H2 Production by Methane Oxy-Reforming: Effect of Catalyst Pretreatment on the Properties and Activity of Rh-Ce0.5Zr0.5O2 Synthetized by Microemulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Rh/Ce0,5Zr0,5O2 Catalysts
2.2. Catalyst Characterizations
2.3. Experimental Set-Up and Description of the Catalytic Tests
3. Results and Discussion
3.1. Catalyst Characterization
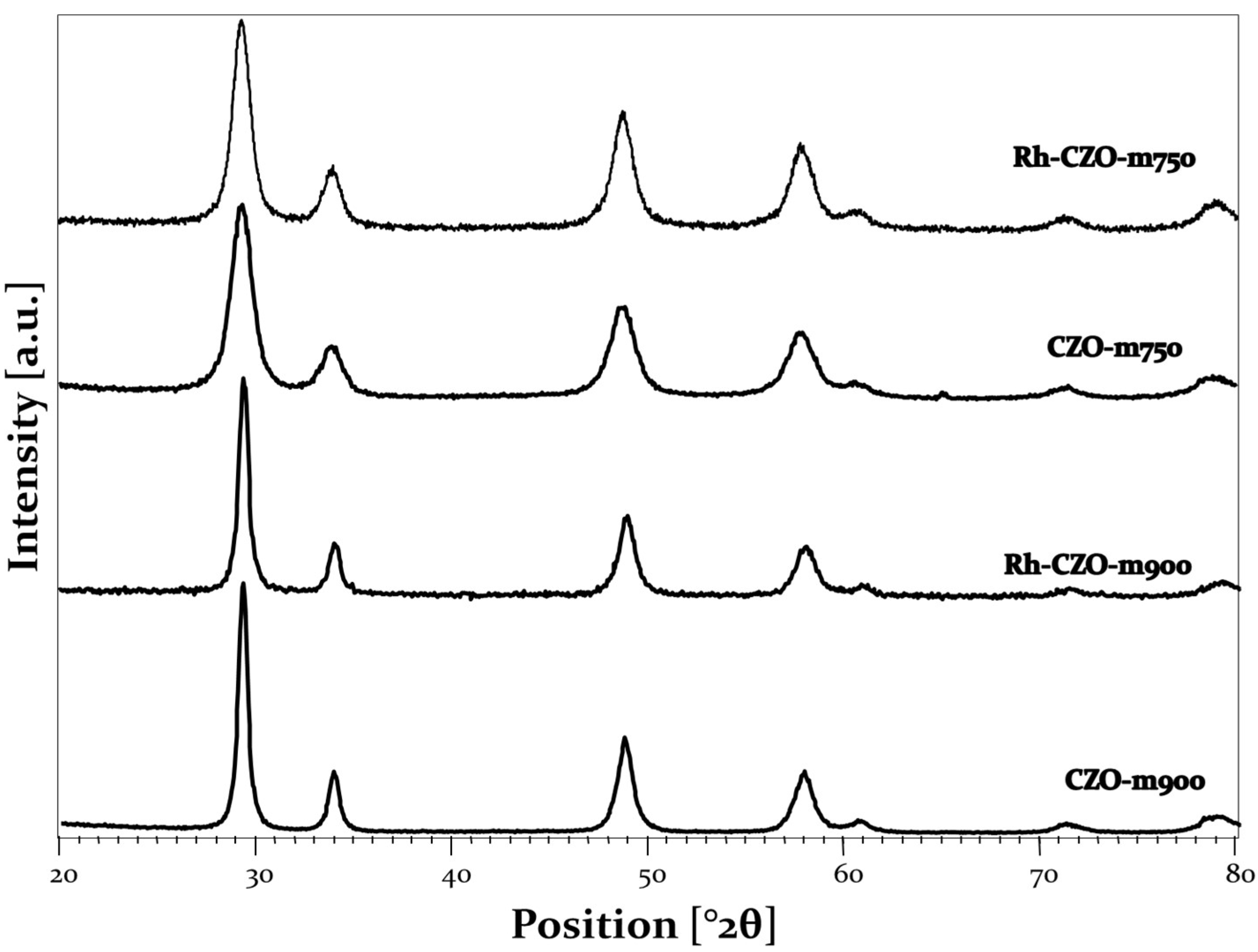
3.1.1. X-ray Diffraction Analysis
3.1.2. Nitrogen Physisorption Analysis
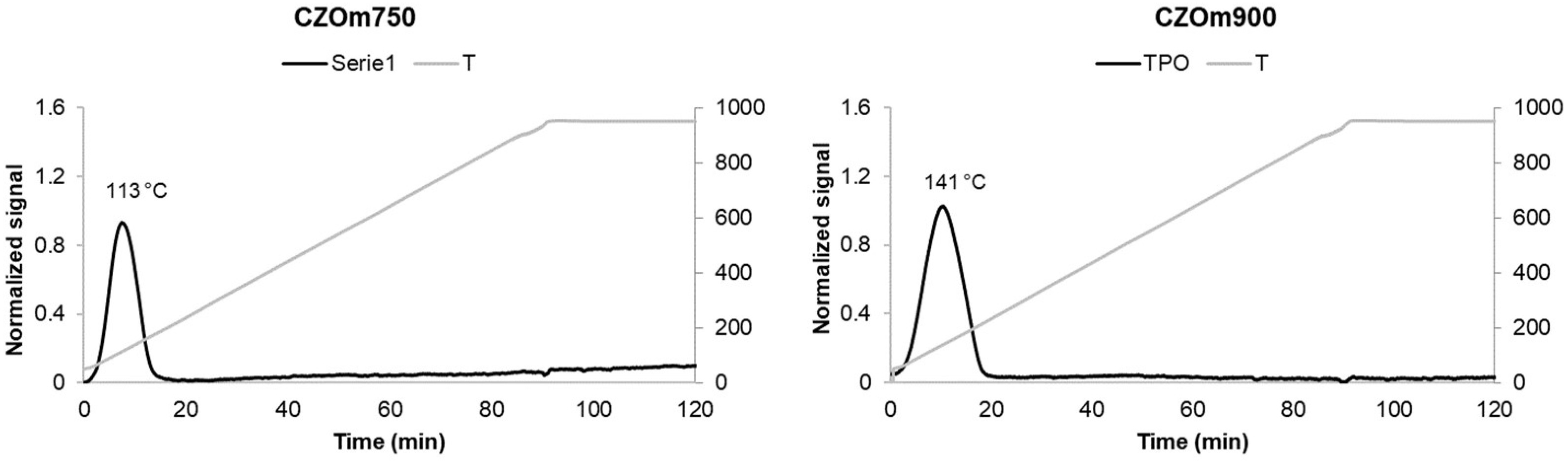
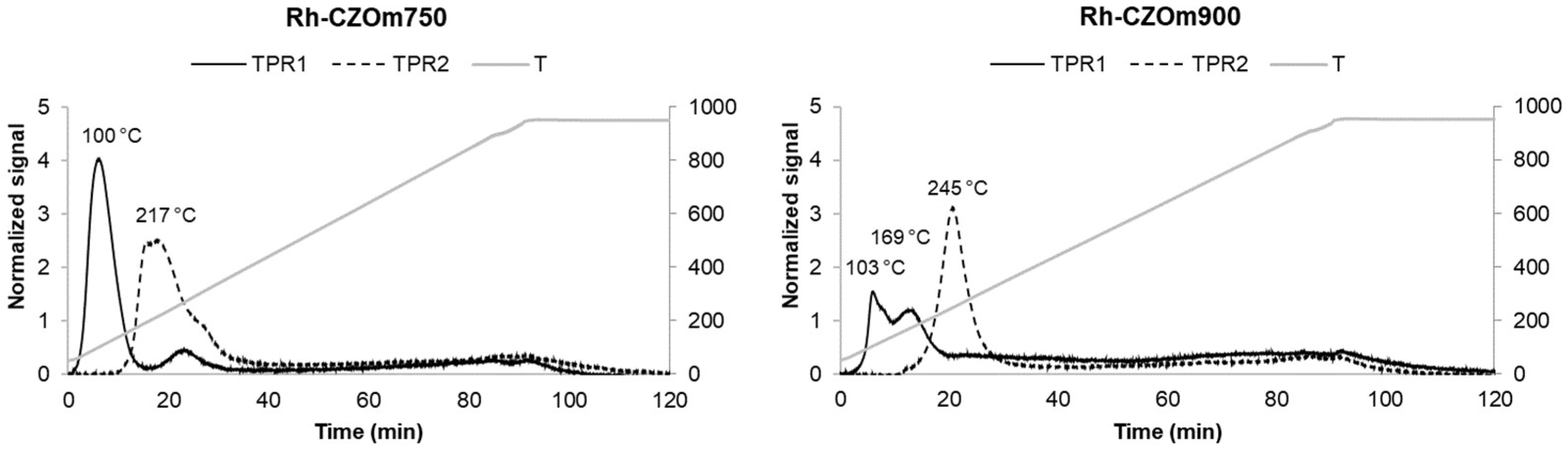
3.1.3. Temperature-Programmed Reduction–Oxidation
3.1.4. Transmission Electron Microscopy
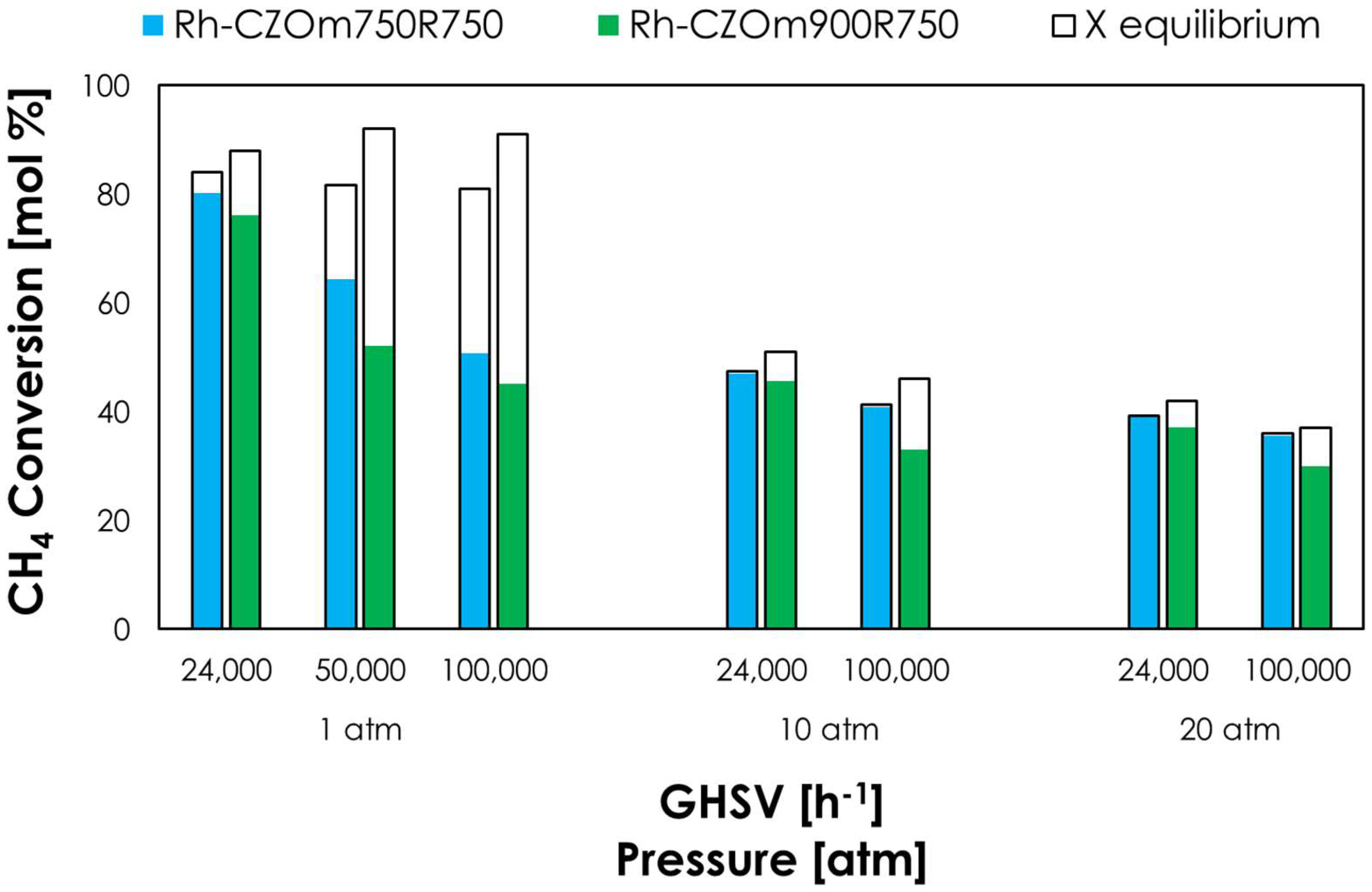
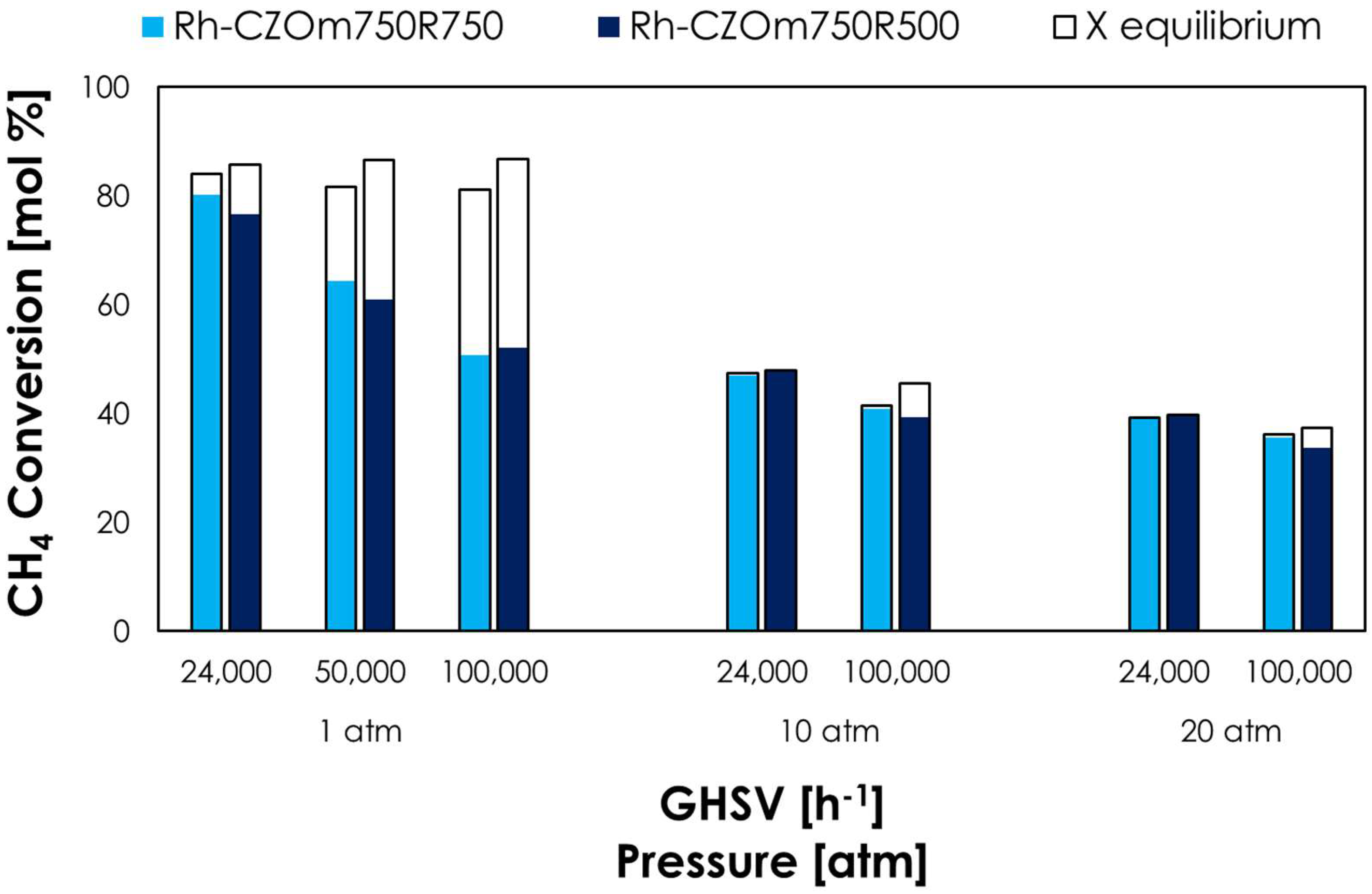
3.2. Catalytic Tests
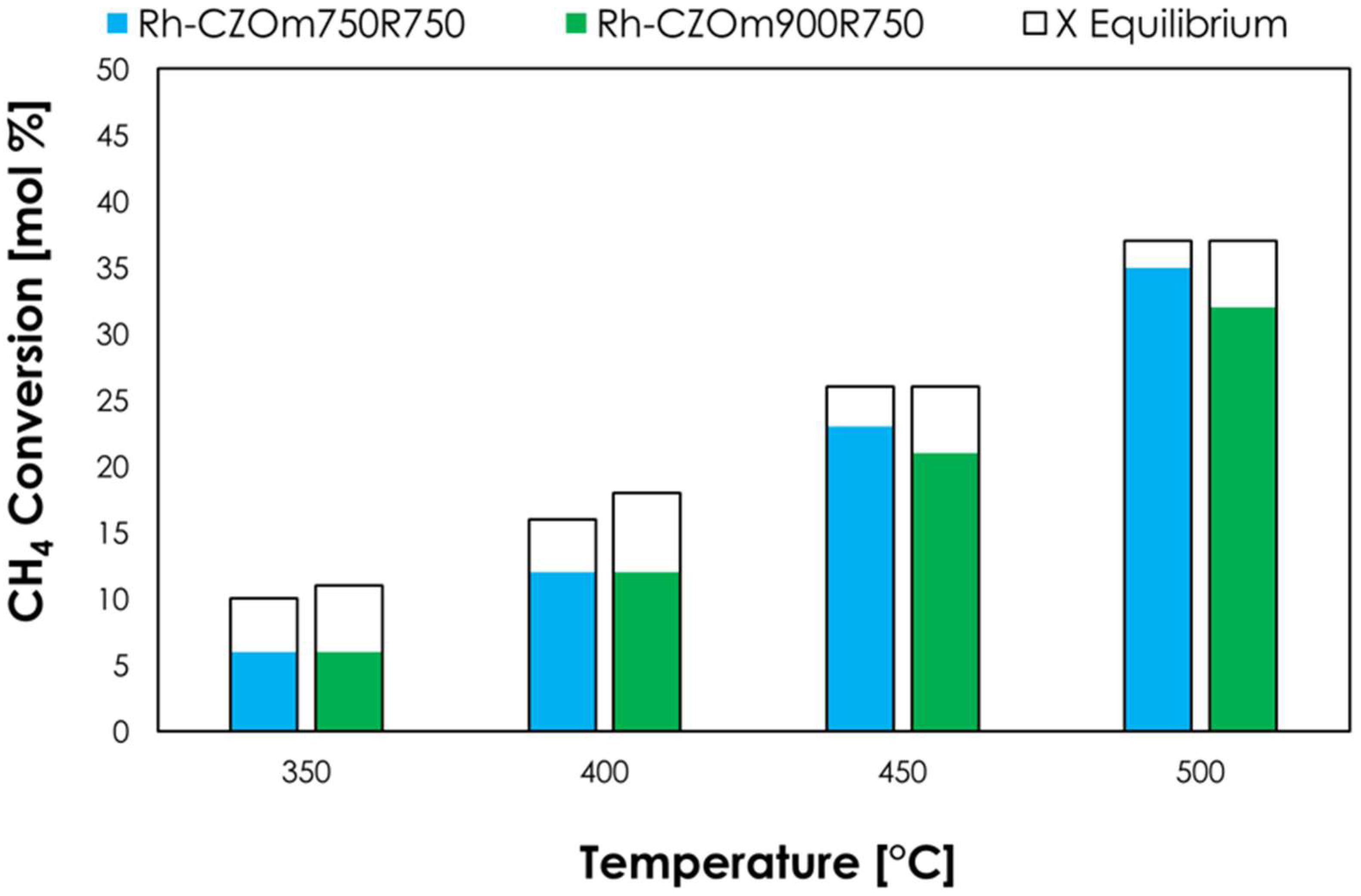
3.2.1. Effect of Calcination Temperature
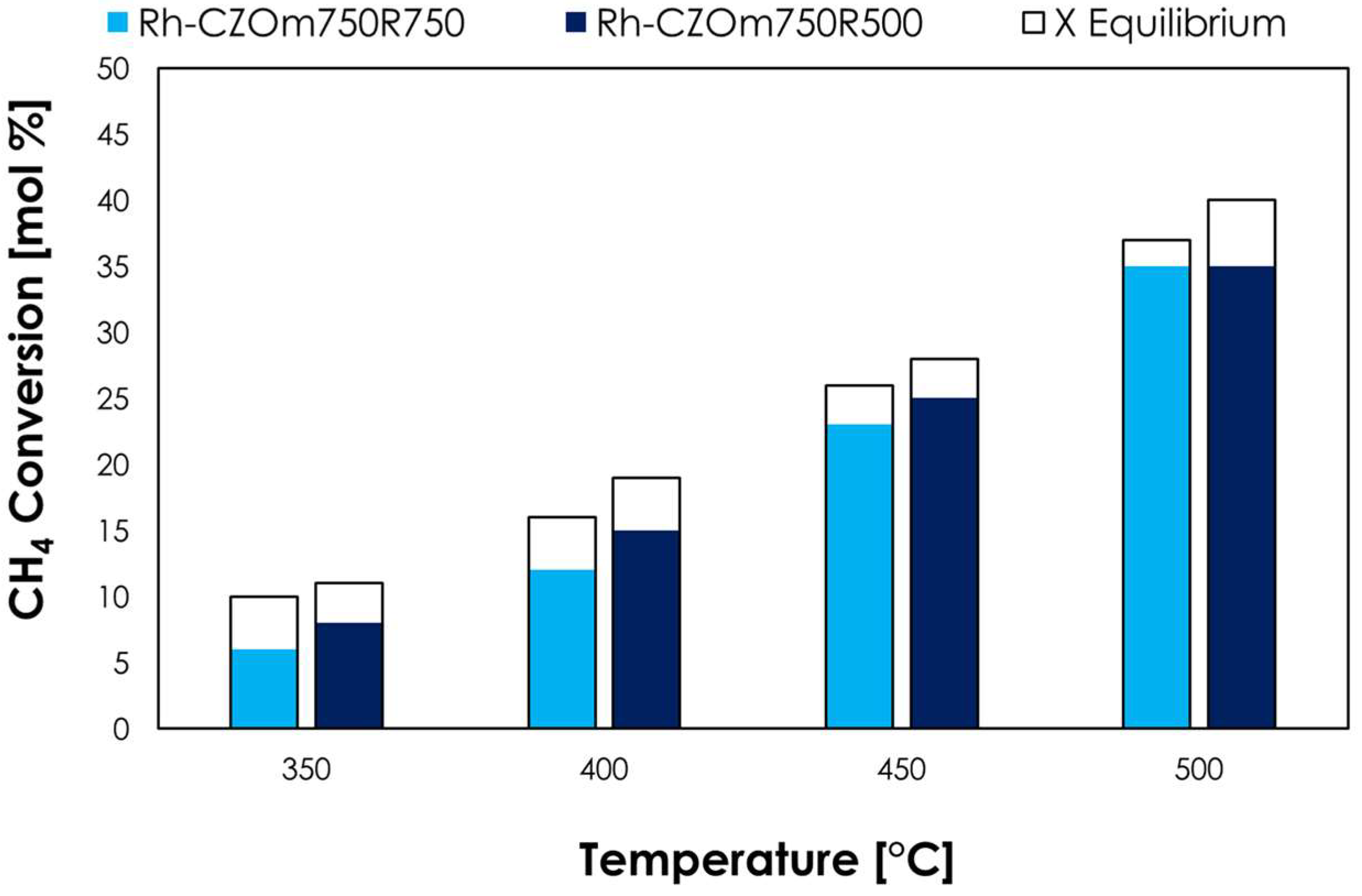
3.2.2. Effect of Reduction Temperature
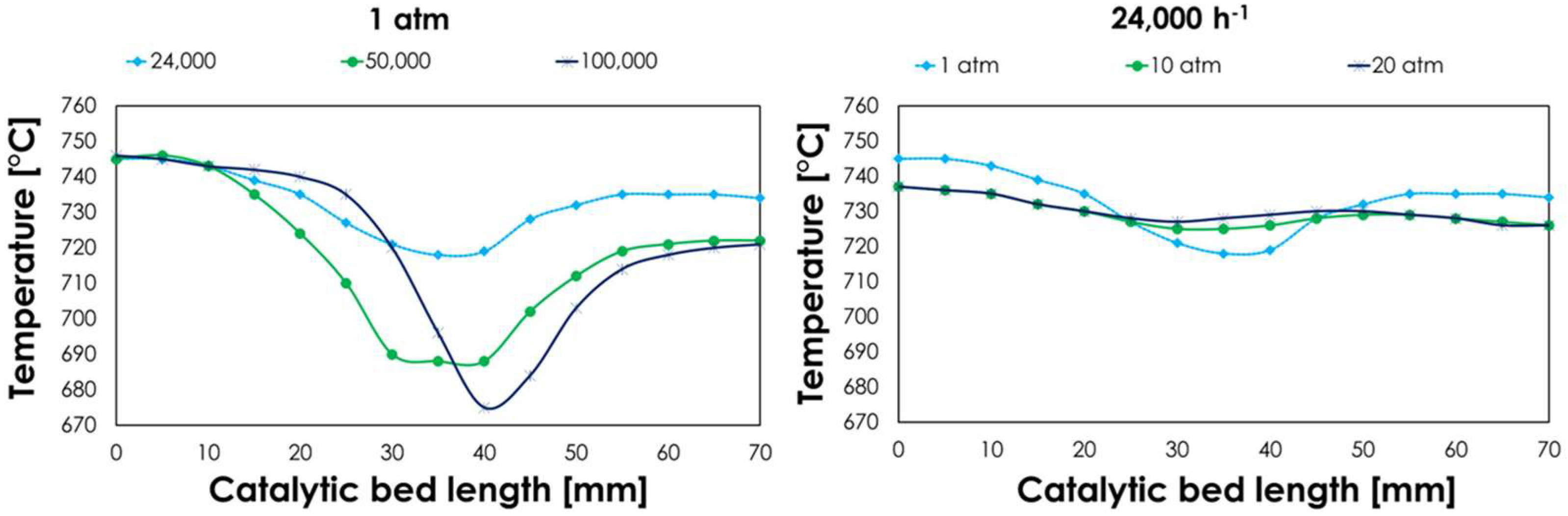
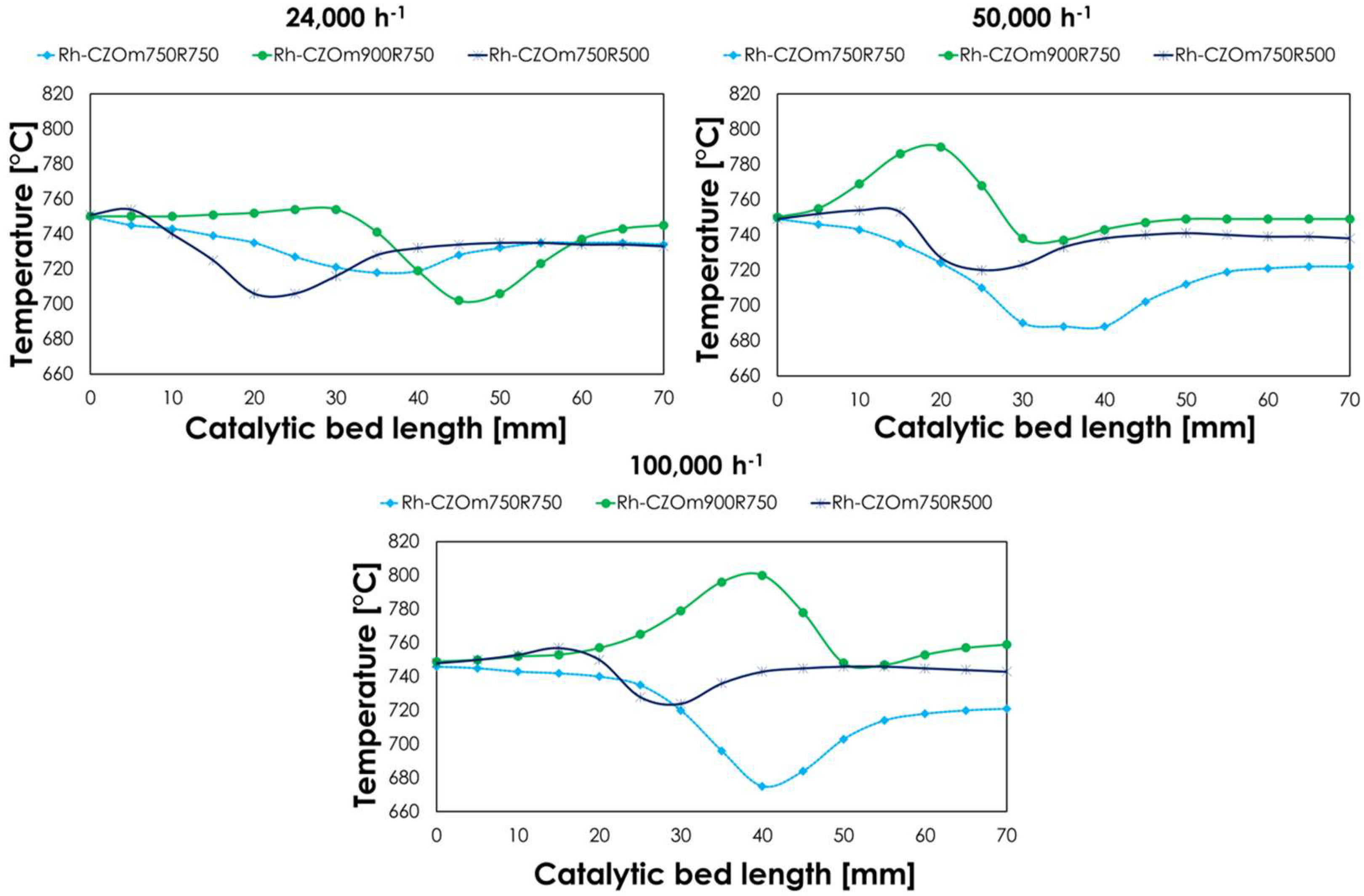
3.2.3. Oxy-Reforming Temperature Profile
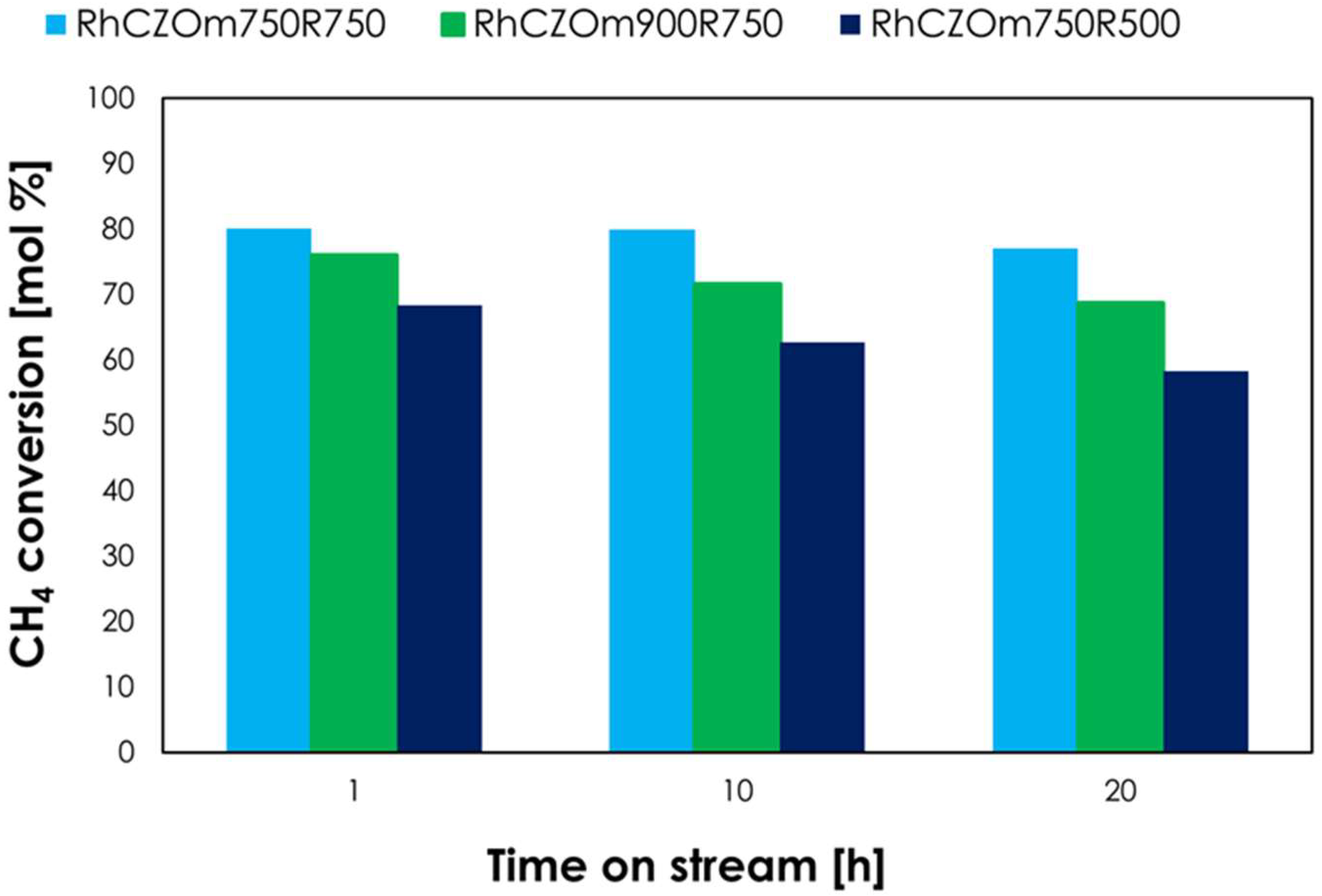
3.2.4. Catalyst Stability
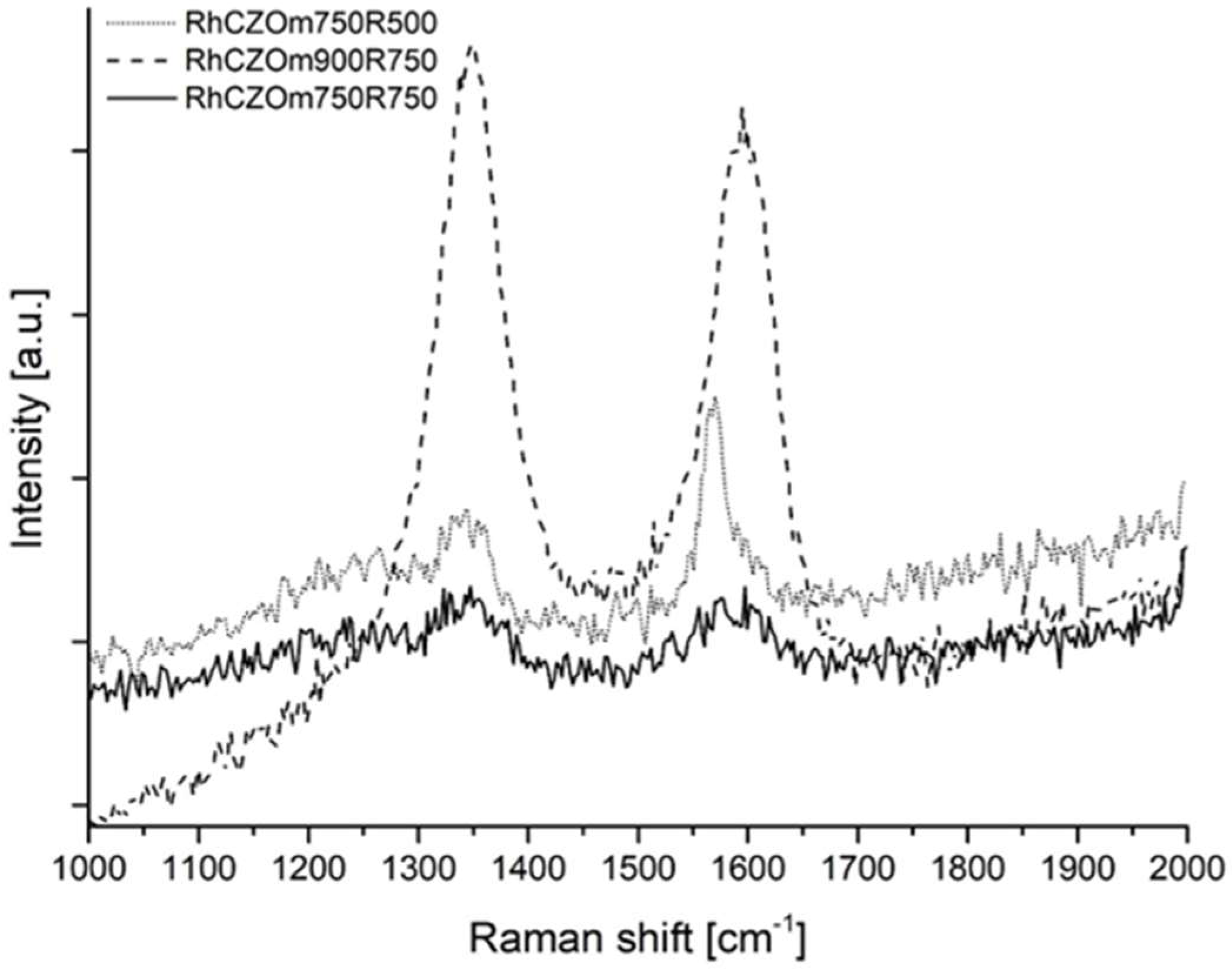
3.2.5. Characterization of the Spent Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Renssen, S. The Hydrogen Solution? Nat. Clim. Chang. 2020, 10, 799–801. [Google Scholar] [CrossRef]
- Dean, N.; Gallagher, J.; Zhang, C. Hydrogen on the Rise. Nat. Energy 2016, 1, 16127. [Google Scholar] [CrossRef] [Green Version]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-Economic Assessment of Various Hydrogen Production Methods—A Review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef] [PubMed]
- Bartels, J. A Feasibility Study of Implementing an Ammonia Economy. Master’s Thesis, Iowa State University, Ames, IA, USA, January 2008. [Google Scholar] [CrossRef]
- Saxe, M.; Alvfors, P. Advantages of Integration with Industry for Electrolytic Hydrogen Production. Energy 2007, 32, 42–50. [Google Scholar] [CrossRef]
- Dolci, F.; European Commission; Joint Research Centre. Green Hydrogen Opportunities in Selected Industrial Processes; Workshop Summary Report; Centre Albert Borschette: Brussels, Belgium, 2018; ISBN 978-92-79-99135-6. [Google Scholar]
- Lange, J.-P. Fuels and Chemicals Manufacturing; Guidelines for Understanding and Minimizing the Production Costs. Cattech 2001, 5, 82–95. [Google Scholar] [CrossRef]
- Dickel, R. Blue Hydrogen as an Enabler of Green Hydrogen: The Case of Germany; Oxford Institute for Energy Studies: Oxford, UK, 2020; ISBN 978-1-78467-159-4. [Google Scholar]
- Basile, F.; Mafessanti, R.; Fasolini, A.; Fornasari, G.; Lombardi, E.; Vaccari, A. Effect of Synthetic Method on CeZr Support and Catalytic Activity of Related Rh Catalyst in the Oxidative Reforming Reaction. J. Eur. Ceram. Soc. 2019, 39, 41–52. [Google Scholar] [CrossRef]
- Fasolini, A.; Abate, S.; Barbera, D.; Centi, G.; Basile, F. Pure H2 Production by Methane Oxy-Reforming over Rh-Mg-Al Hydrotalcite-Derived Catalysts Coupled with a Pd Membrane. Appl. Catal. Gen. 2019, 581, 91–102. [Google Scholar] [CrossRef]
- Mosinska, M.; Szynkowska, M.I.; Mierczynski, P. Oxy-Steam Reforming of Natural Gas on Ni Catalysts—A Minireview. Catalysts 2020, 10, 896. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Salladini, A.; Palo, E.; Centi, G. Catalytic Partial Oxidation Coupled with Membrane Purification to Improve Resource and Energy Efficiency in Syngas Production. ChemSusChem 2015, 8, 717–725. [Google Scholar] [CrossRef]
- Gondolini, A.; Fasolini, A.; Mercadelli, E.; Basile, F.; Sanson, A. Freeze Cast Porous Membrane Catalyst for Hydrogen Production via Oxy-Reforming. Fuel Process. Technol. 2021, 213, 106658. [Google Scholar] [CrossRef]
- Schiaroli, N.; Lucarelli, C.; Sanghez de Luna, G.; Fornasari, G.; Vaccari, A. Ni-Based Catalysts to Produce Synthesis Gas by Combined Reforming of Clean Biogas. Appl. Catal. Gen. 2019, 582, 117087. [Google Scholar] [CrossRef]
- Parsland, C.; Ho, P.H.; Benito, P.; Larsson, A.-C.; Fornasari, G.; Brandin, J. Ba-Ni-Hexaaluminate as a New Catalyst in the Steam Reforming of 1-Methyl Naphthalene and Methane. Catal. Lett. 2020, 150, 1605–1617. [Google Scholar] [CrossRef] [Green Version]
- Chitsazan, S.; Sepehri, S.; Garbarino, G.; Carnasciali, M.M.; Busca, G. Steam Reforming of Biomass-Derived Organics: Interactions of Different Mixture Components on Ni/Al2O3 Based Catalysts. Appl. Catal. B Environ. 2016, 187, 386–398. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Garbarino, G.; Busca, G. Facile Synthesis of a Mesoporous Alumina and Its Application as a Support of Ni-Based Autothermal Reforming Catalysts. Int. J. Hydrogen Energy 2016, 41, 3456–3464. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Garbarino, G.; Busca, G. Preparation and Characterization of Mesoporous Nanocrystalline La-, Ce-, Zr-, Sr-Containing NiAl2O3 Methane Autothermal Reforming Catalysts. Int. J. Hydrogen Energy 2016, 41, 8855–8862. [Google Scholar] [CrossRef]
- Dong, W.-S.; Roh, H.-S.; Jun, K.-W.; Park, S.-E.; Oh, Y.-S. Methane Reforming over Ni/Ce-ZrO2 Catalysts: Effect of Nickel Content. Appl. Catal. Gen. 2002, 226, 63–72. [Google Scholar] [CrossRef]
- Sadykov, V.; Mezentseva, N.; Fedorova, Y.; Lukashevich, A.; Pelipenko, V.; Kuzmin, V.; Simonov, M.; Ishchenko, A.; Vostrikov, Z.; Bobrova, L.; et al. Structured Catalysts for Steam/Autothermal Reforming of Biofuels on Heat-Conducting Substrates: Design and Performance. Catal. Today 2015, 251, 19–27. [Google Scholar] [CrossRef]
- Dekkar, S.; Tezkratt, S.; Sellam, D.; Ikkour, K.; Parkhomenko, K.; Martinez-Martin, A.; Roger, A.C. Dry Reforming of Methane over Ni–Al2O3 and Ni–SiO2 Catalysts: Role of Preparation Methods. Catal. Lett. 2020, 150, 2180–2199. [Google Scholar] [CrossRef]
- Sadykov, V.; Mezentseva, N.; Alikina, G.; Bunina, R.; Rogov, V.; Krieger, T.; Belochapkine, S.; Ross, J. Composite Catalytic Materials for Steam Reforming of Methane and Oxygenates: Combinatorial Synthesis, Characterization and Performance. Catal. Today 2009, 145, 127–137. [Google Scholar] [CrossRef]
- Kim, B.-J.; Seo, J.-C.; Kim, D.-H.; Lee, Y.-L.; Lee, K.; Roh, H.-S. Oxygen Defective Bimodal Porous Ni-CeO2−x-MgO-Al2O3 Catalyst with Multi-Void Spherical Structure for CO2 Reforming of CH4. J. CO2 Util. 2022, 58, 101917. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Gil-Calvo, M.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidative Steam Reforming and Steam Reforming of Methane, Isooctane, and N-Tetradecane over an Alumina Supported Spinel-Derived Nickel Catalyst. Ind. Eng. Chem. Res. 2016, 55, 3920–3929. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Pino, L.; Specchia, S. Syngas Production by Methane Oxy-Steam Reforming on Me/CeO2 (Me=Rh, Pt, Ni) Catalyst Lined on Cordierite Monoliths. Appl. Catal. B Environ. 2015, 162, 551–563. [Google Scholar] [CrossRef]
- Ho, P.H.; Ospitali, F.; Sanghez de Luna, G.; Fornasari, G.; Vaccari, A.; Benito, P. Coating of Rh/Mg/Al Hydrotalcite-Like Materials on FeCrAl Fibers by Electrodeposition and Application for Syngas Production. Energy Technol. 2020, 8, 1901018. [Google Scholar] [CrossRef]
- Tarifa, P.; Schiaroli, N.; Ho, P.H.; Cañaza, F.; Ospitali, F.; Sanghez de Luna, G.; Lucarelli, C.; Fornasari, G.; Vaccari, A.; Monzon, A.; et al. Steam Reforming of Clean Biogas over Rh and Ru Open-Cell Metallic Foam Structured Catalysts. Catal. Today 2022, 383, 74–83. [Google Scholar] [CrossRef]
- Schiaroli, N.; Lucarelli, C.; Iapalucci, M.C.; Fornasari, G.; Crimaldi, A.; Vaccari, A. Combined Reforming of Clean Biogas over Nanosized Ni–Rh Bimetallic Clusters. Catalysts 2020, 10, 1345. [Google Scholar] [CrossRef]
- Men, Y.; Gnaser, H.; Zapf, R.; Hessel, V.; Ziegler, C. Parallel Screening of Cu/CeO2/γ-Al2O3 Catalysts for Steam Reforming of Methanol in a 10-Channel Micro-Structured Reactor. Catal. Commun. 2004, 5, 671–675. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W.; Dong, W.-S.; Park, S.-E.; Baek, Y.-S. Highly Stable Ni Catalyst Supported on Ce–ZrO2 for Oxy-Steam Reforming of Methane. Catal. Lett. 2001, 74, 31–36. [Google Scholar] [CrossRef]
- Halabi, M.H.; de Croon, M.H.J.M.; van der Schaaf, J.; Cobden, P.D.; Schouten, J.C. Low Temperature Catalytic Methane Steam Reforming over Ceria–Zirconia Supported Rhodium. Appl. Catal. Gen. 2010, 389, 68–79. [Google Scholar] [CrossRef]
- Auxéméry, A.; Frias, B.B.; Smal, E.; Dziadek, K.; Philippot, G.; Legutko, P.; Simonov, M.; Thomas, S.; Adamski, A.; Sadykov, V.; et al. Continuous Supercritical Solvothermal Preparation of Nanostructured Ceria-Zirconia as Supports for Dry Methane Reforming Catalysts. J. Supercrit. Fluids 2020, 162, 104855. [Google Scholar] [CrossRef]
- Fasolini, A.; Ruggieri, S.; Femoni, C.; Basile, F. Highly Active Catalysts Based on the Rh4(CO)12 Cluster Supported on Ce0.5Zr0.5 and Zr Oxides for Low-Temperature Methane Steam Reforming. Catalysts 2019, 9, 800. [Google Scholar] [CrossRef] [Green Version]
- Waldvogel, A.; Fasolini, A.; Basile, F.; Thomas, S.; Roger, A.-C. Effect of the Support Synthetic Method on the Activity of Ni/CeZrPr Mixed Oxide in the Co-Methanation of CO2/CO Mixtures for Application in Power-to-Gas with Co-Electrolysis. Energy Fuels 2021, 35, 13304–13314. [Google Scholar] [CrossRef]
- Moretti, E.; Storaro, L.; Talon, A.; Chitsazan, S.; Garbarino, G.; Busca, G.; Finocchio, E. Ceria–Zirconia Based Catalysts for Ethanol Steam Reforming. Fuel 2015, 153, 166–175. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent Progress in Ceria-Based Catalysts for the Dry Reforming of Methane: A Review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Fornasiero, P. The Role of Ceria-Based Nanostructured Materials in Energy Applications. Mater. Today 2014, 17, 349–357. [Google Scholar] [CrossRef]
- Fornasiero, P.; Kašpar, J.; Graziani, M. On the Rate Determining Step in the Reduction of CeO2–ZrO2 Mixed Oxides. Appl. Catal. B Environ. 1999, 22, L11–L14. [Google Scholar] [CrossRef]
- Gatica, J.M.; Baker, R.T.; Fornasiero, P.; Bernal, S.; Blanco, G.; Kašpar, J. Rhodium Dispersion in a Rh/Ce0.68Zr0.32O2 Catalyst Investigated by HRTEM and H2 Chemisorption. J. Phys. Chem. B 2000, 104, 4667–4672. [Google Scholar] [CrossRef]
- Fornasiero, P.; Kašpar, J.; Sergo, V.; Graziani, M. Redox Behavior of High-Surface-Area Rh-, Pt-, and Pd-Loaded Ce0.5Zr0.5O2 Mixed Oxide. J. Catal. 1999, 182, 56–69. [Google Scholar] [CrossRef]
- Fornasiero, P.; Kašpar, J.; Graziani, M. Redox Behavior of High Surface Area Rh-Loaded Ce0.5Zr0.5O2Mixed Oxide. J. Catal. 1997, 167, 576–580. [Google Scholar] [CrossRef]
- Maslova, V.; Quadrelli, E.A.; Gaval, P.; Fasolini, A.; Albonetti, S.; Basile, F. Highly-Dispersed Ultrafine Pt Nanoparticles on Microemulsion-Mediated TiO2 for Production of Hydrogen and Valuable Chemicals via Oxidative Photo-Dehydrogenation of Glycerol. J. Environ. Chem. Eng. 2021, 9, 105070. [Google Scholar] [CrossRef]
- Maslova, V.; Fasolini, A.; Offidani, M.; Albonetti, S.; Basile, F. Solar-Driven Valorization of Glycerol towards Production of Chemicals and Hydrogen. Catal. Today 2021, 380, 147–155. [Google Scholar] [CrossRef]
- Fasolini, A.; Lombardi, E.; Tabanelli, T.; Basile, F. Microemulsion Derived Titania Nanospheres: An Improved Pt Supported Catalyst for Glycerol Aqueous Phase Reforming. Nanomaterials 2021, 11, 1175. [Google Scholar] [CrossRef] [PubMed]
- Delgado, K.H.; Maier, L.; Tischer, S.; Zellner, A.; Stotz, H.; Deutschmann, O. Surface Reaction Kinetics of Steam- and CO2-Reforming as Well as Oxidation of Methane over Nickel-Based Catalysts. Catalysts 2015, 5, 871–904. [Google Scholar] [CrossRef] [Green Version]
- Rostrup-Nielsen, J.R. Production of Synthesis Gas. Catal. Today 1993, 18, 305–324. [Google Scholar] [CrossRef]
- Korup, O.; Goldsmith, C.F.; Weinberg, G.; Geske, M.; Kandemir, T.; Schlögl, R.; Horn, R. Catalytic Partial Oxidation of Methane on Platinum Investigated by Spatial Reactor Profiles, Spatially Resolved Spectroscopy, and Microkinetic Modeling. J. Catal. 2013, 297, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Basile, F.; Fornasari, G.; Trifirò, F.; Vaccari, A. Partial Oxidation of Methane: Effect of Reaction Parameters and Catalyst Composition on the Thermal Profile and Heat Distribution. Catal. Today 2001, 64, 21–30. [Google Scholar] [CrossRef]
- Silva, F.D.A.; Ruiz, J.A.C.; de Souza, K.R.; Bueno, J.M.C.; Mattos, L.V.; Noronha, F.B.; Hori, C.E. Partial Oxidation of Methane on Pt Catalysts: Effect of the Presence of Ceria–Zirconia Mixed Oxide and of Metal Content. Appl. Catal. Gen. 2009, 364, 122–129. [Google Scholar] [CrossRef]
- Eriksson, S.; Rojas, S.; Boutonnet, M.; Fierro, J.L.G. Effect of Ce-Doping on Rh/ZrO2 Catalysts for Partial Oxidation of Methane. Appl. Catal. Gen. 2007, 326, 8–16. [Google Scholar] [CrossRef]
- Maestri, M.; Livio, D.; Beretta, A.; Groppi, G. Hierarchical Refinement of Microkinetic Models: Assessment of the Role of the WGS and r-WGS Pathways in CH4 Partial Oxidation on Rh. Ind. Eng. Chem. Res. 2014, 53, 10914–10928. [Google Scholar] [CrossRef]
- Beretta, A.; Groppi, G.; Lualdi, M.; Tavazzi, I.; Forzatti, P. Experimental and Modeling Analysis of Methane Partial Oxidation: Transient and Steady-State Behavior of Rh-Coated Honeycomb Monoliths. Ind. Eng. Chem. Res. 2009, 48, 3825–3836. [Google Scholar] [CrossRef]
- Ramaswamy, R.C.; Ramachandran, P.A.; Dudukovic, M.P. Modeling Catalytic Partial Oxidation of Methane to Syngas in Short-Contact-Time Packed-Bed Reactors. Ind. Eng. Chem. Res. 2007, 46, 8638–8651. [Google Scholar] [CrossRef]
- Li, B.; Maruyama, K.; Nurunnabi, M.; Kunimori, K.; Tomishige, K. Temperature Profiles of Alumina-Supported Noble Metal Catalysts in Autothermal Reforming of Methane. Appl. Catal. Gen. 2004, 275, 157–172. [Google Scholar] [CrossRef]
- Saenger, K.L.; Tsang, J.C.; Bol, A.A.; Chu, J.O.; Grill, A.; Lavoie, C. In Situ X-ray Diffraction Study of Graphitic Carbon Formed during Heating and Cooling of Amorphous-C/Ni Bilayers. Appl. Phys. Lett. 2010, 96, 153105. [Google Scholar] [CrossRef]




| Operative Parameter | Low-Temperature Steam Reforming | Oxy-Reforming |
|---|---|---|
| Temperature (°C) | 500; 450; 400; 350 | 750 |
| Pressure (atm) | 1 | 1; 10; 20 |
| GHSV (h−1) | 24,000 | 24,000; 50,000; 100,000 |
| Gas feed composition (vol. %) (CH4, O2, H2O) | 25; 75; 0 | 52.04; 11.20; 36.75 |
| Steam-to-carbon ratio (S/C) | 3 | 0.71 |
| Oxygen-to-carbon ratio (O2/C) | 0 | 0.21 |
| Catalyst | Surface Area (m2/g) | Pores Volume (cm3/g) | Avg. Pore Diameter (nm) |
|---|---|---|---|
| CZOm750 | 54.1 | 0.09 | 4.8 |
| CZOm900 | 14.5 | 0.08 | 19.3 |
| Rh-CZOm750 | 34.6 | 0.07 | 5.6 |
| Rh-CZOm900 | 11.5 | 0.06 | 19.7 |
| Catalyst | H2 cons. in TPR1 (mmol/g) | % Ce Reduced | H2 cons. in TPR2 (mmol/g) | % Ce Reduced |
|---|---|---|---|---|
| CZOm750 | 1.49 | 55 | 1.48 | 56 |
| CZOm900 | 1.41 | 60 | 1.44 | 59 |
| Rh-CZOm750 | 1.91 | 82 | 2.03 | 89 |
| Rh-CZOm900 | 1.76 | 74 | 1.67 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Maron, J.; Mafessanti, R.; Gramazio, P.; Orfei, E.; Fasolini, A.; Basile, F. H2 Production by Methane Oxy-Reforming: Effect of Catalyst Pretreatment on the Properties and Activity of Rh-Ce0.5Zr0.5O2 Synthetized by Microemulsion. Nanomaterials 2023, 13, 53. https://doi.org/10.3390/nano13010053
De Maron J, Mafessanti R, Gramazio P, Orfei E, Fasolini A, Basile F. H2 Production by Methane Oxy-Reforming: Effect of Catalyst Pretreatment on the Properties and Activity of Rh-Ce0.5Zr0.5O2 Synthetized by Microemulsion. Nanomaterials. 2023; 13(1):53. https://doi.org/10.3390/nano13010053
Chicago/Turabian StyleDe Maron, Jacopo, Rodolfo Mafessanti, Pio Gramazio, Elisabetta Orfei, Andrea Fasolini, and Francesco Basile. 2023. "H2 Production by Methane Oxy-Reforming: Effect of Catalyst Pretreatment on the Properties and Activity of Rh-Ce0.5Zr0.5O2 Synthetized by Microemulsion" Nanomaterials 13, no. 1: 53. https://doi.org/10.3390/nano13010053





