Synthesis and Characterisation of a Graphene Oxide-Gold Nanohybrid for Use as Test Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide (GO)
2.3. Synthesis of Graphene Oxide-Gold Nanohybrid (GO-Au)
2.4. Characterization of the Nanohybrid and Its Components
2.5. Quantification of Au NPs in the GO-Au Nanohybrid
2.6. Dispersion Stability
3. Results and Discussion
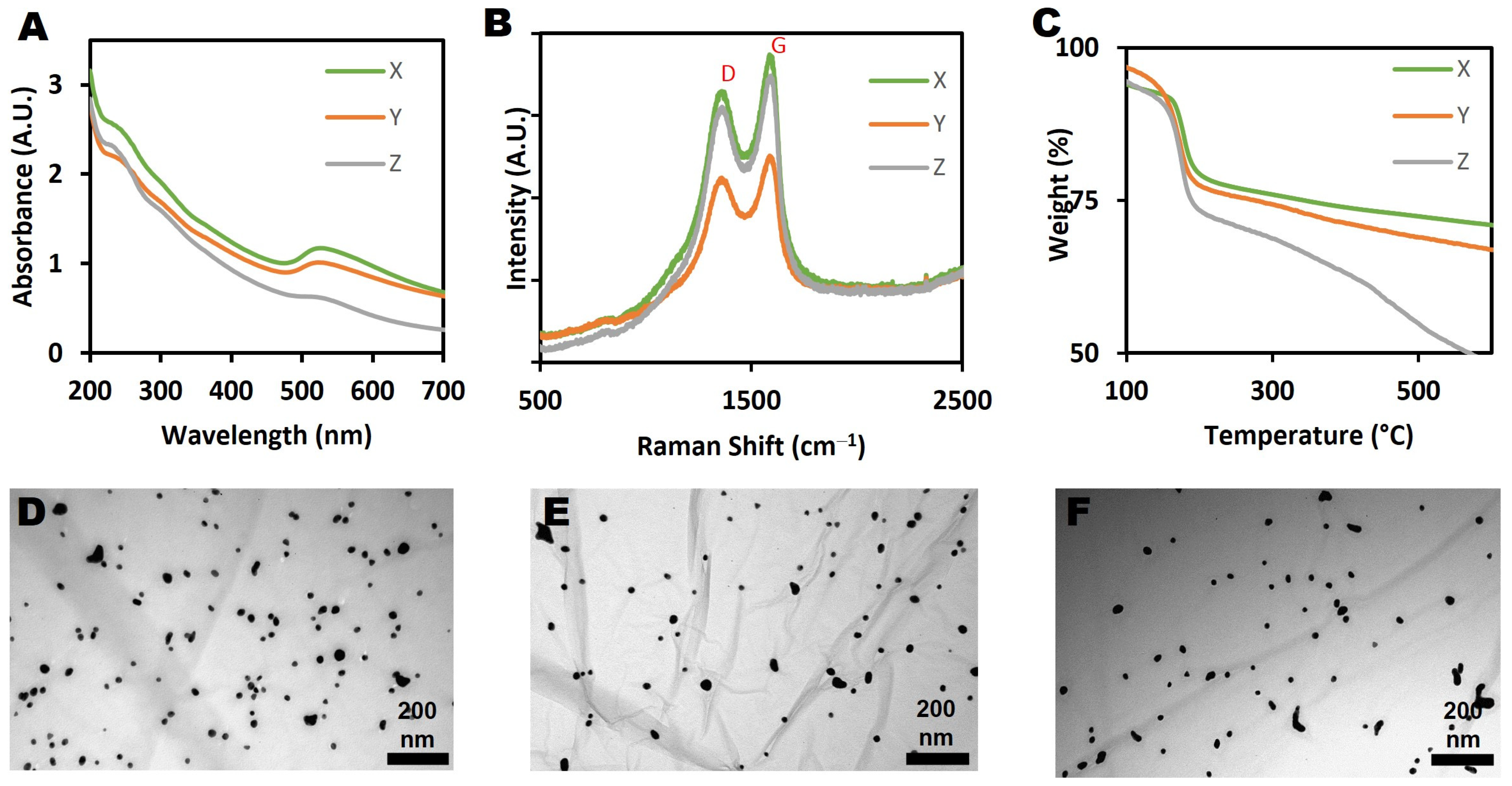
3.1. Characterization of Graphene Oxide
3.2. Characterization of Graphene Oxide-Gold Nanohybrid (GO-Au)
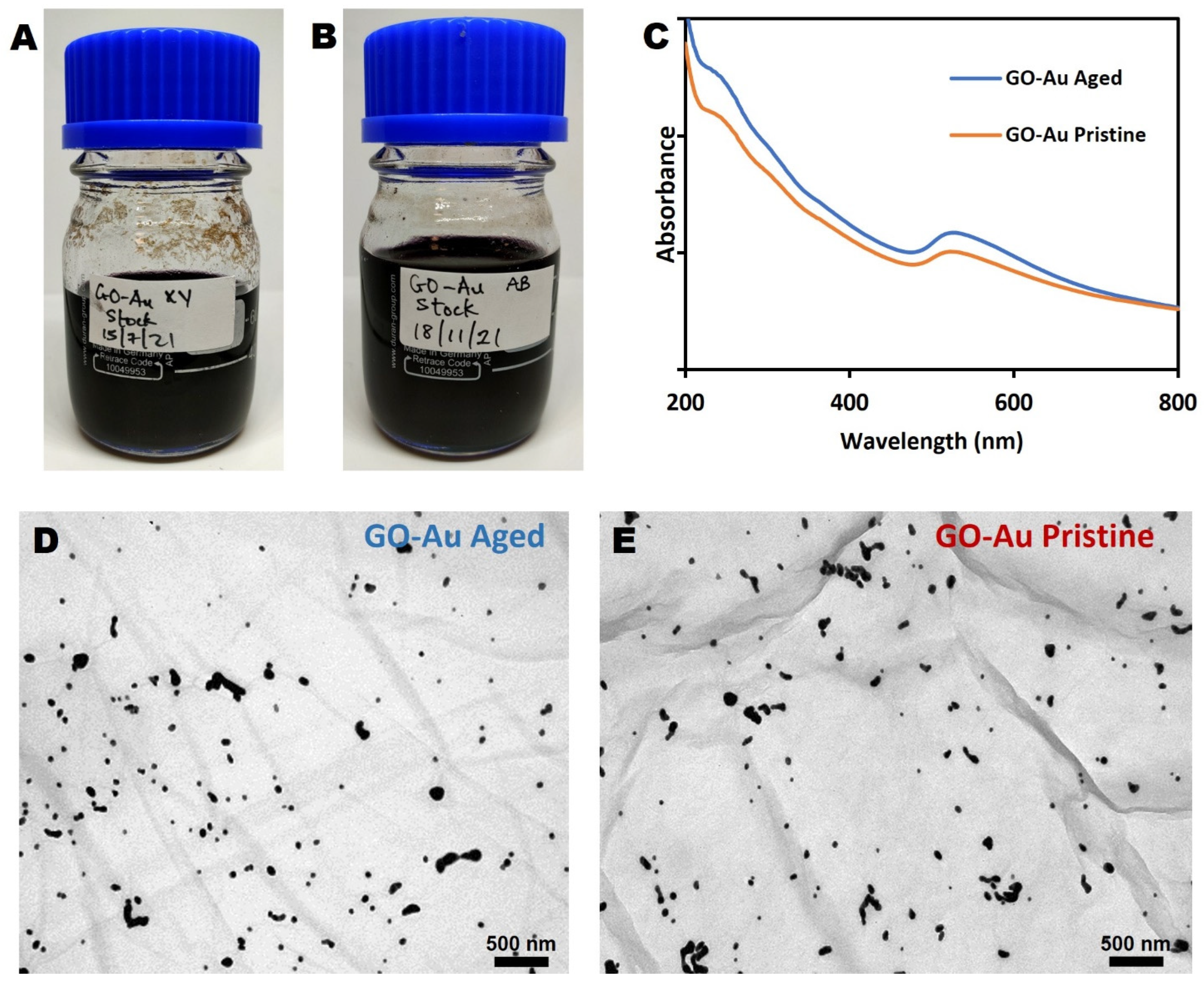
3.3. Evaluation of Nanohybrid Ageing
3.4. Quantification of AuNP on GO-Au Nanohybrid
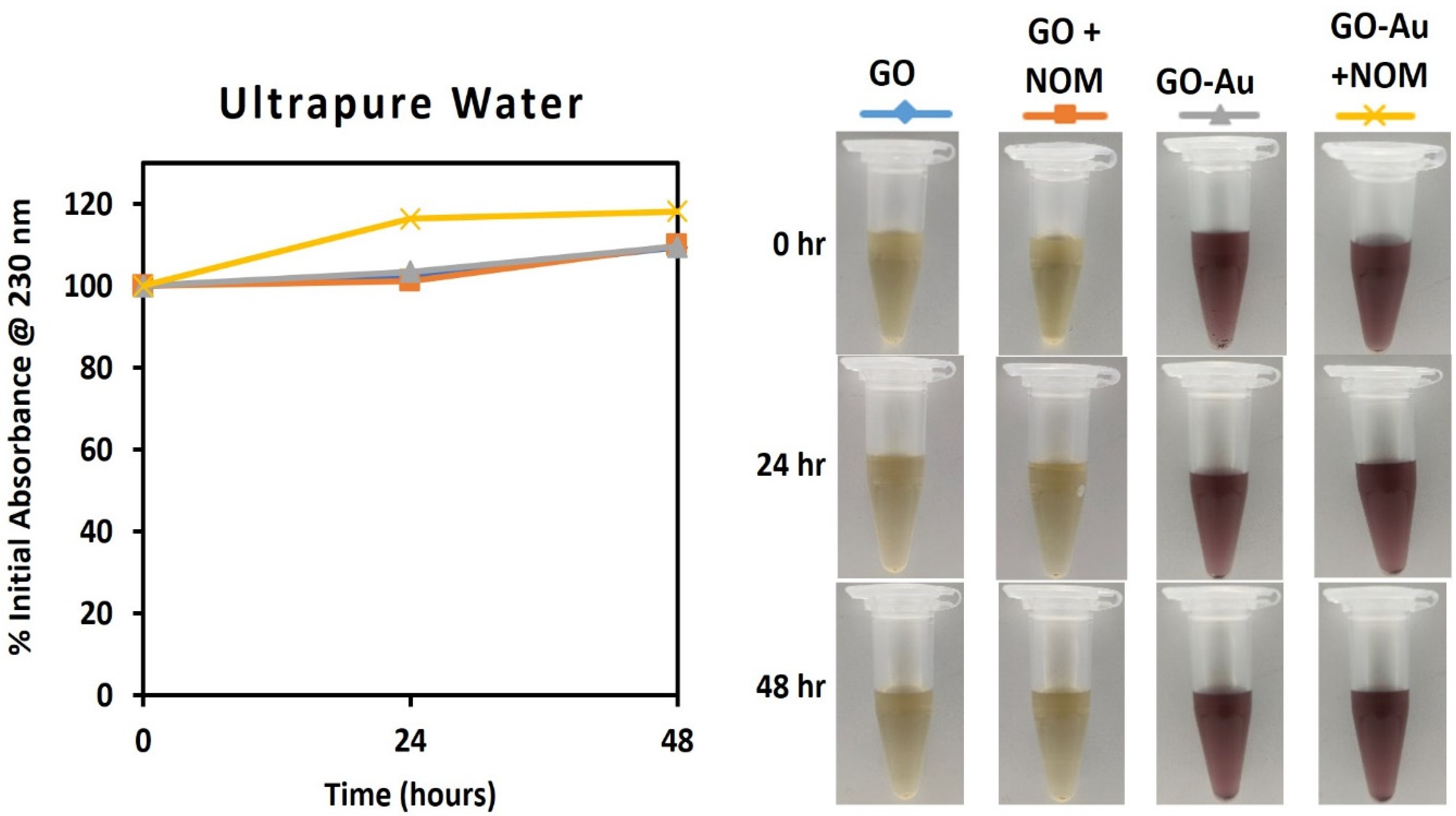
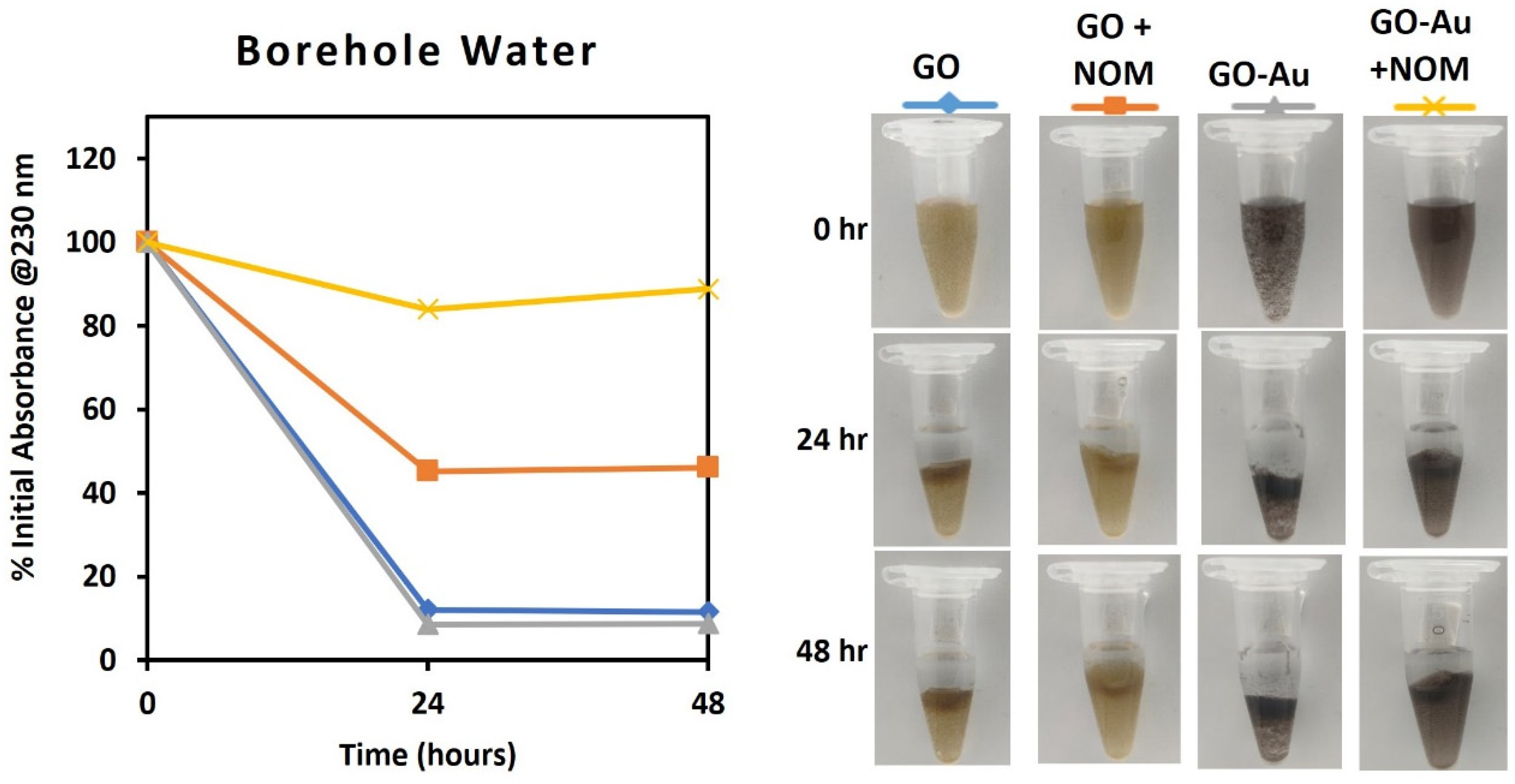
3.5. Assessment of the Impact of NOM on Dispersion Stability of the GO-Au
3.6. Overall Assessment of the GO-Au Nanohybrid as a Model Nano(eco)toxicity Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the Graphene Family—A Recommended Nomenclature for Two-Dimensional Carbon Materials. Carbon N. Y. 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A Decade of Graphene Research: Production, Applications and Outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem.-Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Saleh, N.B.; Sun, W.; Park, C.M.; Shen, C.; Aich, N.; Peijnenburg, W.J.G.M.; Zhang, W.; Jin, Y.; Su, C. Next-Generation Multifunctional Carbon-Metal Nanohybrids for Energy and Environmental Applications. Environ. Sci. Technol. 2019, 53, 7265–7287. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.J. Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications. Chem. Rev. 2018, 119, 1666–1762. [Google Scholar] [CrossRef]
- Fallatah, H.; Elhaneid, M.; Ali-boucetta, H.; Overton, T.W.; El Kadri, H.; Gkatzionis, K. Antibacterial Effect of Graphene Oxide (GO) Nano-Particles against Pseudomonas putida Biofilm of Variable Age. Environ. Sci. Pollut. Res. 2019, 26, 25057–25070. [Google Scholar] [CrossRef]
- Buffet, P.E.; Zalouk-Vergnoux, A.; Châtel, A.; Berthet, B.; Métais, I.; Perrein-Ettajani, H.; Poirier, L.; Luna-Acosta, A.; Thomas-Guyon, H.; Risso-de Faverney, C.; et al. A Marine Mesocosm Study on the Environmental Fate of Silver Nanoparticles and Toxicity Effects on Two Endobenthic Species: The Ragworm Hediste diversicolor and the Bivalve Mollusc Scrobicularia plana. Sci. Total Environ. 2014, 470–471, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandraker, K.; Nagwanshi, R.; Jadhav, S.K.; Ghosh, K.K.; Satnami, M.L. Antibacterial Properties of Amino Acid Functionalized Silver Nanoparticles Decorated on Graphene Oxide Sheets. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2017, 181, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.N.I.; Halilu, A.; Julkapli, N.M.; Ma’amor, A. Gold-Graphene Oxide Nanohybrids: A Review on Their Chemical Catalysis. J. Ind. Eng. Chem. 2020, 83, 1–13. [Google Scholar] [CrossRef]
- Santhosh, C.; Saranya, M.; Ramachandran, R.; Felix, S.; Velmurugan, V.; Grace, A.N. Graphene/Gold Nanocomposites-Based Thin Films as an Enhanced Sensing Platform for Voltammetric Detection of Cr(VI) Ions. J. Naonotechnol. 2014, 2014, 304526. [Google Scholar] [CrossRef] [Green Version]
- Neri, G.; Fazio, E.; Mineo, P.G.; Scala, A.; Piperno, A. SERS Sensing Properties of New Graphene/Gold Nanocomposite. Nanomaterials 2019, 9, 1236. [Google Scholar] [CrossRef] [Green Version]
- Al-Ani, L.A.; AlSaadi, M.A.; Kadir, F.A.; Hashim, N.M.; Julkapli, N.M.; Yehye, W.A. Graphene–Gold Based Nanocomposites Applications in Cancer Diseases; Efficient Detection and Therapeutic Tools. Eur. J. Med. Chem. 2017, 139, 349–366. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Boukherroub, R.; Szunerits, S. Gold-Graphene Nanocomposites for Sensing and Biomedical Applications. J. Mater. Chem. B 2015, 3, 4301–4324. [Google Scholar] [CrossRef]
- Bradley, D. Is Graphene Safe? Mater. Today 2012, 15, 230. [Google Scholar] [CrossRef]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety Considerations for Graphene: Lessons Learnt from Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef]
- Lu, C.-J.; Ma, Y.-B.; Junaid, M.; Jiang, X.-F.; Jia, P.-P.; Wang, H.-B.; Pei, D.-S. Graphene Oxide Nanosheets Induce DNA Damage and Activate the Base Excision Repair (BER) Signaling Pathway Both In Vitro and In Vivo. Chemosphere 2017, 184, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Pretti, C.; Oliva, M.; Di Pietro, R.; Monni, G.; Cevasco, G.; Chiellini, F.; Pomelli, C.; Chiappe, C. Ecotoxicity of Pristine Graphene to Marine Organisms. Ecotoxicol. Environ. Saf. 2014, 101, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, F.; Wang, S.; Peijnenburg, W.J.G.M. Assessment and Prediction of Joint Algal Toxicity of Binary Mixtures of Graphene and Ionic Liquids. Chemosphere 2017, 185, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Xiaohui, L.; Tao, Y.; Zhu, X.; Jiang, Y.; Li, B.; Yang, Y.; Chen, B.; Cai, Z. A Mechanism Study on Toxicity of Graphene Oxide to Daphnia magna: Direct Link between Bioaccumulation and Oxidative Stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef]
- Zou, W.; Zhou, Q.; Zhang, X.; Mu, L.; Hu, X. Characterization of the Effects of Trace Concentrations of Graphene Oxide on Zebrafish Larvae through Proteomic and Standard Methods. Ecotoxicol. Environ. Saf. 2018, 159, 221–231. [Google Scholar] [CrossRef] [PubMed]
- El-Yamany, N.A.; Salaheldin, T.A.; Abd El-Mohsen, W.N.; Amin, A.S.; Mohamed, F.F.; Tohamy, A.A. Graphene Oxide Nanosheets Induced Genotoxicity and Pulmonary Injury in Mice. Exp. Toxicol. Pathol. 2017, 69, 383–392. [Google Scholar] [CrossRef]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene-Gold Nanoparticles Hybrid-Synthesis, Functionalization, and Application in a Electrochemical and Surface-Enhanced Raman Scattering Biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80(6), 1339. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Lee, D.J.; Park, S.S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 2021, 14, 4590. [Google Scholar] [CrossRef]
- Khalili, D. Graphene Oxide: A Promising Carbocatalyst for the Regioselective Thiocyanation of Aromatic Amines, Phenols, Anisols and Enolizable Ketones by Hydrogen Peroxide/KSCN in Water. New J. Chem. 2016, 40, 2547–2553. [Google Scholar] [CrossRef]
- Zhang, Z.; Schniepp, H.C.; Adamson, D.H. Characterization of Graphene Oxide: Variations in Reported Approaches. Carbon N. Y. 2019, 154, 510–521. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Bitounis, D.; Raveendran-Nair, R.; Servant, A.; Van den Bossche, J.; Kostarelos, K. Purified Graphene Oxide Dispersions Lack In Vitro Cytotoxicity and In Vivo Pathogenicity. Adv. Healthc. Mater. 2013, 2, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. Adv. Nanomed. Deliv. Ther. Nucleic Acids 2017, 44–58. [Google Scholar] [CrossRef]
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C.M. The Relationship between PH and Zeta Potential of ∼30 Nm Metal Oxide Nanoparticle Suspensions Relevant to in Vitro Toxicological Evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [Green Version]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-Concentration, Surfactant-Stabilized Graphene Dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Newman, L.; Lozano, N.; Mukherjee, S.P.; Fadeel, B.; Bussy, C.; Kostarelos, K. A Blueprint for the Synthesis and Characterisation of Thin Graphene Oxide with Controlled Lateral Dimensions for Biomedicine. 2D Mater. 2018, 5, 035020. [Google Scholar] [CrossRef] [Green Version]
- Jasim, D.A.; Lozano, N.; Kostarelos, K. Synthesis of Few-Layered, High-Purity Graphene Oxide Sheets from Different Graphite Sources for Biology. 2D Mater. 2016, 3, 014006. [Google Scholar] [CrossRef]
- De Medeiros, A.M.Z.; Khan, L.U.; da Silva, G.H.; Ospina, C.A.; Alves, O.L.; de Castro, V.L.; Martinez, D.S.T. Graphene Oxide-Silver Nanoparticle Hybrid Material: An Integrated Nanosafety Study in Zebrafish Embryos. Ecotoxicol. Environ. Saf. 2021, 209, 111776. [Google Scholar] [CrossRef]
- Martinez, D.S.T.; Da Silva, G.H.; de Medeiros, A.M.Z.; Khan, L.U.; Papadiamantis, A.G.; Lynch, I. Effect of the Albumin Corona on the Toxicity of Combined Graphene Oxide and Cadmium to Daphnia magna and Integration of the Datasets into the Nanocommons Knowledge Base. Nanomaterials 2020, 10, 1936. [Google Scholar] [CrossRef]
- Pham, T.A.; Choi, B.C.; Lim, K.T.; Jeong, Y.T. A Simple Approach for Immobilization of Gold Nanoparticles on Graphene Oxide Sheets by Covalent Bonding. Appl. Surf. Sci. 2011, 257, 3350–3357. [Google Scholar] [CrossRef]
- Mokhtar, M.; El Enein, S.A.; Hassaan, M.; Morsy, M.; Khalil, M. Thermally Reduced Graphene Oxide: Synthesis, Structural and Electrical Properties. Int. J. Nanoparticles Nanotechnol. 2017, 3(1), 8. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Varkani, J.N. Green Synthesis of Reduced Graphene Oxide Decorated with Gold Nanoparticles and Its Glucose Sensing Application. Sens. Actuators B Chem. 2014, 202, 475–482. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Xing, R.; Li, Q.; Zhou, C.; Liu, D.; Song, H. Synthesis of Au/Graphene Oxide Composites for Selective and Sensitive Electrochemical Detection of Ascorbic Acid. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xiang, Y.; Peng, C.; Xu, W.; Banks, M.K.; Wu, R. Synthesis of a Novel Graphene-Based Gold Nanocomposite Using PVEIM-b-PNIPAM as a Stabilizer and Its Thermosensitivity for the Catalytic Reduction of 4-Nitrophenol. Inorg. Chem. Front. 2019, 6, 903–913. [Google Scholar] [CrossRef]
- Caires, A.J.; Alves, D.C.B.; Fantini, C.; Ferlauto, A.S.; Ladeira, L.O. One-Pot in Situ Photochemical Synthesis of Graphene Oxide/Gold Nanorod Nanocomposites for Surface-Enhanced Raman Spectroscopy. RSC Adv. 2015, 5, 46552–46557. [Google Scholar] [CrossRef]
- Spoljaric, S.; Ju, Y.; Caruso, F. Protocols for Reproducible, Increased-Scale Synthesis of Engineered Particles - Bridging the “Upscaling Gap.”. Chem. Mater. 2021, 33, 1099–1115. [Google Scholar] [CrossRef]
- Sitompul, J.P.; Lee, H.W.; Kim, Y.C.; Chang, M.W. A Scaling-up Synthesis from Laboratory Scale to Pilot Scale and to near Commercial Scale for Paste-Glue Production. J. Eng. Technol. Sci. 2013, 45, 9–24. [Google Scholar] [CrossRef]
- Hull, M.S.; Kennedy, A.J.; Steevens, J.A.; Bednar, A.J.; Weiss, C.A.; Vikesland, P.J. Release of Metal Impurities from Carbon Nanomaterials Influences Aquatic Toxicity. Environ. Sci. Technol. 2009, 43, 4169–4174. [Google Scholar] [CrossRef]
- Pan, H.; Low, S.; Weerasuriya, N.; Shon, Y.S. Graphene Oxide-Promoted Reshaping and Coarsening of Gold Nanorods and Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 3406–3413. [Google Scholar] [CrossRef]
- Pan, H.; Low, S.; Weerasuriya, N.; Wang, B.; Shon, Y.S. Morphological Transformation of Gold Nanoparticles on Graphene Oxide: Effects of Capping Ligands and Surface Interactions. Nano Converg. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Bongiorno, A. Origin of the Chemical and Kinetic Stability of Graphene Oxide. Sci. Rep. 2013, 3, 2484. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Ji, Y.; Hui, F.; Wu, H.H.; Lanza, M. Ageing Mechanisms and Reliability of Graphene-Based Electrodes. Nano Res. 2014, 7, 1820–1831. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A.; Enzelberger, M.; Müller, P. Formation and Decomposition of CO2 Intercalated Graphene Oxide. Chem. Mater. 2012, 24, 1276–1282. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of Size, Surface Charge, and Agglomeration State of Nanoparticle Dispersions for Toxicological Studies. J. Nanoparticle Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Gillespie, A.; Jao, D.; Andriola, A.; Duda, T.; Yang, C.F.; Yu, L. Gold Nanoparticle Determination by Inductively Coupled Plasma-Mass Spectrometry, Anodic Stripping Voltammetry, and Flame Atomic Absorption Spectrophotometry. Anal. Lett. 2012, 45, 1310–1320. [Google Scholar] [CrossRef]
- Scheffer, A.; Engelhard, C.; Sperling, M.; Buscher, W. ICP-MS as a New Tool for the Determination of Gold Nanoparticles in Bioanalytical Applications. Anal. Bioanal. Chem. 2008, 390, 249–252. [Google Scholar] [CrossRef]
- Allabashi, R.; Stach, W.; De La Escosura-Muñiz, A.; Liste-Calleja, L.; Merkoçi, A. ICP-MS: A Powerful Technique for Quantitative Determination of Gold Nanoparticles without Previous Dissolving. J. Nanoparticle Res. 2009, 11, 2003–2011. [Google Scholar] [CrossRef]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for Characterizing the Potential Human Health Effects from Exposure to Nanomaterials: Elements of a Screening Strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological Applications of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1909–1930. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Baer, K.N.; Goulden, C.E. Evaluation of a High-Hardness COMBO Medium and Frozen Algae for Daphnia magna. Ecotoxicol. Environ. Saf. 1998, 39, 201–206. [Google Scholar] [CrossRef]
- USEPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 5th ed.; United States Environmental Protection Agency (EPA): Washington, DC, USA, 2002; pp. 1–275. [Google Scholar]
- OECD. Guideline 202: Daphnia Sp., Acute Immobilisation Test. OECD Guidelines for the Testing of Chemicals. Guideline 202: Daphnia sp., Acute Immobilisation Test; OECD: Paris, France, 2004; pp. 1–12. [Google Scholar]
- ISO 6341; Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. International Organization for Standardization: Geneva, Switzerland, 2012; p. 11.
- Rodrigues, A.; Brito, A.; Janknecht, P.; Proena, M.F.; Nogueira, R. Quantification of Humic Acids in Surface Water: Effects of Divalent Cations, PH, and Filtration. J. Environ. Monit. 2009, 11, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, A.; Mungse, H.P.; Khatri, O.P. Surface Chemistry of Graphene and Graphene Oxide: A Versatile Route for Their Dispersion and Tribological Applications. Adv. Colloid Interface Sci. 2020, 283, 102215. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion Behaviour of Graphene Oxide and Reduced Graphene Oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Peng, M.; Huang, Z.; Shi, J.; Huang, T. Stability, Transport and Ecosystem Effects of Graphene in Water and Soil Environments. Nanoscale 2017, 9(17), 5370–5388. [Google Scholar] [CrossRef]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal Properties and Stability of Graphene Oxide Nanomaterials in the Aquatic Environment. Environ. Sci. Technol. 2013, 47, 1359. [Google Scholar] [CrossRef]
- Saleh, N.B.; Pfefferle, L.D.; Elimelech, M. Influence of Biomacromolecules and Humic Acid on the Aggregation Kinetics of Single-Walled Carbon Nanotubes. Environ. Sci. Technol. 2010, 44, 2412–2418. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Influence of Humic Acid on the Aggregation Kinetics of Fullerene (C60) Nanoparticles in Monovalent and Divalent Electrolyte Solutions. J. Colloid Interface Sci. 2007, 309, 126–134. [Google Scholar] [CrossRef]
- Beltran-Huarac, J.; Zhang, Z.; Pyrgiotakis, G.; DeLoid, G.; Vaze, N.; Demokritou, P. Development of Reference Metal and Metal Oxide Engineered Nanomaterials for Nanotoxicology Research Using High Throughput and Precision Flame Spray Synthesis Approaches. NanoImpact 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Orts-Gil, G.; Natte, K.; Österle, W. Multi-Parametric Reference Nanomaterials for Toxicology: State of the Art, Future Challenges and Potential Candidates. RSC Adv. 2013, 3, 18202–18215. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.J.A.; Valsami-Jones, E.; Lynch, I. Exposure Medium and Particle Ageing Moderate the Toxicological Effects of Nanomaterials to Daphnia magna over Multiple Generations: A Case for Standard Test Review? Environ. Sci. Nano 2020, 7, 1136–1149. [Google Scholar] [CrossRef]


| Functional Groups | Binding Energy (eV) | % Atomic |
|---|---|---|
| Aromatic carbon (-C=C-) | 284.13 | 9.98 |
| Aliphatic carbon (-C-C-) | 284.96 | 35.98 |
| Hydroxyl/epoxy (C-O) | 286.93 | 45.68 |
| Ester (COO) | 288.67 | 8.35 |
| Functional Groups | Binding Energy (eV) | % Atomic |
|---|---|---|
| Aromatic carbon (-C=C-) | 288.93 | 7.37 |
| Aliphatic carbon (-C-C-) | 285.03 | 35.04 |
| Hydroxyl/epoxy (C-O) | 287.09 | 45.37 |
| Ester (COO) | 284.12 | 9.92 |
| π→π* transition in aromatic | 290.67 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akere, T.H.; de Medeiros, A.M.Z.; Martinez, D.S.T.; Ibrahim, B.; Ali-Boucetta, H.; Valsami-Jones, E. Synthesis and Characterisation of a Graphene Oxide-Gold Nanohybrid for Use as Test Material. Nanomaterials 2023, 13, 33. https://doi.org/10.3390/nano13010033
Akere TH, de Medeiros AMZ, Martinez DST, Ibrahim B, Ali-Boucetta H, Valsami-Jones E. Synthesis and Characterisation of a Graphene Oxide-Gold Nanohybrid for Use as Test Material. Nanomaterials. 2023; 13(1):33. https://doi.org/10.3390/nano13010033
Chicago/Turabian StyleAkere, Taiwo Hassan, Aline M. Z. de Medeiros, Diego Stéfani T. Martinez, Bashiru Ibrahim, Hanene Ali-Boucetta, and Eugenia Valsami-Jones. 2023. "Synthesis and Characterisation of a Graphene Oxide-Gold Nanohybrid for Use as Test Material" Nanomaterials 13, no. 1: 33. https://doi.org/10.3390/nano13010033






