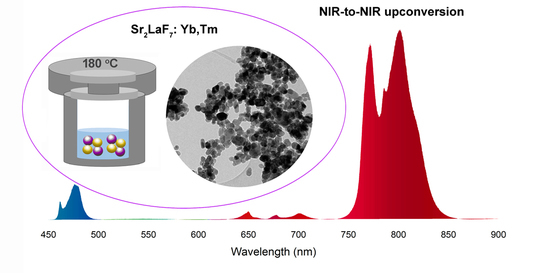
Hydrothermal Synthesis and Properties of Yb3+/Tm3+ Doped Sr2LaF7 Upconversion Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of SLF:Yb,Tm
2.3. Measurement
3. Results and Discussion
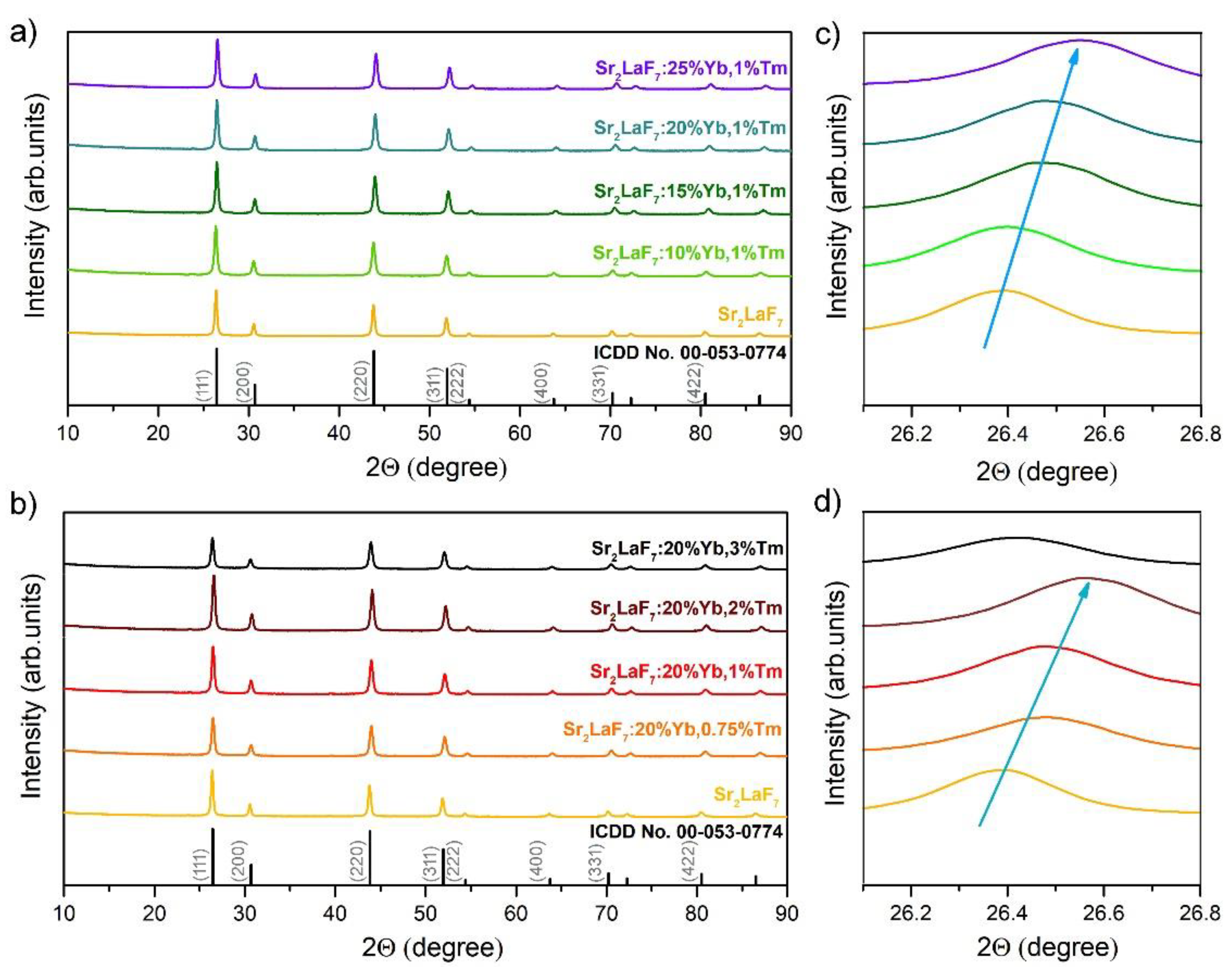
3.1. XRD Analysis
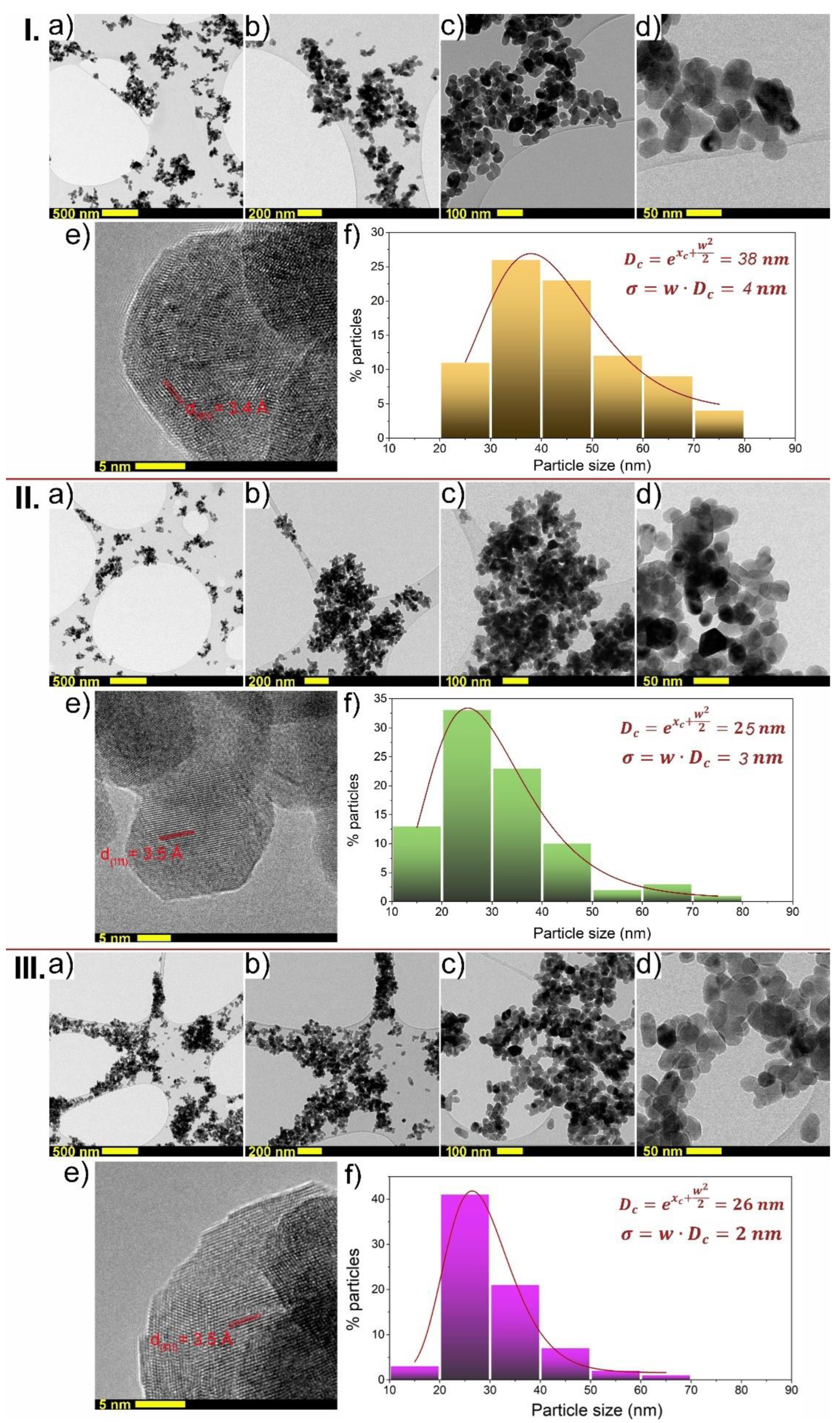
3.2. Morphology Analysis
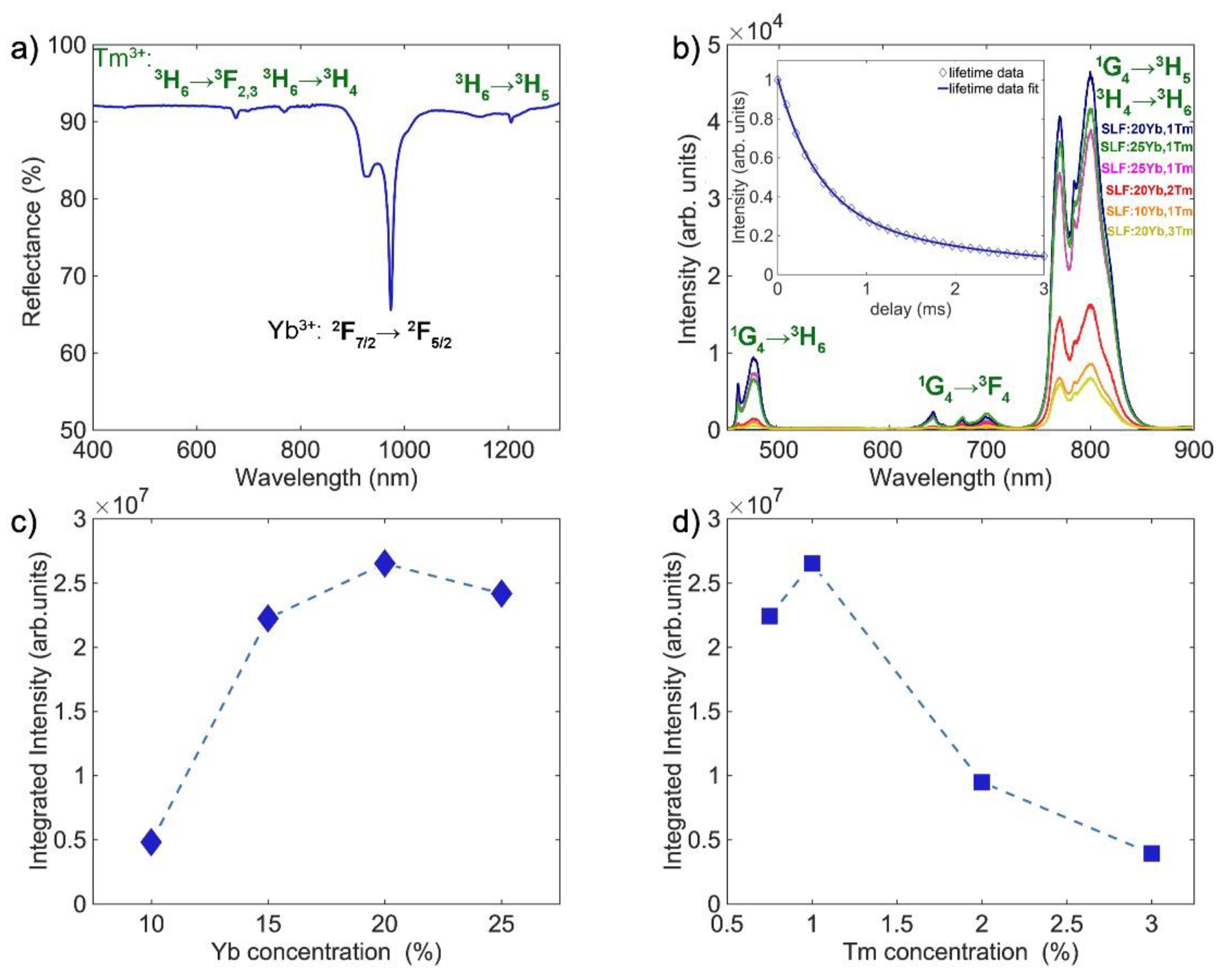
3.3. Spectroscopic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peijzel, P.S.; Meijerink, A.; Wegh, R.T.; Reid, M.F.; Burdick, G.W. A complete 4fn energy level diagram for all trivalent lanthanide ions. J. Solid State Chem. 2005, 178, 448–453. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, B.; Jin, D.; Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 2015, 10, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Visser, C.; Maduro, J.A.; Pshenichnikov, M.S.; Hummelen, J.C. Broadband dye-sensitized upconversion of near-infrared light. Nat. Photonics 2012, 6, 560–564. [Google Scholar] [CrossRef]
- Erol, E.; Vahedigharehchopogh, N.; Kıbrıslı, O.; Ersundu, M.Ç.; Ersundu, A.E. Recent progress in lanthanide-doped luminescent glasses for solid-state lighting applications-a review. J. Phys. Condens. Matter 2021, 33, 483001. [Google Scholar] [CrossRef]
- Chen, E.Y.; Milleville, C.; Zide, J.M.O.; Doty, M.F.; Zhang, J. Upconversion of low-energy photons in semiconductor nanostructures for solar energy harvesting. MRS Energy Sustain. 2018, 5, e16. [Google Scholar] [CrossRef]
- Skwierczyńska, M.; Stopikowska, N.; Kulpiński, P.; Kłonowska, M.; Lis, S.; Runowski, M. Ratiometric Upconversion Temperature Sensor Based on Cellulose Fibers Modified with Yttrium Fluoride Nanoparticles. Nanomaterials 2022, 12, 1926. [Google Scholar] [CrossRef]
- Ryszczyńska, S.; Trejgis, K.; Marciniak, Ł.; Grzyb, T. Upconverting SrF2:Er3+ Nanoparticles for Optical Temperature Sensors. ACS Appl. Nano Mater. 2021, 4, 10438–10448. [Google Scholar] [CrossRef]
- Guo, M.Y.; Shen, L.F.; Pun, E.Y.B.; Lin, H. Sr2GdF7: Er3+/Yb3+ nanocrystal-inlaid pliable fibers for synergistic feedback temperature monitoring. J. Lumin. 2022, 252, 119394. [Google Scholar] [CrossRef]
- Baride, A.; Sigdel, G.; Cross, W.M.; Kellar, J.J.; May, P.S. Near Infrared-to-Near Infrared Upconversion Nanocrystals for Latent Fingerprint Development. ACS Appl. Nano Mater. 2019, 2, 4518–4527. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Yang, M.; Zhang, X.; Yu, A.; Zhu, Y.; Qiu, P.; Mao, C. NIR-induced highly sensitive detection of latent fingermarks by NaYF4:Yb,Er upconversion nanoparticles in a dry powder state. Nano Res. 2015, 8, 1800–1810. [Google Scholar] [CrossRef]
- Kavand, A.; Serra, C.A.; Blanck, C.; Lenertz, M.; Anton, N.; Vandamme, T.F.; Yves Mély, Y.; Przybilla, F.; Chan-Seng, D. Controlled Synthesis of NaYF4:Yb,Er Upconversion Nanocrystals as Potential Probe for Bioimaging: A Focus on Heat Treatment. ACS Appl. Nano Mater. 2021, 4, 5319–5532. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Wang, Y.; Qin, Y.; Peng, Y.; Li, S.; Han, D.; Ren, S.; Qin, K.; Li, S.; Gao, Z.; et al. Latest developments in the upconversion nanotechnology for rapid detection of food safety: A review. Nanotechnol. Rev. 2022, 11, 2110–2122. [Google Scholar] [CrossRef]
- May, P.S.; Baride, A.; Hossan, M.Y.; Berry, M. Measuring the Internal Quantum Yield of Upconversion Luminescence for Ytterbium-Sensitized Upconversion Phosphors Using the Ytterbium-(III) Emission as an Internal Standard. Nanoscale 2018, 10, 17212–17226. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Busko, D.; Joseph, R.; Howard, I.A.; Turshatov, A.; Richards, B.S. Highly Efficient La2O3:Yb3+,Tm3+ Single-Band NIR-to-NIR Upconverting Microcrystals for Anti-Counterfeiting Applications. ACS Appl. Mater. Interfaces 2018, 10, 39851–39859. [Google Scholar] [CrossRef]
- Baride, A.; Meruga, J.M.; Douma, C.; Langerman, D.; Crawford, G.; Kellar, J.J.; Cross, W.M.; May, P.S. A NIR-to-NIR Upconversion Luminescence System for Security Printing Applications. RSC Adv. 2015, 5, 101338–101346. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, J.H.; Jang, J.; Lee, H.; Kim, S.; Hahn, Y.K.; Kim, S.K.; Lee, K.H.; Lee, S.; Pyo, H.; et al. Rapid and Background-free Detection of Avian Influenza Virus in Opaque Sample using NIR-to-NIR Upconversion Nanoparticle-based Lateral Flow Immunoassay Platform. Biosens. Bioelectron. 2018, 112, 209–215. [Google Scholar] [CrossRef]
- Levy, E.S.; Tajon, C.A.; Bischof, T.S.; Iafrati, J.; Fernandez-Bravo, A.; Garfield, D.J.; Chamanzar, M.; Maharbiz, M.M.; Sohal, V.S.; Schuck, P.J. Energy-Looping Nanoparticles: Harnessing Excited-State Absorption for Deep-Tissue Imaging. ACS Nano 2016, 10, 8423–8433. [Google Scholar] [CrossRef]
- Xia, A.; Chen, M.; Gao, Y.; Wu, D.; Feng, W.; Li, F. Gd3+ complex-modified NaLuF4-based upconversion nanophosphors for trimodality imaging of NIR-to-NIR upconversion luminescence, X-Ray computed tomography and magnetic resonance. Biomaterials 2012, 33, 5394–5405. [Google Scholar] [CrossRef]
- Ortgies, D.H.; Tan, M.; Ximendes, E.C.; Del Rosal, B.; Hu, J.; Xu, L.; Wang, X.; Martín Rodríguez, E.; Jacinto, C.; Fernandez, N.; et al. Lifetime-encoded infrared-emitting nanoparticles for in vivo multiplexed imaging. ACS Nano 2018, 12, 4362–4368. [Google Scholar] [CrossRef]
- Ragin, T.; Baranowska, A.; Kochanowicz, M.; Zmojda, J.; Miluski, P.; Dorosz, D. Study of Mid-Infrared Emission and Structural Properties of Heavy Metal Oxide Glass and Optical Fibre Co-Doped with Ho3+/Yb3+ Ions. Materials 2019, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Feifei Huang, F.; Ye, R.; Cai, M.; Lei, R.; Zhang, J.; Shiqing Xu, S.; Zhang, X.H. Structural and fluorescence properties of Ho3+/Yb3+ doped germanosilicate glasses tailored by Lu2O3. J. Alloys Compd. 2018, 746, 540–548. [Google Scholar] [CrossRef]
- Kowalska, K.; Kuwik, M.; Pisarska, J.; Pisarski, W.A. Near-IR Luminescence of Rare-Earth Ions (Er3+, Pr3+, Ho3+, Tm3+) in Titanate–Germanate Glasses under Excitation of Yb3+. Materials 2022, 15, 3660. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Qin, J.; Zhang, J.; Guo, L.; Yang, M.; Wu, X.; You, M.; Peng, H. Recent Progresses in NIR-II Luminescent Bio/Chemo Sensors Based on Lanthanide Nanocrystals. Chemosensors 2022, 10, 206. [Google Scholar] [CrossRef]
- Ledoux, G.; Joubert, M.F.; Mishra, S. Upconversion Phenomena in Nanofluorides. In Photonic & Electronic Properties of Fluoride Materials, 1st ed.; Tressaud, A., Poeppelmeir, K.R., Eds.; Elsevier: Paris, France, 2016; pp. 35–63. [Google Scholar]
- Tiwari, S.P.; Maurya, S.K.; Yadav, R.S.; Kumar, A.; Kumar, V.; Joubert, M.-F.; Swart, H.C. Future prospects of fluoride based upconversion nanoparticles for emerging applications in biomedical and energy harvesting. J. Vac. Sci. Technol. B 2018, 36, 060801. [Google Scholar] [CrossRef]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal Sodium Yttrium Fluoride Based Green and Blue Emitting Upconversion Phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Runowski, M.; Bartkowiak, A.; Majewska, M.; Martín, I.R.; Lis, S. Upconverting Lanthanide Doped Fluoride NaLuF4:Yb3+-Er3+-Ho3+- Optical Sensor for Multi-Range Fluorescence Intensity Ratio (FIR) Thermometry in Visible and NIR Regions. J. Lumin. 2018, 201, 104–109. [Google Scholar] [CrossRef]
- Gonçalves, I.M.; Pessoa, A.R.; Hazra, C.; Correales, Y.S.; Ribeiro, S.J.L.; Menezes, L.d.S. Phonon-assisted NIR-To-Visible Upconversion in Single β-NaYF4 Microcrystals Codoped with Er3+ and Yb3+ for Microthermometry Applications: Experiment and Theory. J. Lumin. 2021, 231, 117801. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, X.; Li, L.; Hao, H. Up-Converting Luminescence and Temperature Sensing of Er3+/Tm3+/Yb3+ Co-Doped NaYF4 Phosphors Operating in Visible and the First Biological Window Range. Nanomaterials 2021, 11, 2660. [Google Scholar] [CrossRef]
- Grzyb, T.; Przybylska, D. Formation Mechanism, Structural, and Upconversion Properties of Alkaline Rare-Earth Fluoride Nanocrystals Doped with Yb3+/Er3+ Ions. Inorg. Chem. 2018, 57, 6410–6420. [Google Scholar] [CrossRef]
- Xia, Z.; Du, P.; Liao, L. Facile hydrothermal synthesis and upconversion luminescence of tetragonal Sr2LnF7:Yb3+/Er3+ (Ln=Y, Gd) nanocrystals. Phys. Status Solidi A 2013, 210, 1734–1737. [Google Scholar] [CrossRef]
- Mao, Y.; Ma, M.; Gong, L.; Xu, C.; Ren, G.; Yang, Q. Controllable synthesis and upconversion emission of ultrasmall near-monodisperse lanthanide-doped Sr2LaF7 nanocrystals. J. Alloys Compd. 2014, 609, 262–267. [Google Scholar] [CrossRef]
- Xie, J.; Bin, J.; Guan, M.; Liu, H.; Yang, D.; Xue, J.; Liao, L.; Mei, L. Hydrothermal Synthesis and Upconversion Luminescent Properties of Sr2LaF7 Doped with Yb3+ and Er3+ Nanophosphors. J. Lumin. 2018, 200, 133–140. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Jiang, Q.; Zeng, H.; Ci, Z.; Sun, L. Pure near-infrared to near-infrared upconversion of multifunctional Tm3+ and Yb3+ co-doped NaGd(WO4)2 nanoparticles. J. Mater. Chem. C 2014, 2, 4495–4501. [Google Scholar] [CrossRef]
- Alkahtani, M.; Alfahd, A.; Alsofyani, N.; Almuqhim, A.A.; Qassem, H.; Alshehri, A.A.; Almughem, F.A.; Hemmer, P. Photostable and Small YVO4:Yb,Er Upconversion Nanoparticles in Water. Nanomaterials 2021, 11, 1535. [Google Scholar] [CrossRef]
- Li, L.; Pan, Y.; Chang, W.; Feng, Z.; Chen, P.; Li, C.; Zeng, Z.; Zhou, X. Near-infrared downconversion luminescence of SrMoO4:Tm3+,Yb3+ phosphors. Mater. Res. Bull. 2017, 93, 144–149. [Google Scholar] [CrossRef]
- Simpson, D.A.; Gibbs, W.E.K.; Collins, S.F.; Blanc, W.; Dussardier, B.; Monnom, G.; Peterka, P.; Baxter, G.W. Visible and near infra-red up-conversion in Tm3+/Yb3+ co-doped silica fibers under 980 nm excitation. Opt. Express 2008, 16, 13781–13799. [Google Scholar] [CrossRef]
- Güell, F.; Solé, R.; Gavaldà, J.; Aguiló, M.; Galán, M.; Díaz, F.; Massons, J. Upconversion luminescence of Tm3+ sensitized by Yb3+ ions in monoclinic KGd(WO4)2 single crystals. Opt. Mater. 2007, 30, 222–226. [Google Scholar] [CrossRef]

| SLF | SLF:10Yb,1Tm | SLF:15Yb,1Tm | SLF:20Yb,1 Tm | SLF:25Yb,1Tm | |
|---|---|---|---|---|---|
| CS (nm) | 27.1 | 23.5 | 25 | 25.4 | 24.4 |
| Strain | 0.144 | 0.254 | 0.254 | 0.272 | 0.258 |
| * Rwp | 6.00 | 4.74 | 4.58 | 4.33 | 4.09 |
| ** Rp | 4.62 | 3.79 | 3.51 | 3.35 | 3.28 |
| *** Re | 3.42 | 3.48 | 3.37 | 3.37 | 3.28 |
| GOF | 1.7569 | 1.3597 | 1.3597 | 1.2843 | 1.2484 |
| a = b = c (Å) | 5.8451 | 5.8372 | 5.8190 | 5.8115 | 5.8045 |
| CV (Å3) | 199.70 | 198.89 | 197.04 | 196.27 | 195.57 |
| SLF | SLF:20Yb,0.75Tm | SLF:20Yb,1Tm | SLF:20Yb,2Tm | SLF:20Yb,3Tm | |
|---|---|---|---|---|---|
| CS (nm) | 27.1 | 22.6 | 25.4 | 21.9 | 17.8 |
| Strain | 0.144 | 0.211 | 0.272 | 0.185 | 0.02 |
| * Rwp | 6.00 | 4.12 | 4.33 | 4.91 | 5.97 |
| ** Rp | 4.62 | 3.14 | 3.35 | 3.90 | 4.41 |
| *** Re | 3.42 | 3.56 | 3.37 | 3.33 | 3.39 |
| GOF | 1.7569 | 1.1589 | 1.2843 | 1.4747 | 1.7607 |
| a = b = c (Å) | 5.8451 | 5.8129 | 5.8115 | 5.8107 | 5.8288 |
| CV (Å3) | 199.70 | 196.42 | 196.27 | 196.20 | 198.03 |
| 0.6887 | 0.4503 | 0.3163 | 2.3607 | 0.0030 | 1.0516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milićević, B.; Periša, J.; Ristić, Z.; Milenković, K.; Antić, Ž.; Smits, K.; Kemere, M.; Vitols, K.; Sarakovskis, A.; Dramićanin, M.D. Hydrothermal Synthesis and Properties of Yb3+/Tm3+ Doped Sr2LaF7 Upconversion Nanoparticles. Nanomaterials 2023, 13, 30. https://doi.org/10.3390/nano13010030
Milićević B, Periša J, Ristić Z, Milenković K, Antić Ž, Smits K, Kemere M, Vitols K, Sarakovskis A, Dramićanin MD. Hydrothermal Synthesis and Properties of Yb3+/Tm3+ Doped Sr2LaF7 Upconversion Nanoparticles. Nanomaterials. 2023; 13(1):30. https://doi.org/10.3390/nano13010030
Chicago/Turabian StyleMilićević, Bojana, Jovana Periša, Zoran Ristić, Katarina Milenković, Željka Antić, Krisjanis Smits, Meldra Kemere, Kaspars Vitols, Anatolijs Sarakovskis, and Miroslav D. Dramićanin. 2023. "Hydrothermal Synthesis and Properties of Yb3+/Tm3+ Doped Sr2LaF7 Upconversion Nanoparticles" Nanomaterials 13, no. 1: 30. https://doi.org/10.3390/nano13010030
APA StyleMilićević, B., Periša, J., Ristić, Z., Milenković, K., Antić, Ž., Smits, K., Kemere, M., Vitols, K., Sarakovskis, A., & Dramićanin, M. D. (2023). Hydrothermal Synthesis and Properties of Yb3+/Tm3+ Doped Sr2LaF7 Upconversion Nanoparticles. Nanomaterials, 13(1), 30. https://doi.org/10.3390/nano13010030