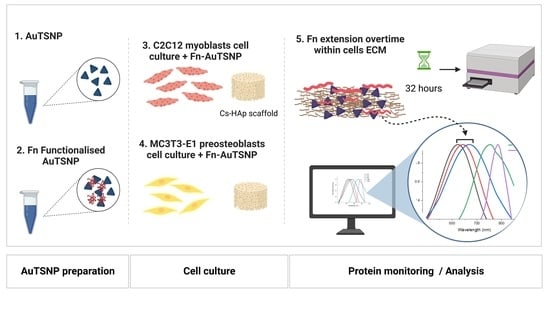
Monitoring In Vitro Extracellular Matrix Protein Conformations in the Presence of Biomimetic Bone-Regeneration Scaffolds Using Functionalized Gold-Edge-Coated Triangular Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
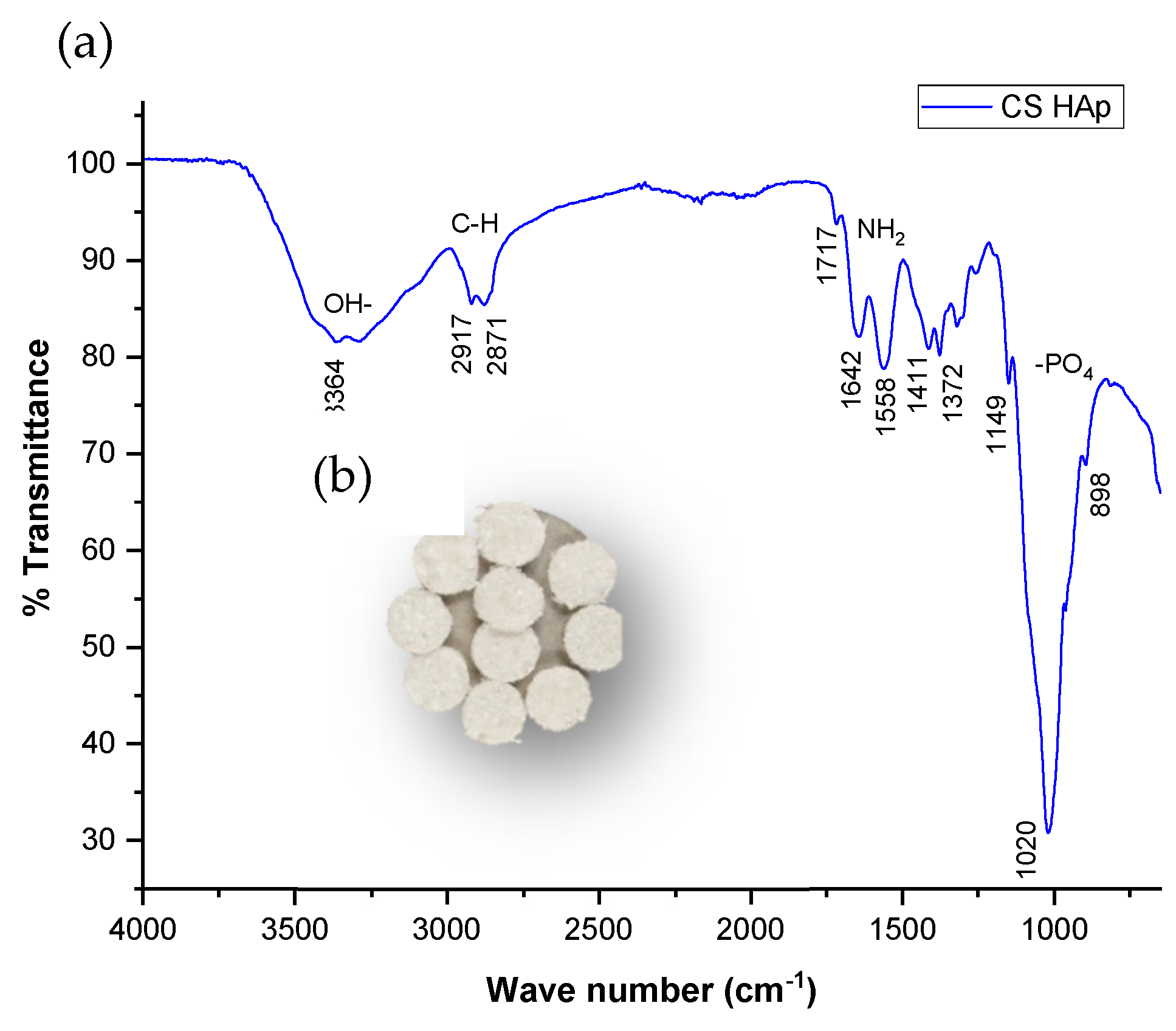
2.1. Fabrication of Chitosan-Based Scaffolds
2.2. Scaffold Characterization
2.3. Gold-Edge Coated Triangular Silver Nanoplates (AuTSNP) Preparation
2.4. Cell Culture
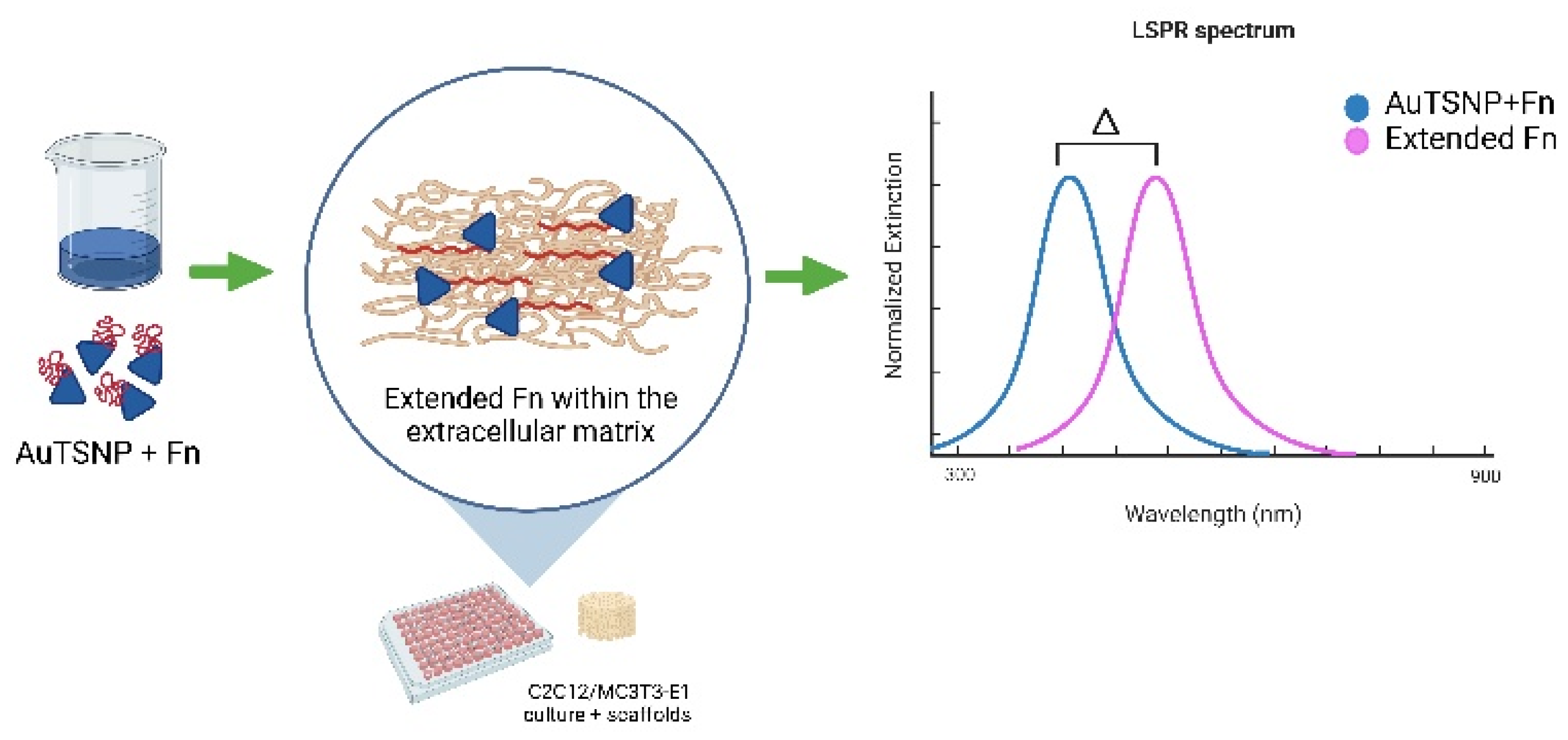
2.5. Protein Monitoring
3. Results and Discussion
3.1. Biomimetic Bone Regeneration Scaffold
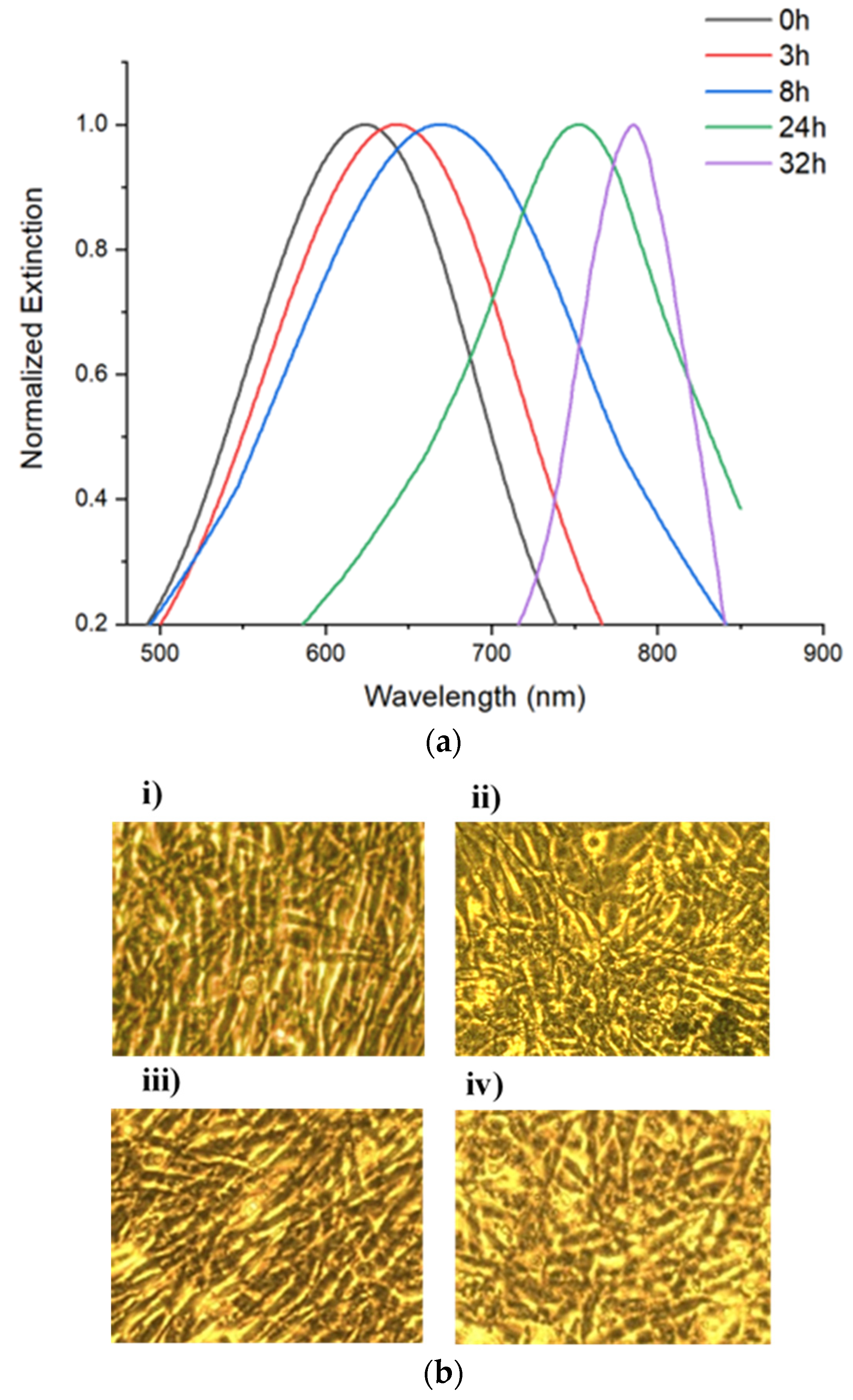
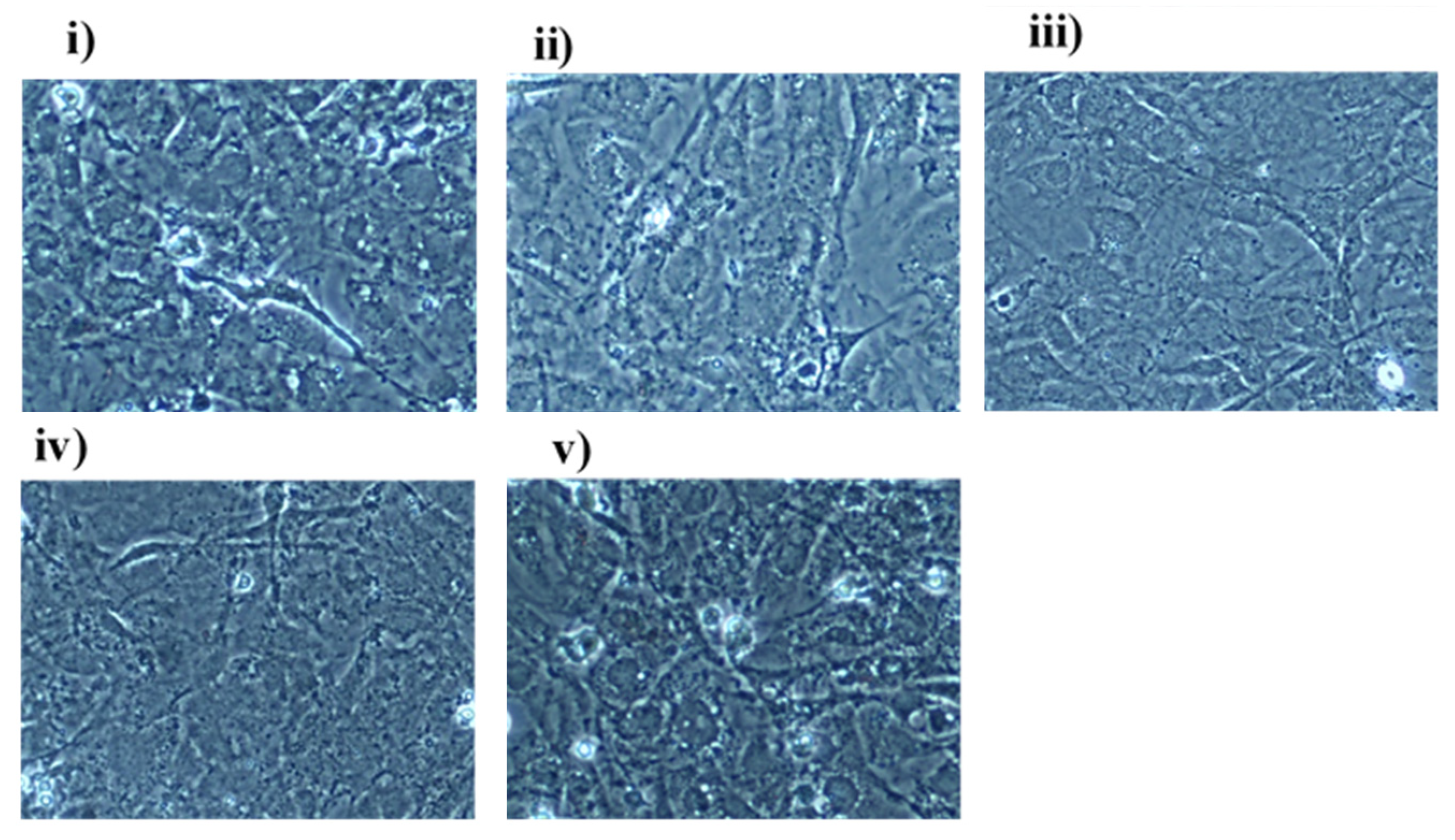
3.2. Fn Monitoring in C2C12 Myoblast Cells
3.3. Fn Monitoring in MC3T3 Pre-Osteoblast Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollock, V. Proteins. Xpharm Compr. Pharmacol. Ref. 2007, 1–11. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Ponnusamy, R. Introduction to proteomics. In Concepts and Techniques in Genomics and Proteomics; Woodhead Publishing Limited: Sawston, UK, 2007; Volume 367, pp. 147–158. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Protein Function. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; pp. 1–19. [Google Scholar]
- Moree, B.; Connell, K.; Mortensen, R.B.; Liu, C.T.; Benkovic, S.J.; Salafsky, J. Protein Conformational Changes Are Detected and Resolved Site Specifically by Second-Harmonic Generation. Biophys. J. 2015, 109, 806–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, D.E.; Aherne, D.; Gara, M.; Ledwith, D.M.; Gun, Y.K.; Kelly, J.M.; Blau, W.J.; Brennan-Fournet, M.E. Silver Nanoplates for Highly Sensitive Plasmon Resonance Sensing. ACS Nano 2010, 4, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, N.; Feig, M.; Trylska, J. Modeling Crowded Environment in Molecular Simulations. Front. Mol. Biosci. 2019, 6, 86. [Google Scholar] [CrossRef]
- Feig, M.; Yu, I.; Wang, P.H.; Nawrocki, G.; Sugita, Y. Crowding in Cellular Environments at an Atomistic Level from Computer Simulations. J. Phys. Chem. B 2017, 121, 8009–8025. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.-T.; Jing, C. Localized Surface Plasmon Resonance Based Nanobiosensors; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-54794-2. [Google Scholar]
- Zhang, Y.; Charles, D.E.; Ledwith, D.M.; Aherne, D.; Cunningham, S.; Voisin, M.; Blau, W.J.; Gun’Ko, Y.K.; Kelly, J.M.; Brennan-Fournet, M.E. Wash-free highly sensitive detection of C-reactive protein using gold derivatised triangular silver nanoplates. RSC Adv. 2014, 4, 29022–29031. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Mauriz, E.; Dey, P.; Lechuga, L.M. Advances in nanoplasmonic biosensors for clinical applications. Analyst 2019, 144, 7105–7129. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing-A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Svystonyuk, D.A.; Mewhort, H.E.M.; Fedak, P.W.M. Using Acellular Bioactive Extracellular Matrix Scaffolds to Enhance Endogenous Cardiac Repair. Front. Cardiovasc. Med. 2018, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable scaffolds for bone regeneration combined with drug-delivery systems in osteomyelitis therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Amariei, G.; Kokol, V.; Boltes, K.; Letón, P.; Rosal, R. Incorporation of antimicrobial peptides on electrospun nanofibres for biomedical applications. RSC Adv. 2018, 8, 28013–28023. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef]
- Brennan-Fournet, M.E.; Huerta, M.; Zhang, Y.; Malliaras, G.; Owens, R.M. Detection of fibronectin conformational changes in the extracellular matrix of live cells using plasmonic nanoplates. J. Mater. Chem. B 2015, 3, 9140–9147. [Google Scholar] [CrossRef]
- Devine, D.M.; Hoctor, E.; Hayes, J.S.; Sheehan, E.; Christopher, H. Extended release of proteins following encapsulation in hydroxyapatite/chitosan composite scaffolds for bone tissue engineering applications. Mater. Sci. Engineering. C Mater. Biol. Appl. 2019, 84, 1–24. [Google Scholar] [CrossRef]
- Azaman, F.A.; Zhou, K.; Blanes-Martínez, M.D.M.; Brennan Fournet, M.; Devine, D.M. Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels 2022, 8, 696. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, J.S.; Chung, Y.S.; Sin, Y.W.; Ryu, K.H.; Lee, J.; You, H.K. Growth and osteogenic differentiation of alveolar human bone marrow-derived mesenchymal stem cells on chitosan/hydroxyapatite composite fabric. J. Biomed. Mater. Res. Part A 2013, 101 A, 1550–1558. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Milan, P.B. Bone Tissue Engineering Using Human Cells: A Comprehensive Review on Recent Trends, Current Prospects, and Recommendations. Appl. Sci. 2019, 9, 174. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Lemos, A.L.; Tavaria, F.K.; Pintado, M. Chitosan and hydroxyapatite based biomaterials to circumvent periprosthetic joint infections. Materials 2021, 14, 804. [Google Scholar] [CrossRef] [PubMed]
- Fern, H.W.; Salimi, M.N. Hydroxyapatite nanoparticles produced by direct precipitation method: Optimization and characterization studies. AIP Conf. Proc. 2021, 2339, 020215. [Google Scholar] [CrossRef]
- Maachou, H.; Bal, K.E.; Bal, Y.; Chagnes, A.; Cote, G.; Alliouche, D. Characterization and in vitro bioactivity of chitosan/hydroxyapatite composite membrane prepared by freeze-gelation method. Trends Biomater. Artif. Organs 2008, 22, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Antia, M.; Baneyx, G.; Kubow, K.E.; Abstract, V.V. Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. NIH Public Access 2008, 139, 229–420. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, D.; Mittal, A.; Malik, D.K. Antimicrobial potential and in vitro cytotoxicity study of polyvinyl pyrollidone-stabilised silver nanoparticles synthesised from Lysinibacillus boronitolerans. IET Nanobiotechnol. 2021, 15, 427–440. [Google Scholar] [CrossRef]
- Hashimoto, M.; Toshima, H.; Yonezawa, T.; Kawai, K.; Narushima, T.; Kaga, M.; Endo, K. Micromorphological cellular responses of MC3T3-E1 and RAW264.7 after exposure to water-dispersible silver nanoparticles stabilized by metal-carbon σ-bonds. Dent. Mater. J. 2013, 32, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]


| Time | Wavelength | |
|---|---|---|
| Active | Denatured | |
| 0 h | 667 | 670 |
| 3 h | 697 | 693 |
| 8 h | 711 | 702 |
| 24 h | 766 | 718 |
| 32 h | 786 | 726 |
| Sample | Peak Wavelength (nm) |
|---|---|
| 0 h | 623 |
| 3 h | 643 |
| 8 h | 669 |
| 24 h | 753 |
| 32 h | 785 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez Barroso, L.G.; Azaman, F.A.; Pogue, R.; Devine, D.; Fournet, M.B. Monitoring In Vitro Extracellular Matrix Protein Conformations in the Presence of Biomimetic Bone-Regeneration Scaffolds Using Functionalized Gold-Edge-Coated Triangular Silver Nanoparticles. Nanomaterials 2023, 13, 57. https://doi.org/10.3390/nano13010057
Rodriguez Barroso LG, Azaman FA, Pogue R, Devine D, Fournet MB. Monitoring In Vitro Extracellular Matrix Protein Conformations in the Presence of Biomimetic Bone-Regeneration Scaffolds Using Functionalized Gold-Edge-Coated Triangular Silver Nanoparticles. Nanomaterials. 2023; 13(1):57. https://doi.org/10.3390/nano13010057
Chicago/Turabian StyleRodriguez Barroso, Laura G., Farah Alwani Azaman, Robert Pogue, Declan Devine, and Margaret Brennan Fournet. 2023. "Monitoring In Vitro Extracellular Matrix Protein Conformations in the Presence of Biomimetic Bone-Regeneration Scaffolds Using Functionalized Gold-Edge-Coated Triangular Silver Nanoparticles" Nanomaterials 13, no. 1: 57. https://doi.org/10.3390/nano13010057
APA StyleRodriguez Barroso, L. G., Azaman, F. A., Pogue, R., Devine, D., & Fournet, M. B. (2023). Monitoring In Vitro Extracellular Matrix Protein Conformations in the Presence of Biomimetic Bone-Regeneration Scaffolds Using Functionalized Gold-Edge-Coated Triangular Silver Nanoparticles. Nanomaterials, 13(1), 57. https://doi.org/10.3390/nano13010057