Concentration | Nanoparticles’ Share on Health Risk Value | Nanoparticles’ Share |
|---|---|---|---|
| Standard method ELCR | 4.7 × 10−7 | 2.7 × 10−8 * | 5.9% * |
| 1.1 × 10−5 | 9.8 × 10−6 | 88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macko, M.; Antoš, J.; Božek, F.; Konečný, J.; Huzlík, J.; Hegrová, J.; Kuřitka, I. Development of New Health Risk Assessment of Nanoparticles: EPA Health Risk Assessment Revised. Nanomaterials 2023, 13, 20. https://doi.org/10.3390/nano13010020
Macko M, Antoš J, Božek F, Konečný J, Huzlík J, Hegrová J, Kuřitka I. Development of New Health Risk Assessment of Nanoparticles: EPA Health Risk Assessment Revised. Nanomaterials. 2023; 13(1):20. https://doi.org/10.3390/nano13010020
Chicago/Turabian StyleMacko, Michal, Jan Antoš, František Božek, Jiří Konečný, Jiří Huzlík, Jitka Hegrová, and Ivo Kuřitka. 2023. "Development of New Health Risk Assessment of Nanoparticles: EPA Health Risk Assessment Revised" Nanomaterials 13, no. 1: 20. https://doi.org/10.3390/nano13010020
APA StyleMacko, M., Antoš, J., Božek, F., Konečný, J., Huzlík, J., Hegrová, J., & Kuřitka, I. (2023). Development of New Health Risk Assessment of Nanoparticles: EPA Health Risk Assessment Revised. Nanomaterials, 13(1), 20. https://doi.org/10.3390/nano13010020








