Fabrication of Novel Heterostructure-Functionalized Graphene-Based TiO2-Sr-Hexaferrite Photocatalyst for Environmental Remediation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
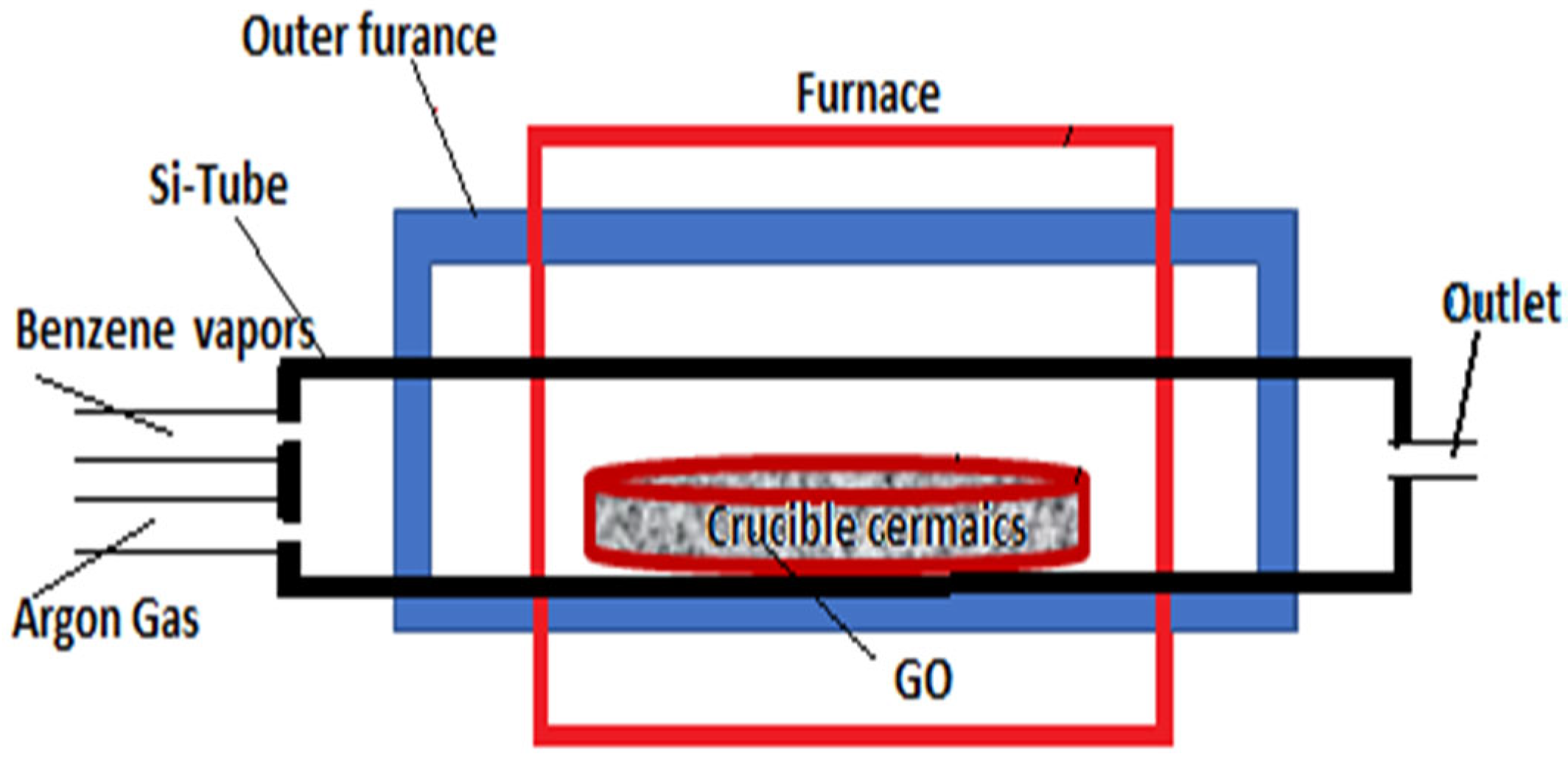
2.2. Preparation of TiO2-Functionalized Graphene
2.3. Fabrication of TiO2-FG/Sr-Hexaferrite Composites
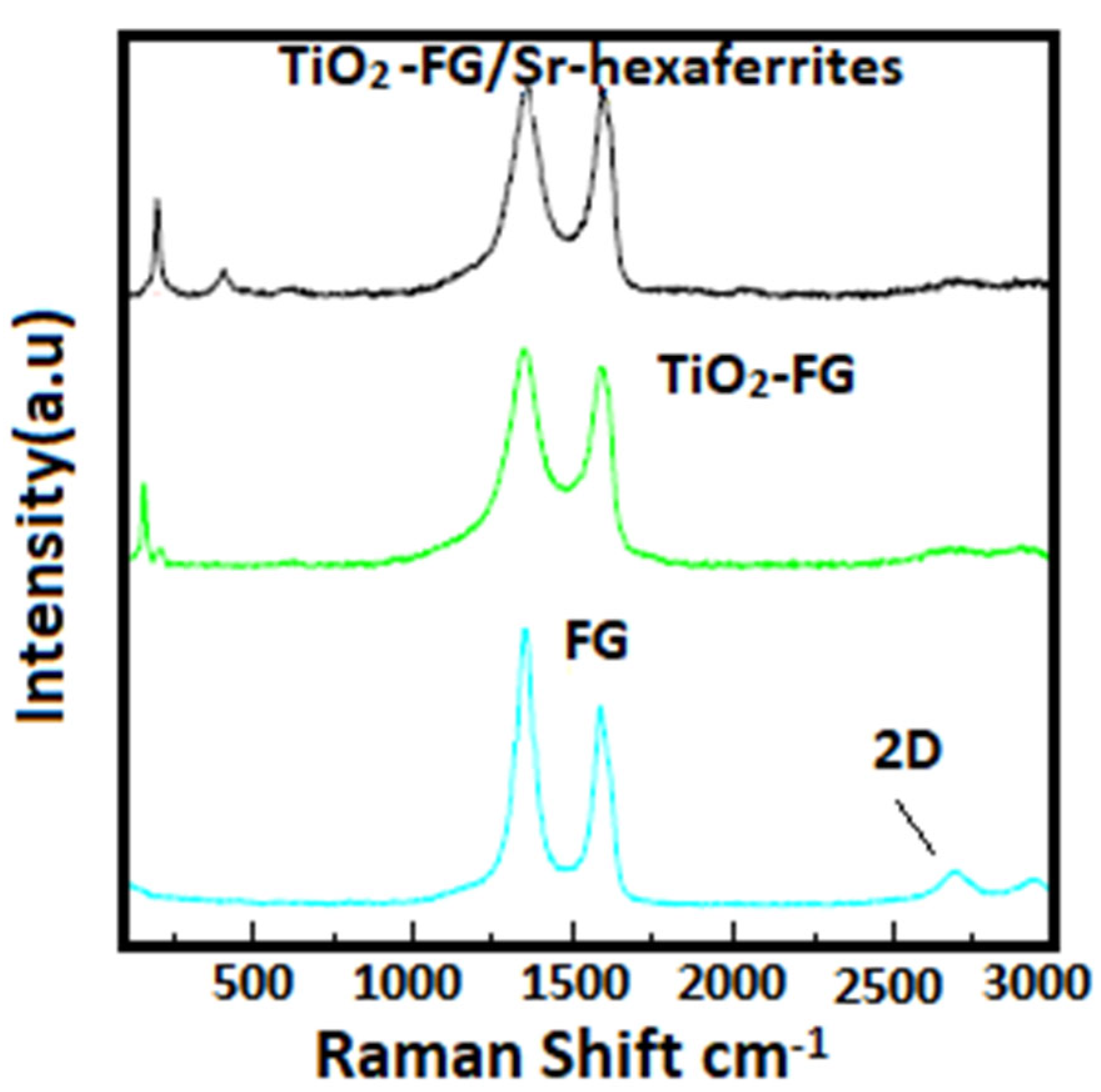
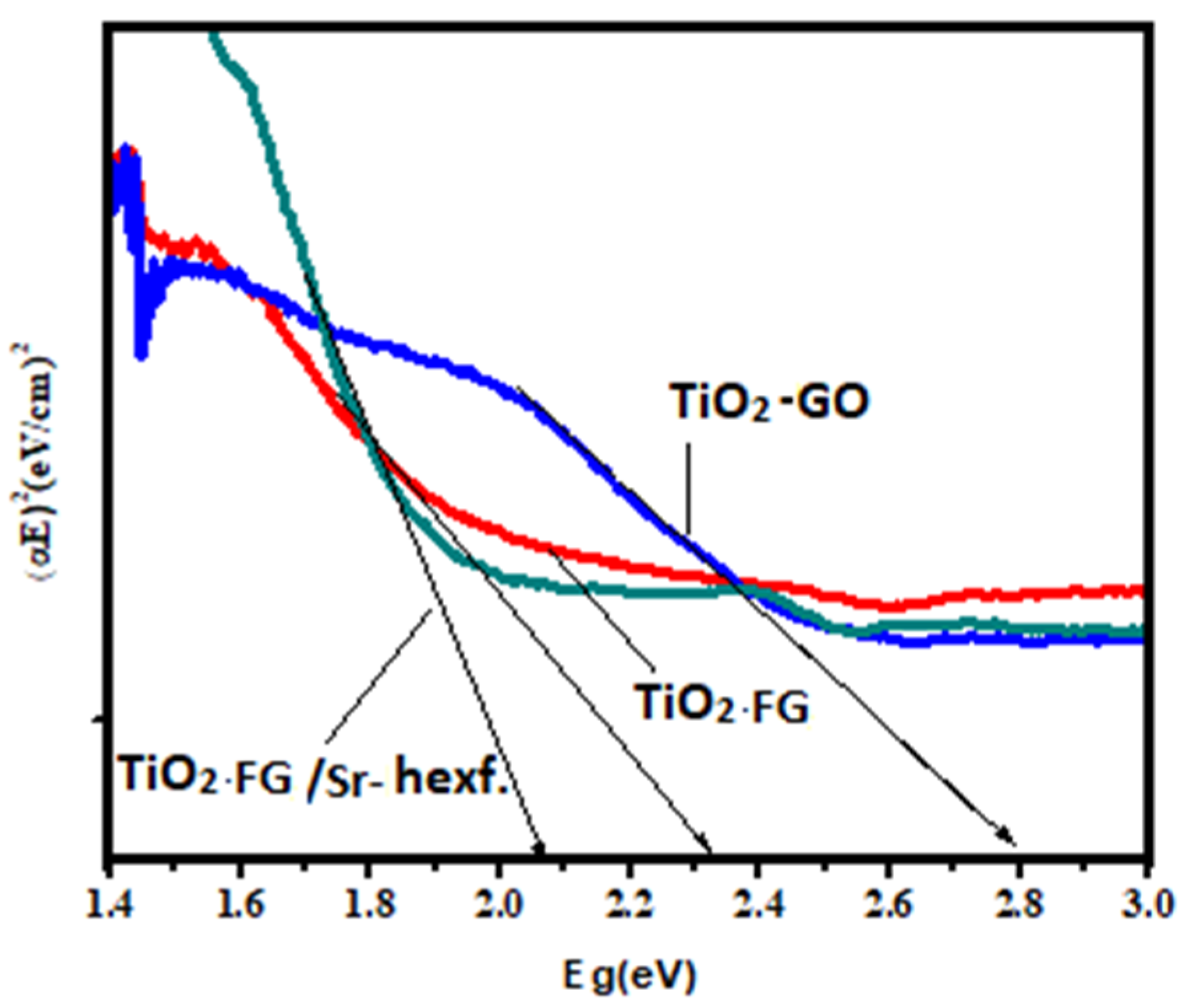
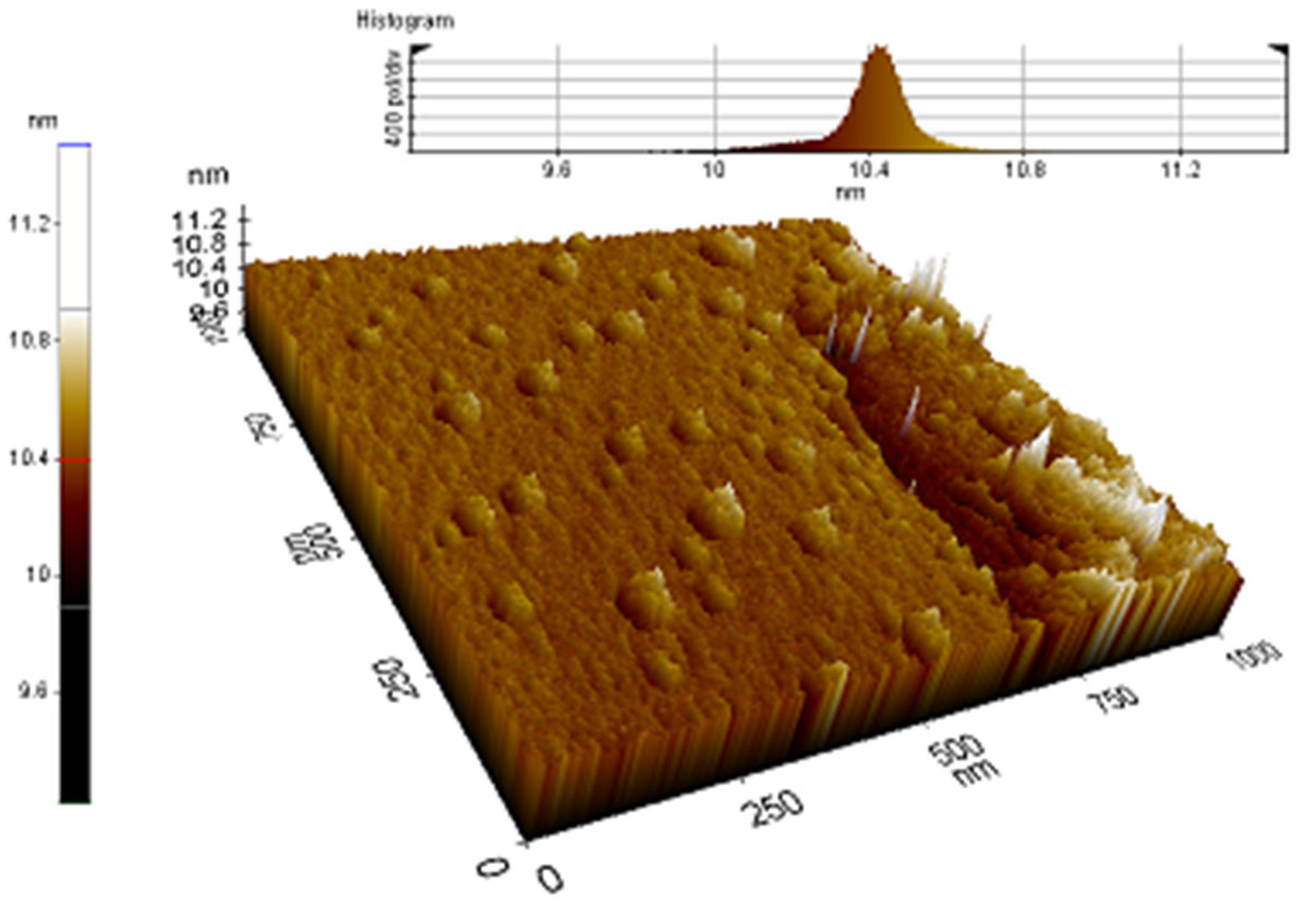
2.4. Characterization
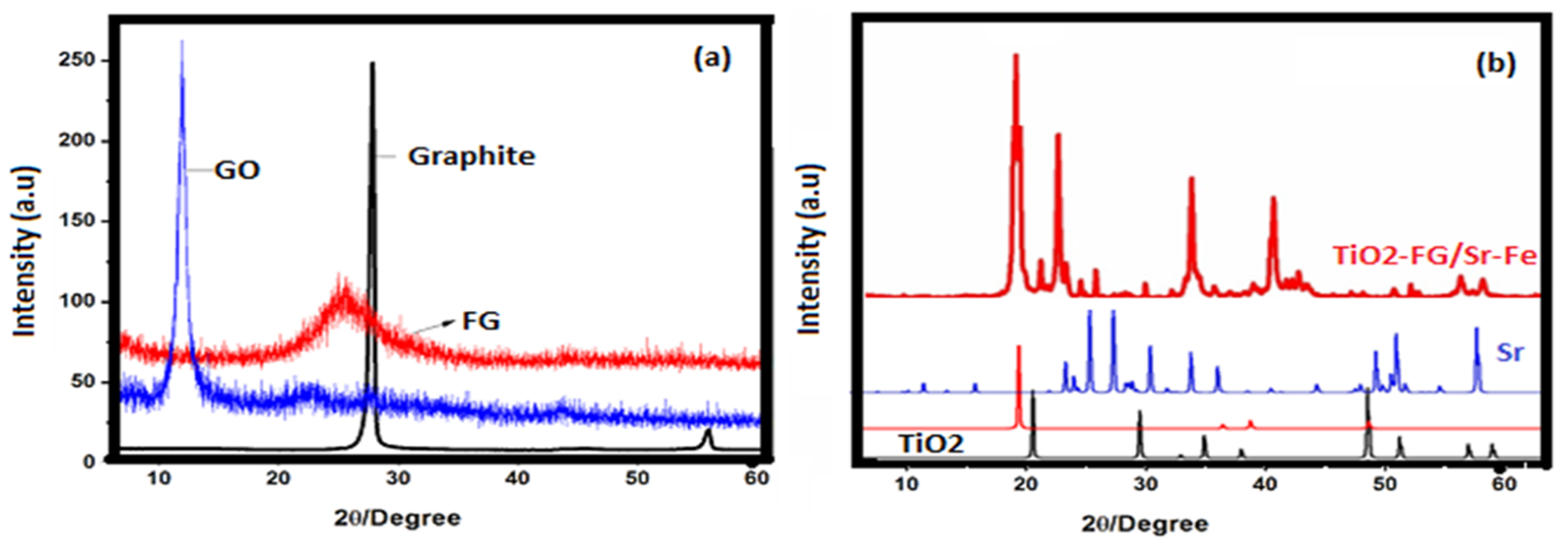
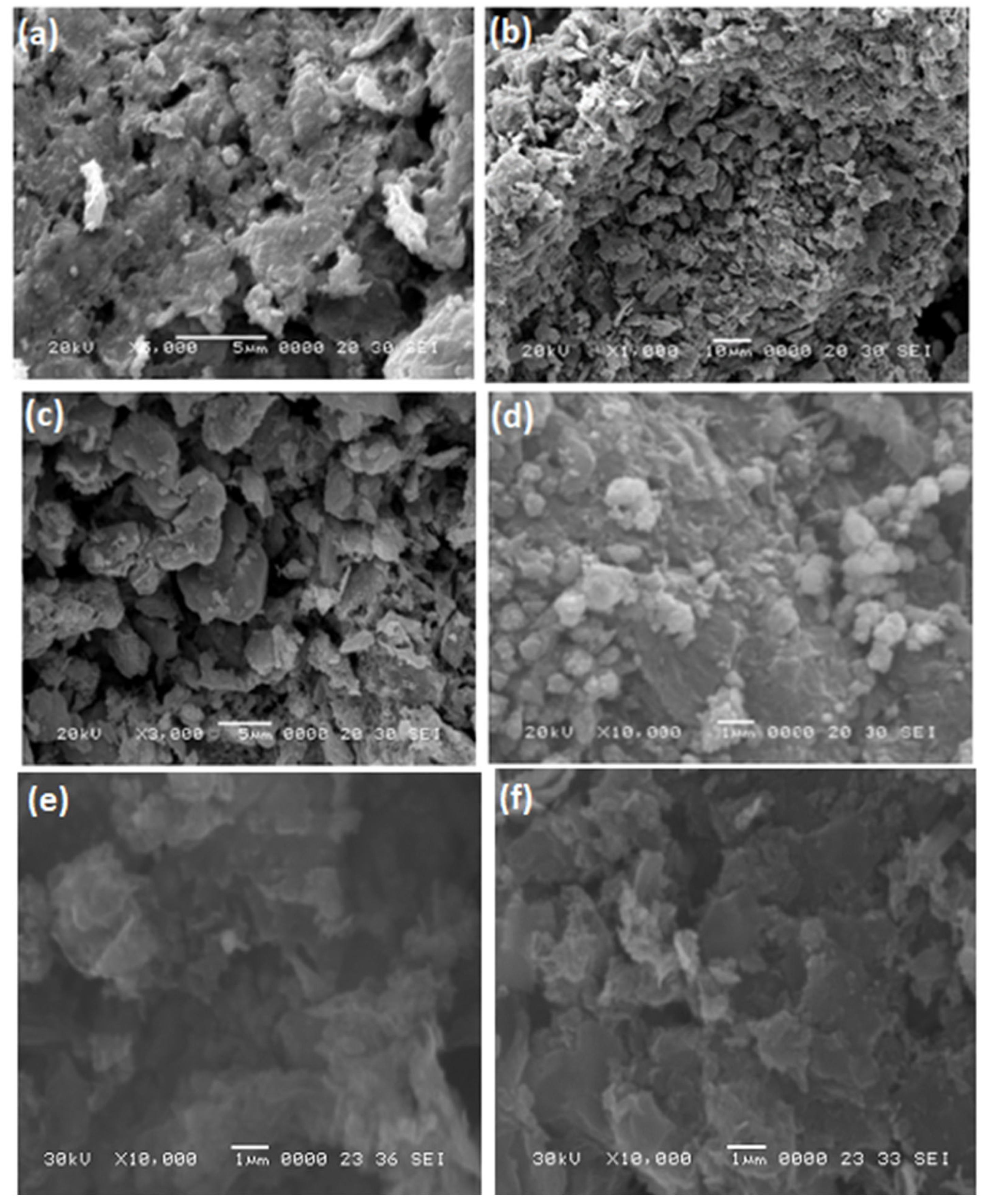
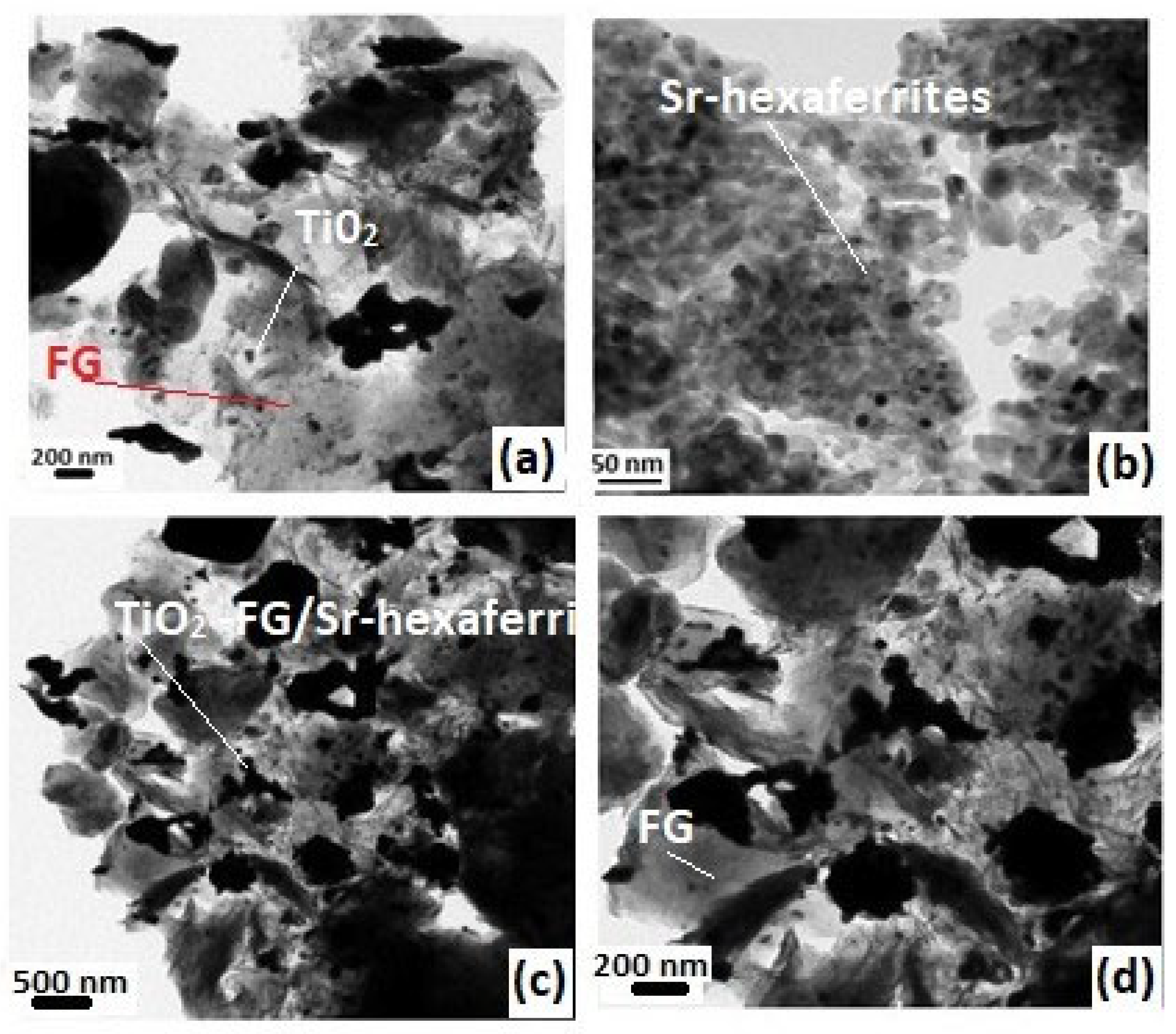
3. Results and Discussion
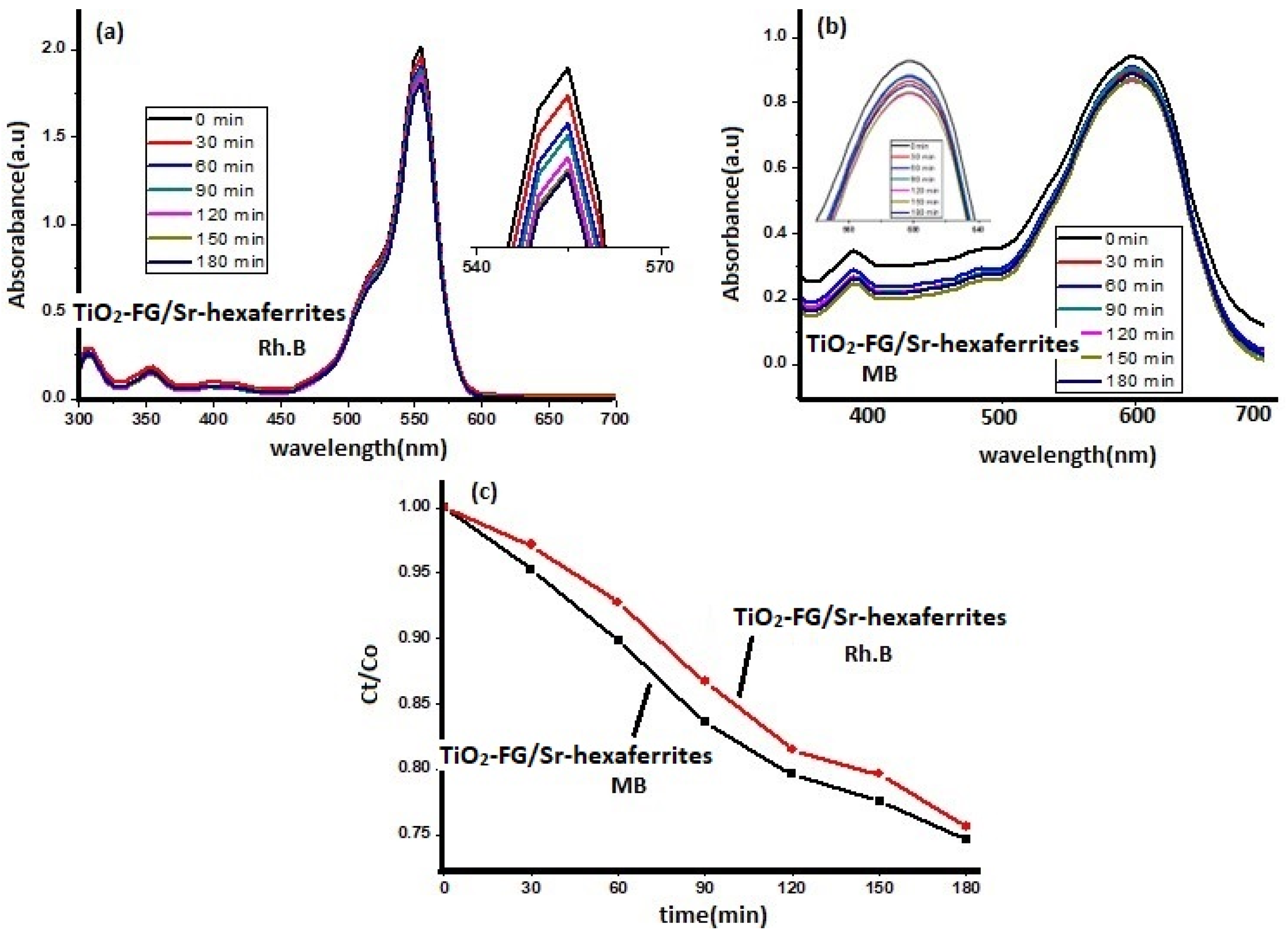
Photocatalytic Decolorization of Rh. B and MB Dyes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Yao, J.; Chen, S.; Zhang, J.; Li, R.; Peng, T. Construction of rGO-coupled C3N4/C3N5 2D/2D Z-scheme heterojunction to accelerate charge separation for efficient visible light H2 evolution. Appl. Catal. B Environ. 2022, 318, 121822. [Google Scholar] [CrossRef]
- Hara, S.; Irie, H. Band structure controls of SrTiO3 towards two-step overall water splitting. Appl. Catal. B Environ. 2012, 115, 330–335. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tang, Z.-R.; Xu, Y.-J. Multifunctional graphene-based composite photocatalysts oriented by multifaced roles of graphene in photocatalysis. Chin. J. Catal. 2022, 43, 708–730. [Google Scholar] [CrossRef]
- Bashir, S.; Jamil, A.; Amin, R.; Ul-hasan, I.; Alazmi, A.; Shahid, M. Hydrothermally synthesized Gd-doped BiSbO4 nanoparticles and their graphene-based composite: A novel photocatalytic material. J. Solid State Chem. 2022, 312, 123217. [Google Scholar] [CrossRef]
- Hara, S.; Yoshimizu, M.; Tanigawa, S.; Ni, L.; Ohtani, B.; Irie, H. Hydrogen and oxygen evolution photocatalysts synthesized from strontium titanate by controlled doping and their performance in two-step overall water splitting under visible light. J. Phys. Chem. C 2012, 116, 17458–17463. [Google Scholar] [CrossRef] [Green Version]
- Rad, T.S.; Khataee, A.; Arefi-Oskoui, S.; Rad, S.S.; Orooji, Y.; Gengec, E.; Kobya, M. Graphene-based ZnCr layered double hydroxide nanocomposites as bactericidal agents with high sonophotocatalytic performances for degradation of rifampicin. Chemosphere 2022, 286, 131740. [Google Scholar]
- Purabgola, A.; Mayilswamy, N.; Kandasubramanian, B. Graphene-based TiO2 composites for photocatalysis & environmental remediation: Synthesis and progress. Environ. Sci. Pollut. Res. 2022, 29, 32305–32325. [Google Scholar]
- Ahmad, S.; Ayoub, M.H.; Khan, A.M.; Waseem, A.; Yasir, M.; Khan, M.S.; Bajwa, T.M.; Shaikh, A.J. Diverse comparative studies for preferential binding of graphene oxide and transition metal oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129057. [Google Scholar] [CrossRef]
- Ullah, K.; Ullah, A.; Aldalbahi, A.; Oh, W.-C. Enhanced visible light photocatalytic activity and hydrogen evolution through novel heterostructure AgI–FG–TiO2 nanocomposites. J. Mol. Catal. A Chem. 2015, 410, 242–252. [Google Scholar] [CrossRef]
- Rehman, G.U.; Tahir, M.; Goh, P.; Ismail, A.; Khan, I.U. Controlled synthesis of reduced graphene oxide supported magnetically separable Fe3O4@ rGO@ AgI ternary nanocomposite for enhanced photocatalytic degradation of phenol. Powder Technol. 2019, 356, 547–558. [Google Scholar] [CrossRef]
- Mandal, S.; Mallapur, S.; Reddy, M.; Singh, J.K.; Lee, D.-E.; Park, T. An overview on graphene-metal oxide semiconductor nanocomposite: A promising platform for visible light photocatalytic activity for the treatment of various pollutants in aqueous medium. Molecules 2020, 25, 5380. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Yi, G.; Zu, X.; Wang, H.; Luo, H.; Tan, M. Preparation of graphene-TiO2 nanocomposite films and its photocatalytic performances on degradation of Rhodamine B. Pigment Resin Technol. 2018, 47, 79–85. [Google Scholar] [CrossRef]
- Tai, X.H.; Lai, C.W.; Yang, T.C.K.; Johan, M.R.; Lee, K.M.; Chen, C.-Y.; Juan, J.C. Highly effective removal of volatile organic pollutants with pn heterojunction photoreduced graphene oxide-TiO2 photocatalyst. J. Environ. Chem. Eng. 2022, 10, 107304. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Ljubas, D.; Mužina, K.; Bačić, I.; Radošević, T.; Podlogar, M.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J. Graphene-based TiO2 nanocomposite for photocatalytic degradation of dyes in aqueous solution under solar-like radiation. Appl. Sci. 2021, 11, 3966. [Google Scholar] [CrossRef]
- Ullah, K.; Ye, S.; Lei, Z.; Cho, K.-Y.; Oh, W.-C. Synergistic effect of PtSe2 and graphene sheets supported by TiO2 as cocatalysts synthesized via microwave techniques for improved photocatalytic activity. Catal. Sci. Technol. 2015, 5, 184–198. [Google Scholar] [CrossRef]
- Cheng, C.; Liang, Q.; Yan, M.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; Liu, Y. Advances in preparation, mechanism and applications of graphene quantum dots/semiconductor composite photocatalysts: A review. J. Hazard. Mater. 2022, 424, 127721. [Google Scholar] [CrossRef]
- Yang, J.; Miao, H.; Jing, J.; Zhu, Y.; Choi, W. Photocatalytic activity enhancement of PDI supermolecular via π-π action and energy level adjusting with graphene quantum dots. Appl. Catal. B Environ. 2021, 281, 119547. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Cao, Y.; Yu, H. Carboxyl-Functionalized Graphene for Highly Efficient H 2-Evolution Activity of TiO2 Photocatalyst. Acta Phys.-Chim. Sin. 2021, 37, 2008047. [Google Scholar]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- García-Martín, E.; Granados-Miralles, C.; Ruiz-Gómez, S.; Pérez, L.; del Campo, A.; Guzmán-Mínguez, J.C.; de Julián Fernández, C.; Quesada, A.; Fernández, J.F.; Serrano, A. Dense strontium hexaferrite-based permanent magnet composites assisted by cold sintering process. J. Alloy. Compd. 2022, 917, 165531. [Google Scholar] [CrossRef]
- Bourzami, R.; Guediri, M.K.; Chebli, D.; Bouguettoucha, A.; Amrane, A. Bottom-up construction of reduced-graphene-oxide-anchored spinel magnet Fe2.02Ni1.01O3.22, anatase TiO2 and metallic Ag nanoparticles and their synergy in photocatalytic water reduction. J. Environ. Chem. Eng. 2021, 9, 105307. [Google Scholar] [CrossRef]
- Yu, H.; Chong, Y.; Zhang, P.; Ma, J.; Li, D. A D-shaped fiber SPR sensor with a composite nanostructure of MoS2-graphene for glucose detection. Talanta 2020, 219, 121324. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chen, R.; Zhao, Z.; Cui, F.; Xu, X. Graphene oxide-supported graphitic carbon nitride microflowers decorated by sliver nanoparticles for enhanced photocatalytic degradation of dimethoate via addition of sulfite: Mechanism and toxicity evolution. Chem. Eng. J. 2021, 425, 131683. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Peng, W.-X.; Jermsittiparsert, K.; Hosseinzadeh, G.; Hosseinzadeh, R. Combination of sonochemical and freeze-drying methods for synthesis of graphene/Ag-doped TiO2 nanocomposite: A strategy to boost the photocatalytic performance via well distribution of nanoparticles between graphene sheets. Ceram. Int. 2020, 46, 7446–7452. [Google Scholar] [CrossRef]
- Potle, V.D.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K. Sonochemical preparation of ternary rGO-ZnO-TiO2 nanocomposite photocatalyst for efficient degradation of crystal violet dye. Optik 2020, 208, 164555. [Google Scholar] [CrossRef]
- Fattahi, A.; Liang, R.; Kaur, A.; Schneider, O.; Arlos, M.J.; Peng, P.; Servos, M.; Zhou, N. Photocatalytic degradation using TiO2-graphene nanocomposite under UV-LED illumination: Optimization using response surface methodology. J. Environ. Chem. Eng. 2019, 7, 103366. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, M.L.; Van Tran, T.T.; Juang, R.-S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, S.; Kumar, V.; Tikoo, K.; Kaushik, A.; Singhal, S. Development of emphatic catalysts for waste water remediation via synchronized free radical and non-free radical routes with composites of strontium hexaferrite, graphene and multi-walled carbon nanotubes. Ceram. Int. 2022, 48, 4795–4811. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, Z.; Zhu, Z.; Zhuang, Q.; Chen, W.; Yu, H.; Liu, Q. Facile synthesis of strontium ferrite nanorods/graphene composites as advanced electrode materials for supercapacitors. J. Colloid Interface Sci. 2021, 588, 795–803. [Google Scholar] [CrossRef]
- Shah, S.; Pandey, O.; Mohammed, J.; Srivastava, A.; Gupta, A.; Basandrai, D. Reduced graphene oxide (RGO) induced modification of optical and magnetic properties of M-type nickel doped barium hexaferrite. J. Sol-Gel Sci. Technol. 2020, 93, 579–586. [Google Scholar] [CrossRef]
- Shakir, I.; Agboola, P.O.; Haider, S. Manganese spinel ferrite-reduced graphene oxides nanocomposites for enhanced solar irradiated catalytic studies. Ceram. Int. 2021, 47, 28367–28376. [Google Scholar] [CrossRef]
- Mungondori, H.H.; Ramujana, S.; Katwire, D.M.; Taziwa, R.T. Synthesis of a novel visible light responsive γ-Fe2O3/SiO2/C-TiO2 magnetic nanocomposite for water treatment. Water Sci. Technol. 2018, 78, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Khalid, S.; Yasin, T.; Mazare, A. A Review on the Progress and Future of TiO2/Graphene Photocatalysts. Energies 2022, 15, 6248. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, M.; Wang, L.; Fei, Y.; Wang, S.; Núñez-Delgado, A.; Bokhari, A.; Race, M.; Khataee, A.; Klemeš, J.J. Photocatalytic degradation of xanthate in flotation plant tailings by TiO2/graphene nanocomposites. Chem. Eng. J. 2022, 431, 134104. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Physica Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Ullah, K.; Oh, W.-C. Fabrication of large size graphene and Ti-MWCNTs/large size graphene composites: Their photocatalytic properties and potential application. Sci. Rep. 2015, 5, 14242. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Liu, X.; Wang, Y.; Li, L.; Zhang, Z. Preparation and characterization of Fe3O4@ SiO2@ TiO2–Co/rGO magnetic visible light photocatalyst for water treatment. RSC Adv. 2019, 9, 20256–20265. [Google Scholar] [CrossRef] [Green Version]
- Otgonbayar, Z.; Cho, K.Y.; Oh, W.-C. Novel Micro and Nanostructure of a AgCuInS2–Graphene–TiO2 Ternary Composite for Photocatalytic CO2 Reduction for Methanol Fuel. ACS Omega 2020, 5, 26389–26401. [Google Scholar] [CrossRef]
- Van Bao, H.; Dat, N.M.; Giang, N.T.H.; Thinh, D.B.; Trinh, D.N.; Hai, N.D.; Khoa, N.A.D.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Behavior of ZnO-doped TiO2/rGO nanocomposite for water treatment enhancement. Surf. Interfaces 2021, 23, 100950. [Google Scholar]
- Banerjee, S.; Benjwal, P.; Singh, M.; Kar, K.K. Graphene oxide (rGO)-metal oxide (TiO2/Fe3O4) based nanocomposites for the removal of methylene blue. Appl. Surf. Sci. 2018, 439, 560–568. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Al Kahtani, A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Singh, N.; Jana, S.; Singh, G.P.; Dey, R. Graphene-supported TiO2: Study of promotion of charge carrier in photocatalytic water splitting and methylene blue dye degradation. Adv. Compos. Hybrid Mater. 2020, 3, 127–140. [Google Scholar] [CrossRef]
- Tian, H.; Wan, C.; Xue, X.; Hu, X.; Wang, X. Effective electron transfer pathway of the ternary TiO2/RGO/Ag nanocomposite with enhanced photocatalytic activity under visible light. Catalysts 2017, 7, 156. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Enhanced photocatalytic activity of hybrid rGO@TiO2/CN nanocomposite for organic pollutant degradation under solar light irradiation. Catalysts 2021, 11, 1023. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Garg, S.; Oh, W.-C.; Hamizi, N.A.; Johan, M.R. Enhanced photocatalytic activity of rGO-CuO nanocomposites for the degradation of organic pollutants. Catalysts 2021, 11, 1008. [Google Scholar] [CrossRef]
- Jing, Z.; Dai, X.; Xian, X.; Zhang, Q.; Zhong, H.; Li, Y. Novel Ternary heterogeneous reduction graphene oxide (RGO)/BiOCl/TiO2 nanocomposites for enhanced adsorption and visible-light induced photocatalytic activity toward organic contaminants. Materials 2020, 13, 2529. [Google Scholar] [CrossRef]
- Vasilaki, E.; Katsarakis, N.; Dokianakis, S.; Vamvakaki, M. rGO functionalized ZnO–TiO2 core-shell flower-like architectures for visible light photocatalysis. Catalysts 2021, 11, 332. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, D.; Hu, X.; Qian, Y.; Li, D. Preparation of TiO2/carbon nanotubes/reduced graphene oxide composites with enhanced photocatalytic activity for the degradation of rhodamine B. Nanomaterials 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Murugananthan, M.; Gu, J.; Zhang, Y. Fabrication of a Z-scheme g-C3N4/Fe-TiO2 photocatalytic composite with enhanced photocatalytic activity under visible light irradiation. Catalysts 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Sun, S.; Xu, H.; Li, M.; Hao, X.; Shao, G.; Wang, H.; Chen, D.; Lu, H.; Zhang, R. Investigation on g-C3N4/rGO/TiO2 nanocomposite with enhanced photocatalytic degradation performance. J. Phys. Chem. Solids 2021, 156, 110181. [Google Scholar] [CrossRef]
- Ye, X.; Chen, L.; Wang, Z.; Wang, Q.; Xiao, X.; Liu, X. Facile in situ hydrothermal synthesis of titania nanosheets on reduced graphene oxide with photocatalytic activity. J. Photochem. Photobiol. A Chem. 2019, 385, 112085. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, K.; Oh, W.-C. Fabrication of Novel Heterostructure-Functionalized Graphene-Based TiO2-Sr-Hexaferrite Photocatalyst for Environmental Remediation. Nanomaterials 2023, 13, 55. https://doi.org/10.3390/nano13010055
Ullah K, Oh W-C. Fabrication of Novel Heterostructure-Functionalized Graphene-Based TiO2-Sr-Hexaferrite Photocatalyst for Environmental Remediation. Nanomaterials. 2023; 13(1):55. https://doi.org/10.3390/nano13010055
Chicago/Turabian StyleUllah, Kefayat, and Won-Chun Oh. 2023. "Fabrication of Novel Heterostructure-Functionalized Graphene-Based TiO2-Sr-Hexaferrite Photocatalyst for Environmental Remediation" Nanomaterials 13, no. 1: 55. https://doi.org/10.3390/nano13010055
APA StyleUllah, K., & Oh, W.-C. (2023). Fabrication of Novel Heterostructure-Functionalized Graphene-Based TiO2-Sr-Hexaferrite Photocatalyst for Environmental Remediation. Nanomaterials, 13(1), 55. https://doi.org/10.3390/nano13010055






