1. Introduction
Experimental detection of heat production in a single living cell attributed to its different compartments, organelles, ionic pumps, and ionic channels has long been a cutting edge challenge for modern experimental approaches in cell physiology research. The proven existence of sharp steady-state temperature gradients (tens of degrees °C) in submicrometer watery volumes near a nanoscale heat source [
1] puts forward a beneficial base for the thermal signaling concept [
2], supposing that living cells are capable of creating significant temperature gradients at nanoscale, engaging these ultralocal thermodynamic events as a variable driving force to govern cascades of morphological, biochemical, and electrical intracellular processes. Therefore, experimental uncovering of the signaling role of the nanoscale thermodynamic events bound to biochemical processes and the thermodynamics around ionic channels and pumps has become an ultimate priority. Being the ubiquitous and specialized factory for transforming the most powerful chemical energy supply of the aerobic respiration process to the universal cellular energy source in the form of macroergic phosphate bonds of ATP, mitochondria are natural producers of heat that is generated due to inefficient (up to 60 percent) energy conversion [
3]. Together with a decoupling mechanism that is used to control the upper value of the proton potential to avoid excessive production of reactive oxygen species [
4], this served as a preadaptation to the development of homeothermy, which maintains the organism’s homeostasis and intensifies metabolism at an unprecedented level. This is taken to the extreme in the brown fat of neonatants and hibernating animals, where mitochondria seem to be solely dedicated to heat production [
5].
Various cellular dysfunctions and pathological conditions lead to changes in the energy metabolism of mitochondria [
6] and, as a result, to changes in their heat production [
7]. There have already been studies on the medical use of the ability of mitochondria to create heat. Recently, thermosensitive nanocarriers have been developed for targeted drug delivery to mitochondria [
8]. These nanocarriers are able to enhance the accumulation of anticancer drugs in mitochondria at a certain endogenous mitochondrial temperature. This increases the selectivity for cancer cells and helps to reverse cancer drug resistance [
9]. Despite these facts, there are still unresolved questions about the absolute temperature of mitochondria in different cells/tissues/organs/organisms, the range of these temperature fluctuations, and how these fluctuations depend on the functional state in normal and pathological conditions.
A comprehensive understanding of mitochondria thermogenesis requires a reliable and robust technique without an external biological impact. Recently, a great variety of techniques and approaches aimed at detecting mitochondrial temperature including fluorescent nanogels [
9,
10], polymers [
11], and proteins [
12,
13,
14]; molecular dyes [
15,
16,
17,
18]; and colloidal quantum dots [
19] were elaborated. A number of them using probes located outside of mitochondria (extramitochondrial) reported increases in temperature of several degrees caused by enhanced mitochondrial activity, generally, due to uncoupling of the respiratory chain which boosts mitochondrial thermogenesis. Some other approaches based on thermosensors located within mitochondria (intramitochondrial) were able to identify an increase in the organelle temperature of several degrees in the same (or similar) conditions, suggesting that the mitochondrial radiators could be warmer than their surroundings. Although Chr’etien et al. [
15] revealed a mitochondrial temperature of more than 10 °C above that of the culture medium in normal conditions (without any stimuli), their study remains so far the only evidence of such a high heat release by mitochondria.
Despite numerous experimental works supporting the hypothesis of “hot mitochondria” [
20,
21], the physical and biological mechanisms for furnishing perceptible thermal bursts remain a matter of controversy. So, Baffou et al. argued that according to Fourier’s law, hardly such a small organelle (typically 500 nm in diameter) could heat the intracellular surrounding by more than 0.1 mK [
21]. Filling the temperature gap would necessitate organelles to produce the heat power
W. Apparently, the mitochondria do not have enough fuel to meet this requirement. Baffou et. al. claimed that the existing fluorescent thermosensors do not directly measure the temperature, and, as with any fluorescent probe, are prone to many artifacts caused by variations in environment (viscosity, pH, ionic strength, quenching). Therefore, the validity of presented results along with their careful interpretation should be questioned.
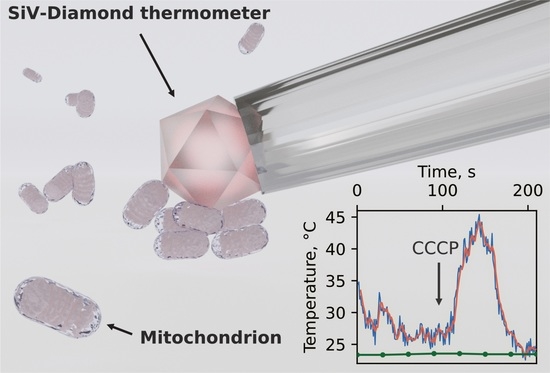
In this paper, we employ a diamond thermometer (DT) designed by us for unambiguous extramitochondrial temperature measurements of isolated mouse brain mitochondria. The DT based on a single fluorescent diamond microparticle fixed at the tip of the glass capillary and pre-calibrated by temperature is absolutely insensitive to external non-thermal parameters allowing for unambiguous temperature detection. By means of DT instrumentation we demonstrate that the mitochondria isolated from the mouse brain could be several tens of degrees warmer than the surrounding environment at room temperature during natural metabolic processes as well as under membrane permeability agent application. The simple physical model is also provided to support the obtained results.
2. Materials and Methods
All animal protocols and experimental procedures were performed in accordance with the requirements of Directive 2010/63/EC of the European Union and Order of the Ministry of Health of Russia of 19 June 2003 No. 267 and were approved by the Ethics Committees for Animal Experimentation at the ITEB RAS. BALB/c mice (25–33 g) were purchased from the Stolbovaya vivarium of laboratory animals (
https://www.pitst.ru (accessed on 1 January 2020), Moscow region, Russia) and housed with food and water ad libitum.
2.1. Mouse Brain Mitochondria Isolation
Adult mice (n = 6) were deeply anesthetized with isoflurane and decapitated. The hemispheres were excised and immediately placed into an ice-cold isolation buffer containing either 125 mM KCl and 10 mM KH2PO4, pH 7.4 (phosphate medium) or 220 mM mannitol, 70 mM sucrose, 10 mM hepes, 1 mM EGTA, and 0.5% bovine serum albumin (sucrose medium). The cerebellum was removed, and the rest of the brain tissue was cut into small pieces and placed in a 7 mL homogenizer (Duran, Wheaton) with 4 mL of the buffer and homogenized manually for 1 min with 20 strokes of tight-fitting pestle. The brain homogenate was centrifuged at 4000× g for 10 min at 4 °C. The pellet was discarded and the supernatant was centrifuged at 12,000× g for 15 min at 4 °C. The resulting pellet was resuspended in 0.5 mL of the isolation medium and stored on ice during the experiment. Below, we apply the absolute mice (M) numeration where sucrose medium was used for M1-4 mice and phosphate medium for M5-6 mice.
2.2. Mitochondria Functional Assay
The mitochondrial functional assay was carried out in the phosphate medium at room temperature (23 °C). A 20 M fluorescent dye TMRM (Tetramethylrhodamine, Methyl Ester, Perchlorate, ThermoFisher, Waltham, MA, USA) was used as the mitochondrial membrane potential indicator (the emission peak at 582 nm); 2 mM pyruvate (Sigma Aldrich, St. Louis, MO, USA) was used as a respiration substrate, 100 M ADP (Sigma Aldrich, USA) was used to activate ATP production, 4 M mitochondrial oxidative phosphorylation uncoupler CCCP (2-[2-(3-Chlorophenyl) hydrazinylyidene] propanedinitrile, ThermoFisher, USA) was used to render mitochondrial inner membrane permeable to protons.
2.3. DT Design
The design principle of the diamond thermometer device was exhaustively described in [
1]. Its backbone consists of a glass microcapillary with a low-strain single diamond crystal fixed at the tip of the capillary. The thermosensitivity of DT is provided by an ensemble of silicon-vacancy centers (SiV-centers) embedded in the diamond crystal during the CVD-synthesis. The position of the maximum of the SiV-fluorescence zero-phonon line (ZPL) is sensitive to temperature and provides the ability to record the temperature of any microsystem once calibrated.
2.4. DT Calibration
The calibration of the developed DT was performed prior to the experiments with mitochondria. The DT was placed in an air environment for a preliminary determination of the power density of optical excitation at a wavelength that did not lead to a shift of the zero-phonon SiV luminescence line, and, consequently, to heating. The measured power density was used to determine the temperature dependence of the spectral position of the zero-phonon line center in a Linkam TS1500 thermostat stabilized at a predetermined level with an accuracy of 1 °C. The temperature in the chamber was changed in increments of 10 °C. At each step, the SiV luminescence spectrum was recorded and the spectral position of the zero-phonon line maximum was determined according to the algorithm proposed in our previous work [
1].
2.5. Experimental Setup
The study of the mitochondrial uncoupling thermal response was performed using a LabRam HR800 (Horiba) confocal spectrometer. The SiV fluorescence was excited with a 473 nm laser light (Laser Quantum) which was focused by the low-NA objective to one of the ends of the multimode optical fiber (Thorlabs) with transmission maximum at 740 nm. Another fiber end laid through the interior of the capillary and located near its tip guided the excitation photons directly to microdiamond. The excitation power of the fiber input was chosen to be 3 mW to provide a sufficient fluorescent signal with no additional heating (see
Supplementary Materials, Figure S1). The fluorescence was collected with a long-focal-length air objective (Olympus x50, NA = 0.55) and directed to the spectrometer. Probing the mitochondrial viability with TMRM dye was implemented with the same optics equipped with a 532 nm laser and bandpass filter (550–700 nm) in the registration path.
3. Results
The mitochondria used for studying the local heat production were isolated from six adult mice according to the protocol described in Methods. To verify the viability of the organelles, we examined their bioenergetic state with membrane potential-sensitive dye. For this, immediately after isolation, mitochondria were loaded with TMRM dye which possesses bright and broadband fluorescence with maximum at 580 nm. The time-track of the dye intensity under application of pyruvate, ADP, and CCCP was then recorded. The mitochondrial oxidative phosphorylation uncoupler CCCP renders mitochondrial inner membrane permeable to protons and thus spill off transmembrane potential with transformation of its energy directly to heat.
Figure 1 illustrates the typical bioenergetic profile for mitochondria isolated in sucrose (a) and phosphate (b) medium. At specified timepoints, the organelles underwent substrate, ADP, and CCCP application. The fluorescent changes
were determined as normalized deviations from the baseline before any additions, while the mitochondria isolated in both media demonstrated relevant physiological response reflected by changing of dye intensity with respect to membrane potential. The value of this response was higher in the sucrose medium, more favorable to mitochondrial physiological conditioning. These measurements were performed once for each mouse to verify that mitochondria survived after isolation and displayed a conventional metabolic response.
Once the viability of the organelles was confirmed, an examination of the mitochondria thermal response to the CCCP electro-chemical action began. A total of 200
L of mitochondrial suspension was applied to the cap of the Petri dish and smeared on its surface. The rest of the suspension was stored on ice throughout the experiment to preserve mitochondrial metabolism. The dish was then placed on the 3D mechanical stage beneath the microscope objective, and an appropriate aggregate of mitochondria with a size ranging from 2 to 10
m was found through the optical CMOS camera (see
Figure 2d; note that not all visible aggregates are mitochondria since some cell debris still remains after isolation). At the next step the DT was immersed in the solution, and the tip with microdiamond accurately touched the top surface of the targeted aggregate, slightly pressing it. Slight pressure is essential to leave the organelles immobilized during temperature protocols. To induce mitochondrial inner membrane permeability to protons and thus transmembrane potential spill off, 2.5
L of CCCP was applied to the suspension, and the temperature change was determined. As a reference indication of the macroscopic temperature we used a conventional thermocouple immersed in the solution. Before starting DT recordings, the temperature in the dish was determined as the steady-state ambient temperature (see
Supplementary Materials, Figure S2).
Figure 3 illustrates the temperature tracks recorded for different dishes within each of the six mice. The time step between DT indications was chosen to be 1 s to achieve the best signal-to-noise ratio while still considering the possible quick thermal dynamics. One can see that the temperature elevation
ranges from several to tens degrees Celsius above the ambient level (green dots) primarily right after CCCP injection (yellow arrow). The maximum thermal burst within a single dish of
°C is observed for M3D1. However, temperature bursts were also detected before CCCP application (e.g., M2D1, M2D6, M3D1). Such spontaneous thermal responses are not lower in amplitude than CCCP-stimulated ones and could be attributed to the mitochondria which may be currently involved in ATP synthesis or controlled potential leaking to avoid reactive oxygen species hyperproduction due to excessive potential value.
The burst duration also varies in a wide range from seconds to hundreds of seconds (
Figure 4b), probably due to inhomogeneous distribution of CCCP action on individual mitochondria within one aggregate: while mitochondria on the edge of aggregate are exposed to the uncoupler action, the deep-dwelling organelles are isolated and remain unperturbed. Thus, the slow penetration of CCCP leads to an asynchronous and heterogeneous time response.
An analysis of the temperature traces allowed us to obtain the
values per dish for a variety of mice (
Figure 4a). The mean thermal response of mitochondria for M1–M4 lies at the level of
11 °C though individual traces show an outstanding temperature breakthrough of 15–22 °C. In contrast, traces for M5 and M6 demonstrate smaller amplitude dynamics with mean
1 °C. The possible explanation of this behavior is based on the difference between mitochondria isolation media. The sucrose- and mannitol-containing medium with EGTA and BSA is more favorable to mitochondrial physiological conditioning during the isolation period due to avoidance of excessive K
+ and phosphate load, Ca
2+ ions chelation, and free lipids absorbance, which altogether leads to a higher yield of intact mitochondria with high metabolic capabilities.
Figure 4b demonstrates the alteration of
in time for M1 and M2 with an apparent decrease for both which is obviously due to mitochondria degradation as the time passed. However, the attenuation rate for M1 is slightly faster than that for M2. So, at 90 min thermal response the amplitude reduces two times for M1 and only 1.3 times for M2. This dissimilarity may be determined by many factors including age and physiological condition of mice or slight differences during isolation, especially at the homogenization phase.
Ultimately, we delve into the kinetics of thermal responses for M1–M4 mice (
Figure 4c). Each burst in temperature traces presented in
Figure 3, either spontaneous or CCCP-stimulated, was carefully treated in order to evaluate temperature amplitude
in accordance with its duration determined as full-width at half-maximum (FWHM). The set of points calculated for each mouse is shown in
Figure 4c. The dependence clearly illustrates the saturation nature of thermal bursts and therefore was fitted (black curve) with
, where
is a temperature long-term limit,
is the saturation duration. An approximation gives
°C and
s implying the principal amplitude of temperature bursts ceases to increase significantly at the duration of ~30 s. Apparently, this behavior implies that the resource of potential energy accumulated in mitochondria is limited and not enough to produce heat permanently.
5. Conclusions
Production of heat by mitochondria is critical for maintaining body temperature, regulating metabolic rate, and preventing oxidative damage to mitochondria and cells. Until now, mitochondrial heat production was measured only by fluorescent probes which are sensitive to environmental variations. Here, the temperature of isolated mitochondria was unambiguously measured by a diamond thermometer that is absolutely indifferent to external non-thermal parameters. Under uncoupling of transmembrane potential by CCCP application, the temperature near the mitochondria rises by 4–22 °C above the ambient with an absolute maximum of 45 °C. Such a broad variation in the temperature response is associated with the heterogeneity of the mitochondria themselves as well as their aggregations in the isolated suspension. Spontaneous temperature bursts with the comparable amplitude were also detected prior to CCCP application which can reflect involvement of some mitochondria to ATP synthesis or membrane potential leaking to avoid hyperproduction of reactive oxygen species.
The proposed diamond temperature sensor as a direct measurement instrument and the data obtained can greatly advance studies of thermodynamics at the subcellular level. In addition to fundamental applications, the new methodological approach may finally reveal the unclear mechanisms underlying temperature dysregulation in normal conditions and pathologies such as those of the central nervous system, which directly depend on the functional state of mitochondria.